Biological Colonization of Carolei’s Nymphaeum (Calabria, Italy)
Abstract
1. Introduction
2. Materials and Methods
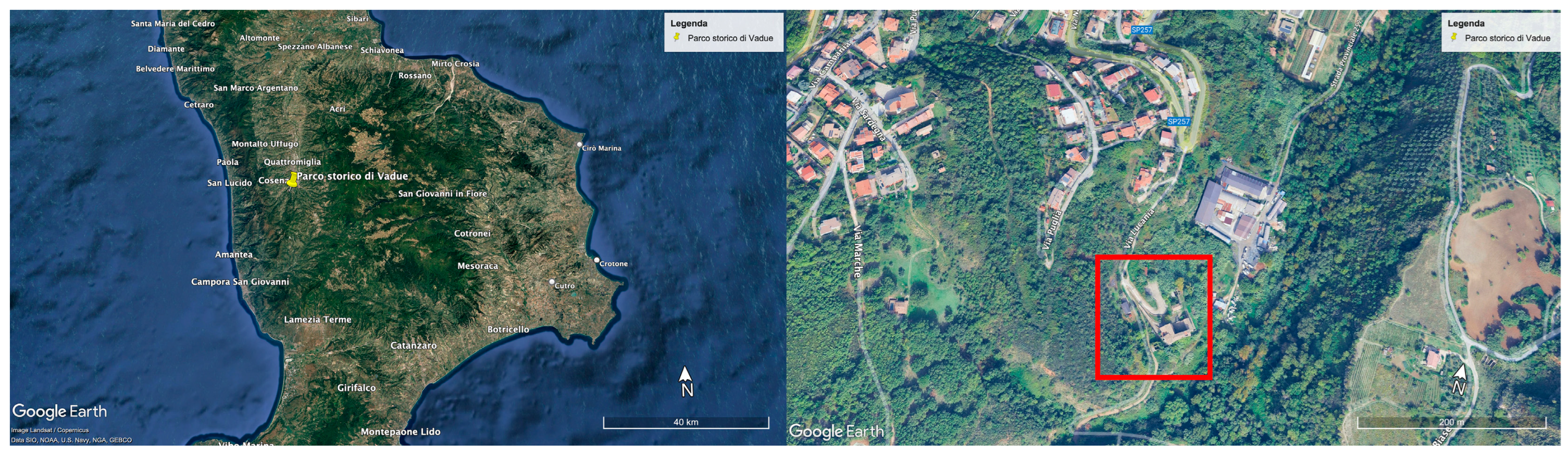
2.1. Site Description
2.2. Conservation Status
2.3. Sampling and Analysis
2.3.1. Vascular Plants
2.3.2. Mosses
2.3.3. Biofilm
2.4. Molecular Analysis
3. Results
3.1. Vascular Plants
3.2. Mosses
3.3. Biofilms
3.3.1. Fungi
3.3.2. Cyanobacteria and Chlorophyta
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caneva, G.; Nugari, M.P.; Salvadori, O. La Biologia Vegetale per i Beni Culturali. Biodeterioramento e Conservazione; Nardini: Firenze, Italy, 2005; Volume 1. [Google Scholar]
- Warscheid, T.; Braams, J. Biodeterioration of Stone: A Review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Krumbein, W.E. Microbial interactions with mineral materials. In Biodeterioration 7; Hougthon, D.R., Eggins, S., Eds.; Elsevier: New York, NY, USA, 1988; pp. 78–100. [Google Scholar]
- Saiz-Jimenez, C. Biodeterioration of stone in historic buildings and monuments. In Mycotoxins, Wood Decay, Plant Stress, Biocorrosion, and General Biodeterioration; Biodeterioration, Research; Llewellyn, G.C., Dashek, W.W., O’Rear, C.E., Eds.; Springer: Boston, MA, USA, 1994; Volume 4, pp. 587–603. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the Rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of Building Stones: A Review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Hueck, H.J. The Biodeterioration of Materials as a Part of Hylobiology. Mater Org. 1965, 1, 5–34. [Google Scholar]
- Hueck, H. The Biodeterioration of Materials—An Appraisal. Int. Biodeterior. Biodegrad. 2001, 48, 5–11. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A New Concept for Building Ecology Studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Cozzolino, A.; Adamo, P.; Bonanomi, G.; Motti, R. The Role of Lichens, Mosses, and Vascular Plants in the Biodeterioration of Historic Buildings: A Review. Plants 2022, 11, 3429. [Google Scholar] [CrossRef]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and Higher Plants on Stone: A Review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Paiva, D.S.; Fernandes, L.; Trovão, J.; Mesquita, N.; Tiago, I.; Portugal, A. Uncovering the Fungal Diversity Colonizing Limestone Walls of a Forgotten Monument in the Central Region of Portugal by High-Throughput Sequencing and Culture-Based Methods. Appl. Sci. 2022, 12, 10650. [Google Scholar] [CrossRef]
- Bilotto, L. Le Serre Cosentine—Itinerari Culturali; Edizioni Santelli: Cosenza, Italy, 1994. [Google Scholar]
- Palmieri, L. Cosenza e le sue Famiglie Attraverso Testi Atti e Manoscritti; Tomi I–II; Pellegrini Editore: Cosenza, Italy, 1999. [Google Scholar]
- Addante, L. Cosenza e i Cosentini. Un Volo Lungo Tre Millenni; Rubbettino Editore: Soveria Mannelli, Catanzaro, Italy, 2001. [Google Scholar]
- De Rose, G. Da Ixia a Carolei Una Passeggiata Storica Ttraverso i Secoli. Cosenza, Italy, 1984. [Google Scholar]
- Cazzato, V.; Fagiolo, M.; Giusti, M.A. Atlante delle Grotte e dei Ninfei in Italia. Toscana, Lazio, Italia Meridionale e Isole; Electa Editore: Milano, Italy, 2001. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Pignatti, S.; Guarino, R.; Rosa, M.L. Flora d’Italia; Flora d’Italia in 4 Volumi: Flora Digitale; Edagricole-New Business Media: Milano, Italy, 2017; ISBN 978-88-506-5243-3. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.; Walters, S.; Webb, D. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1. [Google Scholar]
- Raunkiaer, C. Life Forms of Plants and Statistical Plant Geography. In History of Ecology; Arno Press: New York, NY, USA, 1977; ISBN 978-0-405-10418-3. [Google Scholar]
- Signorini, M.A. Lo Studio e Il Controllo Della Vegetazione Infestante Nei Siti Archeologici. Una Proposta Metodologica. In L’Area Archeologica di Fiesole. Rilievi e Ricerche per la Conservazione; Marino, L., Nenci, C., Eds.; Alinea: Firenze, Italy, 1995; pp. 41–46. [Google Scholar]
- Signorini, M.A. L’indice Di Pericolosità: Un Contributo Del Botanico al Controllo Della Vegetazione Infestante Nelle Aree Monumentali. Inf. Bot. Ital. 1996, 28, 7–14. [Google Scholar]
- Castenholz, R.W. Culturing methods for cyanobacteria. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 1988; Volume 167, pp. 68–93. [Google Scholar]
- Preisig, H.R.; Andersen, R.A. Historical Review of Algal Culturing Techniques. Algal Cult. Tech. 2005, 65, 79–82. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern Approach to the Classification System of Cyanophytes. 1—Introduction. Algol. Stud. 1985, 38, 291–302. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern Approach to the Classification System of Cyanophytes. 3—Oscillatoriales. Arch. Hydrobiol. Algol. Stud. 1988, 50–53 (Suppl. 80), 327–472. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Modern Approach to the Classification System of Cyanophytes. Chroococcales. Algol. Stud. Hydrobiol. 1986, 43, 157–226. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Modern Approach to the Classification System of Cyanophytes 4—Noazstocales. Arch. Hydrobiol. Suppl. Monogr. Beitr. 1989, 82, 247–345. [Google Scholar]
- Komárek, J. Cyanoprokaryota Teil/3rd Part: Heterocytous Genera; Springer Spektrum: Berlin, Germany, 2013. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal Primers for Amplification of Three Non-Coding Regions of Chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Skinner, D.Z.; Liang, G.H. Phylogenetic Analysis of Sorghum and Related Taxa Using Internal Transcribed Spacers of Nuclear Ribosomal DNA. Theor. Appl. Genet. 1994, 89, 26–32. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Nubel, U.; Garcia-Pichel, F.; Muyzer, G. PCR Primers to Amplify 16S rRNA Genes from Cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef]
- Pinna, D. Microbial Growth and its Effects on Inorganic Heritage Materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Pietrini, A.M.; Ricci, S.; Nugari, M.P. Churches and crypts. In Plant Biology for Cultural Heritage. Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Getty Conservation Institute: New York, NY, USA, 2008; pp. 179–183. [Google Scholar]
- Roccardi, A.; Ricci, S.; Pietrini, A.M. Semienclosed environments. In Plant Biology for Cultural Heritage. Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Getty Conservation Institute: New York, NY, USA, 2008; pp. 206–210. [Google Scholar]
- Albertano, P.; Urzì, C. Structural Interactions Among Epilithic Cyanobacteria and Heterotrophic Microorganisms in Roman Hypogea. Microb. Ecol. 1999, 38, 244–252. [Google Scholar] [CrossRef]
- Motti, R.; Stinca, A. Analysis of the Biodeteriogenic Vascular Flora at the Royal Palace of Portici in Southern Italy. Int. Biodeterior. Biodegrad. 2011, 65, 1256–1265. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Stinca, A. Deteriogenic Flora of the Phlegraean Fields Archaeological Park: Ecological Analysis and Management Guidelines. Nord. J. Bot. 2020, 38. [Google Scholar] [CrossRef]
- Dahmani, J.; Benharbit, M.; Fassar, M.; Hajila, R.; Zidane, L.; Magri, N.; Belahbib, N. Vascular Plants Census Linked to the Biodeterioration Process of the Portuguese City of Mazagan in El Jadida, Morocco. J. King Saud Univ. Sci. 2020, 32, 682–689. [Google Scholar] [CrossRef]
- Mascaro, M.E.; Pellegrino, G.; Palermo, A.M. Analysis of Biodeteriogens on Architectural Heritage. An Approach of Applied Botany on a Gothic Building in Southern Italy. Sustainability 2022, 14, 34. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Stinca, A. Biodeteriogens at a Southern Italian Heritage Site: Analysis and Management of Vascular Flora on the Walls of Villa Rufolo. Int. Biodeterior. Biodegrad. 2021, 162, 105252. [Google Scholar] [CrossRef]
- Mascaro, M.E.; Pellegrino, G.; De Rose, I.; Palermo, A.M. Contribution to the Knowledge of Biodeteriogenic Flora on Three Historical Calabrian (Southern Italy) Churches. Open J. Ecol. 2021, 11, 287–300. [Google Scholar] [CrossRef]
- Grbić, M.L.; Simić, G.S.; Stupar, M.; Jelikić, A.; Sabovljević, M.; Dorđević, M.; Vukojević, J. Biodiversity’s Hidden Treasure: Biodeteriorated Archaeological Tombstones of Serbia. Curr. Sci. 2017, 112, 304–310. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionisio, A.; Saiz-Jimenez, C. Biodiversity of Cyanobacteria and Green Algae on Monuments in the Mediterranean Basin: An Overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, B.; Yang, X.; Ge, Q. Deterioration-Associated Microbiome of Stone Monuments: Structure, Variation, and Assembly. Appl. Environ. Microbiol. 2018, 84, e02680-17. [Google Scholar] [CrossRef]
- Rosado, T.; Gil, M.; Caldeira, A.T.; Martins, M.; Dias, C.; Carvalho, L.; Mirão, J.; Candeias, A. Material Characterization and Biodegradation Assessment of Mural Paintings: Renaissance Frescoes from Santo Aleixo Church, Southern Portugal. IJAH 2013, 8, 835–852. [Google Scholar] [CrossRef]
- Berner, M.; Wanner, G.; Lubitz, W. A Comparative Study of the Fungal Flora Present in Medieval Wall Paintings in the Chapel of the Castle Herberstein and in the Parish Church of St Georgen in Styria, Austria. Int. Biodeter. Biodegr. 1997, 40, 53–61. [Google Scholar] [CrossRef]
- Guglielminetti, M.; De Giuli Morghen, C.; Radaelli, A.; Bistoni, F.; Carruba, G.; Spera, G.; Caretta, G. Mycological and Ultrastructural Studies to Evaluate Biodeterioration of Mural Paintings. Detection of Fungi and Mites in Frescos of the Monastery of St Damian in Assisi. Int. Biodeter. Biodegr. 1994, 33, 269–283. [Google Scholar] [CrossRef]
- Soares, F.; Trovão, J.; Portugal, A. Phototrophic and Fungal Communities Inhabiting the Roman Cryptoporticus of the National Museum Machado de Castro (UNESCO site, Coimbra, Portugal). World J. Microbiol. Biotechnol. 2022, 38, 157. [Google Scholar] [CrossRef]
- Tang, Y.; Lian, B. Diversity on Endolithic Fungal Communities in Dolomite and Limestone Rocks from Nanjiand Canyon in Guizhou Larst Area, China. Can. J. Microbiol. 2012, 58, 685–693. [Google Scholar] [CrossRef]
- Caneva, G.; Hosseini, Z.; Bartoli, F. Risk, Hazard, and Vulnerability for Stone Biodeterioration due to Higher Plants: The Methodological Proposal of a Multifactorial Index (RHV). J. Cult. Herit. 2023, 62, 217–229. [Google Scholar] [CrossRef]
- Fitzner, B.; Heinrichs, K.; Kownatzki, R. Weathering Forms at Natural Stone Monuments—Classification, Mapping and Evaluation. Restor. Build. Monum. 1997, 3, 105–124. [Google Scholar] [CrossRef]
- Fitzner, B.; Heinrichs, K. Damage Diagnosis at Stone Monuments-Weathering Forms, Damage Categories and Damage Indices. Acta-Univ. Carol. Geol. 2001, 1, 12–13. [Google Scholar]
- Motti, R.; Bonanomi, G. Vascular Plant Colonization of Four Castles in Southern Italy: Effects of Substrate Bioreceptivity, Local Environment Factors and Current Management. Int. Biodeterior. Biodegrad. 2018, 133, 26–33. [Google Scholar] [CrossRef]
- Tomaselli, L.; Lamenti, G.; Bosco, M.; Tiano, P. Biodiversity of Photosynthetic Micro-Organisms Dwelling on Stone Monuments. Int. Biodeter. Biodegr. 2000, 46, 251–258. [Google Scholar] [CrossRef]
- Liu, X.; Qian, Y.; Wu, F.; Wang, Y.; Wang, W.; Gu, J.D. Biofilms on Stone Monuments: Biodeterioration or Bioprotection? Trends Microbiol. 2022, 30, 816–819. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural Biocides for the Conservation of Stone Cultural Heritage: A Review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Becerra, J.; Mateo, M.P.; Ortiz, P.; Nicolás, G.; Zaderenko, A.P. Evaluation of the Applicability of Nano-biocide Treatments on Limestones Used in Cultural Heritage. J. Cult. Herit. 2019, 38, 126–135. [Google Scholar] [CrossRef]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology Applied to Cultural Heritage: Biorestoration of Frescoes Using Viable Bacterial Cells and Enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef]



| Sampling | Type | |
|---|---|---|
| A1 | Dark green patina |  |
| A2 | Yellow patina |  |
| A3 A4 A7 | Brilliant green patina |  |
| A5 | Black patina |  |
| A6 | Pink patina |  |
| A8 | Green-pink patina |  |
| Denaturation | Annealing | Extension | Cycles | |
|---|---|---|---|---|
| Plants | 30 s at 94 °C | 1 min at 55 °C | 1 min at 72 °C | 30 |
| Mosses | 45 s at 94 °C | 45 s at 61 °C | 1 min at 72 °C | 30 |
| Fungi | 30 s at 94 °C | 1 min at 55 °C | 45 s at 72 °C | 35 |
| Cyanobacteria | 45 s at 94 °C | 45 s at 55 °C | 1 min at 72 °C | 30 |
| Species | Family | Life Form | HI | Position | Identification | |
|---|---|---|---|---|---|---|
| Pteridophyta | ||||||
| Adiantum capillus-veneris L. | Pteridaceae | G rhiz | 3 | I/E | M | |
| Equisetum arvense L. | Equisetaceae | G rhiz | 3 | I/E | M | |
| Angiospermae | ||||||
| Crepis neglecta L. | Asteraceae | T scap | 2 | I/E | S | |
| Ficus carica L | Moraceae | P scap | 10 | E | M | |
| Parietaria judaica L. | Urticaceae | H scap | 5 | I/E | M | |
| Polypogon viridis (Gouan) Breistr. | Poaceae | H caesp | 3 | I/E | S | |
| Quercus robur L. subsp. brutia (Ten.) O. Schwarz | Fagaceae | P scap | 9 | I | M | |
| Samolus valerandi L. | Primulaceae | H scap | 4 | I/E | S | |
| Trachelium caeruleum L. | Campanulaceae | Ch suffr | 4 | I | S | |
| Veronica cymbalaria Bodard | Plantaginaceae | T scap | 0 | I | S |
| Phylum | Genus/Species | Accession Number | bp | Sample |
|---|---|---|---|---|
| Ascomycota | ||||
| Alternaria alternata | PP231814 | 532 | A6/A8 | |
| Aureobasidium pullulans | PP231815 | 579 | A1 | |
| PP231816 | 581 | A5 | ||
| Cladosporium cladosporioides | PP231817 | 517 | A6 | |
| PP231818 | 547 | A1/A6 | ||
| Cladosporium herbarum | PP231819 | 546 | A6 | |
| PP231820 | 536 | A2 | ||
| Parengyodontium album | PP231821 | 562 | A8 | |
| Fusarium sp. | PP231822 | 526 | A2/A3 | |
| Penicillium chrysogenum | PP231823 | 575 | A5 | |
| PP231824 | 580 | A2 | ||
| Sarocladium subulatum | PP231825 | 553 | A8 | |
| Basidiomycota | ||||
| Coprinellus sp. | PP231826 | 676 | A5 | |
| Hyphodermella rosae | PP231827 | 631 | A3 | |
| Phanerochaete martelliana | PP231828 | 681 | A1 | |
| Trametes sp. | PP231829 | 631 | A5 |
| Genus | Accession Number | bp | Sample | Identification | |
|---|---|---|---|---|---|
| Cyanobacteria | |||||
| Chroococcidiopsis | A7 | M | |||
| Leptolyngbya | PP854591 | 379 | A7 | S | |
| Nostoc | PP854592 | 409 | A4 | S | |
| Phormidium | A3 | M | |||
| Oscillatoria | A7 | M | |||
| Chlorophyta | |||||
| Chlorella | A3–A7 | M | |||
| Microspora | A3–A7 | M | |||
| Rhizoclonium | A7 | M | |||
| Stichococcus | A4–A7 | M | |||
| Ulothrix | A4 | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermo, A.M.; Greca, R.; Chiappetta, M. Biological Colonization of Carolei’s Nymphaeum (Calabria, Italy). Sustainability 2025, 17, 8426. https://doi.org/10.3390/su17188426
Palermo AM, Greca R, Chiappetta M. Biological Colonization of Carolei’s Nymphaeum (Calabria, Italy). Sustainability. 2025; 17(18):8426. https://doi.org/10.3390/su17188426
Chicago/Turabian StylePalermo, Anna Maria, Raffaella Greca, and Mattia Chiappetta. 2025. "Biological Colonization of Carolei’s Nymphaeum (Calabria, Italy)" Sustainability 17, no. 18: 8426. https://doi.org/10.3390/su17188426
APA StylePalermo, A. M., Greca, R., & Chiappetta, M. (2025). Biological Colonization of Carolei’s Nymphaeum (Calabria, Italy). Sustainability, 17(18), 8426. https://doi.org/10.3390/su17188426








