1. Introduction
Sugarcane (
Saccharum spp.) is a major C
4 grass cultivated worldwide, historically valued for its exceptional capacity to accumulate sucrose in the stem. Conventional improvement and agronomy have therefore prioritised sugar content and milling performance, typically using long cropping cycles (12–16 months) that allow internodes (the stem segments between nodes) to complete elongation and transition into sucrose storage tissues. Decades of physiological work describe how maturing internodes lose water as the sucrose concentration rises, and how these changes track developmental gradients and vascular architecture within the culm [
1,
2,
3]. However, much of this foundational knowledge derives from a limited set of commercial genotypes under relatively narrow growing conditions. Systematic comparisons across genetically diverse germplasm and contrasting environments remain uncommon, leaving important gaps in our understanding of how development, genotype, and environment jointly shape stem composition.
In parallel, the global context for sugarcane is changing. Climate variability, land and water constraints, and the need for low-emission energy systems are driving interest in crops that deliver both food and renewable materials. Beyond sucrose, sugarcane produces large quantities of lignocellulosic biomass (the fibrous fraction of the stem and leaves) that can serve as a feedstock for second-generation biofuels and an expanding range of bio-based products. Unlocking this value requires a shift in emphasis from viewing sucrose as the sole product to managing the crop as a dual-purpose or even biomass-first system in which total stem yield and biomass quality are co-optimised.
A central determinant of biomass quality for biochemical conversion is digestibility: the ease with which structural carbohydrates in the plant cell wall can be deconstructed to release fermentable sugars. The cell wall is primarily composed of cellulose (a glucose polymer, often referred to as glucan), hemicelluloses such as xylans, and lignin, an aromatic polymer that imparts rigidity and hydrophobicity. During saccharification, pretreatments and enzymes must access cellulose microfibrils; anything that restricts access or resists chemical/enzymatic attack reduces sugar release. Among various compositional indicators, the ratio of glucan to the combined content of lignin and xylan (Glucan/(Lignin + Xylan)) has emerged as a practical proxy for theoretical digestibility [
4]. Conceptually, a higher ratio implies a wall richer in cellulose, the substrate for sugar release, and relatively lower amounts of lignin and xylan. Lignin obstructs enzymes physically and non-productively binds them; xylans form cross-links and can mask cellulose surfaces. While such a ratio does not capture all features relevant to recalcitrance (e.g., cellulose crystallinity, lignin monomer composition, ether vs. ester linkages, or ferulate cross-linking), it condenses major compositional drivers into a single, interpretable metric that correlates with saccharification performance across many grasses.
Evidence supporting this logic is accumulating in sugarcane and related C
4 species. Sugarcane hybrids with reduced lignin and elevated glucan often show improved enzymatic digestibility; lines with altered lignin content can achieve substantially higher glucose conversion under standard treatments [
5]. Comparable trends have been reported in maize (
Zea mays) and switchgrass (
Panicum virgatum), where the absolute and relative amounts of lignin and xylan, as well as their interactions with cellulose, strongly influence recalcitrance [
6,
7]. Importantly, quantitative genetic studies indicate that sugar traits and lignin biosynthesis can be at least partly decoupled, suggesting the feasibility of breeding ideotypes that retain desirable sugar or biomass yields while achieving better digestibility.
Technological advances further elevate the importance of composition. Modern pretreatments, including ionic liquid, hot water, and related thermochemical approaches, improve enzyme access to cellulose, but their efficiency and cost are still influenced by the starting wall chemistry [
4,
8,
9]. Feedstocks with higher glucan and lower lignin/xylan often require milder conditions or less enzyme to reach target conversions, improving overall process economics and environmental footprints. Consequently, agronomic and breeding decisions that shift composition in favourable directions can have outsize downstream benefits in biorefineries.
Despite this potential, the seasonal dynamics of sugarcane cell wall composition remain sparsely characterised at the population level, especially across diverse genotypes and environments. The prevailing production paradigm, which extends the crop to maximize sucrose, does not necessarily align with biomass quality goals. Many reports note that stem digestibility declines with age, broadly coinciding with increased lignification and hemicellulose deposition as tissues mature. This observation raises a critical question: could shorter growth cycles, aligned with vegetative peak biomass, deliver improved digestibility (and, thus, higher convertible sugar per unit time and land area), while maintaining or even increasing annualised biomass yields? Framed differently, the optimal harvest window for sucrose extraction may differ from the window that maximises lignocellulosic quality.
Addressing these questions demands a holistic view of source–sink physiology, development, and environment. As internodes transition from rapid elongation to storage, carbon allocation patterns shift among structural growth, wall thickening, and soluble sugar accumulation. Temperature, water status, and nutrient supply modulate these trajectories, and genotypes differ in both the timing and magnitude of these processes. The result is a moving target: the same genotype may present very different wall chemistries at inland versus coastal sites, or during mid-season vegetative growth versus late-season sucrose storage. Interactions among the genotype (G), environment (E), and developmental stage (S) are therefore expected, and they matter directly for both biomass quality and harvest planning.
Within this broader context, the Glucan/(Lignin + Xylan) ratio serves two complementary roles in the present work. First, it is a screening index that enables fair, interpretable comparisons of digestibility potential across many genotypes, sites, and sampling times without requiring full saccharification assays for every sample. Second, it is a biological lens: shifts in the ratio can be traced back to developmental or environmental drivers (e.g., accelerated lignification under water stress, or delayed wall thickening under cooler conditions), providing mechanistic insight that can guide selection and management. We emphasize that this ratio is not a substitute for direct conversion testing, nor does it capture all dimensions of recalcitrance; rather, it complements process-level measurements by focusing on the major compositional levers that breeders and agronomists can manipulate.
In sugarcane improvement programs, practical constraints also matter. Breeders routinely evaluate large numbers of families across multiple sites, and trait measurements must be robust, scalable, and meaningful for decisions. Incorporating biomass quality metrics that are both informative and field-deployable would allow selection for dual-purpose ideotypes, e.g., lines that either (i) deliver high sucrose without excessively compromising wall digestibility, or (ii) prioritize biomass yield and quality for second-generation products, potentially on shorter cycles or in marginal environments. From a systems perspective, this diversification could stabilise grower income and enhance the value captured from each hectare by linking sugar and fibre markets.
In this study, 17 sugarcane genotypes ranging in sucrose were grown at two contrasting environments (inland and coastal) and analysed at two developmental stages: maximum vegetative growth and peak sucrose accumulation. The aims were to assess how seasonal development affects cell wall composition and digestibility. In addition, to determine whether shorter growth cycles can improve biomass quality in terms of digestibility and yield traits. This integrated approach bridges classical physiology with modern compositional analytics, providing a novel framework for evaluating sugarcane as a multi-use crop that can contribute simultaneously to food, fuel, and fibre value chains.
3. Results and Discussion
3.1. Environmental Conditions and Growth
There was a notable contrast between the two locations in terms of their daily minimum temperatures. The Tablelands site had lower night temperatures compared to the Mossman site, which consequently led to a faster accumulation of heat units at the latter (
Figure S1B,D). However, the accumulation of photosynthetic active radiation remained comparable between the two research sites, as shown in
Figure S1A,C.
Sugarcane height and biomass do not increase in a linear fashion. Instead, growth can be best modelled by the application of a four-parameter logistic (sigmoidal S-curve) function. Growth of the genotypes (expressed as increase in height) is presented in (
Figure S2A). The genotypes differ significantly in the time point where the maximum growth rate is achieved (
Figure S2B). The maximum biomass accumulation rate is reached between 4.5 and 6 months after planting (1050–1250 GDD
18).
3.2. Water and Sucrose Content
A two-way ANOVA was conducted to evaluate the influence of genotype, crop age, and location on moisture content in sugarcane culms (
Table S1). Results revealed that genotype had a highly significant effect (
), confirming inherent genetic variation in water retention capacity among sugarcane cultivars. Crop age also had a significant impact: mature stems (12 months) consistently showed lower moisture content than younger stems (6 months), which was a trend observable across both trial locations (
Figure 1).
This age-dependent decline in stem moisture content is a well-documented physiological transition in sugarcane and other Poaceae. During the early growth phase, sugarcane internodes expanded, were metabolically active, and maintained a high water content (>85% FW) to support turgor-driven elongation and nutrient transport [
1,
3]. As internodes mature, cell wall stiffening and cessation of growth lead to increased turgor pressure (
), which in turn limits further water influx despite continued sugar accumulation and increasingly negative osmotic potential (
).
Significant age effects align with physiological transitions from juvenile to mature tissues, where lignification and reduced cellular hydration are expected.
This apparent paradox (lower water content despite higher sugar concentration) can be explained by changes in the total water potential (
) and a reduction in the driving gradient for water uptake. Furthermore, sugar accumulation physically displaces water in the storage parenchyma, leading to a relative reduction in water content on a dry-weight basis [
2,
23].
In parallel, sucrose content showed an inverse trend. Older internodes (12 months) consistently had higher sucrose concentrations (
Figure 2), and the ANOVA results (
Table S2) highlighted strong effects of genotype, location, and all interaction terms. This supports the physiological observation that as sugar accumulates, water content declines, reflecting the shift from water-rich, expanding tissues to sugar-rich, mature storage tissues [
2,
23].
3.3. Sucrose, Fibre, and Moisture Interaction
The relative contributions of sucrose (DW%), fibre (DW%), and moisture (FW%) for the panel of genotypes grown at two locations (Mossman and Tablelands) were compared. Two complementary visualisations are shown: (i) ternary plots summarising median values per genotype (
Figure S3), and (ii) interaction plots showing genotype traces for each component with a shaded ± SD band (
Figure 3). Young and mature age groups were not separated for this analysis.
The shaded bands (±SD) around the genotype traces capture replicate/within-genotype variability. Moisture (FW%) exhibits relatively small and smooth bands within each location, whereas the sucrose (DW%) bands are wider for a subset of genotypes, especially at Tablelands, indicating greater dispersion for sucrose than for moisture. Fibre (DW%) shows intermediate variability.
Across most genotypes, Tablelands exhibited higher sucrose (DW%) and lower moisture (FW%) than Mossman, with fibre (DW%) tending to be lower at Tablelands. This shift is evident in the ternary panels as a displacement of Tablelands points toward the sucrose vertex and away from the moisture vertex relative to Mossman (
Figure S3)). The data in the interaction plots illustrate that the sucrose content of the cane for Tablelands are higher than that at Mossamn, while moisture levels are higher at Mossman than at Tablelands (
Figure 3).
Although the location trends are consistent on average, several genotypes show clear interactions, i.e., differences in response magnitude and occasional rank changes between locations. For sucrose (DW%), some genotypes express a pronounced elevation at Tablelands whereas others show only a minor shift; similar heterogeneity is visible for fibre. These crossovers indicate that the effect of location is not uniform across the genetic backgrounds and that relative genotype rankings for sucrose and fibre are depended on location.
The clustering of the data in the ternary plots (
Figure S3) reflect a tight range in sucrose and fibre with moderate variation in moisture. The Tablelands cluster is shifted along the sucrose–moisture tradeoff axis compared with Mossman, consistent with the interaction plots. Note that the ternary representation is conceptual: sucrose and fibre are expressed on a dry-weight basis, whereas moisture is on a fresh-weight basis; values are normalised for plotting, so the triangle reflects relative positioning rather than a closed mass balance.
Environmental Modulation of Carbon Partitioning
The higher sucrose (DW%) accompanied by slightly lower moisture (FW%) at Tablelands suggests a shift toward reserve accumulation and away from water content, consistent with environments that favour sucrose storage (e.g., cooler nights or lower humidity) and reduce tissue water content. The small reduction or stability in fibre (DW%) implies that location effects preferentially modulate soluble carbohydrate storage and tissue hydration rather than strongly altering structural biomass in this sampling frame.
The non-parallel genotype traces across locations (crossovers) indicate meaningful G × E for sucrose and, to a lesser extent, fibre. Some genotypes are more location-responsive (large sucrose gain at Tablelands; steeper moisture decline), whereas others remain comparatively stable. Such plasticity is valuable for selection: genotypes with consistently high sucrose across locations are broadly adapted; genotypes with a strong positive response at Tablelands may be specifically adapted to those conditions but less stable elsewhere.
The observed location main effects and G × E interactions underscore the necessity of multi-environment testing when targeting high sugar yield and acceptable fibre. For breeding, emphasising genotypes that combine (i) high sucrose at both locations and (ii) narrow SD (stable performance) will improve predictability. For agronomy, the displacement in the ternary space at Tablelands points to management opportunities (e.g., practices that enhance maturation cues) to nudge partitioning toward sucrose while avoiding undesirable increases in fibre.
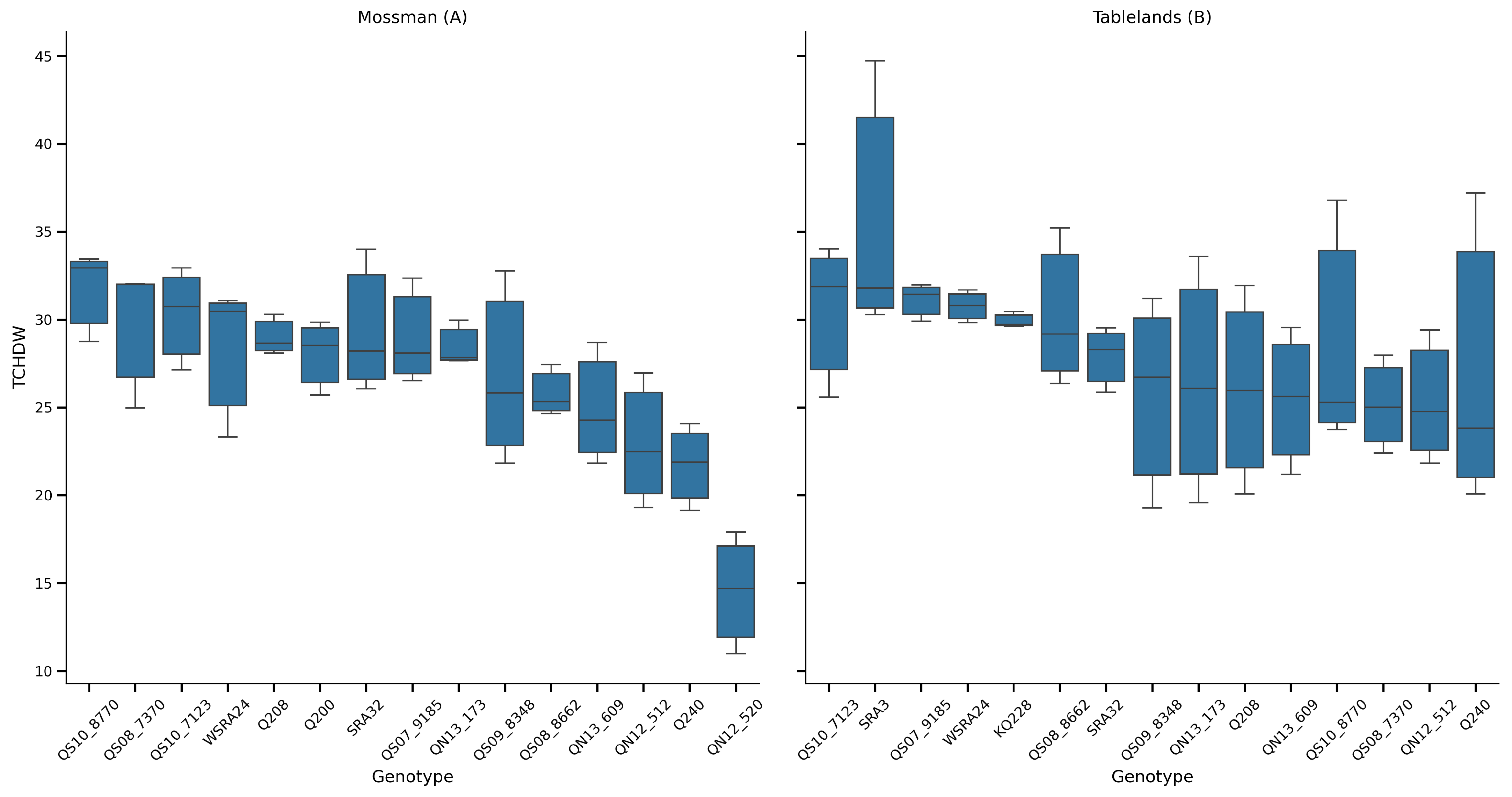
3.4. Biomass Yield
Although it is customary to express sugarcane yield as TCH, the significant variation in moisture content not only at growth stage but also between genotypes can provide misleading information on the true amount of biomass available for processing. As the purpose of this study is focused on the biomass production potential, all yield data have been converted and yield data are expressed as the tonne dry weight of cane per hectare (TCHDW) (
Figure 4).
A two-way ANOVA was conducted to assess the effects of genotype, cycle, and location on TCHDW (
Table 1). The analysis revealed that genotype (G) and cycle (C) had highly significant effects on TCHDW (
p < 0.001), and their interaction (G × C) was also statistically significant (
p = 0.036), suggesting some genotype-specific sensitivity to seasonal conditions. Location effects, when assessed via a genotype × location model, were also highly significant (
p < 0.001), indicating clear environmental influences on trait expression. The residual variance remained comparatively low, confirming good model fit.
3.5. Fibre Content and Yield
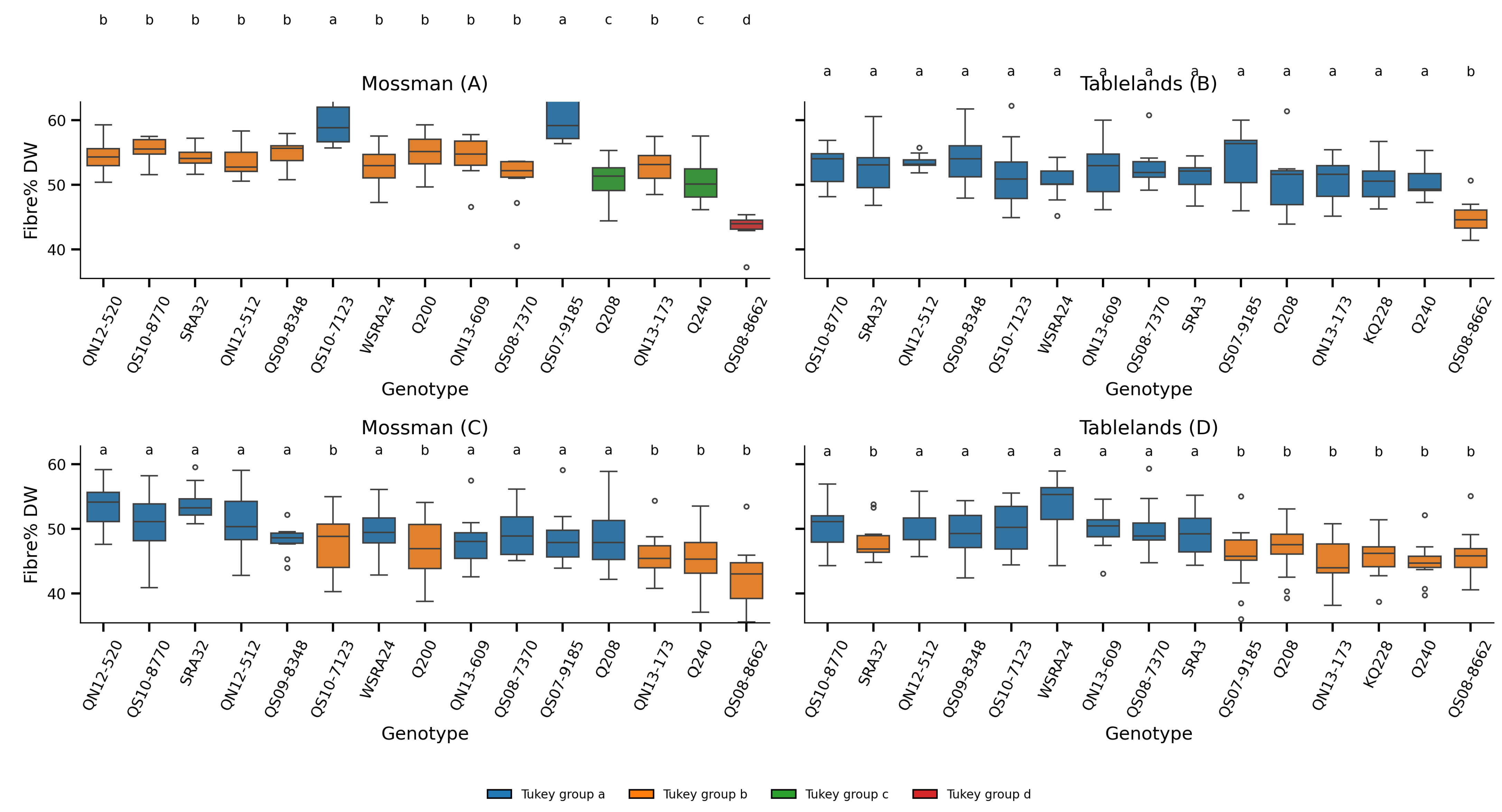
The fibre content in sugarcane genotypes was examined across two locations (Mossman and Tablelands) and developmental ages (young and mature). A combination of two-way ANOVA and post hoc Tukey HSD tests was used to assess main effects and interactions. Boxplots were then used to visualise genotype-specific differences and significance groupings under each condition.
A two-way ANOVA revealed a strong and highly significant effect of genotype on fibre content, expressed as a percentage of total dry weight (F = 231.04,
p < 0.001;
Table S3). Across both cropping cycles and production areas, genotypic differences accounted for a substantial proportion of the observed variance. This was further confirmed by the Tukey HSD test, which showed distinct groupings among genotypes (
Figure 5). High-fibre genotypes such as QS97-9185 and QS07-2122 consistently ranked among the top performers, while others, such as QS08-8682, exhibited significantly lower fibre content.
These results indicate that fibre content is under strong genetic control, with consistent differences between genotypes across environments. Similar findings have been reported in sugarcane and other grasses, where fibre traits are known to be highly heritable and amenable to selection [
24,
25,
26].
The cropping cycle (plant vs ratoon) had no significant effect on the fibre content (p = 0.567), and no significant interaction was detected between genotypes and cycles (p = 0.274). This suggests that fibre content remains stable between the plant and ratoon crops, and that genotypic rankings are largely preserved across cycles. Such stability is advantageous for breeding programs, as it reduces the need to evaluate each genotype in multiple crop cycles to confirm trait performance.
In contrast, the location and developmental stage (age) had significant effects on fibre content (
), as did their interactions with genotype. These findings indicate that while the genotype remains a dominant driver, environmental conditions and tissue maturity modulate fibre content in a genotype-dependent manner. Such genotype × environment (G × E) interactions are well-documented in sugarcane [
25,
27] and highlight the importance of multi-environment testing in breeding programs targeting fibre traits.
The boxplots (
Figure 5C,D) reveal a general trend toward lower fibre content in mature tissues, particularly in later harvest stages. This finding is somewhat counter-intuitive, given that cell wall lignification and pentose deposition typically increase as tissues mature [
28]. However, this apparent paradox may be explained by the disproportionate accumulation of sucrose during the later stages of internode development.
Once internode elongation ceases, sugarcane tissues continue to accumulate biomass, primarily in the form of sucrose, at a much faster rate than cell wall components [
2,
23]. As a result, the proportion of fibre in the dry matter declines, even though the absolute amount of fibre may continue to rise. This suggests that cell wall biosynthesis slows post-elongation, whereas sucrose loading and storage accelerate.
From a breeding perspective, these results emphasise the need to consider both the developmental stage and environmental context when selecting fibre traits. Genotypes like QS97-9185, which maintain high fibre content across stages and environments, may be valuable for bioenergy applications, where lignocellulosic biomass is the target. Conversely, genotypes with low fibre content and high sucrose accumulation in mature tissues, such as QS08-8682, are better suited for conventional sugar production.
Furthermore, the significant G × Age interaction highlights the plasticity of fibre content during development, implying that selection strategies should carefully define the target harvest window. For dual-purpose applications, where both sugar and fibre are valued, intermediate genotypes may offer a balanced solution.
3.6. Fibre Composition
The lignocellulosic biomass (fibre) constitutes the structural component of the sugarcane plant cell walls and comprises mainly cellulose, hemicellulose, and lignin. In grasses like sugarcane, maize, and sorghum, the digestibility of this fraction significantly affects the efficiency of ruminant nutrition and bioethanol conversion processes. Sugarcane is especially important in tropical regions as both a sugar and bioenergy crop, and its fibre digestibility varies substantially across genotypes.
Generally, the sugarcane cell wall composition is described in terms of cellulose (29–44%), hemicellulose (27–30%), lignin (19–43%), and pectins (which include uronic acids at approximately 10%). The hemicellulose fraction of sugarcane is primarily composed of hexosans (polysaccharides made up of hexose sugars like glucose and mannose), pentosans (polysaccharides composed of pentose sugars such as xylose and arabinose), and uronic acids.
Sugarcane hemicellulose primarily consists of glucuronoarabinoxylan (GAX), a complex polysaccharide with a backbone of β-1, 4-linked xylose residues. This backbone is variably substituted with side chains of arabinose and glucuronic acid, among other components. The ratio of these substituents, particularly arabinose to glucuronic acid, influences the structural properties and digestibility of the hemicellulose. The contribution of the major compounds in the lignocellulosic fraction in both young and mature sugarcane from both locations were determined (
Table S4).
Figure 6 and
Figure 7 summarise the genotype-wise distributions of the major cell-wall constituents (glucan, xylan, lignin, and uronic acids) for plant cane at Mossman and Tablelands, which are shown separately for mature and young tissue. Across genotypes, a consistent location shift is evident: Tablelands exhibits higher glucan and xylan and slightly lower uronic acids than Mossman, whereas lignin shows smaller between-location differences. Variation among genotypes is widest for glucan and xylan and narrowest for uronic acids, indicating that most compositional diversity resides in the polysaccharide fraction. Comparing age classes, glucan medians are broadly similar between young and mature tissue, xylan is generally higher in mature tissue, and lignin increases markedly with maturity. Genotype rankings are largely conserved across locations, but several cross-overs are visible, suggesting location-dependent differences in partitioning for some genotypes.
There are varietal and maturity influences on the hemicellulose composition of sugarcane.
An analysis of variance (ANOVA) was performed to assess the influence of location, growth cycle, and their interaction on the concentration of major cell wall components, i.e., glucan, xylan, lignin, and uronic acids in sugarcane. A summary of the results is presented in
Table 2.
Glucan content was significantly affected by both location (F = 40.5,
) and the interaction between location and age (F = 20.1,
). However, the growth cycle (age) alone did not significantly influence glucan levels (
p = 0.2157). These results suggest that environmental conditions between the two locations (Tablelands and Mossman) strongly influence glucan deposition, and that this effect is further modulated by developmental stage. Previous studies have demonstrated that environmental factors such as temperature, soil type, and rainfall can significantly affect cellulose biosynthesis in sugarcane and other C4 grasses [
29,
30,
31].
For xylan, significant effects were observed for location (F = 94.3,
), age (F = 17.8,
), and their interaction (F = 4.7,
p = 0.0304). These findings indicate that hemicellulose content is influenced by both spatial and temporal factors, with evidence of interaction effects implying genotype–environment interplay. Xylan variation was linked to differences in developmental regulation and environmental plasticity, affecting digestibility and cell wall architecture [
32].
Lignin accumulation was significantly influenced by location (F = 9.7,
) and age (F = 82.5,
), but not by their interaction. This result aligns with prior findings that lignin biosynthesis is largely developmentally regulated but can also be modified by environmental stressors such as temperature and water availability [
33]. The strong age effect suggests a marked increase in lignification during stem maturation, which is critical in determining forage quality and biomass recalcitrance.
Uronic acid content was significantly affected by all factors: location, age, and their interaction (
Table 2), indicating highly dynamic regulation. Uronic acids, primarily galacturonic and glucuronic acids, contribute to pectin and hemicellulose structure and are involved in cross-linking of cell wall polysaccharides. The pronounced interaction effect indicates that different locations and growth stages can cause significant shifts in uronic acid levels, possibly through differential activation of pectin-modifying enzymes.
Understanding the variation in cell wall components is crucial for improving sugarcane biomass digestibility for bioenergy. Cell wall traits such as low lignin and optimised polysaccharide content enhance saccharification efficiency [
34]. The observed environmental and developmental effects underscore the importance of considering genotype × environment interactions in breeding programs aimed at producing sugarcane varieties tailored for cell wall-based bioenergy applications.
3.7. Digestibility
Under enzymatic saccharification aimed at fermentable C
6 sugars, the glucose-releasing potential of a lignocellulosic sample scales with its cellulose (glucan)content, whereas conversion is impeded by the surrounding matrix of hemicellulose (predominantly xylan) and lignin. Lignin is non-carbohydrate and strongly recalcitrant; it both sterically shields cellulose microfibrils and sequesters enzymes via non-productive adsorption, reducing effective cellulase activity. Xylan also limits hydrolysis by occluding cellulose surfaces, lowering porosity and accessibility; depending on pretreatment severity and process design, much of this C
5 mass either requires separate valorisation or behaves as an accessibility barrier during cellulase action. Accordingly, a simple, dimensionless indicator of theoretical ease of conversion is
where all terms are mass fractions (g g
−1 DW). This ratio increases when cellulose (the primary enzymatic substrate for glucose release) is high and when the recalcitrant matrix (xylan + lignin) is low, thereby capturing the opposing influences of substrate and barrier in a single index.
Lignin typically exerts the dominant negative effect on enzymatic hydrolysis across grasses, while the contribution of xylan can vary with pretreatment; xylan in the denominator nevertheless retains the accessibility penalty it imposes under mild–moderate conditions. The proxy is thus most informative for biochemical routes prioritising C6 sugar release after standard pretreatments; if a process explicitly valorises C5 streams or achieves extensive delignification, alternative indices (e.g., Glucan/Lignin, or accessibility-based metrics) may be preferred. This study uses the proxy for transparent, genotype-to-genotype comparisons; it does not replace direct saccharification/bioconversion assays.
We retain xylan in the denominator of the Glucan/(Xylan + Lignin) ratio to reflect that hemicelluloses, while generally more labile than lignin, contributes to matrix encapsulation and mass that does not convert to C6 sugars. Nevertheless, lignin exerts the dominant negative influence on bioconversion; thus, genotypes with rising lignin during maturation show the largest declines in proxy. This framing aligns with prior reports that reducing lignin content and/or altering monolignol composition is more impactful for saccharification than marginal shifts in xylan, even though xylan-rich fractions can be valorised to C5 platforms under dedicated conditions.
Digestibility, calculated as the ratio of glucan to the sum of xylan and lignin (digestibility = glucan/[xylan + lignin]), revealed marked genotypic variation across sugarcane genotypes and developmental stages (
Figure 8). The ANOVA (
Table 2) confirmed the highly significant effect of genotype on digestibility across all tested combinations (
), indicating that genetic background is a key determinant of fibre composition and, consequently, the potential for enzymatic degradation.
In all panels of
Figure 8, which represent different combinations of the location and developmental stage (Mossman and Tablelands at both young and mature stages), boxplots are ordered by mean fibre content, with noticeable shifts in genotype rankings. Several genotypes consistently exhibited higher digestibility values, particularly in the young stages (
Figure 8a,b) which corresponds with the reduced lignification and xylan accumulation known to occur earlier in internode development [
35,
36].
The statistical analysis also demonstrated a significant interaction between genotype and age (
), emphasising that the response of fibre digestibility to maturation differs by genotype. This interaction was visually apparent in the contrasting rankings between panels A/B (young) and C/D (mature). For instance, some genotypes ranked highly at early stages but showed significant reductions in digestibility upon maturation, likely due to increased lignin deposition during cell wall thickening [
4].
Digestibility decreases with maturity due to increased lignin deposition, especially in the rind compared to the pith of sugarcane internodes [
37]. Early harvested material often shows better digestibility profiles.
While genotype had a pronounced effect, the production location (Mossman vs. Tablelands) showed no significant main effect on digestibility (p = 0.135), although the genotype–location interaction approached significance of p = 0.001. This suggests that the environmental modulation of digestibility traits is complex and may depend on specific genotype responses rather than broad location-based effects.
A consistent trend was observed where digestibility declined with plant age (
), supporting the notion that fibre becomes more recalcitrant as internodal tissues mature. The significant genotype × age interaction (
) underlines the importance of evaluating fibre traits at different developmental stages to fully capture genotypic potential. This has practical implications for breeding programs targeting bioenergy applications, as genotypes maintaining higher digestibility at maturity would be particularly valuable for reducing preprocessing requirements in lignocellulosic biomass conversion [
34,
38].
The Tukey HSD test further delineated groupings within each panel (
Figure 6), identifying genotypes that were not significantly different at
p = 0.05. These groupings offer practical value for identifying statistically robust top performers within each developmental and environmental context. Notably, some genotypes consistently clustered among the most digestible across multiple panels, indicating stable expression of favourable cell wall composition traits.
3.8. Sustainability Implications
This work contributes to global sustainability efforts under the UN Sustainable Development Goals, particularly goals related to clean energy (SDG 7) and climate action (SDG 13), by suggesting pathways to optimize sugarcane for bioenergy production.
Shorter cropping cycles can reduce input demands (e.g., irrigation and fertiliser) while enhancing land-use efficiency.
Dual-purpose genotypes support diversified end uses (sugar, fibre, and bioenergy), enhancing economic resilience for growers and processors.
Improved digestibility translates to reduced pretreatment energy and chemical inputs in biorefineries, lowering environmental impacts.
Seasonal harvesting strategies can facilitate the year-round biomass supply, stabilising the feedstock availability for biorefineries and minimising the idle infrastructure time.
These factors align with broader goals of climate mitigation, sustainable intensification, and the development of circular, bio-based economies.
4. Conclusions
This multi-site, 17-genotype assessment study provides several evidence-based patterns that directly inform varietal choice, harvest timing, and end-use positioning. ANOVA showed strong genotype effects for sucrose %DW, fibre %DW, and digestibility, with weaker and less consistent effects of cropping cycle and interactions. This supports selection concerning lignocellulose quality within breeding pipelines, which is typical of the sugarcane cropping cycle optimised for sugar yield, as the moisture content decreased with development as sucrose %DW increased. A long cropping cycle is therefore a necessity to optimise sucrose yields. The Glucan/(Xylan + Lignin) proxy declined from young to mature cane in both production environments, primarily tracking increases in lignin with age. Accumulating fibre mass does not translate to higher fermentable yield when the lignin fraction increases; the quality penalty offsets quantity gains. Thus, although biomass and fibre continues to increase during a long cropping cycle, cell-wall recalcitrance also rises. Several genotypes maintained above-median sucrose while avoiding the lowest digestibility values across sites and stages in the cropping cycle. These are immediate candidates for dual-use contexts (sugar + bioenergy), whereas fibre-rich, lower-sucrose lines better suit dedicated bioenergy.The integration of physiological, agronomic, and compositional analyses supports the feasibility of selecting sugarcane genotypes optimised for both high biomass yield and improved digestibility. For sugar markets, later harvests leverage higher sucrose %DW but coincide with lower theoretical digestibility; for bioenergy, earlier harvests or genotypes with slower lignification offer more tractable feedstock. These findings highlight the potential of sugarcane as a dual-purpose crop for sugar and bioenergy, particularly when grown under shortened cycles to capitalise on peak digestibility windows. The results reinforce the strategic potential of sugarcane as a sustainable dual-purpose crop that can contribute to a circular bioeconomy. By optimising harvest timing and selecting genotypes with improved fibre digestibility at earlier growth stages, it is possible to increase biomass yield per unit time, reduce processing inputs, and extend the operational window of biorefineries. These findings support the diversification of sugarcane value chains toward more resilient and climate-smart agriculture systems.