Analysis of the Main Hydrogen Production Technologies
Abstract
1. Introduction
2. Technologies for Hydrogen Production
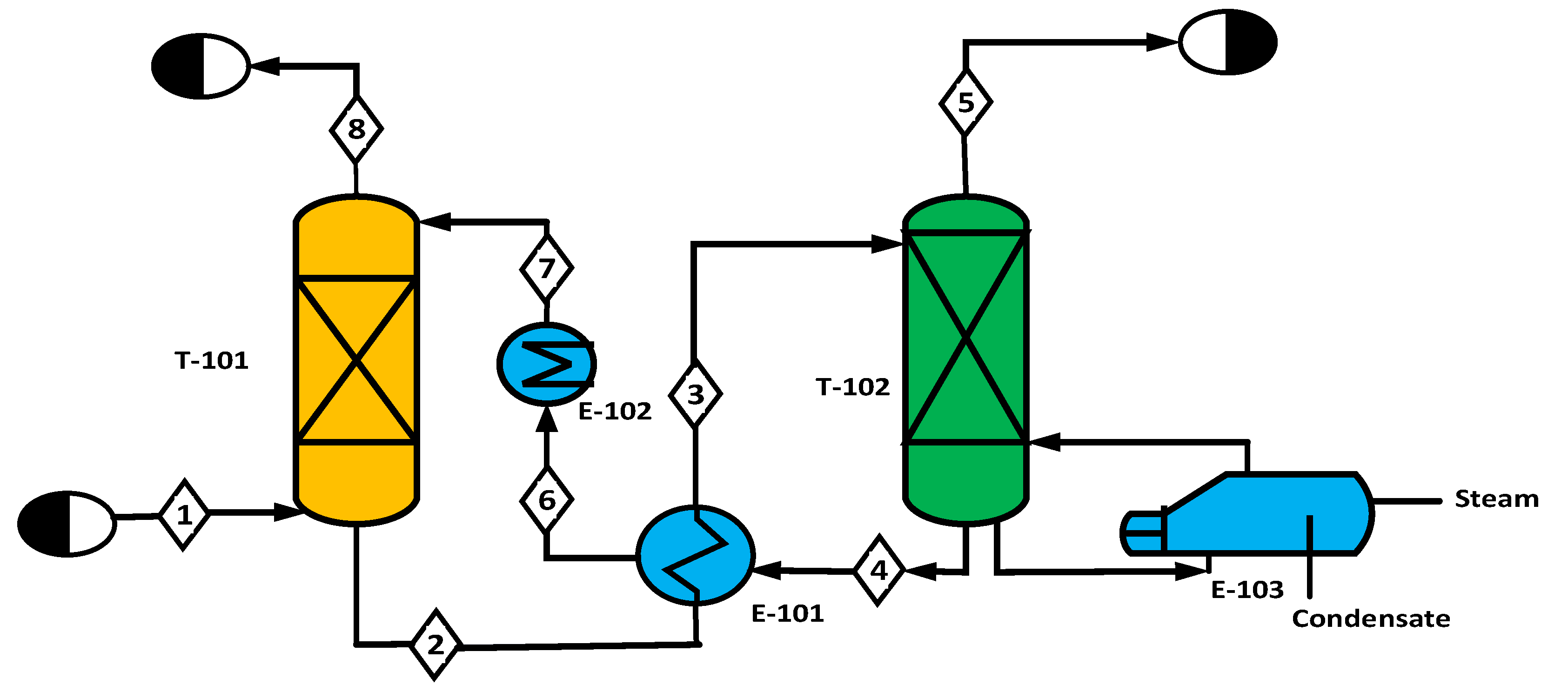
2.1. Hydrogen Production Using Steam Methane Reforming (SMR)
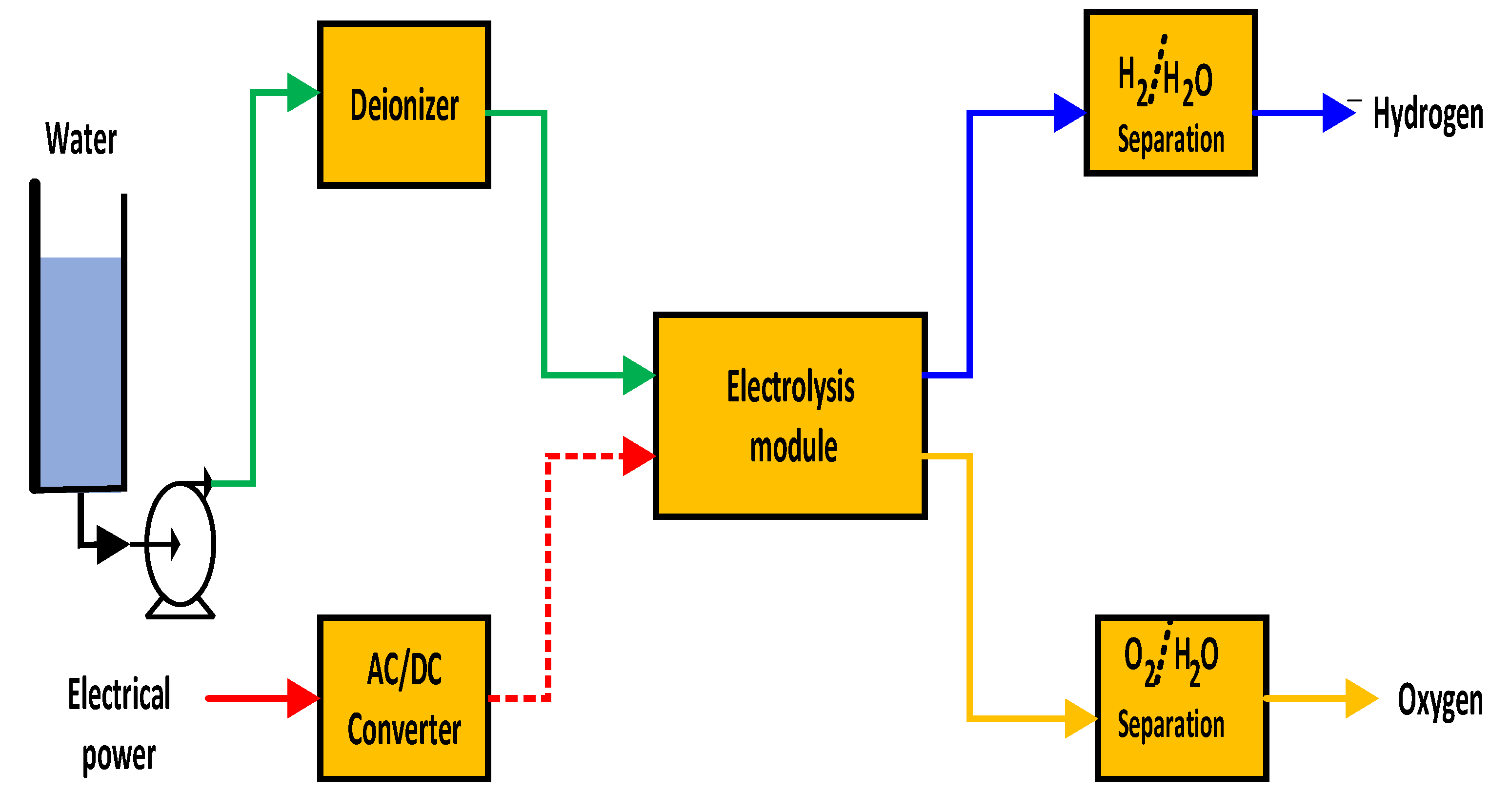
2.2. Hydrogen Production by Electrolysis of Water
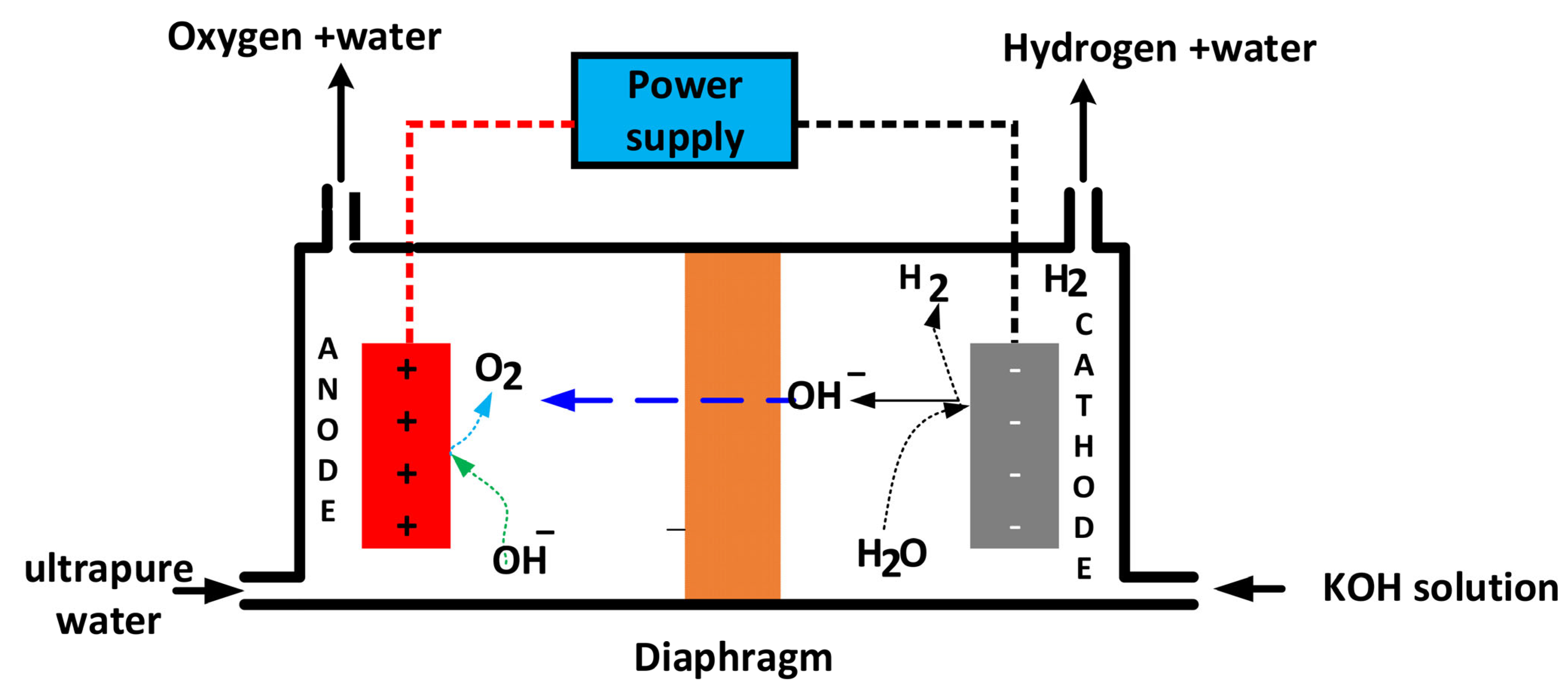
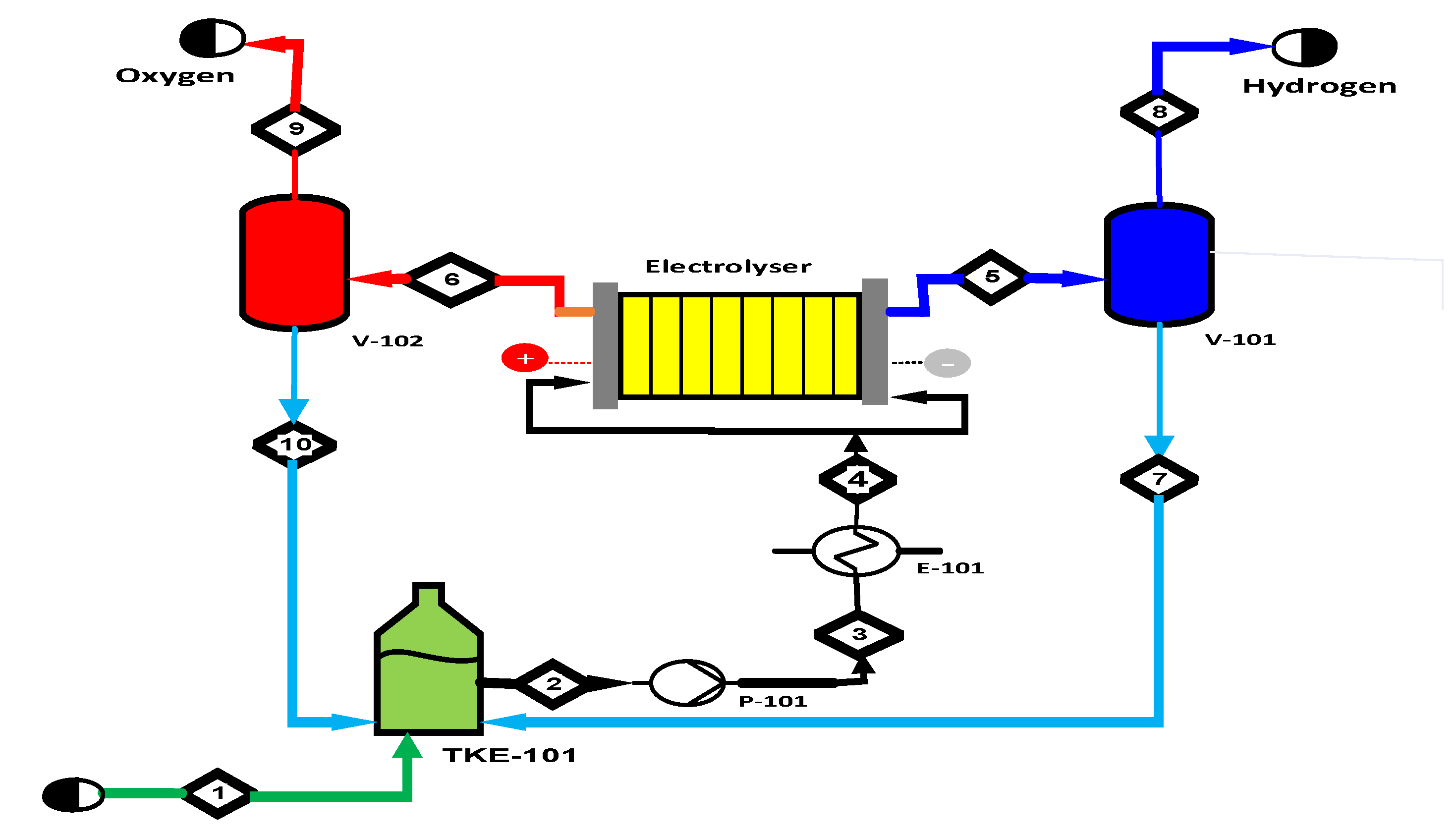
2.2.1. Alkaline Water Electrolysis (AWE)
2.2.2. Proton Exchange Membrane Electrolysis (PEMWE)
2.2.3. Anion Exchange Membrane Electrolysis (AEMWE)
| N° | Characteristics | Alkaline Water Electrolysis (AWE) | Proton Exchange Membrane Electrolysis (PEMWE) | Anion Exchange Membrane Electrolysis (AEMWE) |
|---|---|---|---|---|
| 1 | Principle | Hydrogen gas is generated at the cathode by the action of direct current, while oxygen gas is generated at the anode and the diaphragm separates the two electrodes. | Water decomposes to oxygen and protons (H+) at the anode, the protons migrate across the membrane to the cathode to generate hydrogen gas | Water decomposes on the cathode producing hydrogen gas and OH−, which crosses the membrane to the anode to form oxygen and water. |
| 2 | Electrolyte | Concentrated alkaline liquor (20–40 wt% KOH) [54] | Solid polymer electrolyte (Perfluoro sulfonic acid (PFSA)), usually Nafion. electrolyte membranes (Fumapem) [55]. | Deionized water /1% K2CO3/KHCO3 [56] Pure water [57] |
| 3 | Separator | diaphragm (usually Zirfon); Asbestos/Zirfon/Ni | polymeric membrane Nafion | Fumatech, Selemion AMV [56] |
| 4 | Anode | Ni, alloys Ni-Co | Iridium of RuO2, IrO2 Ti/RuO2, IrO2 | Nickel or NiFeCo alloys; Ni, Fe, Co oxides |
| 5 | Cathode | alloys of Ni, Ni-Mo Steel + Ni | Pt, Pt-Pd | Nickel and Nialloys |
| 6 | Current density (A cm−2) | 0.2–0.4 [55] | 0.6–2.0 [55] | Not specified [56]. |
| 0.2–0.8 [30] | 1–2 [30] | 0.2–2 [30] | ||
| 7 | Cell voltage (V) | 1.8–2.4 [55] | 1.75–2.20 [55] | 1.8–2.20 |
| 8 | Operating temperature (°C) | 60–80 [55] | 50–80 | 40–90 °C |
| 30–60 [58] | ||||
| 9 | Operating pressure (bar) | 2–35 | 15–40 | <70 bar |
| 10 | Gas purity (%) | >99.5 [59] | 99.99 [55] | 99.9–99.999% |
| 11 | Electrolysis energy consumption (kWh/kg H2) | 4.6–4.8 [60] | 4.1–4.3 [60] | Hydrogen production of 61.13 mL/min and 48.25 kWh/kg [61] |
| 12 | Efficiency | 60–70% | 65–75% | 60–70% |
| 13 | Development status | Mature | Commercialized | R&D [30] |
2.3. Hydrogen Production by Photocatalysis and Photoelectrocatalysis
2.3.1. Production of Hydrogen by Splitting Water Through Photocatalysis
2.3.2. Photoelectrocatalytic Hydrogen Production by Water Splitting
2.4. Production of Hydrogen as a By-Product
- Mercurycell
- Diaphragmcell
- Membranecell
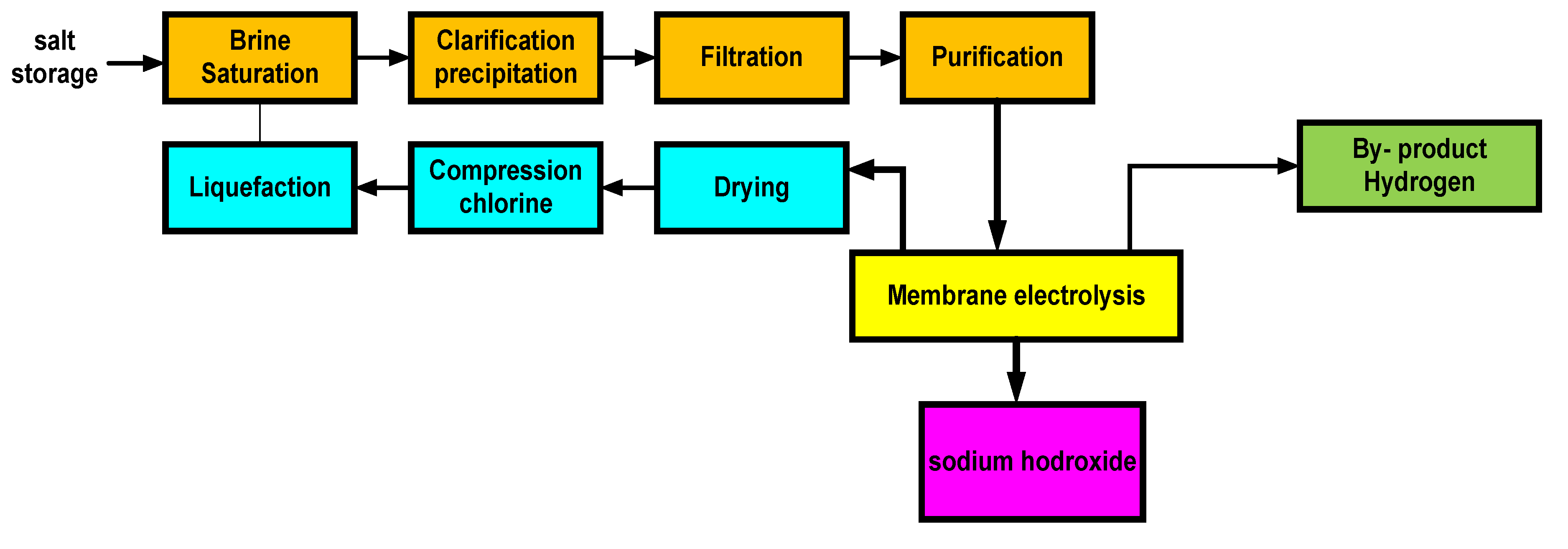
2.4.1. Brine Purification for Chlor-Alkalis Production
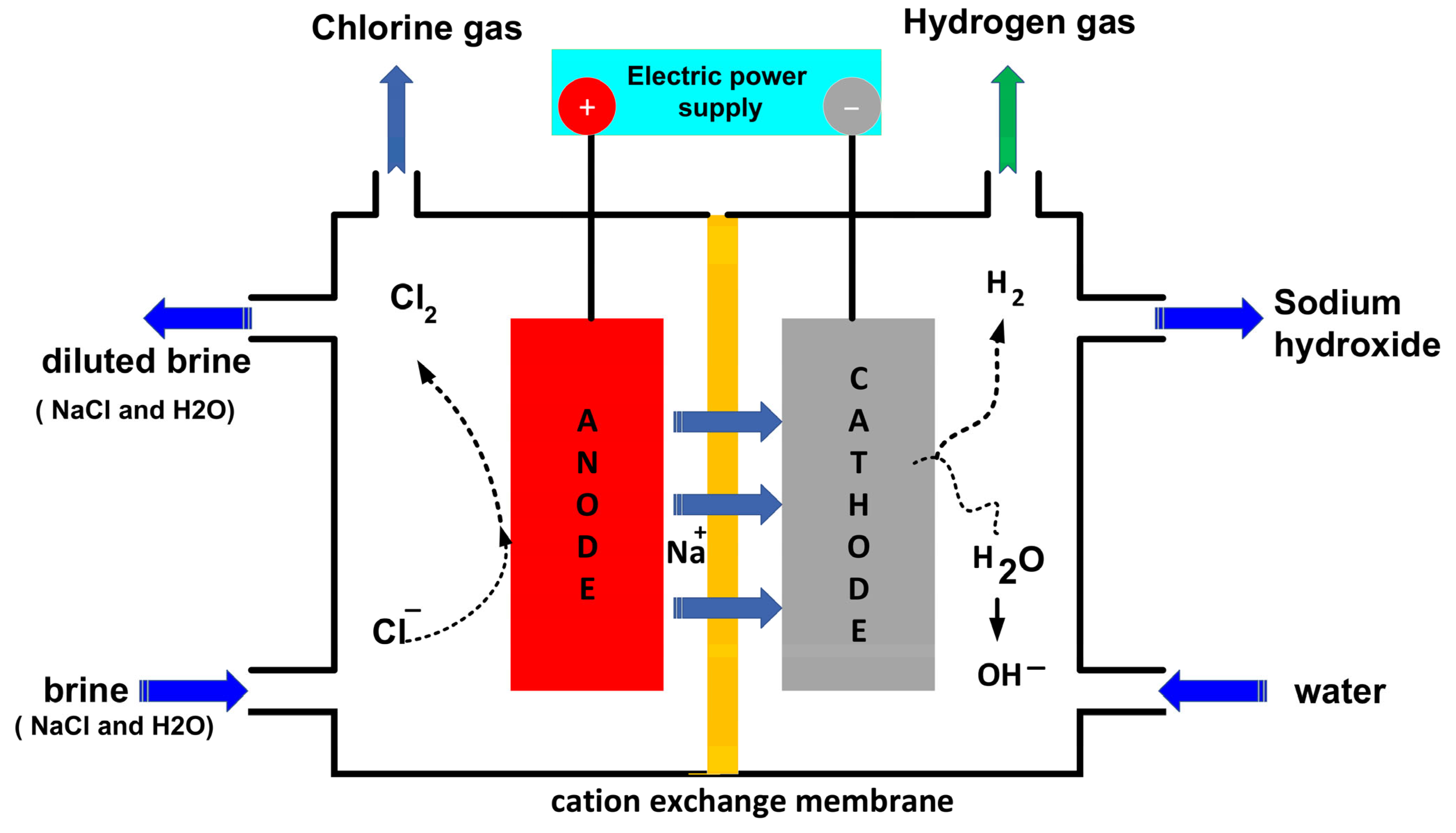
2.4.2. Membrana Cell
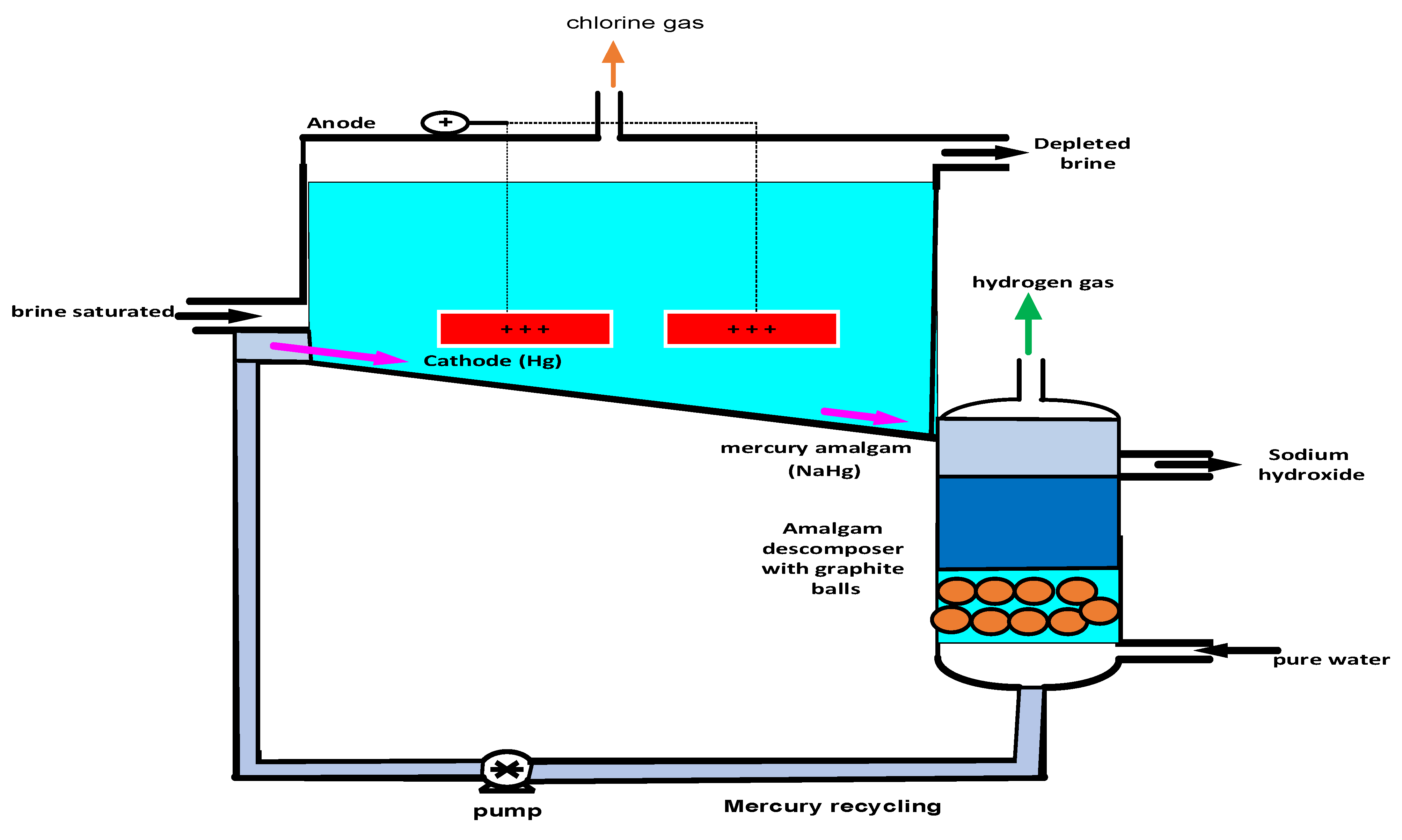
2.4.3. Mercury Cell
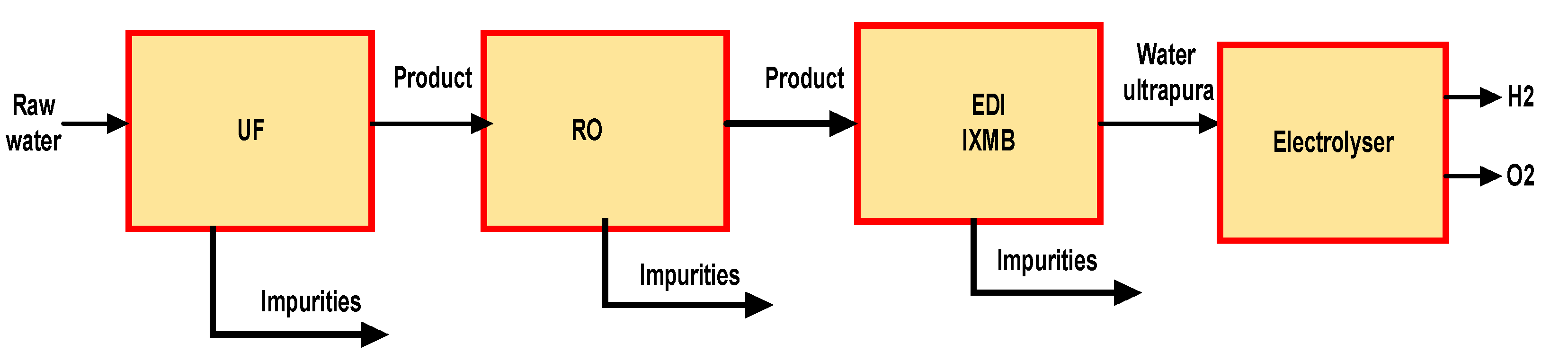
2.5. Water Purification
2.6. Types of Hydrogen Colors
2.7. Carbon Footprint Assessments
2.8. Hydrogen Use
2.9. Hydrogen Transport
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AEM | Anion exchange membrane |
| PEM | Proton exchange membrane |
| CCS | Carbon capture and storage |
| KOH | Potassium hydroxide |
| UF | Ultrafiltration |
| RO | Reverse osmosis |
| IXMB | Ion exchange mixed bed |
| EDI | Electrodeionization |
| GHG | High greenhouse gas |
References
- Xia, W.; Zhang, J.; Xu, G.; Jin, T.; Wang, Q.; Jiao, L. Recent advances and challenges in single-atom catalysts for proton exchange membrane water electrolysis. Next Mater. 2025, 8, 100553. [Google Scholar] [CrossRef]
- Roucham, B.; Zaghdoud, O. Mapping Green Hydrogen and Renewable Energy Research in Extended BRICS (Brazil, Russia, India, China, South Africa and Others): A Bibliometric Approach with a Future Agenda. Hydrogen 2025, 6, 33. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Li, X.; Ye, T.; Meng, X.; He, D.; Li, L.; Song, K.; Jiang, J.; Sun, C. Advances in the Application of Sulfonated Poly(Ether Ether Ketone) (SPEEK) and Its Organic Composite Membranes for Proton Exchange Membrane Fuel Cells (PEMFCs). Polymers 2024, 16, 2840. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, D.-C.; Lee, J.-J.; Kim, C.-W. Optimization for maximum specific energy density of a lithium-ion battery using progressive quadratic response surface method and design of experiments. Sci. Rep. 2020, 10, 15586. [Google Scholar] [CrossRef]
- Peng, W.; Xu, Y.; Li, G.; Song, J.; Xu, G.; Xu, X.; Pan, Y. Research on typical operating conditions of hydrogen production system with off-grid wind power considering the characteristics of proton exchange membrane electrolysis cell. Glob. Energy Interconnect. 2024, 7, 642–652. [Google Scholar] [CrossRef]
- Mandal, M. Novel Electrocatalyst for Alkaline Membrane Water Electrolysis. ChemElectroChem 2020, 7, 4303–4305. [Google Scholar] [CrossRef]
- Mostafaeipour, A.; Sedeh, A.S. Investigation of solar energy utilization for production of hydrogen and sustainable chemical fertilizer: A case study. Int. J. Energy Res. 2019, 43, 8314–8336. [Google Scholar] [CrossRef]
- Yang, X.; Nielsen, C.P.; Song, S.; McElroy, M.B. Breaking the hard-to-abate bottleneck in China’s path to carbon neutrality with clean hydrogen. Nat. Energy 2022, 7, 955–965. [Google Scholar] [CrossRef]
- Edwards, R.L.; Font-Palma, C.; Howe, J. The status of hydrogen technologies in the UK: A multi-disciplinary review. Sustain. Energy Technol. Assess. 2021, 43, 100901. [Google Scholar] [CrossRef]
- Azam, A.; Rafiq, M.; Shafique, M.; Yuan, J. Mitigating Carbon Emissions in China: The Role of Clean Energy, Technological Innovation, and Political-Institutional Quality. Front. Environ. Sci. 2022, 10, 814439. [Google Scholar] [CrossRef]
- Aravindan, M.; Kumar, P. Hydrogen towards sustainable transition: A review of production, economic, environmental impact and scaling factors. Results Eng. 2023, 20, 101456. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Jin, Z.; Gu, D.; Li, P.; Ye, G.; Zhu, H.; Wei, K.; Li, C.; Zhong, W.; Du, W.; Zhu, Q. Artificial intelligence-driven catalyst design for electrocatalytic hydrogen production: Paradigm innovation and challenges in material discovery. Sustain. Chem. Energy Mater. 2025, 2, 100010. [Google Scholar] [CrossRef]
- Gomonov, K.; Permana, C.T.; Handoko, C.T. The growing demand for hydrogen: Current trends, sectoral analysis, and future projections. Unconv. Resour. 2025, 6, 100176. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef]
- Madon, R.H.; Ali, M.F.M.; Khairul, L.; Sarwani, K.L.; Osman, S.A.; Razali, M.A.; Wahab, A.; Baru, P.A. Effect of Reaction Temperature on Steam Methane Reforming’s yield over Coated Nickel Aluminide (Ni 3 Al) Catalyst in Micro Reactor. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 50, 170–177. [Google Scholar]
- Railkar, R.; Kwak, Y.; Vlachos, D.G. Intensifying steam methane reforming and water-gas shift in tandem via rapid pulsed Joule heating. Chem. Eng. J. 2025, 512, 162700. [Google Scholar] [CrossRef]
- Davies, W.G.; Babamohammadi, S.; Yang, Y.; Soltani, S.M. The rise of the machines: A state-of-the-art technical review on process modelling and machine learning within hydrogen production with carbon capture. Gas Sci. Eng. 2023, 118, 205104. [Google Scholar] [CrossRef]
- Yan, Y.; Thanganadar, D.; Clough, P.T.; Mukherjee, S.; Patchigolla, K.; Manovic, V.; Anthony, E.J. Process simulations of blue hydrogen production by upgraded sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 222, 113144. [Google Scholar] [CrossRef]
- Gil, A.G.; Wu, Z.; Chadwick, D.; Li, K. Ni/SBA-15 Catalysts for combined steam methane reforming and water gas shift—Prepared for use in catalytic membrane reactors. Appl. Catal. A Gen. 2015, 506, 188–196. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Grande, C.A.; Lopes, F.V.; Loureiro, J.M.; Rodrigues, A.E. A parametric study of layered bed PSA for hydrogen purification. Chem. Eng. Sci. 2008, 63, 5258–5273. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Loachamin, D.; Casierra, J.; Calva, V.; Palma-Cando, A.; Ávila, E.E.; Ricaurte, M. Amine-Based Solvents and Additives to Improve the CO2 Capture Processes: A Review. Chemengineering 2024, 8, 129. [Google Scholar] [CrossRef]
- Choi, W.-J.; Seo, J.-B.; Jang, S.-Y.; Jung, J.-H.; Oh, K.-J. Removal characteristics of CO2 using aqueous MEA/AMP solutions in the absorption and regeneration process. J. Environ. Sci. 2009, 21, 907–913. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.I.; Kim, J.; Park, S.; Kim, H.S.; Woo, M.; Kim, J.R.; Lim, D.-H. Simple and efficient fabrication of cathode using activated polyaniline as binder for alkaline water electrolysis. Appl. Surf. Sci. 2025, 693, 162785. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Husaini, M.A.; Darmanto, P.S.; Juangsa, F.B. Integration of geothermal power plant, water treatment plant, AWE, and PEM electrolyzer for green hydrogen production: A techno-economic study. Next Energy 2025, 7, 100288. [Google Scholar] [CrossRef]
- Wang, C.R.; Stansberry, J.M.; Mukundan, R.; Chang, H.-M.J.; Kulkarni, D.; Park, A.M.; Plymill, A.B.; Firas, N.M.; Liu, C.P.; Lang, J.T.; et al. Proton Exchange Membrane (PEM) Water Electrolysis: Cell-Level Considerations for Gigawatt-Scale Deployment. Chem. Rev. 2025, 125, 1257–1302. [Google Scholar] [CrossRef] [PubMed]
- Hembach-Stunden, K.; Banning, M.; Becker, L.; Lutz, C.; Matschoss, P.; Klann, U.; Horst, J. Future Installation, Production and Global Trade of Clean Energy Technologies. Sustainability 2024, 16, 10482. [Google Scholar] [CrossRef]
- Clapp, M.; Zalitis, C.M.; Ryan, M. Perspectives on current and future iridium demand and iridium oxide catalysts for PEM water electrolysis. Catal. Today 2023, 420, 114140. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy 2024, 107, 218–240. [Google Scholar] [CrossRef]
- Bayat, A.; Das, P.K.; Saha, G.; Saha, S.C. Proton Exchange Membrane Electrolysis Revisited: Advancements, Challenges, and Two-Phase Transport Insights in Materials and Modelling. Eng 2025, 6, 72. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, Y.; Yin, F.; Li, J.; Kang, F.; Lv, R. Stabilizing non-iridium active sites by non-stoichiometric oxide for acidic water oxidation at high current density. Nat. Commun. 2023, 14, 7644. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, G. Seawater electrolysis for hydrogen production: A solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Ribeiro, C.; Santos, D.M.F. Transitioning Ammonia Production: Green Hydrogen-Based Haber–Bosch and Emerging Nitrogen Reduction Technologies. Clean Technol. 2025, 7, 49. [Google Scholar] [CrossRef]
- Mirshekari, G.; Ouimet, R.; Zeng, Z.; Yu, H.; Bliznakov, S.; Bonville, L.; Niedzwiecki, A.; Capuano, C.; Ayers, K.; Maric, R. High-performance and cost-effective membrane electrode assemblies for advanced proton exchange membrane water electrolyzers: Long-term durability assessment. Int. J. Hydrogen Energy 2021, 46, 1526–1539. [Google Scholar] [CrossRef]
- Sezer, N.; Bayhan, S.; Fesli, U.; Sanfilippo, A. A comprehensive review of the state-of-the-art of proton exchange membrane water electrolysis. Mater. Sci. Energy Technol. 2025, 8, 44–65. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Grimmer, I.; Ranz, M.; Höglinger, M.; Kartusch, S.; Rauh, J.; Macherhammer, M.-G.; Grabner, B.; Trattner, A. A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: Insights for stack application, development, and research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Poimer, F.; Köll, R.; Macherhammer, M.-G.; Trattner, A. Main degradation mechanisms of polymer electrolyte membrane fuel cell stacks—Mechanisms, influencing factors, consequences, and mitigation strategies. Int. J. Hydrogen Energy 2023, 50, 1159–1182. [Google Scholar] [CrossRef]
- Chang, S.H.; Rajuli, M.F. An overview of pure hydrogen production via electrolysis and hydrolysis. Int. J. Hydrogen Energy 2024, 84, 521–538. [Google Scholar] [CrossRef]
- Kombargi, A.; Bao, B.; Ellis, E.; Hart, D.P. Life-cycle assessment and cost analysis of hydrogen production via aluminum-seawater reactions. Cell Rep. Sustain. 2025, 2, 100407. [Google Scholar] [CrossRef]
- Bodkhe, R.G.; Shrivastava, R.L.; Soni, V.K.; Chadge, R.B. A review of renewable hydrogen generation and proton exchange membrane fuel cell technology for sustainable energy development. Int. J. Electrochem. Sci. 2023, 18, 100108. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- A Lee, S.; Kim, J.; Kwon, K.C.; Park, S.H.; Jang, H.W. Anion exchange membrane water electrolysis for sustainable large-scale hydrogen production. Carbon Neutralization 2022, 1, 26–48. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Riaz, M.A.; Trogadas, P.; Aymé-Perrot, D.; Sachs, C.; Dubouis, N.; Girault, H.; Coppens, M.-O. Water electrolysis technologies: The importance of new cell designs and fundamental modelling to guide industrial-scale development. Energy Environ. Sci. 2025, 18, 5190–5214. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. Water Electrolysis Technologies and Their Modeling Approaches: A Comprehensive Review. Eng 2025, 6, 81. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kamaroddin, M.F.A.; Sabli, N.; Abdullah, T.A.T.; Siajam, S.I.; Abdullah, L.C.; Jalil, A.A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weissgaerber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Ferreira, A.P.R.A.; Oliveira, R.C.P.; Mateus, M.M.; Santos, D.M.F. A Review of the Use of Electrolytic Cells for Energy and Environmental Applications. Energies 2023, 16, 1593. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, J.; Ye, D.; Wang, Y.; Zhang, L.; Zhu, X.; Liao, Q. A comprehensive study of parameters distribution in a short PEM water electrolyzer stack utilizing a full-scale multi-physics model. Energy 2024, 300, 131565. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, Q.; Zhang, Z.; Li, H.; Li, S. Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells. Eng 2025, 6, 111. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, C.; Tu, J.; Zhan, Z.; Zhang, B.; Liu, Q.; Zhang, Z.; Hu, H.; Liu, T. The multi-scenario projection of cost reduction in hydrogen production by proton exchange membrane (PEM) water electrolysis in the near future (2020–2060) of China. Fuel 2023, 354, 129409. [Google Scholar] [CrossRef]
- Bagheri, B.; Kumagai, H.; Hashimoto, M.; Sugiyama, M. Techno-Economic Assessment of Green Hydrogen Production in Australia Using Off-Grid Hybrid Resources of Solar and Wind. Energies 2025, 18, 3285. [Google Scholar] [CrossRef]
- Yin, C.; Jin, L. Estimating Hydrogen Price Based on Combined Machine Learning Models by 2060: Especially Comparing Regional Variations in China. Sustainability 2025, 17, 1049. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Pan, J.; Cross, J.L.; Zou, X.; Zhang, B. To tax or to trade? A global review of carbon emissions reduction strategies. Energy Strat. Rev. 2024, 55, 101508. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; SangmeshB; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Emblemsvåg, J. Rethinking the “Levelized Cost of Energy”: A critical review and evaluation of the concept. Energy Res. Soc. Sci. 2024, 119, 103897. [Google Scholar] [CrossRef]
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The renewable energy role in the global energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Sachse, D.; Glüsen, A.; Wippermann, K.; Müller, M.; Rau, U.; Peters, R. The Ammonia Adsorption and Desorption Behavior of Nafion. Membranes 2025, 15, 149. [Google Scholar] [CrossRef]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Lavorante, M.J.; Reynoso, C.Y.; Franco, J.I. Water electrolysis with Zirfon® as separator and NaOH as electrolyte. Desalination Water Treat. 2015, 56, 3647–3653. [Google Scholar] [CrossRef]
- Lee, H.I.; Mehdi, M.; Kim, S.K.; Cho, H.S.; Kim, M.J.; Cho, W.C.; Rhee, Y.W.; Kim, C.H. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode. J. Membr. Sci. 2020, 616, 118541. [Google Scholar] [CrossRef]
- Ali, M.F.; Lee, H.I.; Bernäcker, C.I.; Weißgärber, T.; Lee, S.; Kim, S.-K.; Cho, W.-C. Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer. Polymers 2022, 14, 1173. [Google Scholar] [CrossRef]
- Luo, X.; Xu, N.; Zhou, Y.; Yang, X.; Yang, W.; Liu, G.; Lee, J.K.; Qiao, J. Porous PVA skin-covered thin Zirfon-type separator as a new approach boosting high-rate alkaline water electrolysis beyond 1000 hours’ lifespan. eScience 2024, 4, 100290. [Google Scholar] [CrossRef]
- Chand, K.; Paladino, O. Recent developments of membranes and electrocatalysts for the hydrogen production by anion exchange membrane water electrolysers: A review. Arab. J. Chem. 2023, 16, 104451. [Google Scholar] [CrossRef]
- Lange, H.; Klose, A.; Lippmann, W.; Urbas, L. Technical evaluation of the flexibility of water electrolysis systems to increase energy flexibility: A review. Int. J. Hydrogen Energy 2023, 48, 15771–15783. [Google Scholar] [CrossRef]
- Kim, J.H.; Jo, H.J.; Han, S.M.; Kim, Y.J.; Kim, S.Y. Recent advances in electrocatalysts for anion exchange membrane water electrolysis: Design strategies and characterization approaches. Energy Mater. 2025, 5, 500099. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, B.; Yan, K.; Luo, J.; Haq, M.U.; Zeng, L. Long-term stability for anion exchange membrane water electrolysis: Recent development and future perspectives. Futur. Batter. 2025, 5, 100024. [Google Scholar] [CrossRef]
- Zheng, Y.; Colón, L.N.I.; Hassan, N.U.; Williams, E.R.; Stefik, M.; LaManna, J.M.; Hussey, D.S.; Mustain, W.E. Effect of membrane properties on the carbonation of anion exchange membrane fuel cells. Membranes 2021, 11, 102. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Sun, H.; Yang, L.; Huang, L. Advancements and Policy Implications of Green Hydrogen Production from Renewable Sources. Energies 2024, 17, 3548. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Juarez-García, M.; Sánchez-Ramírez, E. Green hydrogen production for sustainable development: A critical examination of barriers and strategic opportunities. RSC Sustain. 2024, 3, 134–157. [Google Scholar] [CrossRef]
- Jeje, S.O.; Marazani, T.; Obiko, J.O.; Shongwe, M.B. Advancing the hydrogen production economy: A comprehensive review of technologies, sustainability, and future prospects. Int. J. Hydrogen Energy 2024, 78, 642–661. [Google Scholar] [CrossRef]
- Eikeng, E.; Makhsoos, A.; Pollet, B.G. Critical and strategic raw materials for electrolysers, fuel cells, metal hydrides and hydrogen separation technologies. Int. J. Hydrogen Energy 2024, 71, 433–464. [Google Scholar] [CrossRef]
- Razmjooei, F.; Morawietz, T.; Taghizadeh, E.; Hadjixenophontos, E.; Mues, L.; Gerle, M.; Wood, B.D.; Harms, C.; Gago, A.S.; Ansar, S.A.; et al. Increasing the performance of an anion-exchange membrane electrolyzer operating in pure water with a nickel-based microporous layer. Joule 2021, 5, 1776–1799. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-scale green hydrogen production using alkaline water electrolysis based on seasonal solar radiation. Energy Harvest. Syst. 2024, 11. [Google Scholar] [CrossRef]
- Mazumder, G.C.; Parvez, S.; Zishan, S.R.; Rahman, H. Quantitative Analysis of Green H2 Production Costs: A Comparison between Domestic Developed and Imported Electrolyzers. Emerg. Sci. Innov. 2024, 3, 12–26. [Google Scholar] [CrossRef]
- Titheridge, L.J.; Marshall, A.T. Techno-economic modelling of AEM electrolysis systems to identify ideal current density and aspects requiring further research. Int. J. Hydrogen Energy 2024, 49, 518–532. [Google Scholar] [CrossRef]
- Krishnan, S.; Koning, V.; de Groot, M.T.; de Groot, A.; Mendoza, P.G.; Junginger, M.; Kramer, G.J. Present and future cost of alkaline and PEM electrolyser stacks. Int. J. Hydrogen Energy 2023, 48, 32313–32330. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Touili, S.; Merrouni, A.A.; El Hassouani, Y.; Amrani, A.-I.; Rachidi, S. Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones. Int. J. Hydrogen Energy 2020, 45, 26785–26799. [Google Scholar] [CrossRef]
- Krishnan, S.; Corona, B.; Kramer, G.J.; Junginger, M.; Koning, V. Prospective LCA of alkaline and PEM electrolyser systems. Int. J. Hydrogen Energy 2024, 55, 26–41. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Feliczak-Guzik, A. Hydrogen Production Using Modern Photocatalysts. Coatings 2024, 14, 366. [Google Scholar] [CrossRef]
- Pokrant, S.; Dilger, S.; Landsmann, S.; Trottmann, M. Size effects of cocatalysts in photoelectrochemical and photocatalytic water splitting. Mater. Today Energy 2017, 5, 158–163. [Google Scholar] [CrossRef]
- Jakhar, M.; Kumar, A.; Ahluwalia, P.K.; Tankeshwar, K.; Pandey, R. Engineering 2D Materials for Photocatalytic Water-Splitting from a Theoretical Perspective. Materials 2022, 15, 2221. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Qanugo, K.; Bose, D.; Thakur, K.K. A Review on Photocatalytic Water Splitting. In Proceedings of the 3rd International Conference on Design and Manufacturing Aspects for Sustainable Energy (ICMED-ICMPC 2021), Hyderabad, India, 24–26 December 2021. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Bratovčić, A.; Tomašić, V. Hydrogen Production Through Newly Developed Photocatalytic Nanostructures and Composite Materials. Processes 2025, 13, 1813. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Wei, Y.-C.; Torimoto, T.; Chien, Y.-A.; Chen, C.-Y.; Chang, T.-F.M.; Sone, M.; Hsieh, P.-Y.; Hsu, Y.-J. Yolk@Shell Nanostructures for Water Splitting: Current Development and Future Prospects. ACS Mater. Lett. 2024, 6, 4066–4089. [Google Scholar] [CrossRef]
- Sun, X.; Han, J.; Guo, R. A Mini Review on Yolk-Shell Structured Nanocatalysts. Front. Chem. 2020, 8, 606044. [Google Scholar] [CrossRef]
- Shu, Z.; Zhao, Z.; Zhou, J.; Wang, Y.; Wang, W.; Li, T. Natural molybdenite mineral enhanced polymeric carbon nitride nano-composites for efficient noble-metal-free photocatalytic hydrogen evolution. Mater. Res. Bull. 2021, 136, 111158. [Google Scholar] [CrossRef]
- Qiao, F. Photoelectrocatalytic hydrogen production: Hydrogen production principle, performance optimization strategy, application and prospect. Nano Res. Energy 2025, 4, e9120132. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, N.K.; Kumar, R.S.; Singh, R. Production of Green Hydrogen through Photocatalysis. Am. Chem. Soc. 2024, 1468, 1–24. [Google Scholar] [CrossRef]
- Xue, J.; Yang, Q.; Guan, R.; Shen, Q.; Liu, X.; Jia, H.; Li, Q. High-performance ordered porous Polypyrrole/ZnO films with improved specific capacitance for supercapacitors. Mater. Chem. Phys. 2020, 256, 123591. [Google Scholar] [CrossRef]
- Li, K.; Dong, W.J.; Mi, Z. Photoelectrochemical water splitting under concentrated sunlight: Best practices and protocols. Front. Energy Res. 2025, 13, 1550153. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhu, Q.; Qin, W.; Han, G.; Shen, J.; Zong, X.; Li, C. Spatially Separated Photosystem II and a Silicon Photoelectrochemical Cell for Overall Water Splitting: A Natural–Artificial Photosynthetic Hybrid. Angew. Chem. Int. Ed. Engl. 2016, 55, 9229–9233. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach. Sci. Total. Environ. 2017, 580, 147–157. [Google Scholar] [CrossRef]
- Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. When life cycle thinking is necessary for decision making: Emerging cleaner technologies in the chlor-alkali industry. Chem. Eng. Trans. 2016, 52, 475–480. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, X.; Xiao, C.; Yin, X.; Wang, X.; Wang, Z.; Yu, H.; Han, Z.; Lin, L. Advancements in purification and holistic utilization of industrial by-product hydrogen: Progress, challenges, and prospects. Green Energy Resour. 2024, 2, 100098. [Google Scholar] [CrossRef]
- Khan, J.R.; Muhammad, S.; Feroze, N.; Mustsfa Ali Bukhari Yasir Khurshid, S.; Wahab Malik, A. Brine Puri-fication for Chlor-Alkalis Production Based on Membrane Technology. Pak. J. Eng. Appl. Sci. 2015, 16, 17–24. Available online: https://journal.uet.edu.pk/ojs_old/index.php/pjeas/article/view/67/25 (accessed on 8 September 2025).
- Ali, W.; Jiang, C.; Dehghanpour, H. A Critical Review of Produced Water Management Using the Chlor-Alkali Process: Challenges and Future Prospects. Water Environ. Res. 2025, 97, e70124. [Google Scholar] [CrossRef]
- Aziz, H.; Ghazali, M.; Yusoff, M.; Hung, Y. Waste Treatment and Management in Chlor-Alkali Industries. In Waste Treatment in the Service and Utility Industries; CRC Press: Boca Raton, FL, USA, 2017; pp. 99–144. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Hydrogen Production by Electrical Energy. In Sustainable Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–161. [Google Scholar] [CrossRef]
- Samiee, L.; Goodarzvand-Chegini, F.; Ghasemikafrudi, E.; Kashefi, K. Hydrogen recovery in an industrial chlor-alkali plant using alkaline fuel cell and hydrogen boiler techniques: Techno-economic assessment and emission estimation. J. Renew. Energy Environ. 2021, 18, 49–57. [Google Scholar] [CrossRef]
- Islam, T.; Qadir, S.A.; Ali, A.; Khan, M.W. Economic and environmental impact assessment of renewable energy integration: A review and future research directions. Clean. Energy Syst. 2024, 9, 100162. [Google Scholar] [CrossRef]
- Beswick, R.R.; Oliveira, A.M.; Yan, Y. Does the Green Hydrogen Economy Have a Water Problem? ACS Energy Lett. 2021, 6, 3167–3169. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.; de Leão, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Holst, M.; Aschbrenner, S.; Smolinka, T.; Voglstätter, C.; Grimm, G. Cost Forecast for Low Temperature Electrolysis-Technology Driven Bottom-Up Prognosis for PEM and Alkaline Water Electrolysis Systems; Fraunhofer Institute for Solar Energy Systems ISE: Freiburg, Germany, 2021; 79p. [Google Scholar]
- Wood, J.; Gifford, J.; Arba, J.; Shaw, M. Production of ultrapure water by continuous electrodeionization. Desalination 2010, 250, 973–976. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Electrodeionization theory, mechanism and environmental applications. A review. Environ. Chem. Lett. 2020, 18, 1209–1227. [Google Scholar] [CrossRef]
- Grimmig, R.; Gillemot, P.; Stucki, S.; Günther, K.; Baltruschat, H.; Witzleben, S. Operating an ozone-evolving PEM electrolyser in tap water: A case study of water and ion transport. Sep. Purif. Technol. 2022, 292, 121063. [Google Scholar] [CrossRef]
- Lin, N.; Arzumanyan, M.; Calzado, E.R.; Nicot, J.-P. Water Requirements for Hydrogen Production: Assessing Future Demand and Impacts on Texas Water Resources. Sustainability 2025, 17, 385. [Google Scholar] [CrossRef]
- Henriksen, M.S.; Matthews, H.S.; White, J.; Walsh, L.; Grol, E.; Jamieson, M.; Skone, T.J. Tradeoffs in Life Cycle Water Use and Greenhouse Gas Emissions of Hydrogen Production Pathways. Int. J. Hydrogen Energy 2023, 49, 1221–1234. [Google Scholar] [CrossRef]
- Anaya, K.; Oni, A.O.; Kumar, A. Water intensity for hydrogen production with and without carbon capture and sequestration. J. Environ. Chem. Eng. 2025, 13, 117572. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Reichartz, T.; Jacobs, G.; Rathmes, T.; Blickwedel, L.; Schelenz, R. Optimal position and distribution mode for on-site hydrogen electrolyzers in onshore wind farms for a minimal levelized cost of hydrogen (LCoH). Wind. Energy Sci. 2024, 9, 281–295. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the climate impacts of blue hydrogen production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Dumančić, A.; Vlahinić, N.; Skok, M. Replacing Gray Hydrogen with Renewable Hydrogen at the Consumption Location Using the Example of the Existing Fertilizer Plant. Sustainability 2024, 16, 6437. [Google Scholar] [CrossRef]
- Patel, G.H.; Havukainen, J.; Horttanainen, M.; Soukka, R.; Tuomaala, M. Climate change performance of hydrogen production based on life cycle assessment. Green Chem. 2024, 26, 992–1006. [Google Scholar] [CrossRef]
- Díaz, M.T.M.; Oróstica, H.C.; Guajardo, J. Economic Analysis: Green Hydrogen Production Systems. Processes 2023, 11, 1390. [Google Scholar] [CrossRef]
- Camargo, L.; Comas, D.; Escorcia, Y.C.; Alviz-Meza, A.; Caballero, G.C.; Portnoy, I. Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021. Energies 2023, 16, 87. [Google Scholar] [CrossRef]
- Alizadeh, S.M.; Khalili, Y.; Ahmadi, M. Comprehensive Review of Carbon Capture and Storage Integration in Hydrogen Production: Opportunities, Challenges, and Future Perspectives. Energies 2024, 17, 5330. [Google Scholar] [CrossRef]
- Ali, D.N.H.A.P.H.O.; Suhaimi, H.; Abas, P.E. Membrane-Based Hydrogen Production: A Techno-Economic Evaluation of Cost and Feasibility. Hydrogen 2025, 6, 9. [Google Scholar] [CrossRef]
- Galusnyak, S.; Petrescu, L.; Cormos, C.-C. Techno-economic and environmental assessment of hydrogen production based on natural gas steam reforming process. Stud. Univ. Babeș-Bolyai Chem. 2020, 65, 7–19. [Google Scholar] [CrossRef]
- Roy, R.; Antonini, G.; Hayibo, K.S.; Rahman, M.; Khan, S.; Tian, W.; Boutilier, M.S.; Zhang, W.; Zheng, Y.; Bassi, A.; et al. Comparative techno-environmental analysis of grey, blue, green/yellow and pale-blue hydrogen production. Int. J. Hydrogen Energy 2025, 116, 200–210. [Google Scholar] [CrossRef]
- Bento, C.; Lopes, T.F.; Rodrigues, P.; Gírio, F.; Silva, C. Biogas reforming as a sustainable solution for hydrogen production: Comparative environmental metrics with steam-methane reforming and water electrolysis in the Portuguese context. Int. J. Hydrogen Energy 2024, 66, 661–675. [Google Scholar] [CrossRef]
- European Union. Estimation of the Global Average GHG Emission Intensity of Hydrogen Production; JRC Technical Report; European Union: Brussels, Belgium, 2023. [Google Scholar] [CrossRef]
- Weinand, J.M.; McKenna, R.; Kleinebrahm, M.; Scheller, F.; Fichtner, W. The impact of public acceptance on cost efficiency and environmental sustainability in decentralized energy systems. Patterns 2021, 2, 100301. [Google Scholar] [CrossRef]
- Badruzzaman, A.; Karagoz, S.; Eljack, F. Sustainable-green hydrogen production through integrating electrolysis, water treatment and solar energy. Front. Chem. Eng. 2025, 7, 1526331. [Google Scholar] [CrossRef]
- Roucham, B.; Lefilef, A.; Zaghdoud, O.; Mohammed, K.S. The evolution of green hydrogen in renewable energy research: Insights from a bibliometric perspective. Energy Rep. 2025, 13, 576–593. [Google Scholar] [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Ampah, J.D.; Wilberforce, T.; Afrane, S.; Nutakor, C. Research Progress, Trends, and Current State of Development on PEMFC-New Insights from a Bibliometric Analysis and Characteristics of Two Decades of Research Output. Membranes 2022, 12, 1103. [Google Scholar] [CrossRef]
- Lipiäinen, S.; Lipiäinen, K.; Ahola, A.; Vakkilainen, E. Use of existing gas infrastructure in European hydrogen economy. Int. J. Hydrogen Energy 2023, 48, 31317–31329. [Google Scholar] [CrossRef]
- Hassan, M.A.; El-Amary, N.H. Economic and technical analysis of hydrogen production and transport: A case study of Egypt. Sci. Rep. 2025, 15, 9002. [Google Scholar] [CrossRef]
- Lipiäinen, S.; Vakkilainen, E. Feasibility of the transportation options of renewable hydrogen for moderate distances: Insights from a remote production in Finland. Energy Convers. Manag. X 2025, 27, 101158. [Google Scholar] [CrossRef]
- Jia, G.; Lei, M.; Li, M.; Xu, W.; Li, R.; Lu, Y.; Cai, M. Hydrogen embrittlement in hydrogen-blended natural gas transportation systems: A review. Int. J. Hydrogen Energy 2023, 48, 32137–32157. [Google Scholar] [CrossRef]
- Chung, S.-M.; Jeon, G.-M.; Park, J.-C. Numerical approach to analyze fluid flow in a type C tank for liquefied hydrogen carrier (part 1: Sloshing flow). Int. J. Hydrogen Energy 2022, 47, 5609–5626. [Google Scholar] [CrossRef]
- Amjath, M.; Eljack, F.; Haouari, M. Optimizing post-production alternate hydrogen supply chain pathways—An integrated TEA and LCA approach. Int. J. Hydrogen Energy 2025, 100, 1421–1443. [Google Scholar] [CrossRef]

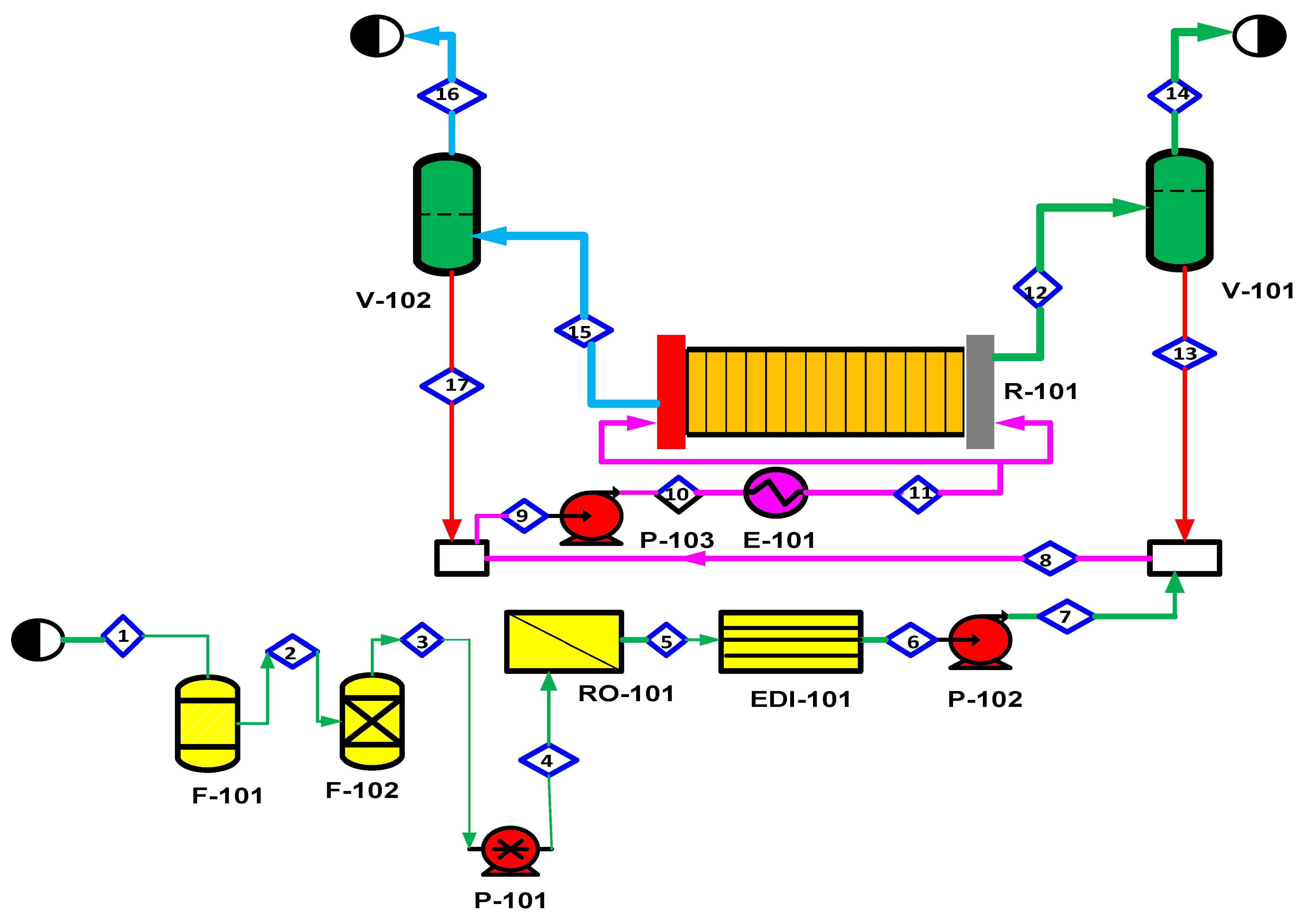
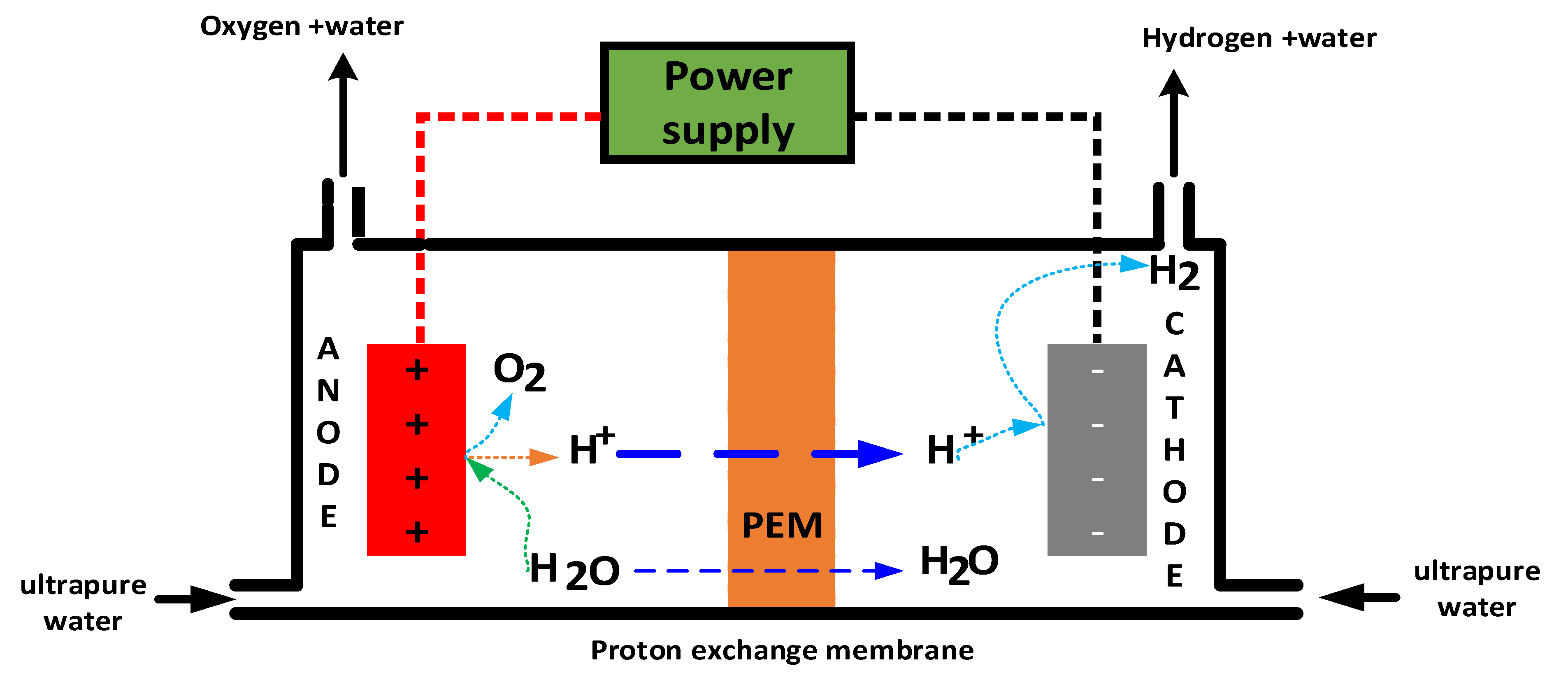
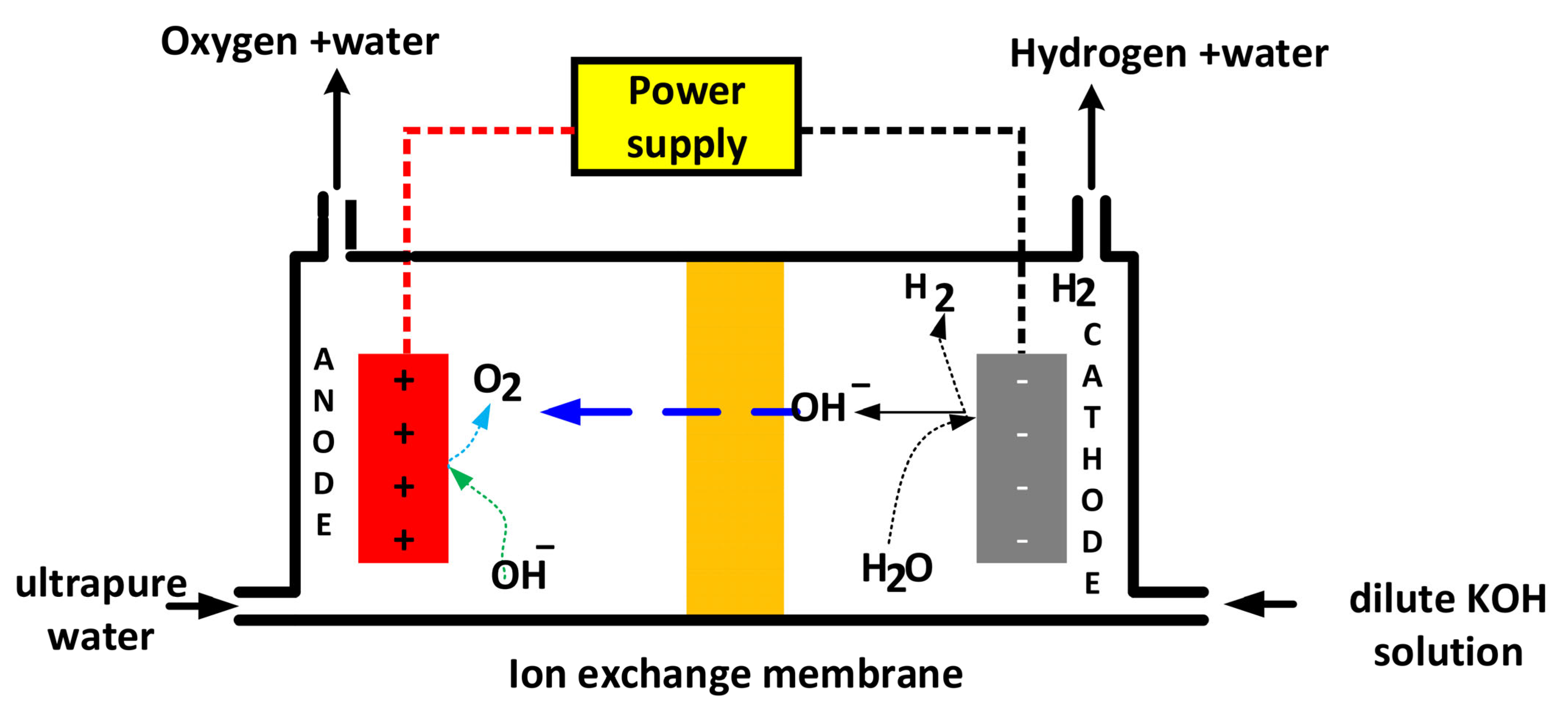
| Electrolysis Technology | Additional Raw Materials/Components | Remarks/Notes | References (R) |
|---|---|---|---|
| PEM (Proton Exchange Membrane) | Anode catalyst: typically IrO2 (Iridium oxide)—Cathode catalyst: typically Pt (Platinum)—Acidic membrane electrolyte (Nafion membrane) | Noble metal catalysts (Ir, Pt) are critical; acidic membrane is a solid electrolyte; pure water feed | [57] |
| WE (Alkaline Water Electrolysis) | Alkaline electrolyte (e.g., KOH solution)—Electrodes: typically Ni-based or other transition metals as catalysts—Supporting electrolyte materials | Mature, low cost, highly safe technology; uses liquid alkaline electrolyte; intermittent renewable energy integration is common | [87] |
| AEM (Anion Exchange Membrane Electrolysis) | Anion exchange membrane with quaternary ammonium ion exchange groups (e.g., A201 and A901 types of Tokuyama membranes)—Catalyst: PGM-free transition metal catalysts (Ni(OH)2, Fe(OOH), NiCoOx, CuCoOx, Ni/(CeO2-La2O3)/C)—Low concentration alkaline solutions or pure water feed—supporting electrolytes such as 1% K2CO3, KHCO3—Membrane binder materials (quaternized polystyrene) | Combines advantages of AWE and PEM; uses lower cost membranes and catalysts; electrolyte can be pure water or diluted alkaline solutions; research on catalysts and membrane stability ongoing; no corrosive liquid electrolyte | [57] |
| Parameter | Alkaline Water Electrolysis (AWE) | Proton Exchange Membrane (PEM) Electrolysis | Anion Exchange Membrane (AEM) Electrolysis | R |
|---|---|---|---|---|
| Cost of capital (Electrolyzer Stack, USD/kW) | Approx. 270 (current), target < 100 by 2050 | Approx. 1500–2200 (current) | Approximately (USD 444–USD 460/kW), Installation costs for AEM electrolysis systems ranging from 1 MW to 5 MW | [57] |
| Cost of capital (USD/kW) | USD 800–1200/kW (typical large scale) | USD 1500–2200/kW (higher due to the materials) | Currently TRL 6, ~USD 500–1000/kW, expected to decrease | [88] |
| Operating Cost | Electricity cost assumption ~USD 0.03/kWh; chemical costs due to KOH electrolyte are notable | Lower electricity consumption than AWE, no chemical costs | Potential cost advantage with cheaper catalysts and stainless steel components, but technology less mature | [91] |
| Annual Fixed Costs | Example: USD 228,115 per year (developed AE) | USD 191 990 per year (PEM) | For 1 MW plants, costs range from approximately USD 922 and USD 1279 kW. | [57] |
| Hydrogen Production Cost | Around USD 3.64–USD 4.76/kg (varies by scale and location) | Typically USD 4–USD 6/kg but can decrease to ~USD 2.15/kg in 10 years due to tech learning | 0.62–0.89 (USD/kg H2) Future and far-future projected costs per National Renewable Energy Lab (NREL) analysis | [90] |
| Electrical efficiency (kWh/kg H2) | 50–78 (system), objective < 45 para 2050 | 50–60 (improvement with higher current density) | Objective comparable to PEM, with ongoing R&D to achieve voltage efficiency > 70% | [92] |
| Current density (A/cm2) | 0.2–0.8 (present), objective > 2 | Generally around 2 | >1 currently, with the aim of reaching a higher level to match the PEM | [57] |
| Purity of hydrogen | 99.9–99.9998%, objective > 99.9999 | High purity (99.999%) | Comparable to PEM purity objectives | [88] |
| Raw materials | Nickel-based, widely available | Contains expensive Pt, Ir, and Ti as critical components | Nickel-based raw materials, more abundant and cheaper | [92] |
| System footprint | Bigger | 20–24% lower on similar scales | Potentially compact design as PEM, but requires expansion | [93] |
| Hydrogen Production Route | (kg Water/kg H2) | (kg CO2/kg H2) | Environmental Compensation | R |
|---|---|---|---|---|
| Steam methane reforming (SMR) without CCS | 15–40 (primarily cooling water) | ~10.4–12.4 | Moderate water use, high emissions of GHG | [125] |
| SMR with CCS | 18–45 | Reduction in GEI emissions compared to no CCS | Carbon capture and storage reduces emissions, but increases energy and water consumption. | [124] |
| Electrolysis (PEM) grid electricity | 220~280 | ~25–31 | Very high-water consumption, high dependence on electricity source; high GHG levels if grid electricity is used. | [126] |
| Electrolysis (PEM) renewable electricity (solar/wind) | (~30–40) | ~2–3 | Much lower water and GEG footprint when using renewable energy. | [124] |
| Hydrogen | Technology | Notes/Status | USD/kg H2 | g de CO2/g de H2 | Ref |
|---|---|---|---|---|---|
| Green | Electrolysis using renewables (wind/solar) | Cleanest, currently expensive | 2.3–7.4 [131] 3.6–5.8 [30] | ~0.3 g–1.5 g CO2/g H2 | [132] |
| 1.6 g–1.8 g CO2/g de H2 | [132] | ||||
| 2.5–9.5 [133] | ~0 g CO2/g H2 | [134] | |||
| 0 g CO2/g H2 | [36] | ||||
| Blue | SMR with carbon capture and storage (CCS) | Lower emissions, higher cost | 1.8–4.7 [132] 1.5–2.9 [30] | (200–300) g CO2/g de H2 | [135] |
| 1000–4000 | [132] | ||||
| ~8 [136] | 410 | [137] | |||
| 1000–2000 | [135] | ||||
| Grey | Steam Methane Reforming (SMR) from natural gas | Most common, high emissions | 1–2 [138] 0.7–2.5 [133] 1–2.1 [30] | 8000–9000 | [139] |
| 9000 | [20] | ||||
| (1000–1200) g CO2/g H2 | [140] | ||||
| 9000–11,000 | [141] | ||||
| 9000–13,000 | [138] |
| Category of Use | Description | R |
|---|---|---|
| Energy carrier and storage | Seasonal energy storage to complement intermittent wind and solar power. | [142] |
| Industrial raw material | Fertilizer production, refining, and chemical manufacturing. | [143] |
| Power generation | Fuel cells for electricity generation in stationary and portable applications. | [144] |
| heating medium | Decarbonizing building heating, including residential and commercial heating systems industrial process heating, boiler technologies | [145] |
| Transport fuel | Cars, buses, trucks, trains, ships, and potentially aircraft powered by hydrogen fuel cells. | [143] |
| Applications in space | Rocket fuel for propulsion (green hydrogen as a clean combustion propellant) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Collana, J.T.; Carrasco-Venegas, L.; Ancieta-Dextre, C.; Rodriguez-Taranco, O.; Gabriel-Hurtado, D.; Montaño-Pisfil, J.; Rodriguez-Aburto, C.; Chávez-Sánchez, W.; Santos-Mejía, C.; Morcillo-Valdivia, P.; et al. Analysis of the Main Hydrogen Production Technologies. Sustainability 2025, 17, 8367. https://doi.org/10.3390/su17188367
Medina Collana JT, Carrasco-Venegas L, Ancieta-Dextre C, Rodriguez-Taranco O, Gabriel-Hurtado D, Montaño-Pisfil J, Rodriguez-Aburto C, Chávez-Sánchez W, Santos-Mejía C, Morcillo-Valdivia P, et al. Analysis of the Main Hydrogen Production Technologies. Sustainability. 2025; 17(18):8367. https://doi.org/10.3390/su17188367
Chicago/Turabian StyleMedina Collana, Juan Taumaturgo, Luis Carrasco-Venegas, Carlos Ancieta-Dextre, Oscar Rodriguez-Taranco, Denis Gabriel-Hurtado, Jorge Montaño-Pisfil, Cesar Rodriguez-Aburto, Wilmer Chávez-Sánchez, Cesar Santos-Mejía, Pablo Morcillo-Valdivia, and et al. 2025. "Analysis of the Main Hydrogen Production Technologies" Sustainability 17, no. 18: 8367. https://doi.org/10.3390/su17188367
APA StyleMedina Collana, J. T., Carrasco-Venegas, L., Ancieta-Dextre, C., Rodriguez-Taranco, O., Gabriel-Hurtado, D., Montaño-Pisfil, J., Rodriguez-Aburto, C., Chávez-Sánchez, W., Santos-Mejía, C., Morcillo-Valdivia, P., & Herrera-Espinoza, N. (2025). Analysis of the Main Hydrogen Production Technologies. Sustainability, 17(18), 8367. https://doi.org/10.3390/su17188367






