Ammonia from Hydrogen: A Viable Pathway to Sustainable Transportation?
Abstract
1. Introduction
2. NH3 as an H2 Carrier
- -
- Gray NH3: NH3 is generated using natural gas or coal by the Haber–Bosch process. It is the most commonly used method, and it emits high CO2 emissions.
- -
- Blue NH3: NH3 is produced using natural gas through the Haber–Bosch process with carbon capture storage technology. It emits less CO2 compared to gray NH3, but it relies on fossil fuels.
- -
- Green NH3: Electrolysis of water, operated by renewable energy sources, is used to generate green H2, which then combines with nitrogen from the air to generate NH3. This process is considered the most sustainable method to generate NH3.
- -
- Turquoise NH3: A thermal decomposition of methane through methane pyrolysis is used to generate H2 and solid carbon. The generated H2 combines with the nitrogen from the air to generate NH3. This process generates solid carbon, which is easier to manage compared to gaseous CO2.
- -
- Yellow NH3: This type of NH3 is generated from yellow H2 combined with nitrogen. The yellow H2 is produced by water electrolysis operated by grid electricity that is supplied from a mix of renewable and non-renewable sources.
- -
- Pink NH3: The H2 used to generate NH3 is generated using water electrolysis powered by a nuclear energy source.
3. Review Methods
- -
- Inclusion: Peer-reviewed articles and conference papers; publications in English; studies focusing on the use of NH3 as a fuel for transportation (e.g., maritime, road, and aviation); papers discussing NH3 production pathways, infrastructure, safety, and policy implications related to its use as a fuel; and publications from the last two decades (2005–2025) to guarantee the review captures the most recent developments.
- -
- Exclusion: Publications not directly related to NH3 as a fuel or energy carrier and papers focused solely on NH3’s traditional uses (e.g., fertilizer and chemical feedstock).
4. Current and Future Use of NH3
5. Technologies Used to Generate NH3
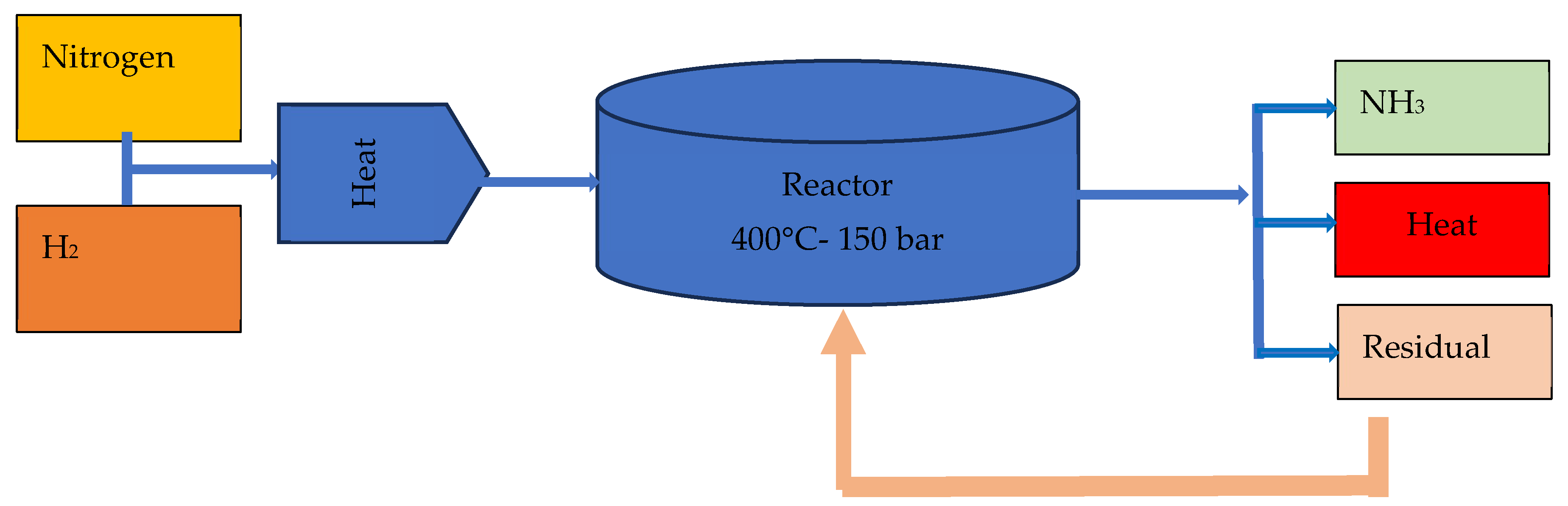
5.1. Haber–Bosch Process
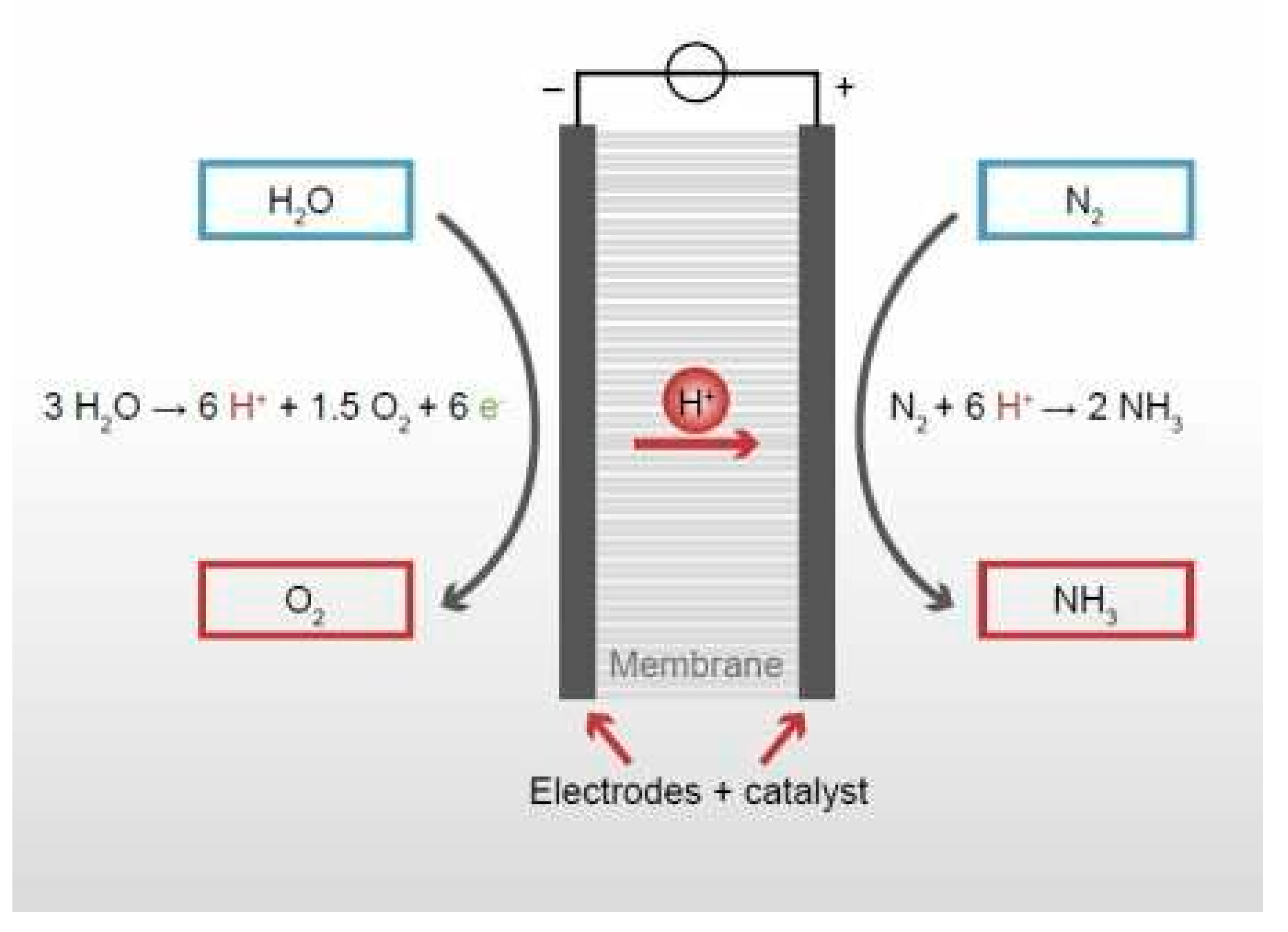
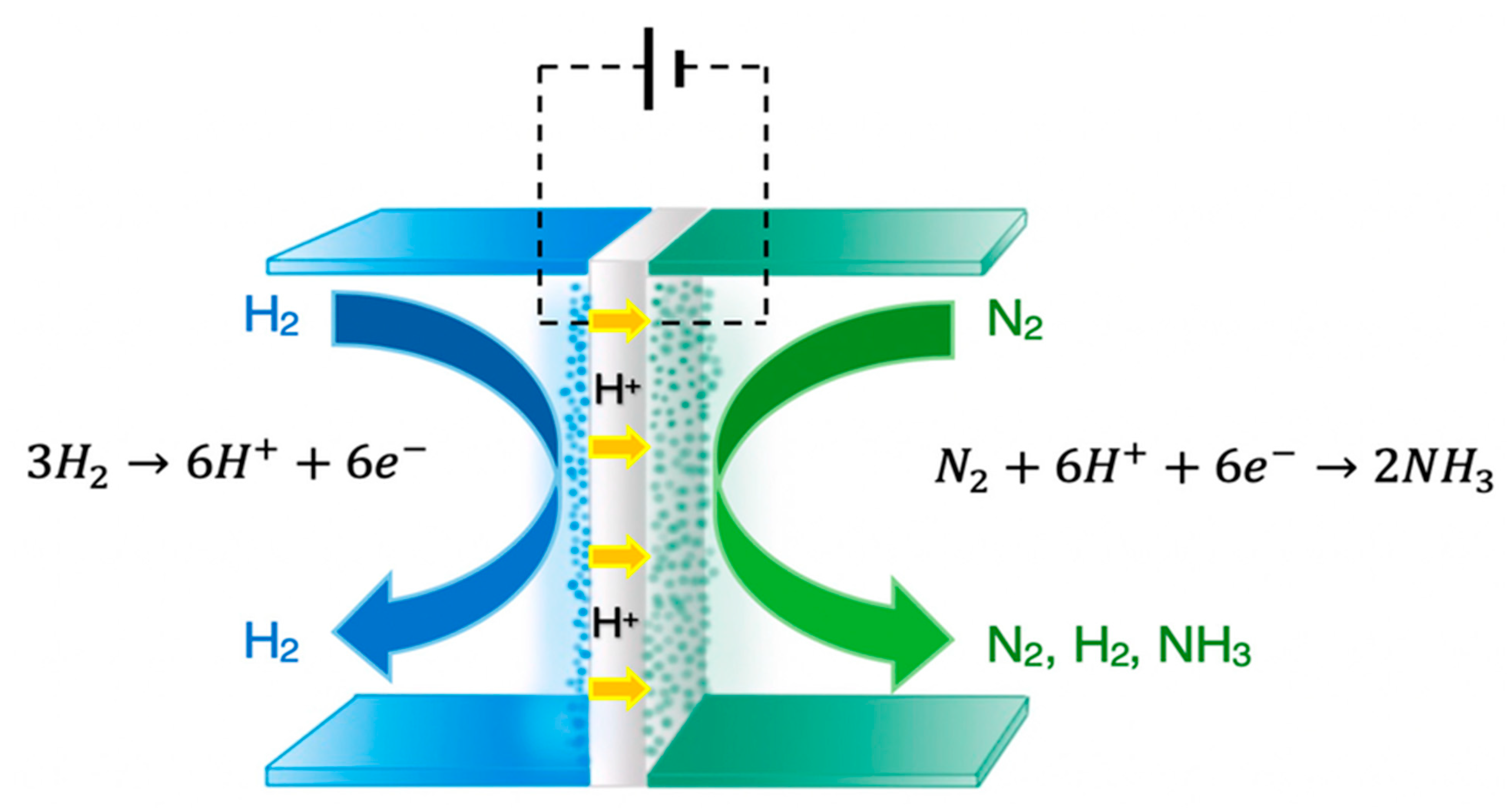
5.2. Electrochemical Synthesis
5.3. Solid-State NH3 Synthesis (SSAS)
5.4. Biological Nitrogen Fixation
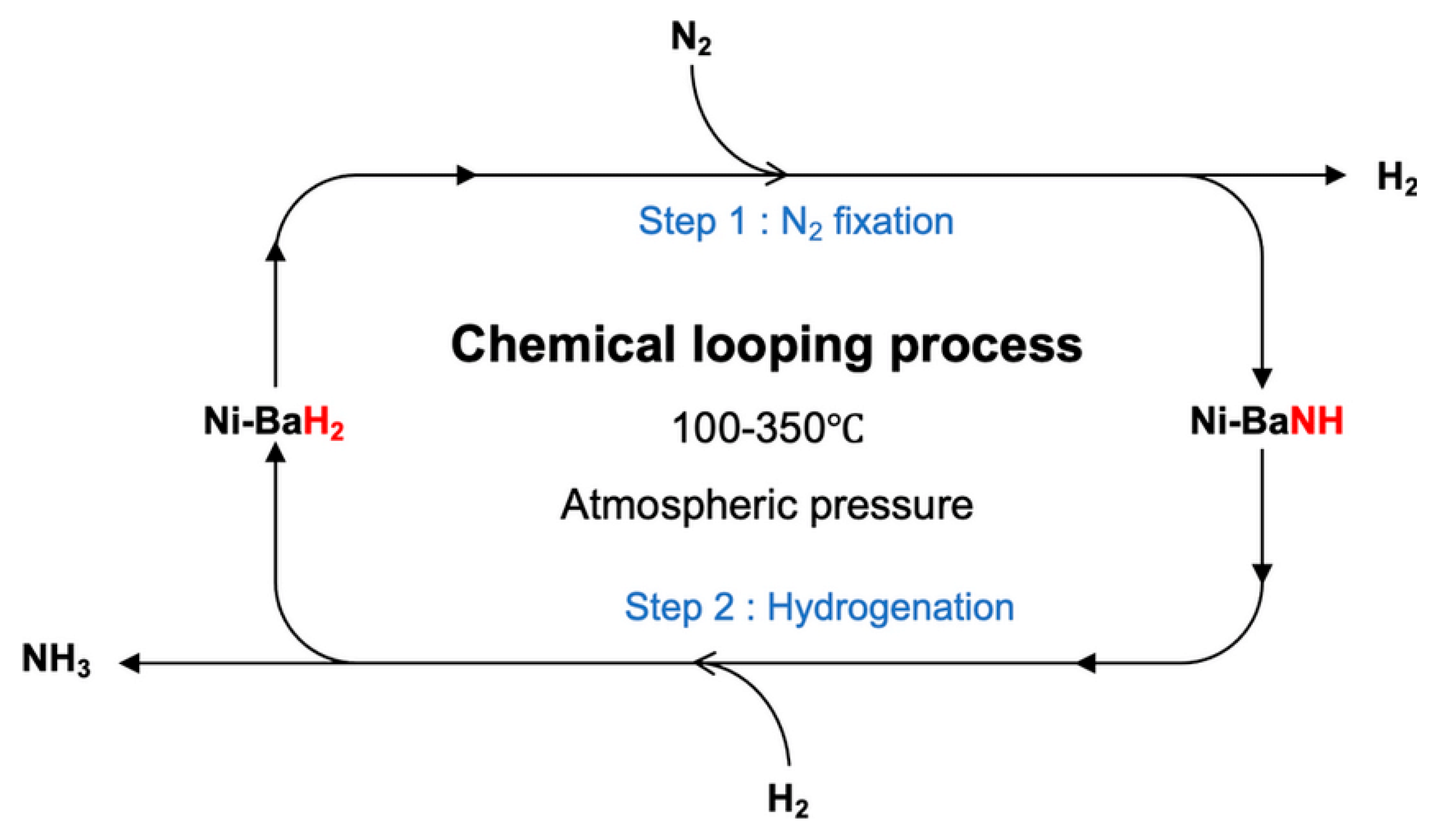
5.5. Thermochemical Looping
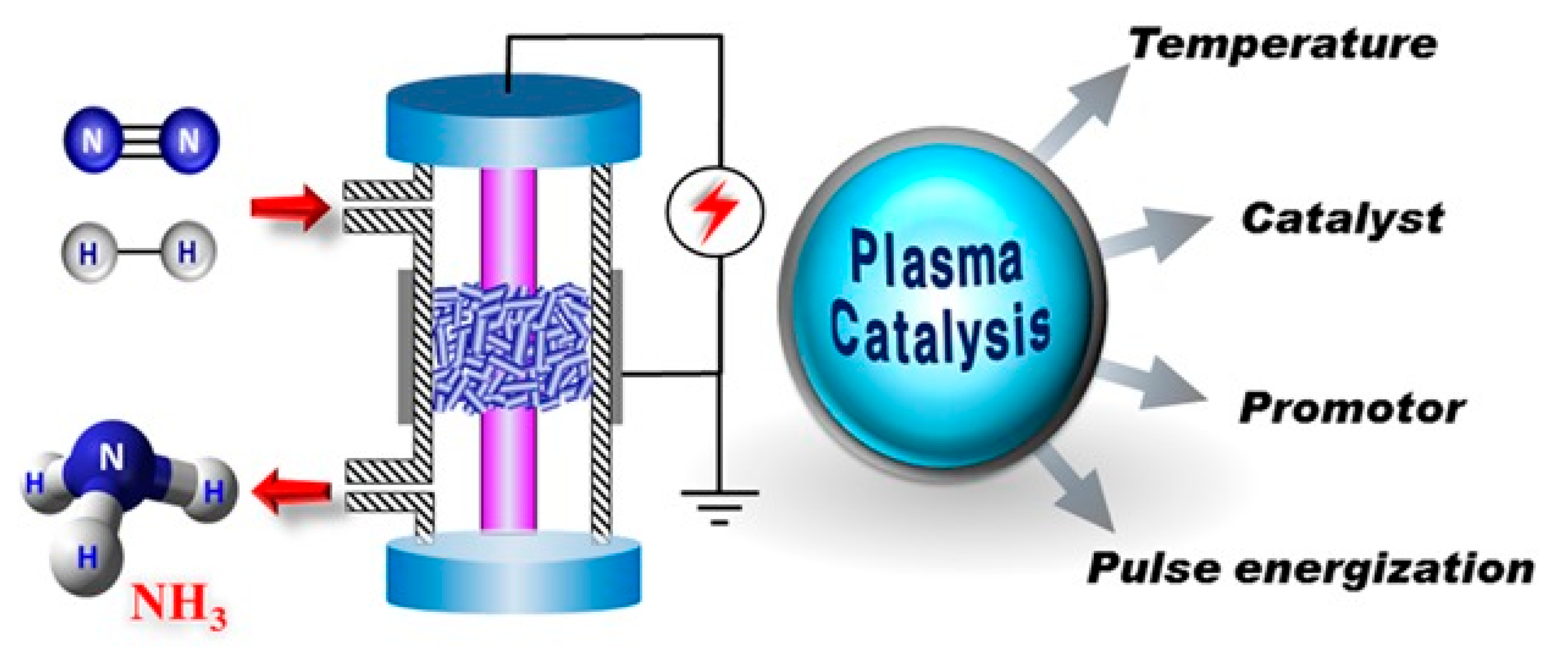
5.6. Plasma Synthesis
5.7. Photocatalytic Synthesis
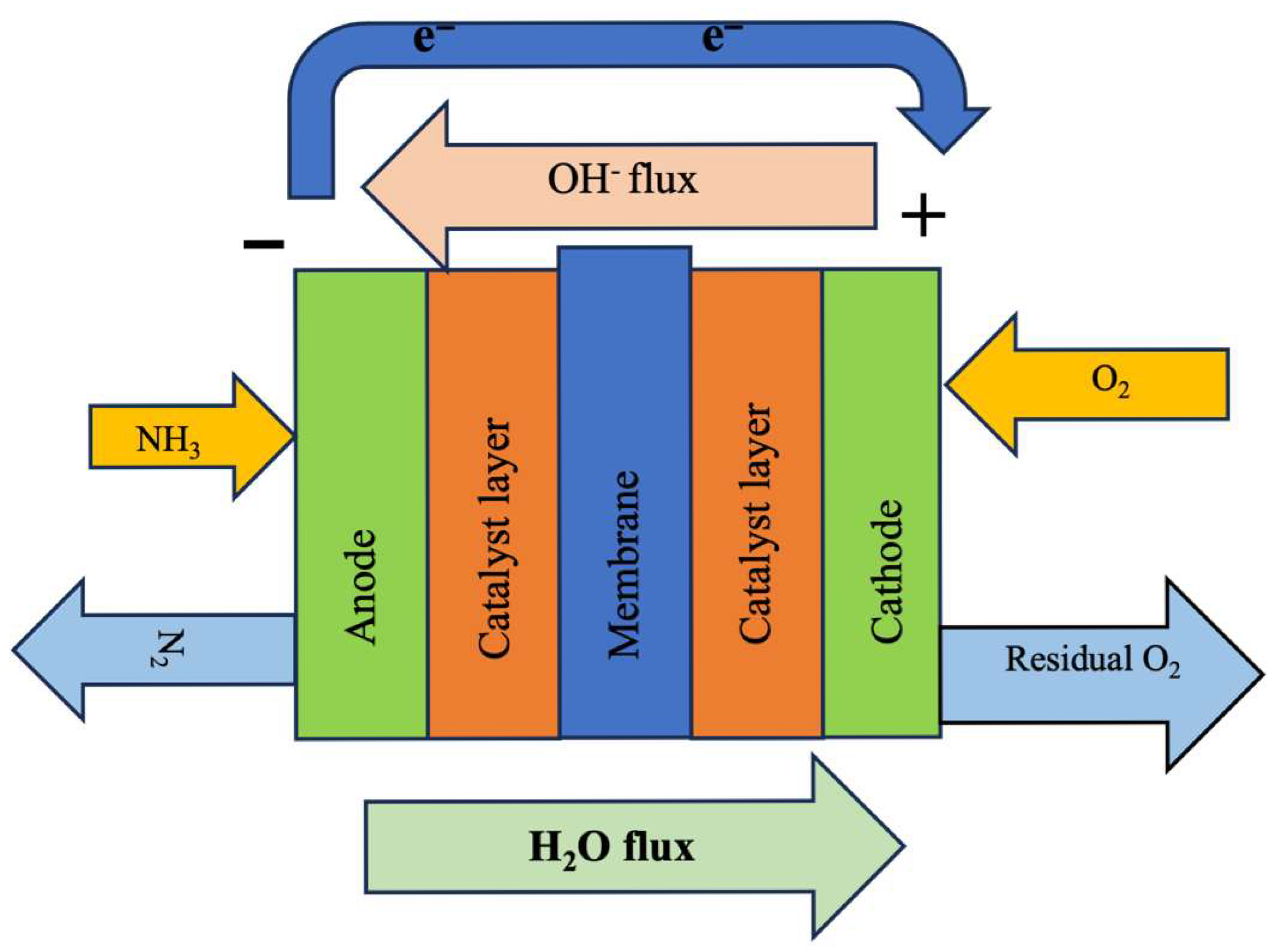
5.8. Hybrid Plasma–Electrochemical Synthesis
5.9. A Comparison Between the Different Technologies Used to Generate NH3
6. NH3 Utilization in Transportation
6.1. Ways of Using NH3 in Transportation
6.1.1. Direct Use in Internal Combustion Engines
6.1.2. Use of NH3 in Fuel Cells
6.1.3. Hybrid Systems
6.2. A Comparison Between the Various NH3 Utilization Technologies in the Transportation Sector
6.3. The Current State of Technology for NH3-Powered Vehicles
- -
- Marine transport: NH3 has a promising potential to be used in internal combustion engines and fuel cells in the maritime industry with less environmental impact compared to conventional fuels [64]. In internal combustion engines, NH3 could be used directly in a modified internal combustion engine or in dual-fuel systems, where NH3 is mixed with other fuel to enhance its combustion efficiency. Also, NH3 could be used as a fuel cell by implementing an onboard cracking system to decompose NH3 into H2 and nitrogen to generate electricity to power electric propulsion systems. Another way to use direct NH3 fuel cells is to directly convert NH3 into electricity without the need for an NH3 cracking system, but this technology is still under development. The benefits and challenges facing the use of NH3 in marine transport are the same as those in the aviation sector.
- -
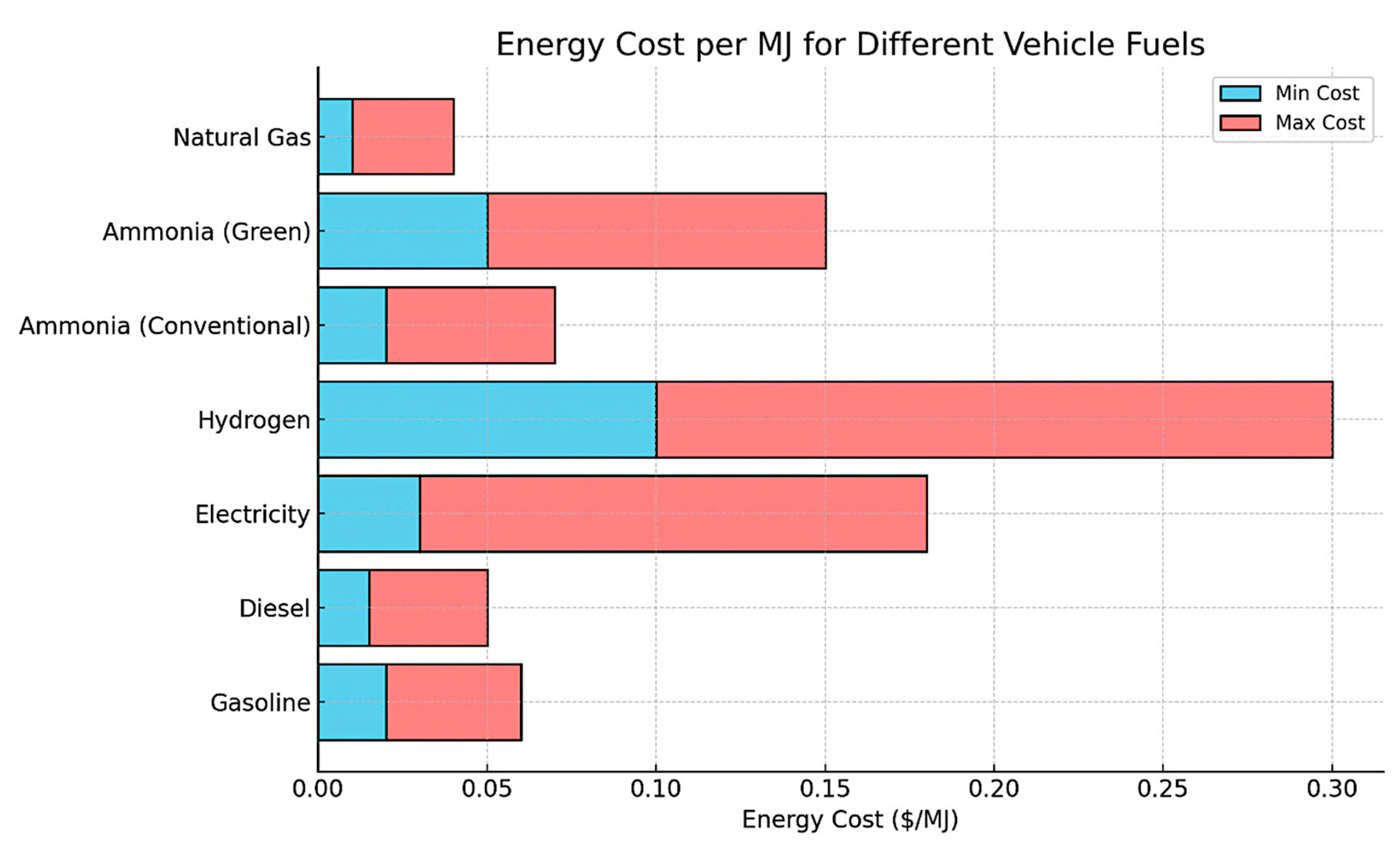
- Heavy-duty vehicles: The use of NH3 in trucks and buses can be either in internal combustion engines or in fuel cells. The internal combustion engines should be designed to burn NH3, and the fuel cell should be adjusted to convert NH3 into electricity [65]. Ongoing research and development are being conducted to adjust the design of engines to deal with the different barriers associated with efficiencies and emissions. The cost of different vehicle fuels in terms of energy cost is displayed in Figure 9, including conventional NH3, green NH3, and H2. Natural gas is the cheapest fuel with a narrow cost range, while green NH3 and H2 are more expensive, derived from the high cost of renewable energy. Conventional NH3 is cheaper but more costly than fossil fuels.
- -
- Aviation: NH3 is being viewed as a potential aviation fuel owing to its high energy density, existing infrastructure, storage and handling, and smaller carbon footprint [69]. In terms of energy density, NH3 has a higher energy density by volume compared to liquid H2, making it more convenient for long-trip flights. The existing infrastructure to generate and distribute NH3 for agriculture can be used for aviation. Additionally, NH3 can be stocked as a liquid state under moderate temperatures and pressures, resulting in easier storage and handling. However, some challenges are encountered in the use of NH3 in aviation, including toxicity and corrosivity, combustion efficiency, and the weight of the onboard cracking system. Stricter safety regulations are required to avoid any leak of NH3 [70]. The combustion efficiency of NH3 is lower compared to traditional jet fuels, requiring a substantial engine adaptation. The use of onboard cracking systems adds weight to the aircraft, negatively affecting the performance and the efficiency of the aircraft.
| Fuel | Energy Consumption (MJ/km) | Fuel Consumption (kg/tkm) |
|---|---|---|
| Kerosene (jet fuel A) | 343.5 | 0.21 |
| Natural Gas | 460 | 0.25 |
| Methanol | 360 | 0.49 |
| Ethanol | 360 | 0.33 |
| H2 | 316.5 | 0.07 |
| NH3 | 350 | 0.51 |
- -
- Rail transport: NH3 can be used to replace the use of diesel to power trains where electrification of railways is not practicable, offering a significant benefit. While promising, its broad implementation is challenged by engineering constraints, mainly attributed to its energy density; liquid NH3 possesses a volumetric energy density of approximately 12.7 MJ/L, significantly less than diesel’s 36 MJ/L, implying that NH3-powered locomotives would need either larger fuel tanks or more frequent refueling to achieve similar ranges [74]. NH3 provides approximately 18.6 MJ/kg of energy, significantly lower than diesel’s 42 MJ/kg. Regarding emissions, while NH3 combustion is carbon-free, it can lead to NOₓ emissions from the fuel’s nitrogen content. Nonetheless, its lower combustion temperatures may help limit thermal NOₓ from air nitrogen. Optimizing these variables necessitates sophisticated combustion technologies and exhaust after-treatment systems to control total NOx and potent nitrous oxide (N2O) emissions. Although technical hurdles remain, companies including Fortescue Future Industries (FFI) and leading locomotive manufacturers are actively pursuing research and conceptual design work to explore NH3’s integration into rail propulsion systems, frequently exploring both direct combustion in adapted engines and integration with high-efficiency fuel cells.
7. Challenges and Potential Solutions for NH3 as a Transportation Fuel
7.1. NOx Emissions Reduction
7.2. NH3 Slip
7.3. Engine/Fuel Cell Modifications
7.4. Fuel Efficiency
8. Infrastructure and Safety for NH3 as a Transportation Fuel
8.1. NH3 Refueling Infrastructure Requirements
8.2. Safety Considerations of Using NH3 as a Transportation Fuel
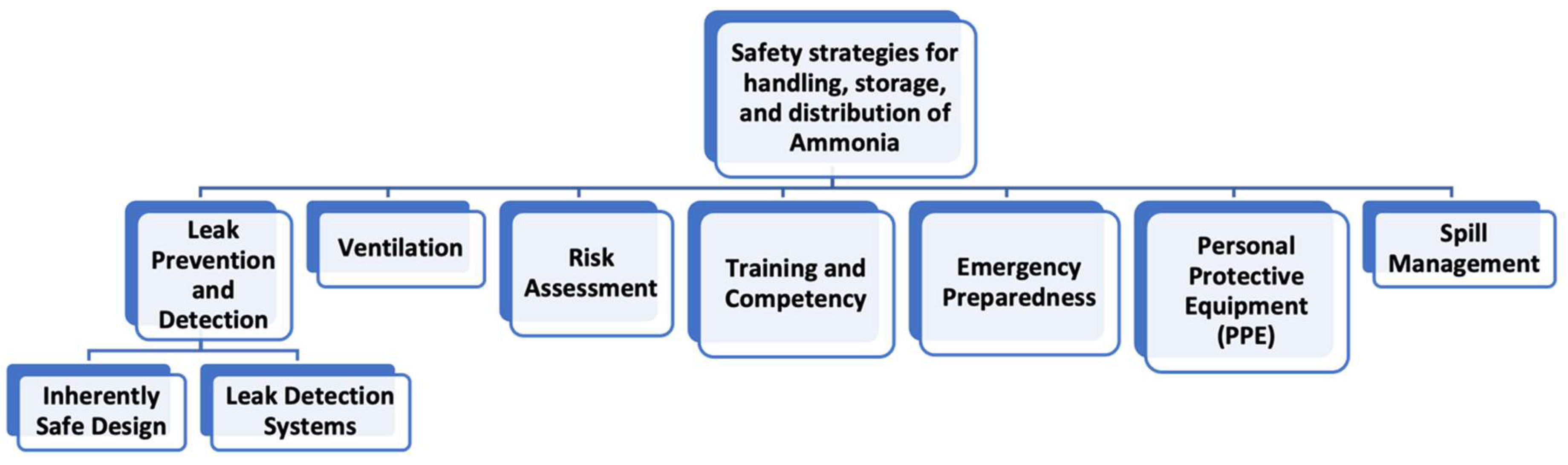
8.3. Strategies for Safe Handling, Storage, and Distribution of NH3
9. Environmental and Economic Analysis of NH3 as a Transportation Fuel
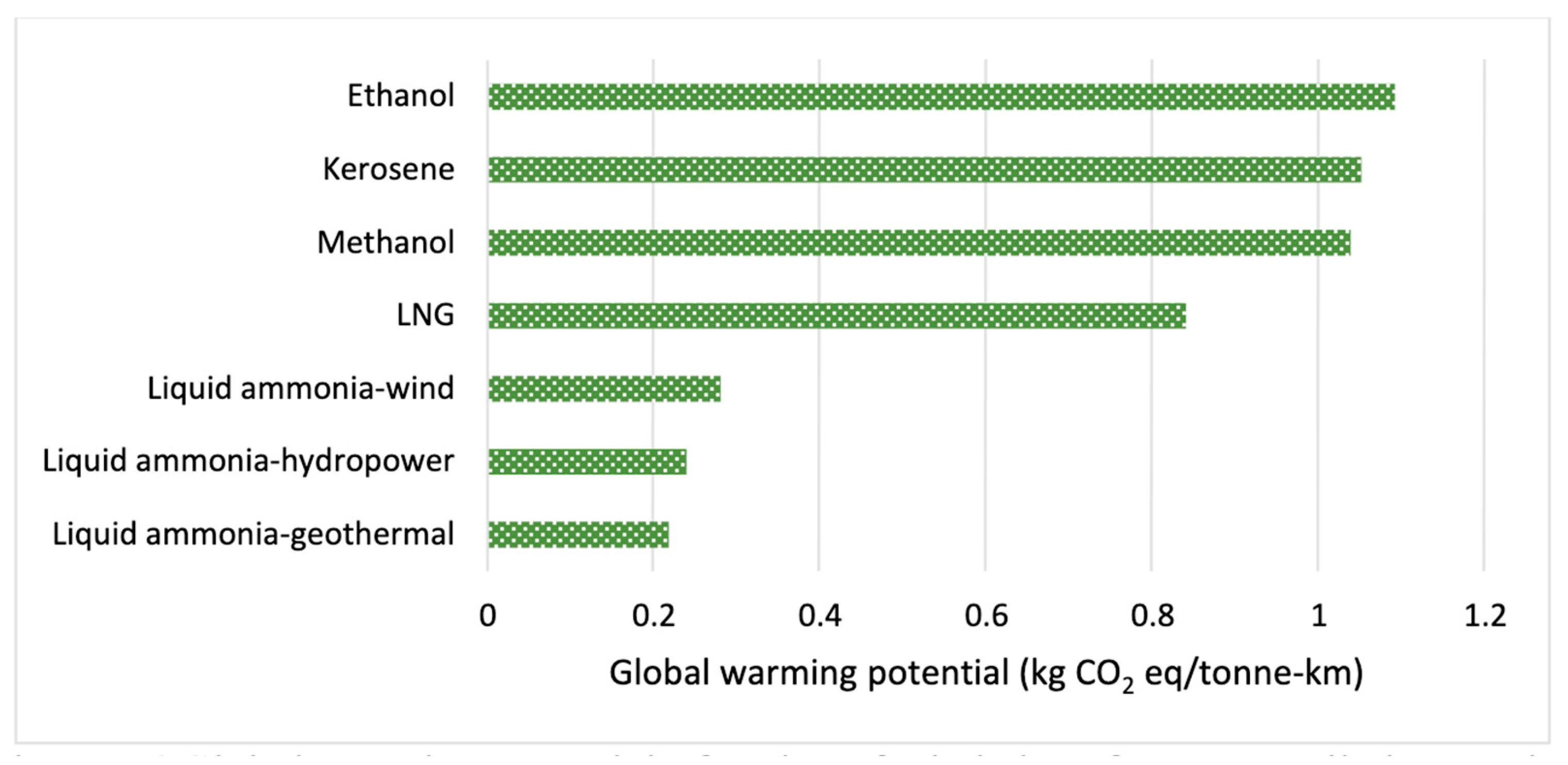
9.1. Environmental Analysis of NH3
- -
- Well-to-tank (WtT): This phase accounts for emissions resulting from H2 generation and NH3 synthesis through the Haber–Bosch process. The H2 source determines its environmental effect. The generation of brown or gray H2 is energy intensive and emits a significant amount of CO2 emissions, since it is generated using natural gas and coal through steam methane reforming and coal gasification. This emission is reduced in the case of blue H2 owing to the use of carbon capture and storage. However, green H, generated from water using water electrolysis powered by renewable energy, provides the most promising pathway to near-zero lifecycle emissions. The synthesis of NH3 through the Haber–Bosch process necessitates substantial energy even when using green H2 [106]. Thus, the entire process should rely on renewable energy to enable a low carbon footprint.
- -
- Tank-to-wheel (TtW): This phase addresses combustion-related emissions or using NH3 in vehicles. The combustion of NH3 does not contribute to CO2 emissions, but it emits nitrogen oxides (NOx), nitrous oxide (N2O), and unburned NH3 [107]. N2O is a major heat-retaining gas in the atmosphere, and nitrogen oxide (NOx) leads to air quality degradation and acid rain formation. The engine technology and the functional settings, including fuel mix, air–fuel ratio, and combustion chamber design, highly influence the emission levels. Additionally, the unburned NH3 and NOx may result in the creation of fine particulate matter (PM 2.5) in the atmosphere, presenting health hazards, particularly arising from pure NH3 engines in the absence of cutting-edge emission management solutions [108].
9.2. Economic Analysis of NH3 as a Transportation Fuel
- -
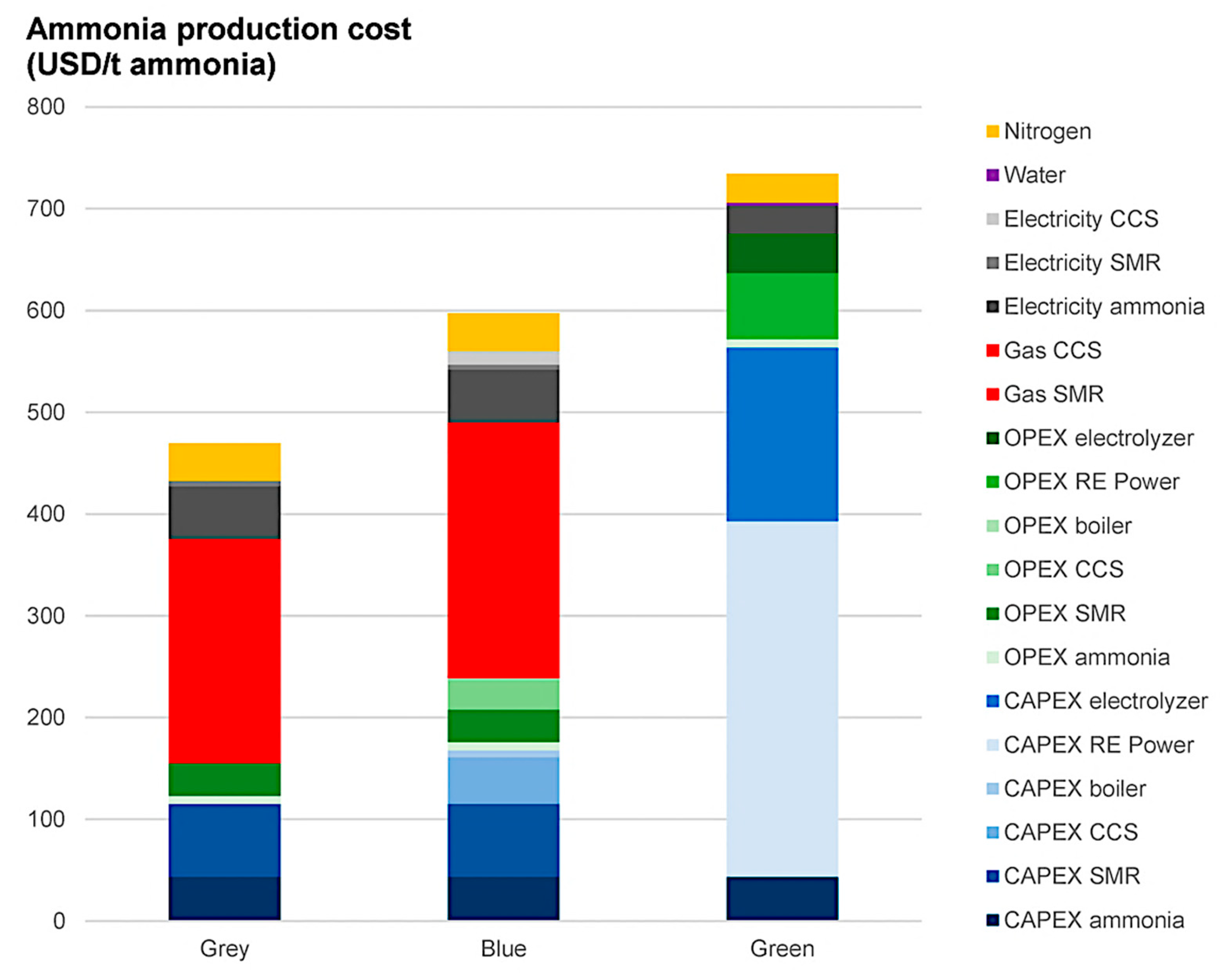
- Production costs: The production costs of brown and gray NH3 are the most cost-effective, implementing mature and efficient procedures through natural gas or coal. However, the production costs of green NH3 are more expensive due to considerable upfront capital investment (CAPEX) in renewable energy infrastructure (e.g., wind farms and solar panels) and electrolyzers. Advancements in technology, economies of scale, and supportive renewable energy policies are anticipated to drive down these costs. The current production costs of green NH3 range from USD 600 to 800 per ton, with forecasts indicating a reduction to approximately USD 250 per ton by 2030 if green H2 production scales effectively [110] (Figure 12).
- -
- Distribution costs: NH3 takes advantage of a worldwide infrastructure already in place for its role as a fertilizer and chemical. NH3 could be stored and transported as a liquid under moderate operating conditions (−33 °C or ~10 bar), requiring substantially less energy and expense than liquefying and transporting H2 (which requires −253 °C or very high pressures) [112]. The volumetric energy density of NH3 is higher compared to liquid H2 (12,822 MJ/m3 for liquid NH3 vs. 8496 MJ/m3 for liquid H2) [113]. These lead to lower spatial requirements for storage, resulting in reduced distribution costs per energy unit. The transportation of H2 as NH3 is significantly more affordable than transporting gaseous H2 across extended distances.
- -
- Vehicle costs: Changes in internal combustion engines or the design of new NH3-specific fuel cells are needed to adopt NH3 as fuel. These adjustments, mainly for internal combustion engines, involve modification of fuel injection systems, higher compression ratios, and material selection to avoid corrosion. This could result in increasing initial investment in vehicles; additionally, dual-fuel engine scenarios, which are based on mixing different fuels like NH3 with diesel or with H2, are being evaluated to improve combustion and control emission, potentially impacting vehicle system complexity and expenses.
- -
- Fuel costs: The total cost will encompass production, distribution, and requisite onboard processing. The cost of renewable energy and electrolyzer technology significantly increases the cost of green NH3.
9.3. Comparison of Costs with Other Alternative Fuels
9.4. Economies of Scale Driving Cost Reduction
9.5. Commercialization Path and Cost Reduction Potential of Green NH3 in Different Markets
10. Public Policy Perspective and Practical Implications
11. Future Prospects
- -
- Technological innovations: Several research studies will need to be conducted to enhance NH3 combustion technologies, emission control systems, and fuel cells with the aim of boosting the feasibility of NH3 as a transportation fuel and minimizing its emission. The main research areas are NH3 cracking and direct NH3 fuel cell technologies, including single-step conversion, elimination of cracking infrastructure, and new cracking technologies [124].
- -
- Policy support and impulse: National incentives and policies to foster carbon-neutral fuels and minimize emissions will speed up the adoption of using NH3 as fuel in the transportation sector.
- -
- Green NH3 generation: Shifting to green NH3, generated using renewable energy resources, is essential for the sustainability aspect of NH3 as fuel. Currently, several research studies are focusing on enhancing the performance and cost-effectiveness of green NH3 generation methods [125].
- -
- Infrastructure development: There is a need to invest in infrastructure for generation, storage, and distribution of NH3 to promote its use as fuel in the transportation sector.
- -
- Safety protocols and standards: The public widespread adoption of NH3 requires the development of sturdy safety protocols and standards.
- -
- Collaboration between different stakeholders: Cooperation and partnership between different stakeholders, including government, research centers, and industry players, will be essential to address the different economic and technical challenges and promote the expansion of NH3-based transportation solutions.
- -
- Pilot projects: Demonstration projects could supply useful data and perception, leading to cultivated technologies and boosted confidence in NH3 as a feasible transportation fuel.
- -
- Investment and funding: The development of NH3-based energy requires a rise in investment and funding in research, pilot projects, infrastructure development, and technological innovations.
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlZohbi, G.; Almoaikel, A.; AlShuhail, L. An overview on the technologies used to store hydrogen. Energy Rep. 2023, 9, 28–34. [Google Scholar] [CrossRef]
- Brown, T. Updating the Literature: Ammonia Consumes 43% of Global Hydrogen; Ammonia Energy Association: New York, NY, USA, 2020. [Google Scholar]
- Yüzbaşıoğlu, A.E.; Avşar, C.; Gezerman, A.O. The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Curr. Res. Green Sustain. Chem. 2022, 5, 100307. [Google Scholar] [CrossRef]
- Djinović, P.; Schüth, F. Energy carriers made from hydrogen. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 183–199. [Google Scholar]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Aziz, M.; Oda, T.; Kashiwagi, T. Comparison of liquid hydrogen, methylcyclohexane and ammonia on energy efficiency and economy. Energy Procedia 2019, 158, 4086–4091. [Google Scholar] [CrossRef]
- Wolny, P.; Tuśnio, N.; Mikołajczyk, F. Explosion risks during firefighting operations in storage rooms and the transport of ammonium nitrate-based fertilizers. Sustainability 2022, 14, 8565. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, C.; Shen, Y.; Tao, X.; Gan, Z. Thermodynamic and economic analysis of new coupling processes with large-scale hydrogen liquefaction process and liquid air energy storage. Energy 2024, 286, 129563. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.; Munnings, C.; Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.; Hodgetts, R.Y.; Bakker, J.M.; Vallana, F.M.F.; Simonov, A.N. A roadmap to the ammonia economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Mashruk, S.; Pugh, D.; Bowen, P. Ammonia. In Renewable Fuels: Sources, Conversion, and Utilization; Cambridge University Press: Cambridge, UK, 2022; pp. 245–274. [Google Scholar]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef]
- Pattabathula, V.; Richardson, J. Introduction to ammonia production. Chem. Eng. Prog. 2016, 112, 69–75. [Google Scholar]
- David, W. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store. Policy Brief; The Royal Society: London, UK, 2020; Available online: https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf (accessed on 1 June 2025).
- IEA. Ammonia Technology Roadmap; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Mu, B.-S.; Xu, Y.; Tu, Z.; Zhang, Y.; Liang, W.; Li, J.; Wang, X.; Shen, S.; Chen, J.; Liu, Z. Radiocatalytic ammonia synthesis from nitrogen and water. Natl. Sci. Rev. 2024, 11, nwae302. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.; Lan, R.; Tao, S. Development and recent progress on ammonia synthesis catalysts for Haber–Bosch process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Kaur, G.; Zhu, H.; Dhawale, D.S.; Ju, H.; Biswas, S.; Kim, J.H.; Yoon, H.C.; Giddey, S. A review on intermediate temperature electrochemical synthesis of ammonia. Appl. Energy 2025, 393, 126092. [Google Scholar] [CrossRef]
- Deibert, W.; Ivanova, M.E.; Baumann, S.; Guillon, O.; Meulenberg, W.A. Ion-conducting ceramic membrane reactors for high-temperature applications. J. Membr. Sci. 2017, 543, 79–97. [Google Scholar] [CrossRef]
- Wu, T.; Fan, W.; Zhang, Y.; Zhang, F. Electrochemical synthesis of ammonia: Progress and challenges. Mater. Today Phys. 2021, 16, 100310. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, S.; Wu, R.; Su, L.; Yang, Y.; Zhu, X.; Hu, Y.; Zhang, J.; Si, J.; Zeng, W. A capacitance-enhanced MXene anode for high-performance and dual-functional quasi-solid-state ammonium ion hybrid supercapacitor. J. Alloys Compd. 2025, 1022, 179788. [Google Scholar] [CrossRef]
- Garagounis, I.; Vourros, A.; Stoukides, D.; Dasopoulos, D.; Stoukides, M. Electrochemical synthesis of ammonia: Recent efforts and future outlook. Membranes 2019, 9, 112. [Google Scholar] [CrossRef]
- Makrakis, C. Optimal Decarbonization of Ammonia Production in the Netherlands: MILP-Based Modeling and Analysis of a Multi-Energy System. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2023. [Google Scholar]
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Fattah, I.R.; Handayani, F.; Ong, H.C.; Silitonga, A. A comprehensive review on the recent development of ammonia as a renewable energy carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Ebenezer, J.; Velayudham, P.; Schechter, A. Peroxide-Driven Nitrogen Fixation Reactions for Energy Storage Applications. Adv. Energy Mater. 2025, 15, 2501583. [Google Scholar] [CrossRef]
- Threatt, S.D.; Rees, D.C. Biological nitrogen fixation in theory, practice, and reality: A perspective on the molybdenum nitrogenase system. FEBS Lett. 2023, 597, 45–58. [Google Scholar] [CrossRef]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Sarker, N.C.; Hammed, A. Trends in biological ammonia production. BioTech 2023, 12, 41. [Google Scholar] [CrossRef]
- Moon, J.; Cheng, Y.; Daemen, L.; Novak, E.; Ramirez-Cuesta, A.J.; Wu, Z. On the structural transformation of Ni/BaH2 during a N2-H2 chemical looping process for ammonia synthesis: A joint in situ inelastic neutron scattering and first-principles simulation study. Top. Catal. 2021, 64, 685–692. [Google Scholar]
- Al-Qadri, A.A.; Ahmed, U.; Ali, H.M.; Farooqi, A.S. A combined gasification-chemical looping approach for valorizing waste tires into ammonia: Techno-economic analysis. Waste Manag. 2025, 205, 115020. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.; Gao, P.-X.; Bollas, G.M. Thermodynamic feasibility analysis of distributed chemical looping ammonia synthesis. Chem. Eng. J. 2021, 426, 131421. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 2017, 14, 1600157. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zheng, J.; Du, M.; Wu, X.; Song, J.; Cheng, C.; Li, T.; Yang, W. Non-thermal plasma-assisted ammonia production: A review. Energy Convers. Manag. 2023, 293, 117482. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, R.; Zhang, S.; Zhou, R.; Ding, J.; Li, F.; Hong, J.; Dou, L.; Shao, T.; Murphy, A.B. Sustainable ammonia synthesis from nitrogen and water by one-step plasma catalysis. Energy Environ. Mater. 2023, 6, e12344. [Google Scholar] [CrossRef]
- Choe, S.; Kim, S.M.; Lee, Y.; Seok, J.; Jung, J.; Lee, J.S.; Jang, Y.J. Rational design of photocatalysts for ammonia production from water and nitrogen gas. Nano Converg. 2021, 8, 22. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, R.; Shen, S.; Wang, J.; Sun, Y.; Dong, F. NH3 Synthesis from N1 Compounds by Photocatalytic Technology: Promotion Mechanism, Reaction Pathways, and Efficiency Evaluation Criteria. ACS Catal. 2024, 14, 15721–15742. [Google Scholar] [CrossRef]
- Sun, J.; Alam, D.; Daiyan, R.; Masood, H.; Zhang, T.; Zhou, R.; Cullen, P.J.; Lovell, E.C.; Jalili, A.; Amal, R. A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ. Sci. 2021, 14, 865–872. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Tu, X. Tandem plasma electrocatalysis: An emerging pathway for sustainable ammonia production. Curr. Opin. Green Sustain. Chem. 2025, 51, 100986. [Google Scholar] [CrossRef]
- Sánchez, A.P.; Fernández, H.M.A.; Almanza, G.I.V.; Rodríguez, N.C.; Julián, A.M.G. Designing a packed column for ammonia recovery: Evaluating two packing types. Rev. Cienc. Tecnol. 2025, 43, 119–131. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as green fuel in internal combustion engines: State-of-the-art and future perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, W.; Liu, S.; Wang, W.; Peng, Y.; Wang, Z. A review on ammonia-hydrogen fueled internal combustion engines. eTransportation 2023, 18, 100288. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, P.; Ji, Q.; Ni, X.; Wang, M. Investigation on combustion and emissions of ammonia-diesel dual-fuel engine in relation to ammonia energy ratio and injection parameters. Int. J. Hydrogen Energy 2025, 100, 713–726. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A perspective on the use of ammonia as a clean fuel: Challenges and solutions. Int. J. Energy Res. 2021, 45, 4827–4834. [Google Scholar] [CrossRef]
- Kalinci, Y.; Dincer, I. Analysis and performance assessment of NH3 and H2 fed SOFC with proton-conducting electrolyte. Int. J. Hydrogen Energy 2018, 43, 5795–5807. [Google Scholar] [CrossRef]
- Oh, S.; Oh, M.J.; Hong, J.; Yoon, K.J.; Ji, H.I.; Lee, J.H.; Kang, H.; Son, J.W.; Yang, S. A comprehensive investigation of direct ammonia-fueled thin-film solid-oxide fuel cells: Performance, limitation, and prospects. iScience 2022, 25, 105009. [Google Scholar] [CrossRef]
- Ur Rehman, M.M.; Mehdi, A.M.; Kazmi, W.W.; Bukhari, S.A.H.; Javed, R.; Mumtaz, H.; Al-Khulaifi, F.M.; Hussain, A.; Khan, M.Z.; Raza, R. Review on Ammonia-Powered SOFCs: Fundamentals, Thermodynamics, Degradation Mechanisms, and Future Perspectives. Energy Fuels 2025, 39, 6097–6117. [Google Scholar] [CrossRef]
- Yu, J.; Jing, H.; Zhao, P.; Lu, K.; Song, J.; Wu, Z.; Wu, H.; Liu, B.; Lei, W.; Hao, Q. Defect-rich walnut-like copper-doped Ni (PO3)2 catalyst towards ammonia borane electrooxidation reaction with high performance. J. Mater. Chem. A 2022, 10, 2035–2044. [Google Scholar] [CrossRef]
- Eluwah, C.; Fennell, P.S. Novel onboard ammonia cracker for light-duty automotive fuel cell vehicles. Energy Adv. 2025, 4, 796–809. [Google Scholar] [CrossRef]
- Krishnan, V.; Hussain, K. Hydrogen production from ammonia cracking. In Sustainable and Green Catalytic Processes for Renewable Fuel Production with Net-Zero Emissions; Elsevier: Amsterdam, The Netherlands, 2025; pp. 261–286. [Google Scholar]
- Kim, H.Y.; Kim, J.; Lee, E.; Choi, H.; Chun, H.; Kundu, J.; Choi, S.-I.; Lee, K.; Kim, J.Y. Electrocatalyst design strategies towards high performance anion-exchange membrane-based direct ammonia fuel cells. J. Mater. Chem. A 2025, 13, 6176–6204. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Miyashita, K.; Nagasawa, Y.; Suzuki, R.; Hara, M. Ammonia Synthesis Over an Iron Catalyst with an Inverse Structure. Adv. Sci. 2025, 12, 2410313. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Chen, P. Transition-metal-free ammonia synthesis. Nat. Chem. 2025, 17, 636–637. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fan, J.L.; Liu, S.B.; Sun, S.P.; Lou, Y.Y. Copper-Based Electrocatalysts for Nitrate Reduction to Ammonia. Materials 2023, 16, 4000. [Google Scholar] [CrossRef]
- Tripodi, A.; Conte, F.; Rossetti, I. Process Intensification for Ammonia Synthesis in Multibed Reactors with Fe-Wustite and Ru/C Catalysts. Ind. Eng. Chem. Res. 2021, 60, 908–915. [Google Scholar] [CrossRef]
- Bagheri, A.; Cabral, T.O.; Pourkargar, D.B. Integrated learning-based estimation and nonlinear predictive control of an ammonia synthesis reactor. AIChE J. 2025, 71, e18732. [Google Scholar] [CrossRef]
- Cai, S.; Yang, L.; Yang, J.; Li, S.; Pei, H.; Tu, Z. Regulating of a hybrid system using ammonia-reformed hydrogen for a proton exchange membrane fuel cell integrated with an internal combustion engine: A large-scale power supply scenario. Energy Convers. Manag. 2025, 327, 119559. [Google Scholar] [CrossRef]
- Elkafas, A.G.H. Feasibility Assessment of Retrofitting Ships with Fuel Cell-Based Hybrid Systems to Decarbonize the Domestic Maritime Sector. Ph.D. Thesis, University of Genoa, Genoa, Italy, 2025. [Google Scholar]
- Wang, G.; Chen, C.; Beshiwork, B.A.; Lin, B. Developing a low-carbon hybrid of ammonia fuel cell and internal combustion engine for carbon neutrality. Appl. Energy Combust. Sci. 2023, 16, 100214. [Google Scholar] [CrossRef]
- Ishaq, H.; Crawford, C. Review of ammonia production and utilization: Enabling clean energy transition and net-zero climate targets. Energy Convers. Manag. 2024, 300, 117869. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, M.A.; Khaligh, V.; Liu, J. Comparative Analysis of Direct Ammonia and Hydrogen Utilization: A Holistic Sustainability Perspective for Decarbonizing Transport and Power Sectors. ACS Sustain. Chem. Eng. 2025, 13, 13071–13084. [Google Scholar] [CrossRef]
- Azim, R.M.; Torii, S. Exploring the potential of ammonia and hydrogen as alternative fuels for transportation. Open Eng. 2024, 14, 20240024. [Google Scholar] [CrossRef]
- Elçiçek, H. Bibliometric analysis on hydrogen and ammonia: A comparative evaluation for achieving IMO’s decarbonization targets. Int. J. Environ. Sci. Technol. 2024, 21, 7039–7060. [Google Scholar] [CrossRef]
- Jalava, R. The Potential of Ammonia as a Marine Fuel: Seafarers Perspective on Ammonia’s Potential and Future. Bachelor’s Thesis, Novia University of Applied Sciences, Vaasa, Finland, 2025. [Google Scholar]
- Wu, X.; Jiang, H.; Zhao, H.; Lai, Y.; Zu, L.; Wang, Y.; Fu, M.; Su, S.; Ding, Y. Characteristics and influence factors of ammonia emissions from light-duty vehicles under real-world driving conditions. Fuel 2025, 393, 135038. [Google Scholar] [CrossRef]
- Chorowski, M.; Lepszy, M.; Machaj, K.; Malecha, Z.; Porwisiak, D.; Porwisiak, P.; Rogala, Z.; Stanclik, M. Challenges of application of green ammonia as fuel in onshore transportation. Energies 2023, 16, 4898. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Bréquigny, P.; Medina, A.V.; Boulet, E.; Emberson, D.; Løvås, T. Ammonia as fuel for transportation to mitigate zero carbon impact. In Engines and Fuels for Future Transport; Springer: Singapore, 2022; pp. 257–279. [Google Scholar]
- El-Shafie, M.; Kambara, S. Recent advances in ammonia synthesis technologies: Toward future zero carbon emissions. Int. J. Hydrogen Energy 2023, 48, 11237–11273. [Google Scholar] [CrossRef]
- Massaro, M.C.; Aluia, F.; Biga, R.; Accardo, G.; Monteverde, A.H.A. Potential of ammonia as hydrogen storage for future electrified aircraft. Energy Convers. Manag. X 2025, 26, 101034. [Google Scholar] [CrossRef]
- Hohman, L.A.; McKaskle, M.; Cannon, T.; Layhew, G.J.; Schafer, D.; Kramer, T.J.; Roberson, C.J.; Roberts, R.A.; Freemon, A. Investigation of Various Electrofuels for Potential Use in Aviation. In Proceedings of the AIAA Aviation Forum and Ascend 2025, Las Vegas, NV, USA, 21–25 July 2025. [Google Scholar]
- Sasi, S.; Mourouzidis, C.; Rajendran, D.J.; Roumeliotis, I.; Pachidis, V.; Norman, J. Ammonia for civil aviation: A design and performance study for aircraft and turbofan engine. Energy Convers. Manag. 2024, 307, 118294. [Google Scholar] [CrossRef]
- Adler, E.J.; Martins, J.R. Reprint of: Energy demand comparison for carbon-neutral flight. Prog. Aerosp. Sci. 2025, 153, 101084. [Google Scholar] [CrossRef]
- Filipe, M.; Afonso, F.; Suleman, A. A Study on Thermal Management Systems for Fuel-Cell Powered Regional Aircraft. Energies 2025, 18, 3074. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Masum, F.H.; Jiang, Y.; Li, S.; Curran, S.J.; Biruduganti, M.S.; Hawkins, T.R. Techno-Economic Analysis and Life Cycle Assessment of Alternative Fuels for Locomotives in the US Freight Rail Sector. Environ. Sci. Technol. 2025, 59, 15741–15750. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Sun, Y.; Wang, H.; Wang, J.; Sun, L.; Shen, J. Life cycle assessment of ammonia co-firing power plants: A comprehensive review and analysis from a whole industrial chain perspective. Adv. Appl. Energy 2024, 14, 100178. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Effects of inter-stage mixing on the NOx emission of staged ammonia combustion. Int. J. Hydrogen Energy 2022, 47, 9791–9799. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, B.-S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Zhuang, Z.; Guan, B.; Chen, J.; Zheng, C.; Zhou, J.; Su, T.; Chen, Y.; Zhu, C.; Hu, X.; Zhao, S. Review of nitrous oxide direct catalytic decomposition and selective catalytic reduction catalysts. Chem. Eng. J. 2024, 486, 150374. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Zhang, H.; Wang, X.; Qin, H.; Wu, K.; Zhao, C.; Fan, W.; Xu, J. Ammonia Co-firing with Coal: A Review of the Status and Prospects. Energy Fuels 2024, 38, 15861–15886. [Google Scholar] [CrossRef]
- Li, T.; Duan, Y.; Wang, Y.; Zhou, M.; Duan, L. Research progress of ammonia combustion toward low carbon energy. Fuel Process. Technol. 2023, 248, 107821. [Google Scholar] [CrossRef]
- Kannan, C.; Vijayakumar, T. Application of exhaust gas recirculation for NOx reduction in CI engines. In NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 2022; pp. 189–222. [Google Scholar]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef]
- Marchuk, V.; Sharapa, D.I.; Grunwaldt, J.-D.; Doronkin, D.E. Surface states governing the activity and selectivity of Pt-based ammonia slip catalysts for selective ammonia oxidation. ACS Catal. 2024, 14, 1107–1120. [Google Scholar] [CrossRef]
- Wei, Z.; Guan, B.; Zhuang, Z.; Chen, J.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Shu, K. Review on solid selective catalytic reduction (SSCR) technology: Excellent optimization of selective catalytic reduction technology. Catal. Sci. Technol. 2025, 15, 647–668. [Google Scholar] [CrossRef]
- Lastumäki, F. Ammonia as a Fuel in Maritime Decarbonization. Bachelor’s Thesis, Turku University of Applied Sciences, Turku, Finland, 2024. [Google Scholar]
- Tornatore, C.; Sementa, P.; Catapano, F. Ammonia–Hydrogen Dual-Fuel Combustion: Strategies for Optimizing Performance and Reducing Emissions in Internal Combustion Engines. Energies 2025, 18, 3159. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Sunden, B.A.; Wang, S. Advanced Technologies in Flow Dynamics and Combustion in Propulsion and Power, Volume II; Frontiers Media SA: Lausanne, Switzerland, 2023; Volume 16648714. [Google Scholar]
- Chavando, A.; Silva, V.; Cardoso, J.; Eusebio, D. Advancements and challenges of ammonia as a sustainable fuel for the maritime industry. Energies 2024, 17, 3183. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Zhong, L.; Wei, H. Enhanced combustion of ammonia engine based on novel air-assisted pre-chamber turbulent jet ignition. Energy Convers. Manag. 2023, 276, 116526. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Shan, Y.; Chen, T.; Zhao, Y.; Yu, C.; Pang, H. Metal-organic frameworks nanocomposites with different dimensionalities for energy conversion and storage. Adv. Energy Mater. 2022, 12, 2100346. [Google Scholar] [CrossRef]
- Fan, K.; Xu, P.; Li, Z.; Shao, M.; Duan, X. Layered double hydroxides: Next promising materials for energy storage and conversion. Next Mater. 2023, 1, 100040. [Google Scholar] [CrossRef]
- Asif, M.; Bibi, S.S.; Ahmed, S.; Irshad, M.; Hussain, M.S.; Zeb, H.; Khan, M.K.; Kim, J. Recent advances in green hydrogen production, storage and commercial-scale use via catalytic ammonia cracking. Chem. Eng. J. 2023, 473, 145381. [Google Scholar] [CrossRef]
- Tutak, W. Co-combustion of ammonia and hydrogen in spark ignition engines-State-of-the-art and challenges. Int. J. Hydrogen Energy 2024, 80, 188–205. [Google Scholar] [CrossRef]
- Alnajideen, M.; Shi, H.; Northrop, W.; Emberson, D.; Kane, S.; Czyzewski, P.; Alnaeli, M.; Mashruk, S.; Rouwenhorst, K.; Yu, C. Ammonia combustion and emissions in practical applications: A review. Carbon Neutrality 2024, 3, 13. [Google Scholar] [CrossRef]
- Togun, H.; Aljibori, H.S.S.; Abed, A.M.; Biswas, N.; Alshamkhani, M.T.; Niyas, H.; Mohammed, H.I.; Rashid, F.L.; Paul, D. A review on recent advances on improving fuel economy and performance of a fuel cell hybrid electric vehicle. Int. J. Hydrogen Energy 2024, 89, 22–47. [Google Scholar] [CrossRef]
- Oladosu, T.L.; Pasupuleti, J.; Kiong, T.S.; Koh, S.P.J.; Yusaf, T. Energy management strategies, control systems, and artificial intelligence-based algorithms development for hydrogen fuel cell-powered vehicles: A review. Int. J. Hydrogen Energy 2024, 61, 1380–1404. [Google Scholar] [CrossRef]
- Sivaraman, S.; Makarov, D.; Molkov, V. Flash boiling and pressure recovery phenomenon during venting from liquid ammonia tank ullage. Process Saf. Environ. Prot. 2024, 182, 880–893. [Google Scholar] [CrossRef]
- O’Connor, D.R. Liquid Ammonia Fuel Storage Alternatives and Minimizing Integration Impact on Ship Design. Master’s Thesis, University of Liège, Liège, Belgium, 2023. [Google Scholar]
- Hofste, P. Design of an Ammonia Bunker Installation for Sea-Going Vessels. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2013. [Google Scholar]
- Yoes, M.D. Hazards of Nitrogen and Other Inert Gases: How They Can Be Safely Managed; John Wiley & Sons: Hoboken, NJ, USA, 2025. [Google Scholar]
- Böllinghaus, T.; Rhode, M.; Falkenreck, T. Corrosion and Corrosion Resistance. In Springer Handbook of Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 185–213. [Google Scholar]
- Duong, P.A.; Lee, J.; Ryu, B.R.; Kang, H. A preliminary safety assessment of fuel gas supply system in the engine room of the ammonia fuelled ship. J. Mar. Eng. Technol. 2025, 1–20. [Google Scholar] [CrossRef]
- Naeem, R.M.S. Safety of Ammonia Fuel in Maritime Sector for Transportation, Operation, Fueling and Use in Satakunta-Region Focusing on Aspects of Sustainability. Master’s Thesis, Satakunta University of Applied Sciences, Pori, Finland, 2024. [Google Scholar]
- Feng, W.; Wang, Z.; Dai, X.; Dong, S.; Qiao, W.; Ma, X. Ship-To-Ship Liquefied Natural Gas Bunkering Risk Assessment by Integrating Fuzzy Failure Mode and Effect Analysis and the Technique for Order Preference by Similarity to an Ideal Solution. J. Mar. Sci. Eng. 2025, 13, 710. [Google Scholar] [CrossRef]
- Tripathi, S.; Gorbatenko, I.; Garcia, A.; Sarathy, M. Sustainability of Future Shipping Fuels: Well-to-Wake Environmental and Techno-Economic Analysis of Ammonia and Methanol. In Proceedings of the 16th International Conference on Engines & Vehicles for Sustainable Transport, Capri, Naples, Italy, 10–14 September 2023. [Google Scholar]
- Himona, E.; Poullikkas, A. Comparative Review of Natural Gas Vehicles During the Energy Transition. Energies 2025, 18, 3512. [Google Scholar] [CrossRef]
- Heo, J.; Kim, P.; Park, J.; Lee, J.Y. Source identification of ultrafine particulate matter and ammonia emissions in the metropolitan area. Environ. Eng. Res. 2025, 30, 240074. [Google Scholar] [CrossRef]
- Wong, A.Y.; Selin, N.E.; Eastham, S.D.; Mounaïm-Rousselle, C.; Zhang, Y.; Allroggen, F. Climate and air quality impact of using ammonia as an alternative shipping fuel. Environ. Res. Lett. 2024, 19, 084002. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F.; Ni, B.-J. Green ammonia production technologies: A review of practical progress. J. Environ. Manag. 2023, 342, 118348. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia production from clean hydrogen and the implications for global natural gas demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Seo, Y.; Han, S. Economic evaluation of an ammonia-fueled ammonia carrier depending on methods of ammonia fuel storage. Energies 2021, 14, 8326. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging materials and methods toward ammonia-based energy storage and conversion. Adv. Mater. 2021, 33, 2005721. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Parsapur, R.K.; Huang, K.-W. Limitations of ammonia as a hydrogen energy carrier for the transportation sector. ACS Energy Lett. 2021, 6, 4390–4394. [Google Scholar] [CrossRef]
- Palazzi, C.; Nayak-Luke, R.; Verschuur, J.; Salmon, N.; Hall, J.W.; Bañares-Alcantara, R. Green ammonia imports could supplement long-duration energy storage in the UK. Environ. Res. Energy 2024, 1, 043001. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R.; Nayak-Luke, R. Optimization of green ammonia distribution systems for intercontinental energy transport. iScience 2021, 24, 102903. [Google Scholar] [CrossRef]
- Wang, H.; Lin, N.; Arzumanyan, M. The Market for Low-Carbon-Intensity Ammonia. Gases 2024, 4, 224–235. [Google Scholar] [CrossRef]
- Smith, C.; Torrente-Murciano, L. Cost efficiency versus energy utilization in green ammonia production from intermittent renewable energy. Nat. Chem. Eng. 2025, 2, 261–272. [Google Scholar] [CrossRef]
- Jaiswall, C. Europe Green Ammonia Market Overview; Market Research Future: New York, NY, USA, 2025; Available online: https://www.marketresearchfuture.com/reports/europe-green-ammonia-market-49774 (accessed on 1 June 2025).
- Section 45V: Credit for Production of Clean Hydrogen; Taxpayers for Common Sense: Washington, DC, USA, 2024.
- Balci, G.; Phan, T.T.N.; Surucu-Balci, E.; Iris, Ç. A roadmap to alternative fuels for decarbonising shipping: The case of green ammonia. Res. Transp. Bus. Manag. 2024, 53, 101100. [Google Scholar] [CrossRef]
- Huang, Y.; Nie, Z.; Zhang, L.; Liu, X.; Feng, J.; Yin, Y.; Wang, H.; Tian, G.; Li, J.; Duan, F. Ammonia Fuel: A Pathway for Carbon-Neutral of the Transportation Sector. Chain 2024, 1, 113–137. [Google Scholar] [CrossRef]
- Vigneswaran, V.S.; Gowd, S.C.; Ravichandran, V.; Karthikeyan, M.; Ganeshan, P.; Kandasamy, S.; Lee, J.; Barathi, S.; Rajendran, K. Green ammonia as hydrogen carrier: Current status, barriers, and strategies to achieve sustainable development goals. Sci. Total Environ. 2025, 982, 179646. [Google Scholar] [CrossRef]
- Dolan, R.H.; Anderson, J.E.; Wallington, T.J. Outlook for ammonia as a sustainable transportation fuel. Sustain. Energy Fuels 2021, 5, 4830–4841. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]

| Liquid H2 | NH3 | |
|---|---|---|
| Gravimetric energy density (MJ/kg) | 120 | 18.6 |
| Volumetric energy density (MJ/L) | 8.5 | 12.7 |
| Boiling point (°C) | −252.87 | −33.34 |
| Flammability range | 4–75% | 15–28% |
| Health hazard | Non-toxic, risk of frostbite and cold burns because it has a low boiling point | Highly toxic, corrosive, and strong, with a pungent odor |
| Technology | Pros | Cons |
|---|---|---|
| Haber–Bosch | High generation rates, well established | High energy consumption, operation under high pressure and temperature, high carbon footprint |
| Electrochemical synthesis | Operates under ambient conditions, low carbon footprint | Low efficiency and generation rate, under research and development |
| Solid-state NH3 synthesis | Possibility for renewable energy integration, minimizes liquid handling problems | Operates under high temperature |
| Biological nitrogen fixation | Low energy input because it is based on microorganisms, eco-friendly | Lower production rate |
| Thermochemical looping | Possibility for use of renewable energy and waste heat, high efficiency | Operates under high temperature, a complex process with different stages |
| Plasma synthesis | Operate under atmospheric pressure, possibility for renewable energy integration | High energy input, scalability issues |
| Criteria | Direct Combustion (ICEs) | Fuel Cells (e.g., SOFCs) | Hybrid Systems (e.g., ICE + Cracker) |
|---|---|---|---|
| Costs | Lower initial capital cost due to engine adaptation | High initial cost (catalysts, complex systems) | High initial cost (multiple components) |
| Efficiency | Medium (typically < 50%) | High (up to 60%) | High (improved by blending with H2) |
| Emissions | High NOx emissions, requires aftertreatment | Zero direct NOx emissions | Moderate NOx emissions, requires aftertreatment |
| Infrastructure | Utilizes existing fuel handling but needs specialized storage. | Requires new, specialized fuel cell technology | Complex, requires onboard cracking and storage |
| Key challenges | Managing NOx and unburnt NH3 emissions | High cost, performance at varying loads | System integration and complexity |
| Type of Vehicle Fuel | CO2 Emission | |
|---|---|---|
| Gasoline | 2.31 kg CO2/Liter | |
| Diesel | 2.68 kg CO2/Liter | |
| Gray H2 | 10 kg CO2/kg | |
| Blue H2 | 2 kg CO2/kg | |
| Green H2 | 0.01 kg CO2/kg | |
| Gray NH3 | 2.75 kg CO2/kg | |
| Green NH3 | 0.01 kg CO2/kg | |
| CNG | 2.75 kg CO2/kg | |
| Electricity | Renewable energy | Nearly zero kg CO2/kWh |
| Coal | 0.4–0.5 kg CO2/kWh | |
| Natural gas | 0.82 kg CO2/kWh | |
| Technology | Transportation Sector | Description | Technology Readiness Level (TRL) |
|---|---|---|---|
| Direct combustion | Maritime, road, rail | NH3 is directly used as fuel in internal combustion engines (ICEs) or gas turbines. Requires modifications to the engine and aftertreatment systems to handle its low flammability and mitigate NOx and unburnt NH3 emissions. | 6–7 (large-scale prototype or pre-commercial demonstration, particularly in the maritime sector). |
| Fuel cells | Maritime, heavy-duty transport | Solid oxide fuel cells (SOFCs): Use high temperatures to directly convert NH3 to electricity, considered more mature than other fuel cell types for NH3. Proton exchange membrane fuel cells (PEMFCs): Cannot use NH3 directly. Requires a reformer (cracker) to first convert NH3 to hydrogen, adding complexity and cost. | SOFCs: 5–6 (system-level validation in a relevant environment). PEMFCs: 3–5 (proof of concept to component and subsystem validation). |
| Hybrid systems | Maritime, heavy-duty transport | Combines different technologies, such as an internal combustion engine running on an NH3–hydrogen blend. Hydrogen is often produced onboard via an NH3 cracker, which improves the combustion properties of NH3 and increases efficiency. | 5–7 (system-level validation to pre-commercial demonstration, often seen in maritime and power generation). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlZohbi, G. Ammonia from Hydrogen: A Viable Pathway to Sustainable Transportation? Sustainability 2025, 17, 8172. https://doi.org/10.3390/su17188172
AlZohbi G. Ammonia from Hydrogen: A Viable Pathway to Sustainable Transportation? Sustainability. 2025; 17(18):8172. https://doi.org/10.3390/su17188172
Chicago/Turabian StyleAlZohbi, Gaydaa. 2025. "Ammonia from Hydrogen: A Viable Pathway to Sustainable Transportation?" Sustainability 17, no. 18: 8172. https://doi.org/10.3390/su17188172
APA StyleAlZohbi, G. (2025). Ammonia from Hydrogen: A Viable Pathway to Sustainable Transportation? Sustainability, 17(18), 8172. https://doi.org/10.3390/su17188172