Creating a National Coral-Focused Climate Change Adaptation Plan for Fiji to Prevent Coral Species Extinction in the Face of Rapid Climate Change: Applying the UNESCO-Endorsed “Reefs of Hope” Ocean Decade Action
Abstract
1. Introduction
1.1. The Immense Value of Coral Reefs and Their Ongoing Loss
1.2. Buying Time for Coral Reefs
- Further loss of coral genetic diversity must be prevented by securing what remains.
- Sexual reproduction of rare and declining coral species must be restored and maintained.
- Restoration efforts must focus on and include heat-adapted, bleaching-resistant corals.
- Coral-focused work must support natural coral reef regeneration and adaptation processes.
1.3. The Need for National Coral-Focused Adaptation Plans
1.4. Promoting Coral-Focused Adaptation
1.5. Mainstreaming ROH Strategies into Existing Programs and Policies
1.6. Background Important to Developing an Adaptation Plan for Fiji’s Coral Reefs
1.7. Fiji’s Resilient Reefs in Peril
1.8. An Ideal Crucible for the Evolution of Thermal Tolerance in Corals
1.9. Breakthroughs in Facilitating Natural Processes of Adaptation and Recovery
2. Materials and Methods
2.1. Reefs Hope Operational Strategy
2.2. Nursery Design and Construction
2.3. Planting Corals to Table Nurseries
2.4. Regeneration Patch Design and Construction
3. Results and Discussion
3.1. Proof of Concept for Proactive Coral Rescue in the Face of a Marine Heat Wave
3.2. Challenges of Establishing Gene Bank Nurseries
3.3. Mass Coral Bleaching as a Major Selection Event for Bleaching-Resistant Corals
4. Developing a Fiji National Coral Reef Adaptation Plan
4.1. Phases of a Fiji-Wide National Coral Reef Adaptation Plan
4.1.1. Phase One: Coral Species Rescue and Stabilization
4.1.2. Phase Two: Restoring Sexual Reproduction
4.1.3. Phase Three: Facilitating Natural Recovery Processes
4.1.4. Phase Four: Additional Measures to Support Coral Reef Health
- Captive Breeding of Corals
- 2.
- Including Tridacnid Clams in the Coral Reef Adaptation Work
- 3.
- Coral-focused Measures to Adapt to Ocean Acidification
5. Recommendations Based on Lessons Learned
5.1. Repurposing Restoration Methods for Adaptation
5.2. Predator Removal and Control as a Facilitated Adaptation Strategy
5.3. Strategies for Collecting and Moving Corals
5.4. Guidance for Selecting Cooler Water Gene Bank Nursery Sites
6. Support for Implementation
6.1. Government Incentives and Policies Are Needed to Support Coral Reef Adaptation
6.2. Representative Estimated Budget
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drake, J.L.; Mass, T.; Stolarski, J.; Von Euw, S.; van de Schootbrugge, B.; Falkowski, P.G. How corals made rocks through the ages. Glob. Change Biol. 2020, 26, 31–53. [Google Scholar] [CrossRef]
- Barnett, J.; Jarillo, S.; Swearer, S.E.; Lovelock, C.E.; Pomeroy, A.; Konlechner, T.; Waters, E.; Morris, R.L.; Lowe, R. Nature-based solutions for atoll habitability. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210124. [Google Scholar] [CrossRef]
- Ferrario, F.; Beck, M.W.; Storlazzi, C.D.; Micheli, F.; Shepard, C.C.; Airoldi, L. The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 2014, 5, 3794. [Google Scholar] [CrossRef]
- Hasan, M.M.; Kabir, M.B.; Prodhan, M.S.R.; Anik, M.S.; Zubaer, A.; Hasan, M.R.; Sami, M.N. The Physical and Mechanical Properties of Coral Sand. Eur. J. Theor. Appl. Sci. 2024, 2, 313–337. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017, 4, 252954. [Google Scholar] [CrossRef]
- Spalding, M.; Burke, L.; Wood, S.A.; Ashpole, J.; Hutchison, J.; zu Ermgassen, P. Mapping the global value and distribution of coral reef tourism. Mar. Policy 2017, 82, 104–113. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Voolstra, C.R.; Peixoto, R.S.; Ferrier-Pagès, C. Mitigating the ecological collapse of coral reef ecosystems. EMBO Rep. 2023, 24, e56826. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Program. Why Are Coral Reefs Dying? 12 November 2021. Available online: https://www.unep.org/news-and-stories/story/why-are-coral-reefs-dying (accessed on 15 June 2025).
- Najeeb, S.; Khan, R.A.A.; Deng, X.; Wu, C. Drivers and consequences of degradation in tropical reef island ecosystems: Strategies for restoration and conservation. Front. Mar. Sci. 2025, 12, 1518701. [Google Scholar] [CrossRef]
- Mulà, C.; Bradshaw, C.J.A.; Cabeza, M.; Manca, F.; Montano, S.; Strona, G. Restoration cannot be scaled up globally to save reefs from loss and degradation. Nat. Ecol. Evol. 2025, 9, 822–832. [Google Scholar] [CrossRef]
- Leggat, W.P.; Camp, E.F.; Suggett, D.J.; Heron, S.F.; Fordyce, A.J.; Gardner, S.; Deakin, L.; Turner, M.; Beeching, L.J.; Kuzhiumparambil, U.; et al. Rapid Coral Decay Is Associated with Marine Heatwave Mortality Events on Reefs. Curr. Biol. 2019, 29, 2723–2730.e4. [Google Scholar] [CrossRef]
- Bowden-Kerby, A. Coral-focused climate change adaptation and restoration based on accelerating natural processes: Launching the “Reefs of Hope” paradigm. Oceans 2023, 4, 13–26. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- IPCC. Special Report on the Ocean and Cryosphere in a Changing Climate; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; Available online: https://www.ipcc.ch/srocc/ (accessed on 15 June 2025).
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. A Research Review of Interventions to Increase the Persistence and Resilience of Coral Reefs; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Bay, R.A.; Rose, N.H.; Logan, C.A.; Morikawa, M.K.; Palumbi, S.R. Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Proc. Natl. Acad. Sci. USA 2017, 116, 11042–11050. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.A.; Palumbi, S.R. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 2011, 30, 429–440. [Google Scholar] [CrossRef]
- Safaie, A.; Silbiger, N.J.; McClanahan, T.R.; Pawlak, G.; Barshis, D.J.; Hench, J.L.; Rogers, J.S.; Williams, G.J.; Davis, K.A. High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 2018, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Rose, N.H.; Bay, R.A.; Lopez, E.H.; Morikawa, M.K.; Ruiz-Jones, L.; Palumbi, S.R. Mechanisms of thermal tolerance in reef-building corals across a fine-grained environmental mosaic: Lessons from Ofu. American Samoa. Front. Mar. Sci. 2018, 5, 434. [Google Scholar] [CrossRef]
- Morikawa, M.K.; Palumbi, S.R. Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. USA 2019, 116, 10586–10591. [Google Scholar] [CrossRef]
- van Oppen, M.J.H.; Oliver, J.K.; Putnam, H.M.; Gates, R.D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef]
- Wood, S.; Stephen, J.; Pandolfi, J.M.; Palumbi, S.R. Potential for conservation via relocation: Spatial genetic structure of thermally tolerant corals and implications for assisted migration. Glob. Change Biol. 2023, 29, 999–1013. [Google Scholar]
- Reefs of Hope: Coral-Focused Climate Adaptation. Available online: https://oceandecade.org/actions/reefs-of-hope/ (accessed on 15 June 2025).
- Pears, R.J.; Devlin, M.; Hammerman, N.; Allen, C.E.; Vains, J.; Gibbs, M.; Karisa, J.; van Oppen, M.; Baker, A.; Al Sawalmih, A. CORDAP R&D Technology Roadmap on Managing the Ecological Risks of Coral Reef Interventions. CORDAP. 2024. Available online: https://cordap.org (accessed on 1 June 2025).
- Daily Sea Surface Temperature. Available online: https://climatereanalyzer.org/clim/sst_daily/. (accessed on 1 June 2025).
- Bowden-Kerby, A.; Romero, L.; Kirata, T. Chapter 17: Line Islands Case Study. In Active Coral Restoration: Techniques for a Changing Planet; Vaughn, D., Ed.; J. Ross Publishing: Plantation, FL, USA, 2021; 660p. [Google Scholar]
- NOAA. Unprecedented Marine Heatwave Impacts Florida Coral Reefs. National Oceanic and Atmospheric Administration Coral Reef Watch. 2023. Available online: https://coralreefwatch.noaa.gov (accessed on 20 June 2025).
- Lindsey, R. NOAA and Partners Race to Rescue Remaining Florida Corals from Historic Ocean Heat Wave. 2023. Available online: https://www.climate.gov/news-features/event-tracker/noaa-and-partners-race-rescue-remainig-florida-corals-historic-ocean (accessed on 20 June 2025).
- GCRMN. Unprecedented Marine Heat Wave on Caribbean Coral Reefs. 2023. Available online: https://gcrmn.net/2023/10/13/marine-heatwave-caribbean-coral/ (accessed on 20 June 2025).
- Florida Keys National Marine Sanctuary. 2023 Coral Rescue Operation Summary; NOAA Office of National Marine Sanctuaries: Silver Spring, MD, USA, 2023. [Google Scholar]
- AGRRA (Atlantic and Gulf Rapid Reef Assessment). Preliminary Report on Coral Mortality in the Caribbean Following 2023 Heatwaves. 2023. Available online: https://www.agrra.org (accessed on 20 June 2025).
- Hernández-Delgado, E.A.; Rodríguez-González, Y.M. Runaway Climate Across the Wider Caribbean and Eastern Tropical Pacific in the Anthropocene: Threats to Coral Reef Conservation, Restoration, and Social–Ecological Resilience. Atmosphere 2025, 16, 575. [Google Scholar] [CrossRef]
- IPCC. AR6 Synthesis Report: Climate Change 2023; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2023. [Google Scholar]
- Copernicus Climate Change Service. Global Climate Highlights 2023: Hottest Year on Record European Union, ECMWF. 2024. Available online: https://climate.copernicus.eu (accessed on 15 June 2025).
- Pacific Solutions to Save Our Ocean: An Integrated Ocean Science Programme Towards A Healthy Blue Pacific Continent to Sustain Future Generations. Available online: https://oceandecade.org/actions/pacific-solutions-to-save-our-ocean/. (accessed on 1 June 2025).
- Bowden-Kerby, A. Community-based Management of Coral Reefs: An Essential Requisite for Certification of Marine Aquarium Products Harvested from Reefs Under Customary Marine Tenure. In Marine Ornamental Species: Collection, Culture and Conservation; Cato, J., Brown, C., Eds.; Iowa State Press/Blackwell Scientific Publications: New York, NY, USA, 2003; Chapter 11; pp. 139–166. [Google Scholar]
- Govan, H.; Tawake, A.; Tabunakawai, K. Community-based marine resource management in the South Pacific. SPC Tradit. Mar. Resour. Manag. Knowl. Inf. Bull. 2009, 25, 11–23. [Google Scholar]
- Jupiter, S.D.; Cohen, P.J.; Weeks, R.; Tawake, A.; Govan, H. Locally-managed marine areas: Multiple objectives and diverse strategies. Pacific. Conserv. Biol. 2014, 20, 165–179. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Darling, E.S.; Maina, J.; Muthiga, N.A.; Beger, M.; Fox, H.E.; Graham, N.A.J.; McLeod, E.; Wilson, S.K.; Jupiter, S.D.; et al. Climate change and coral reef fisheries: Shared challenges, converging solutions. Proc. R. Soc. B 2021, 288, 20210927. [Google Scholar]
- Bowden-Kerby, A. Coral restoration for climate change adaptation in the South Pacific. In Coral Reef Restoration as a Strategy to Improve Ecosystem Services—A Guide to Coral Restoration Methods; Hein, M.Y., Interna-tional Coral Reef Initiative, Eds.; UNEP Report: Nairobi, Kenya, 2020; pp. 54–57. 60p. [Google Scholar]
- Lovell, E.R.; McLardy, C. Annotated checklist of the CITES-listed corals of Fiji with reference to Vanuatu, Tonga, Samoa and American Samoa. JNCC Rep. 2008, 415, 82. [Google Scholar]
- Seeto, J.; Baldwin, W.J. A Checklist of the Fishes of Fiji and a Bibliography of Fijian Fish; Division of Marine Studies Technical Report No. 2010/01; The University of the South Pacific: Suva, Fiji, 2010; p. 102. [Google Scholar]
- Eastwood, E.K.; López, E.H.; Drew, J.A. Population Connectivity Measures of Fishery-Targeted Coral Reef Species to Inform Marine Reserve Network Design in Fiji. Sci. Rep. 2016, 6, 19318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Harding, S.P.; Solandt, J.L.; Walker, R.C.; Walker, D.; Taylor, J.; Haycock, S.; Davis, M.T.; Raines, P. Reef Check Data Reveal Rapid Recovery from Coral Bleaching in the Mamanucas, Fiji. Silliman J. 2022, 44, 2. [Google Scholar]
- WCS 2020, Research Expedition to Assess Coral Reef Health and Recovery from Tropical Cyclone Winston. Available online: https://fiji.wcs.org/News-Room/ID/15026/ (accessed on 10 June 2025).
- Rowlands, G.; Comley, J.; Raines, P. The Coral Coast Viti Levu Fiji AMarine Resource Assessment; Coral Cay Conservation Ltd.: Surrey, UK, 2005; 124p. [Google Scholar]
- Bruckner, A.W. Global Reef Expedition: Lau Province, Fiji. Field Report; Khaled bin Sultan Living Oceans Foundation: Landover, MD, USA, 2014; 33p. [Google Scholar]
- Lequeux, C.; Dumas, P.; Andréfouët, S. Connectivity of coral reefs in the Fiji Islands: Insights from oceanographic modeling and implications for conservation. Mar. Pollut. Bull. 2018, 135, 684–697. [Google Scholar]
- Moritz, C.; Vii, J.; Lee Long, W.; Tamelander, J.; Thomassin, A.; Planes, S. (Eds.) Status and Trends of Coral Reefs of the Pacific; Global Coral Reef Monitoring Network, 2018. 218p. Available online: https://gcrmn.net/wp-content/uploads/2022/06/Status-and-Trends-of-Coral-Reefs-of-the-Pacific-2018.pdf (accessed on 1 June 2025).
- Chand, S.; Aung, T.; Rao, S. The 1997-98 El Niño and 1999 La Niña evolution of current around the Fiji Islands. South Pac. J. Nat. Sci. 2007, 2, 10–15. [Google Scholar] [CrossRef]
- Fiji. Available online: https://coralreefwatch.noaa.gov/data/vs/ts_figures/ts_multi_year/vs_ts_multiyr_fiji.png (accessed on 28 June 2025).
- Jamaica. Available online: https://coralreefwatch.noaa.gov/data/vs/ts_figures/ts_multi_year/vs_ts_multiyr_jamaica.png (accessed on 28 June 2025).
- Northern Line Islands Kiribati. Available online: https://coralreefwatch.noaa.gov/data/vs/ts_figures/ts_multi_year/vs_ts_multiyr_northern_line_islands.png (accessed on 28 June 2025).
- Mumby, P.J.; Sartori, G.; Buccheri, E.; Alessi, C.; Allan, H.; Doropoulos, C.; Rengiil, G.; Ricardo, G. Allee effects limit coral fertilization success. Proc. Natl. Acad. Sci. USA. 2024, 121, e2418314121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mellin, C.; Brown, S.; Cantin, N.; Klein-Salas, E.; Mouillot, D.; Heron, S.F.; Fordham, D.A. Cumulative risk of future bleaching for the world’s coral reefs. Sci. Adv. 2024, 10, eadn9660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoepf, V.; Stat, M.; Falter, J.L.; McCulloch, M.T. Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob. Change Biol. 2019, 25, 968–982. [Google Scholar]
- Brown, K.T.; Martynek, M.P.; Barott, K.L. Local habitat heterogeneity rivals regional differences in coral thermal tolerance. Coral Reefs 2024, 43, 571–585. [Google Scholar] [CrossRef]
- Klepac, C.N.; Barshis, D.J. Reduced thermal tolerance of massive coral species in a highly variable environment. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201379. [Google Scholar] [CrossRef]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Bourne, D.G.; Morrow, K.M.; Webster, N.S. Insights into the coral microbiome: Underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 2016, 70, 317–340. [Google Scholar] [CrossRef]
- Suggett, D.J.; Smith, D.J. Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Change Biol. 2020, 26, 68–79. [Google Scholar] [CrossRef]
- Apprill, A. The role of symbioses in the adaptation and resilience of coral reefs to environmental change. Annu. Rev. Mar. Sci. 2020, 12, 489–516. [Google Scholar] [CrossRef]
- Ziegler, M.; Arif, C.; Burt, J.A.; Dobretsov, S.; Roder, C.; LaJeunesse, T.C.; Voolstra, C.R. Biogeography and molecular diversity of coral symbionts in the genus Symbiodinium around the Arabian Peninsula. J. Biogeogr. 2017, 44, 674–686. [Google Scholar] [CrossRef]
- Rowan, R. Thermal adaptation in reef coral symbionts. Nature 2004, 430, 742. [Google Scholar] [CrossRef]
- Berkelmans, R.; van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 2006, 273, 2305–2312. [Google Scholar] [CrossRef]
- Cunning, R.; Silverstein, R.N.; Baker, A.C. Investigating the causes and consequences of symbiont shuffling in a multi-partner coral symbiosis under environmental stress. Proc. R. Soc. B 2015, 282, 20141725. [Google Scholar] [CrossRef]
- Barshis, D.J.; Ladner, J.T.; Oliver, T.A.; Seneca, F.O.; Traylor-Knowles, N.; Palumbi, S.R. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Middlebrook, R.; Anthony, K.R.N.; Hoegh-Guldberg, O. Thermal priming affects symbiont photosynthesis but does not alter bleaching susceptibility in Acropora millepora. J. Exp. Mar. Biol. Ecol. 2008, 357, 40–45. [Google Scholar] [CrossRef]
- Bay, R.A.; Palumbi, S.R. Multilocus adaptation associated with heat resistance in reef-building corals. Curr. Biol. 2014, 24, 2952–2956. [Google Scholar] [CrossRef]
- Dixon, G.B.; Davies, S.W.; Aglyamova, G.A.; Meyer, E.; Bay, L.K.; Matz, M.V. Genomic determinants of coral heat tolerance across latitudes. Science 2015, 348, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, V.; Carrion, S.A.; Pfeifer, S.M.; Naugle, M.; Dugal, L.; Bruyn, J.; McCulloch, M.T. Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 2019, 10, 4031. [Google Scholar] [CrossRef]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, C.D.; Meyer, E.; Matz, M.V. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol. Ecol. 2013, 22, 4322–4334. [Google Scholar] [CrossRef] [PubMed]
- Quigley, K.M.; Bay, L.K.; van Oppen, M.J.H. Selective breeding of corals provides insight into the genetic basis of thermal tolerance. Front. Mar. Sci. 2020, 7, 796. [Google Scholar]
- Quigley, K.M.; Warner, P.A.; Bay, L.K. The genetic architecture of inherited variation in thermal tolerance in the reef-building coral Acropora millepora. Mol. Ecol. 2019, 28, 3864–3880. [Google Scholar]
- Schwarz, J.A.; Brokstein, P.B.; Voolstra, C.; Coffroth, M.A. Late larval development and onset of symbiosis in the coral Acropora palmata. Biol. Bull. 1999, 196, 70–79. [Google Scholar] [CrossRef]
- Hirose, M.; Kinzie, R.A.; Hidaka, M. Vertical transmission of symbiotic dinoflagellates in the planulae of the coral Pocillopora damicornis. Zool. Sci. 2001, 18, 515–518. [Google Scholar]
- Apprill, A.; Marlow, H.Q.; Martindale, M.Q.; Rappé, M.S. The onset of microbial associations in the coral Pocillopora meandrina. ISME J. 2009, 3, 685–699. [Google Scholar] [CrossRef]
- Leite, D.C.A.; Salles, J.F.; Calderon, E.N.; Castro, C.B.; Bianchini, A.; Marques, J.A.; Parente, T.E.; Tsai, S.M.; Peixoto, R.S. Broadcast spawning coral species associate with specific bacterial communities during early developmental stages. Ecol. Indic. 2017, 81, 48–57. [Google Scholar]
- Putnam, H.M.; Mayfield, A.B.; Fan, T.Y.; Chen, C.S.; Gates, R.D. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Mar. Biol. 2012, 160, 2157–2173. [Google Scholar] [CrossRef]
- Voolstra, C.R.; Buitrago-López, C.; Perna, G.; Cárdenas, A.; Hume, B.C.C.; Rädecker, N.; Barshis, D.J. Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Change Biol. 2021, 27, 4307–4319. [Google Scholar] [CrossRef] [PubMed]
- Stat, M.; Gates, R.D. Clade D Symbiodinium in Scleractinian Corals: A “Nugget” of Hope, a Selfish Opportunist, an Ominous Sign, or All of the Above? J. Mar. Biol. 2011, 2011, 730715. [Google Scholar] [CrossRef]
- Barott, K.L.; Huffmyer, A.S.; Davidson, J.M.; Lenz, E.A.; Matsuda, S.B.; Hancock, J.R.; Innis, T.; Drury, C.; Putnam, H.M.; Gates, R.D. Coral bleaching response is unaltered by community composition in long-term warmed reefs. Proc. Natl. Acad. Sci. USA 2021, 118, e2025435118. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- NOAA. Record-Breaking Marine Heatwave Devastates Florida Coral Reefs; Coral Reef Watch; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2023. [Google Scholar]
- Thiem, H. The Future of Coral Restoration in the Florida Keys After Unprecedented Marine Heat Wave of 2023. 2024. Available online: https://www.climate.gov/news-features/event-tracker/future-coral-restoration-florida-keys-after-unprecedented-marine-heat (accessed on 15 June 2025).
- Vardi, T.; Williams, D.E.; Sandin, S.A.; Zgliczynski, B.J. Shifting paradigms in coral restoration: Building resilience in the age of climate change. Ecol. Appl. 2021, 31, e02315. [Google Scholar]
- Shearer, T.L.; Porto, I.; Zubillaga, A.L. Restoration of coral populations in light of genetic diversity estimates. Coral Reefs 2009, 28, 727–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kyeema Foundation and Corals for Conservation. Constructing Coral Nurseries. Available online: https://www.youtube.com/watch?v=DGeUrs5pJDA (accessed on 28 June 2025).
- BULA Reef. Available online: https://icriforum.org/bula-reef/ (accessed on 1 June 2025).
- BULA Reef First Anniversary World Ocean’s Day. 2025. Available online: https://www.youtube.com/watch?v=C9OmNWZEt94. (accessed on 12 June 2025).
- McDougall, A. Territorial Damselfishes as Coral Gardening Partners during a Bloom of the Cyanobacterium Lyngbya majuscula. Master’s Thesis, University of Miami, Coral Gables, FL, USA, 2020. [Google Scholar]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Environ. Microbiol. Rep. 2012, 4, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, M.J.A.; Marhaver, K.L.; Huijbers, C.M.; Nagelkerken, I.; Simpson, S.D. Coral larvae move toward reef sounds. PLoS ONE 2010, 5, e10660. [Google Scholar] [CrossRef]
- Lillis, A.; Eggleston, D.B.; Bohnenstiehl, D.R. Soundscape variation from a larval perspective: The role of habitat identity and temporal dynamics in a coral reef. Mar. Ecol. Prog. Ser. 2013, 472, 1–16. [Google Scholar]
- Mason, B.; Beard, M.; Miller, M.W. Coral larvae settle at a higher frequency on red surfaces. Coral Reefs 2011, 30, 667–676. [Google Scholar] [CrossRef]
- Erwin, P.M.; Szmant, A.M. Settlement induction of Acropora palmata planulae by a GLW-amide neuropeptide. Coral Reefs 2010, 29, 929–939. [Google Scholar] [CrossRef]
- Takahashi, T.; Hatta, M. The Importance of GLWamide Neuropeptides in Cnidarian Development and Physiology. J. Amino Acids 2011, 2011, 424501. [Google Scholar] [CrossRef]
- Pacific Coral Reef Action Plan. Available online: https://www.sprep.org/sites/default/files/30-SPREP-Meeting/Officials/Eng/WP-8.2.3_Att.1.rev_.1-Pacific_Coral_Reef_Action_Plan_Members_Endorsement.pdf (accessed on 1 June 2025).
- Coral Reef Rescue Initiative. Available online: https://coralreefrescueinitiative.org/ (accessed on 28 June 2025).
- Fiji Launches First National Hub for Coral Reef Conservation. Available online: https://icriforum.org/fiji-coral-hub/ (accessed on 28 June 2025).
- Morishima, S.Y.; Yamashita, H.; Ohara, S.; Nakamura, Y.; Quek, V.Z.; Yamauchi, M.; Koike, K.; Thuesen, E.V. Study on expelled but viable zooxanthellae from giant clams, with an emphasis on their potential as subsequent symbiont sources. PLoS ONE 2019, 14, e0220141. [Google Scholar] [CrossRef]
- Wooldridge, S.A.; Pratchett, M.S. Coral predators exert selection pressure on corals with heat tolerance traits: Implications for reef recovery under climate change. Front. Ecol. Evol. 2019, 7, 221. [Google Scholar]
- Kayal, M.; Vercelloni, J.; Lison de Loma, T.; Bosserelle, P.; Chancerelle, Y.; Geoffroy, S.; Stievenart, C.; Michonneau, F.; Penin, L.; Planes, S.; et al. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 2012, 7, e47363. [Google Scholar] [CrossRef] [PubMed]
- Obligations of States in respect of Climate Change. Available online: https://www.icj-cij.org/sites/default/files/case-related/187/187-20250723-pre-01-00-en.pdf (accessed on 28 June 2025).
- Kleypas, J.A.; Anthony, K.R.N.; Castruccio, F.S.; Allemand, D.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.P.; et al. Designing a blueprint for coral reef survival under rapid climate change. Glob. Change Biol. 2021, 27, 1186–1200. [Google Scholar]
- Rahmstorf, S. Is the Atlantic overturning circulation approaching a tipping point? Oceanography 2024, 37, 16–29. [Google Scholar] [CrossRef]
- Orihuela-Pinto, B.; England, M.; Taschetto, A. Interbasin and interhemispheric impacts of a collapsed Atlantic Circulation. Nat. Clim. Change 2022, 12, 558–565. [Google Scholar] [CrossRef]
- Heinze, C.; Blenckner, T.; Martins, H.; Rusiecka, D.; Döscher, R.; Gehlen, M.; Gruber, N.; Holland, E.; Hov, Ø.; Joos, F.; et al. The quiet crossing of ocean tipping points. Proc. Natl. Acad. Sci. USA 2021, 118, e2008478118. [Google Scholar] [CrossRef]
- Lowe, R.J.; Falter, J.L.; Monismith, S.G.; Atkinson, M.J. Wave-driven circulation of a coastal reef–lagoon system. J. Phys. Oceanogr. 2009, 39, 873–893. [Google Scholar] [CrossRef]
- van Woesik, R.; Houk, P.; Isechal, A.L.; Idechong, J.W.; Victor, S.; Golbuu, Y. Climate-change refugia in the sheltered bays of Palau: Analogs of future reefs. Ecol. Evol. 2012, 2, 2474–2484. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.L.; Wisshak, M.; Form, A.U.; Carricart-Ganivet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 2020, 30, 912–918. [Google Scholar] [CrossRef] [PubMed]
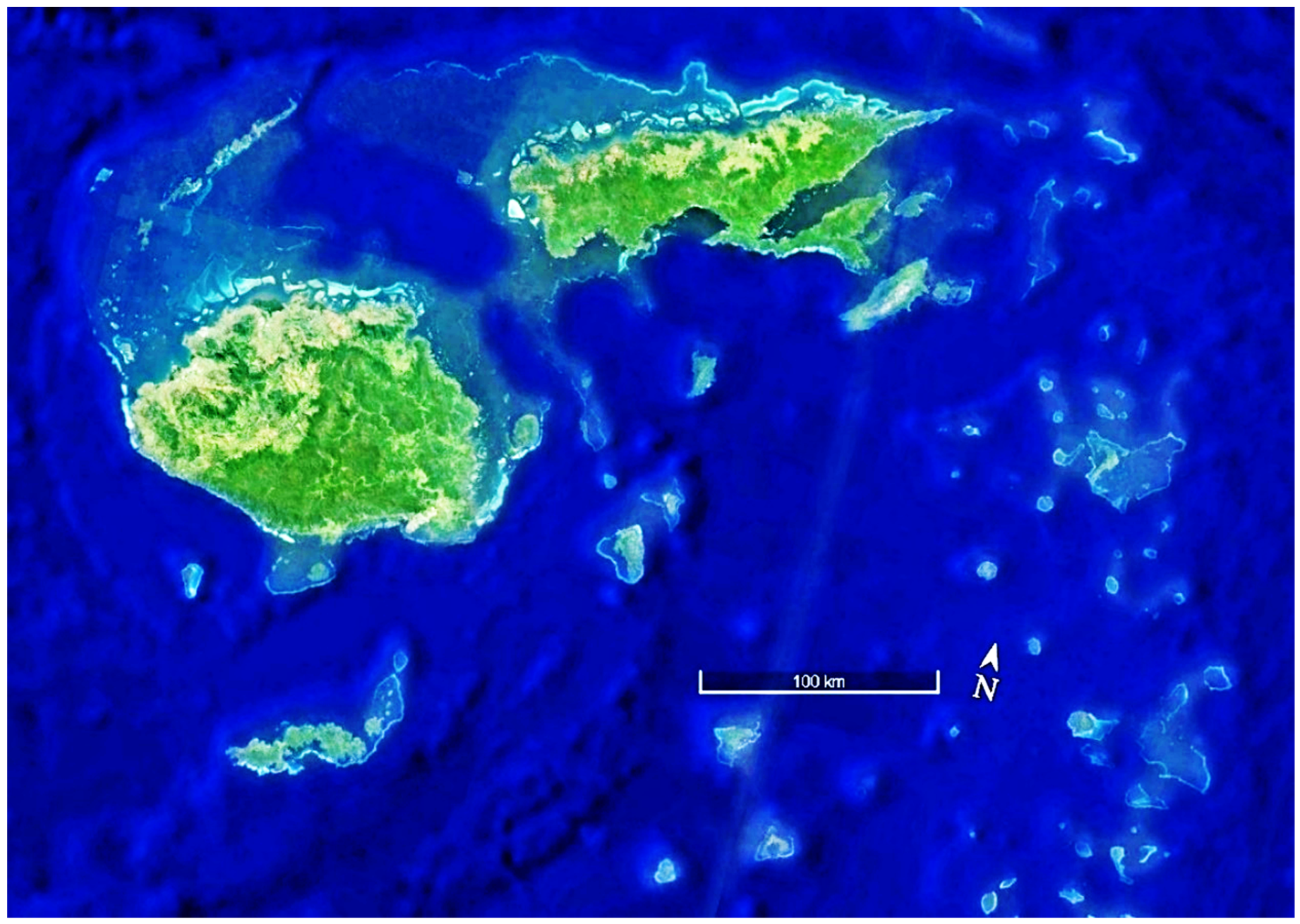

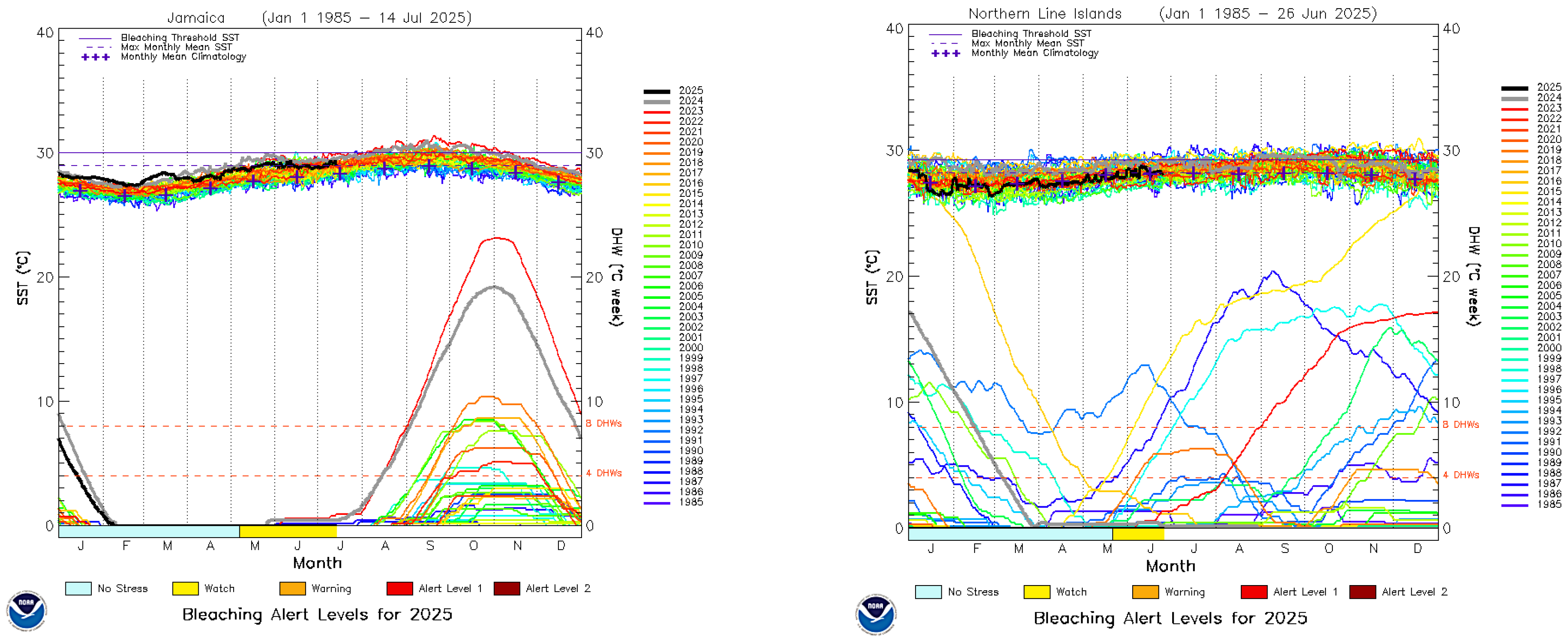

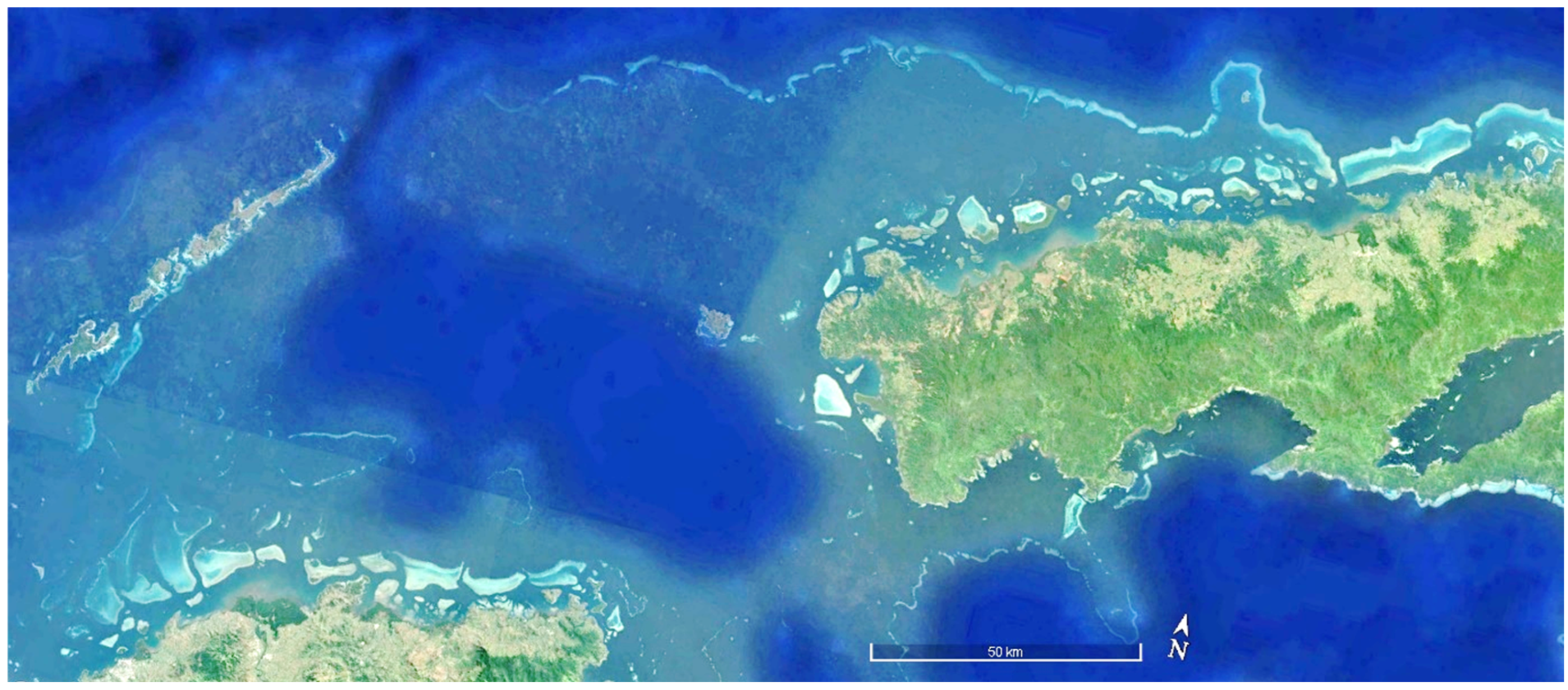
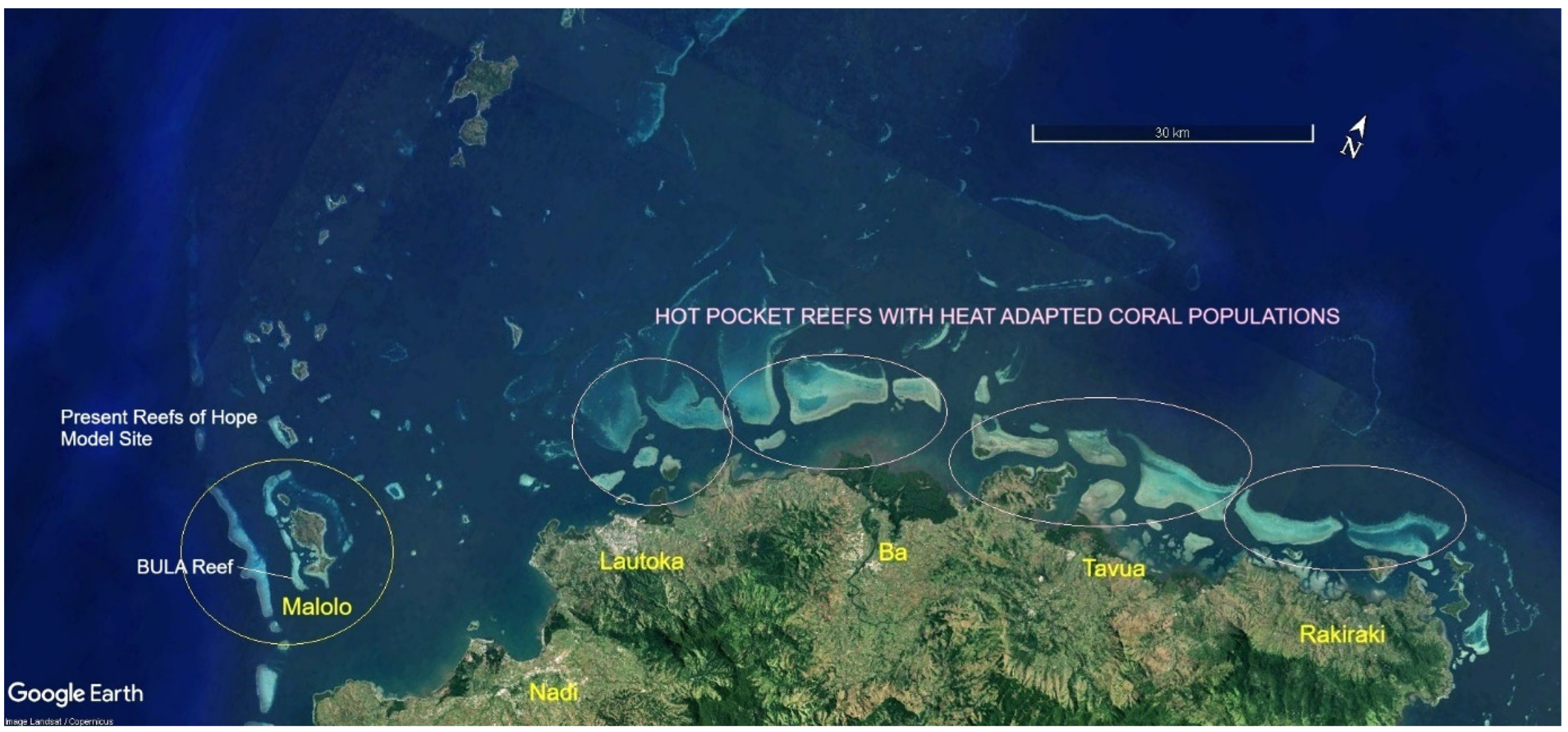
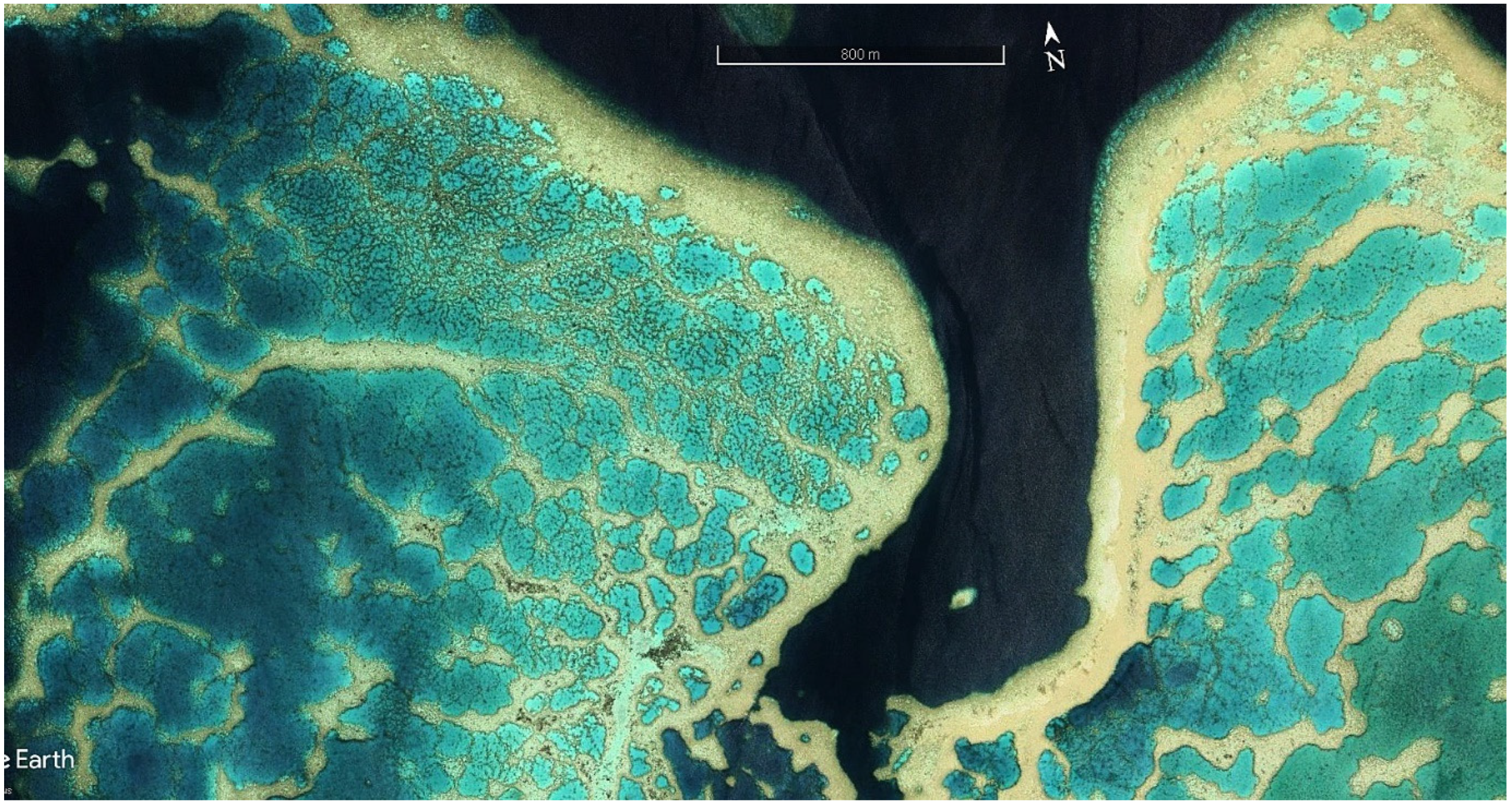

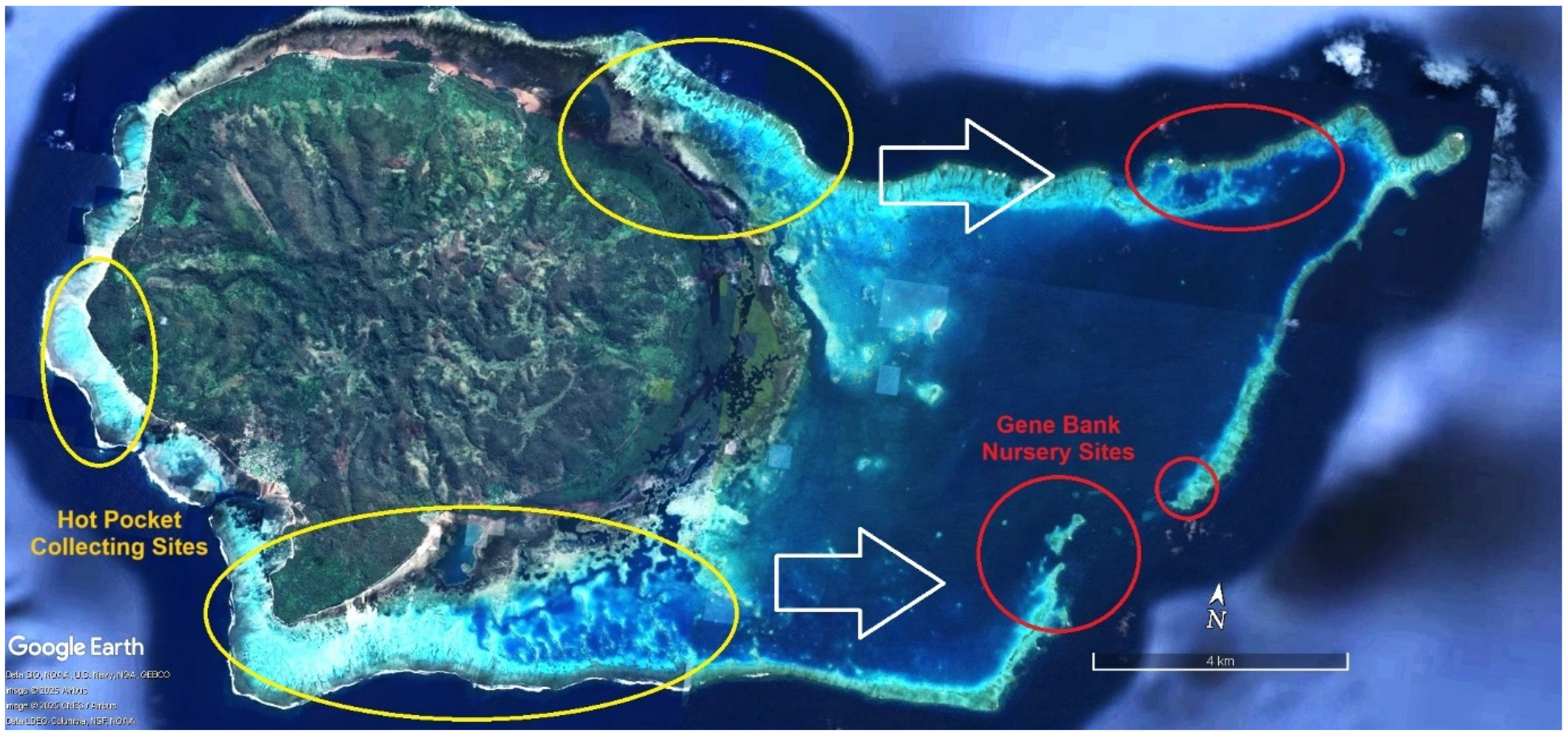

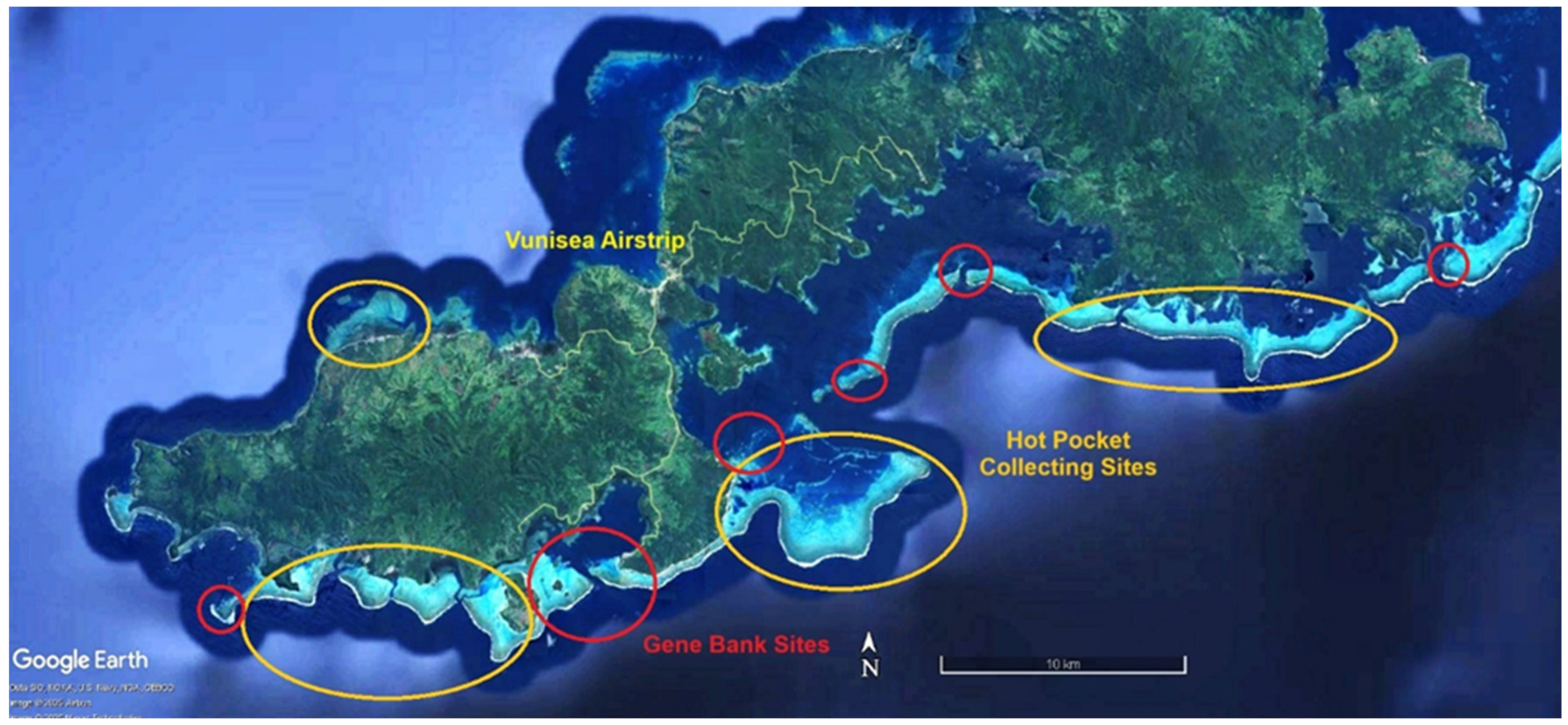








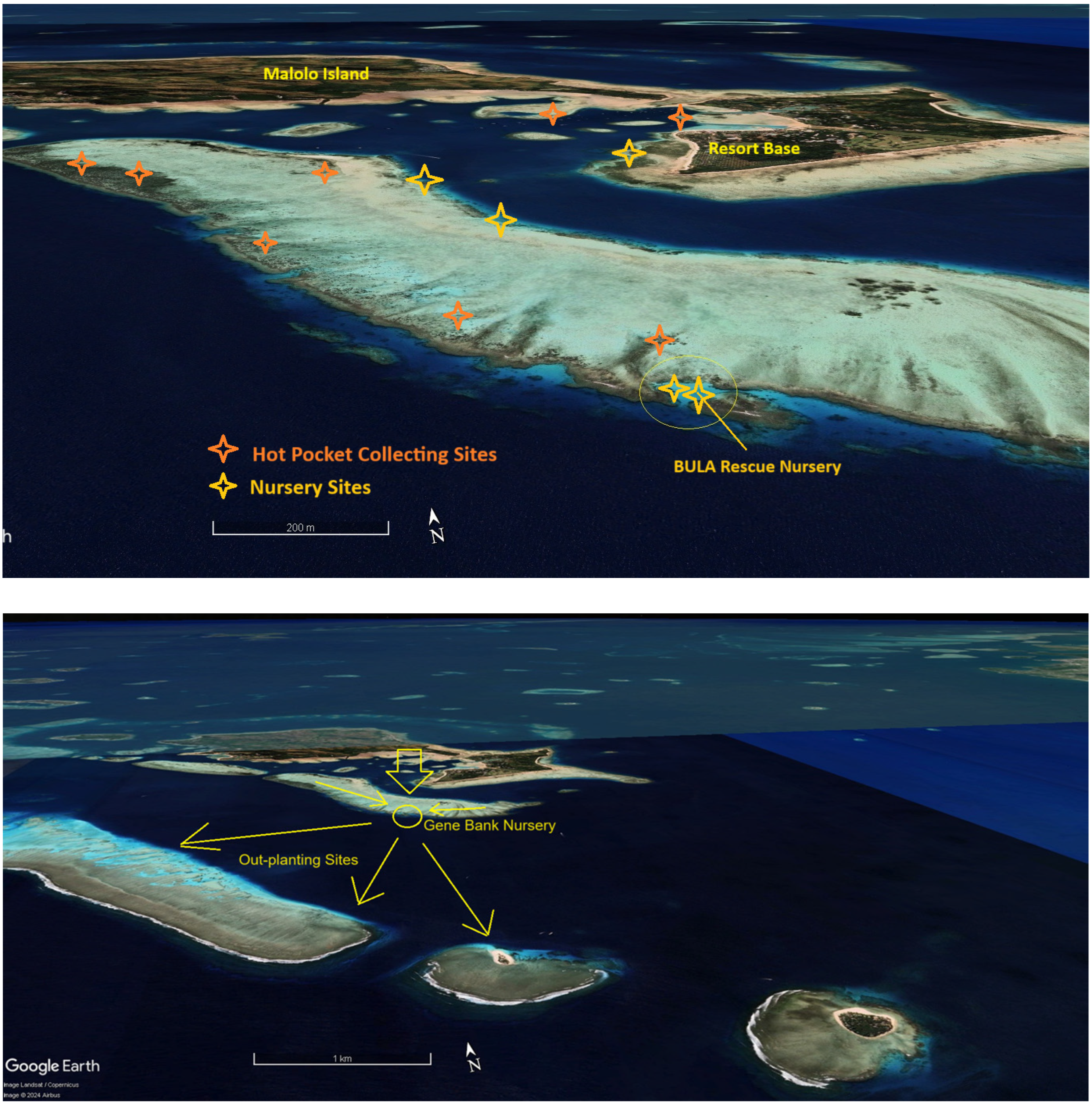


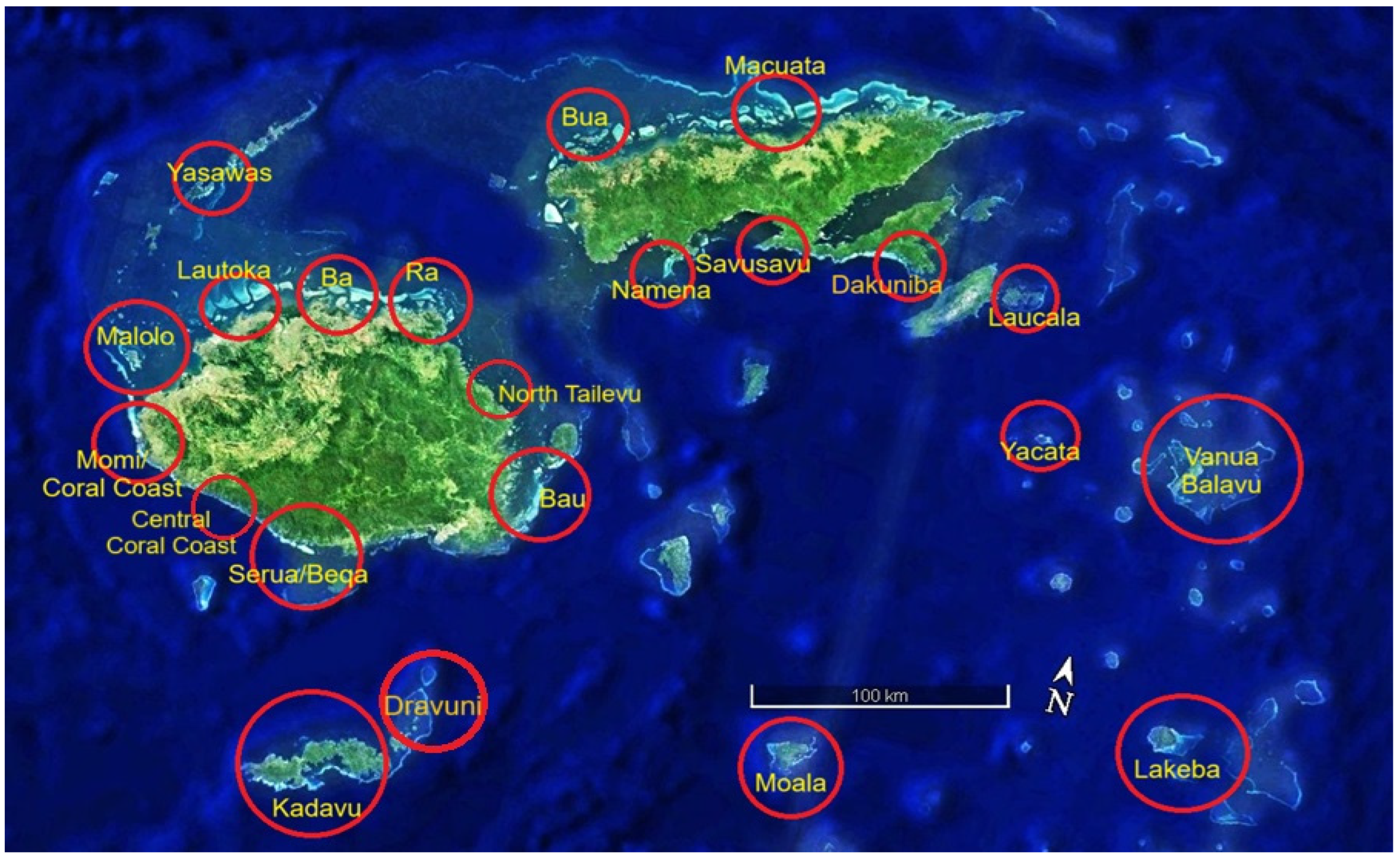
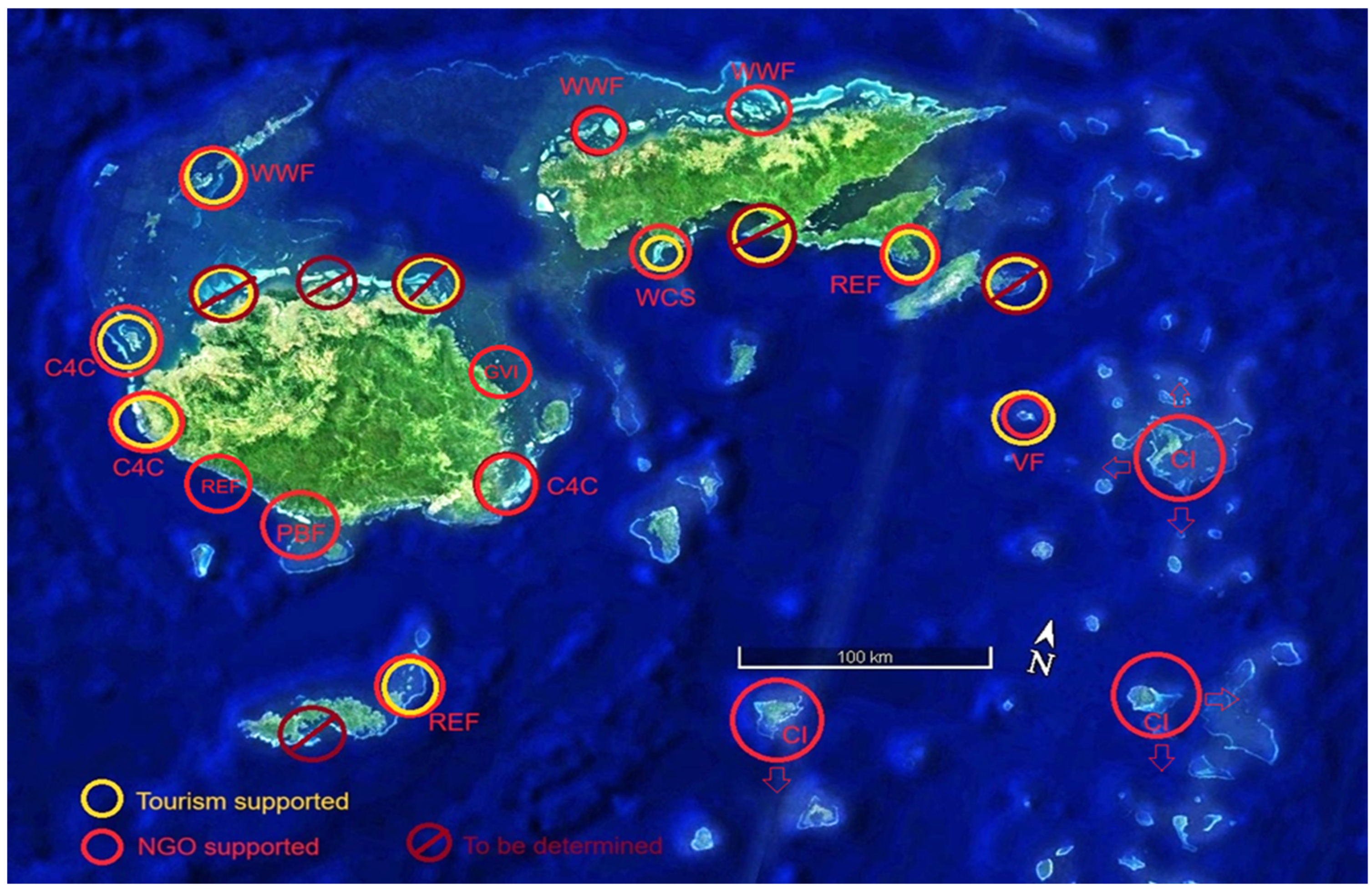

| ITEM | Per Year Site Cost | 20 Sites/Year | Ten Years |
|---|---|---|---|
| Personnel | €100,000 | €2,000,000 | €20,000,000 |
| Boats, Fuel, Travel | 50,000 | 1,000,000 | 10,000,000 |
| Nursery Materials | 10,000 | 200,000 | 2,000,000 |
| Out-planting Materials | 10,000 | 200,000 | 2,000,000 |
| Community Workshops | 10,000 | 200,000 | 2,000,000 |
| Subtotals | €180,000 | €3,600,000 | €36,000,000 |
| Additional support for coral spawning systems to double as giant clam hatcheries | 100,000 | Five sites/year 500,000 | Ten Years €5,000,000 |
| Totals | €275,000 | €4,100,000 | €41,000,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowden-Kerby, A. Creating a National Coral-Focused Climate Change Adaptation Plan for Fiji to Prevent Coral Species Extinction in the Face of Rapid Climate Change: Applying the UNESCO-Endorsed “Reefs of Hope” Ocean Decade Action. Sustainability 2025, 17, 8430. https://doi.org/10.3390/su17188430
Bowden-Kerby A. Creating a National Coral-Focused Climate Change Adaptation Plan for Fiji to Prevent Coral Species Extinction in the Face of Rapid Climate Change: Applying the UNESCO-Endorsed “Reefs of Hope” Ocean Decade Action. Sustainability. 2025; 17(18):8430. https://doi.org/10.3390/su17188430
Chicago/Turabian StyleBowden-Kerby, Austin. 2025. "Creating a National Coral-Focused Climate Change Adaptation Plan for Fiji to Prevent Coral Species Extinction in the Face of Rapid Climate Change: Applying the UNESCO-Endorsed “Reefs of Hope” Ocean Decade Action" Sustainability 17, no. 18: 8430. https://doi.org/10.3390/su17188430
APA StyleBowden-Kerby, A. (2025). Creating a National Coral-Focused Climate Change Adaptation Plan for Fiji to Prevent Coral Species Extinction in the Face of Rapid Climate Change: Applying the UNESCO-Endorsed “Reefs of Hope” Ocean Decade Action. Sustainability, 17(18), 8430. https://doi.org/10.3390/su17188430





