Valorization of Coffee Pulp: Spray-Dried Hemp Oil Microcapsules Stabilized with Coffee Pectin and Maltodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hemp Seed Oil Emulsions
2.3. Analysis of Emulsion Properties
2.3.1. Particle Size, Electrical Conductivity, and Zeta Potential
2.3.2. Color and Optical Light Microscopy Images
2.3.3. Viscosity
2.4. Spray-Drying Process for Microencapsulation
2.5. Analysis of Microencapsulated Powders
2.5.1. Moisture, Water Activity, and Particle Size Distribution
2.5.2. Encapsulation Efficiency
2.5.3. Wettability and Solubility
2.5.4. Release Characteristics
2.5.5. Peroxide Values
2.5.6. Bulk Density and Tapped Density
2.5.7. Particle Morphology
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Emulsions
3.1.1. Viscosity, Electrical Conductivity, Zeta Potential, and Particle Size
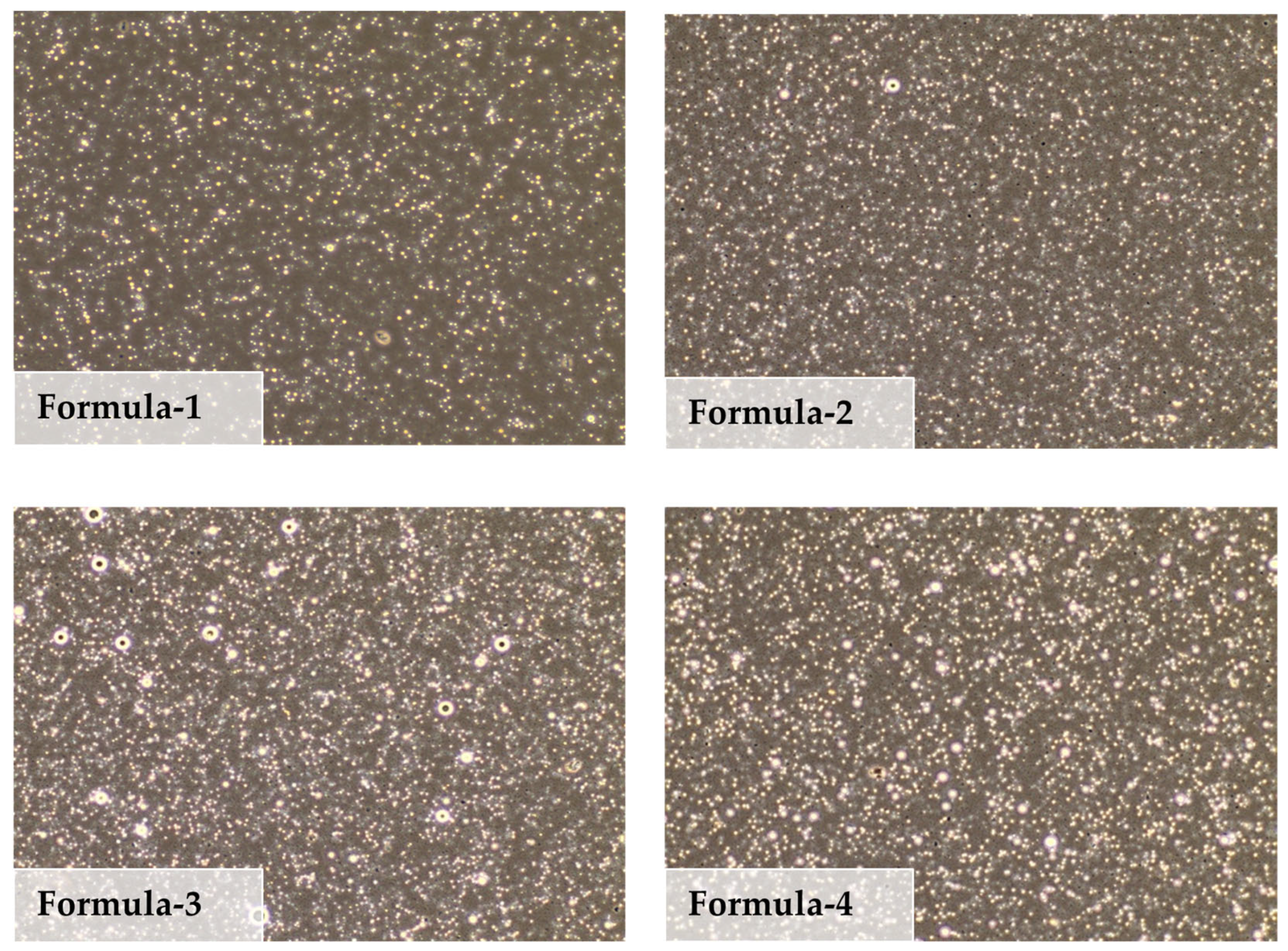
3.1.2. Color and Light Microscopic Images
3.2. Physicochemical Properties of Encapsulated Powders
3.2.1. Moisture Content, Water Activity, Particle Size, Wettability, Solubility, Bulk, and Tapped Densities
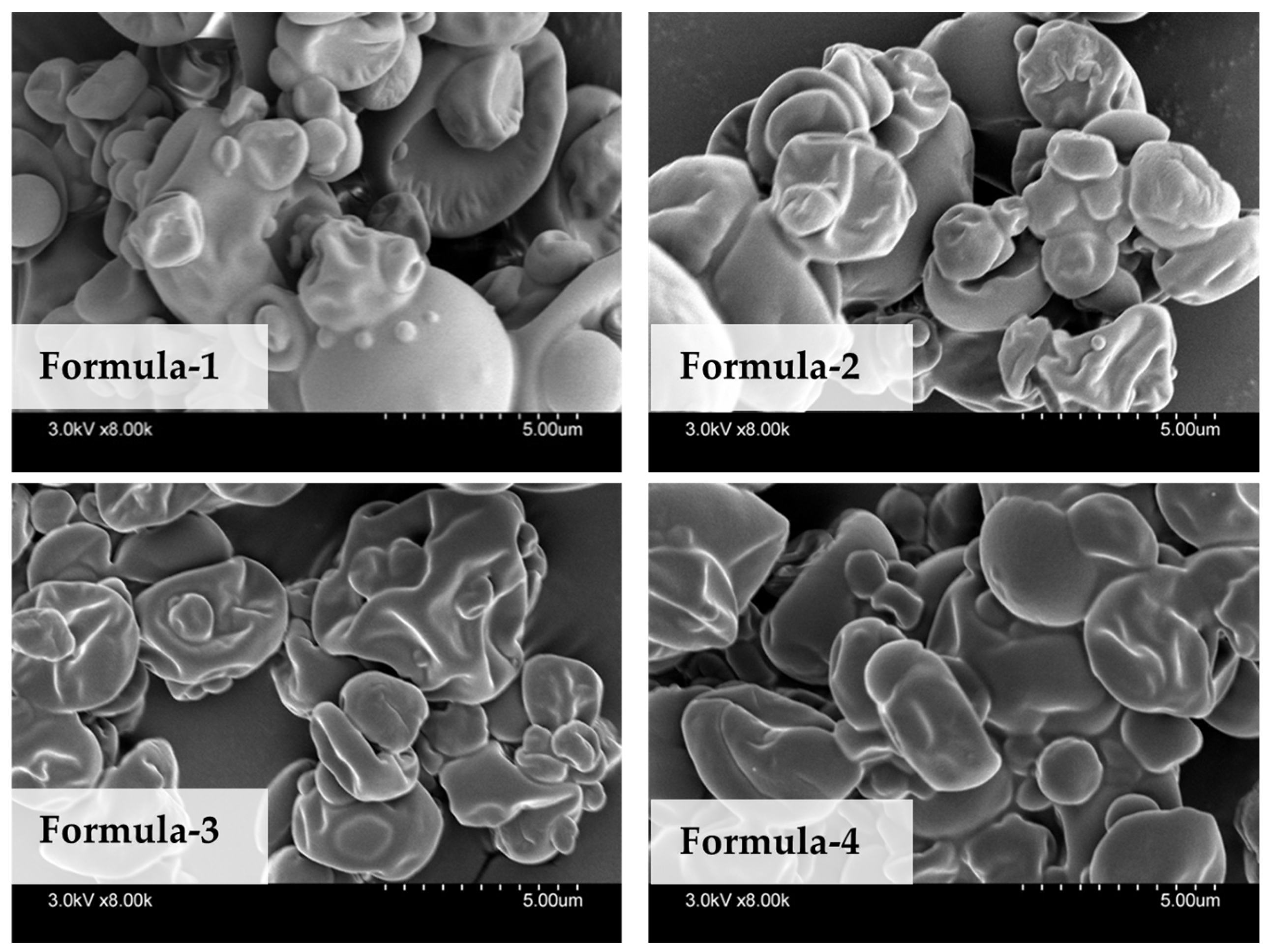
3.2.2. Particle Size Distributions and Morphology
3.2.3. Encapsulation Efficiencies, Oil Release Characteristics, and Peroxide Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOCS | American Oil Chemists’ Society |
| ANOVA | Analysis of variance |
| aw | Water activity |
| CI | Carr index |
| CP | Coffee pectin |
| DE | Dextrose equivalent |
| DI | Deionized (water) |
| D10, D50, D90 | Particle diameters at 10%, 50%, and 90% of cumulative volume, respectively |
| DLS | Dynamic light scattering |
| EE | Encapsulation efficiency |
| ELS | Electrophoretic light scattering |
| HSD | Tukey’s honestly significant difference (post hoc test) |
| L*, a*, b* | CIE color coordinates (lightness, green–red, blue–yellow) |
| MD | Maltodextrin |
| PDI | Polydispersity index |
| PV | Peroxide value |
| SEM | Scanning electron microscopy/microscope |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| S.D. | Standard deviation |
| TD | Tapped density |
References
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the secrets of industrial hemp protein as emerging functional food ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Kahraman, O.; Petersen, G.E.; Fields, C. Physicochemical and functional modifications of hemp protein concentrate by the application of ultrasonication and pH shifting treatments. Foods 2022, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Kahraman, O.; Pandalaneni, K.; Kapoor, R.; Feng, H. A comprehensive review on hempseed protein: Production, functional and nutritional properties, novel modification methods, applications, and limitations. Int. J. Biol. Macromol. 2023, 253, 127240. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Gallo, M.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Encapsulation of active compounds in fruit and vegetable juice processing: Current state and perspectives. J. Food Sci. 2017, 82, 1291–1301. [Google Scholar] [CrossRef]
- Naidu, H.; Kahraman, O.; Feng, H. Novel applications of ultrasonic atomization in the manufacturing of fine chemicals, pharmaceuticals, and medical devices. Ultrason. Sonochem. 2022, 86, 105984. [Google Scholar] [CrossRef]
- Jafari, S.; Jafari, S.M.; Ebrahimi, M.K.; Kijpatanasilp, I.; Assatarakul, K. A decade overview and prospect of spray drying encapsulation of bioactives from fruit products: Characterization, food application and in vitro gastrointestinal digestion. Food Hydrocoll. 2023, 134, 108062. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Steth, S.; Ahmed, I.A.M.; Manzoor, M.F.; Wei, W.; Wang, X. Effects of maltodextrin combination with gum Arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Reichembach, L.H.R.; Petkowicz, C.L.O. Extraction and characterization of a pectin from coffee (Coffea arabica L.) pulp with gelling properties. Carbohydr. Polym. 2020, 245, 116473. [Google Scholar] [CrossRef]
- Chamyuang, S.; Duangphet, S.; Owatworakit, A.; Intatha, U.; Nacha, J.; Kerdthong, P. Preparation of pectin films from coffee cherry and its antibacterial activity. Trends Sci. 2021, 18, 34. [Google Scholar] [CrossRef]
- Biratu, G.; Gonfa, G.; Bekele, M.; Woldemariam, H.W. Extraction and characterization of pectin from coffee (Coffea arabica L.) pulp obtained from four different coffee producing regions. Int. J. Biol. Macromol. 2024, 274, 133321. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; Jorge, L.M.M.; Jorge, R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef]
- Misono, T.; Dynamic Light Scattering (DLS); Abe, M. (Eds.) Measurement Techniques and Practices of Colloid and Interface Phenomena; Springer: Singapore, 2019; pp. 65–69. [Google Scholar] [CrossRef]
- Lekjing, S.; Keawpeng, I.; Venkatachalam, K.; Karrila, S. Impact of different sugar types and their concentrations on salted duck egg white based meringues. Foods 2022, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Fuchs, M.; Turchiuli, C.; Bohin, M.; Cuvelier, M.E.; Ordonnaud, C.; Maillard, M.N.; Dumoulin, E. Encapsulation of oil in powder using spray drying and fluidized bed agglomeration. J. Food Eng. 2006, 75, 27–35. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Encapsulation of flaxseed oil using a benchtop spray dryer for legume protein-maltodextrin microcapsule preparation. J. Agric. Food Chem. 2013, 61, 5148–5155. [Google Scholar] [CrossRef]
- AOCS. AOCS Official method Cd 8-53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society Method Cd 8-53. Peroxide Value Acetic Acid–Chloroform Method, 4th ed.; Gunstone, F., Ed.; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- Saifullah, M.; Yusof, Y.A.; Aziz, M.G. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Borges, S.V.; Silva, E.K.; Da Silva, Y.F.; De Souza, H.J.B.; Do Carmo, E.L.; De Oliveira, C.R.; Yoshida, M.I.; Botrel, D.A. Study of ultrasound-assisted emulsions on microencapsulation of ginger essential oil by spray drying. Ind. Crops Prod. 2016, 94, 413–423. [Google Scholar] [CrossRef]
- Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin, and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Choudhary, P.; Moses, J.; Anandharamakrishnan, C. Emulsion electrospraying and spray drying of whey protein nano and microparticles with curcumin. Food Hydrocoll. Health 2023, 3, 100122. [Google Scholar] [CrossRef]
- Noello, C.; Carvalho, A.; Silva, V.; Hubinger, M. Spray dried microparticles of chia oil using emulsion stabilized by whey protein concentrate and pectin by electrostatic deposition. Food Res. Int. 2016, 89, 549–557. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Sanchez, M.D.R.H.; Cuvelier, M.; Turchiuli, C. Design of liquid emulsions to structure spray dried particles. J. Food Eng. 2015, 167, 99–105. [Google Scholar] [CrossRef]
- Taboada, M.L.; Heiden-Hecht, T.; Brückner-Gühmann, M.; Karbstein, H.P.; Drusch, S.; Gaukel, V. Spray drying of emulsions: Influence of the emulsifier system on changes in oil droplet size during the drying step. J. Food Process. Preserv. 2021, 45, e15753. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Z.; Zhao, Y.; Prakash, S.; Liu, W.; Han, J.; Wang, Z. Effects of lutein particle size in embedding emulsions on encapsulation efficiency, storage stability, and dissolution rate of microencapsules through spray drying. LWT 2021, 146, 111430. [Google Scholar] [CrossRef]
- Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Córdoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J. Physical characteristics of spray-dried dairy powders containing different vegetable oils. J. Food Eng. 2014, 122, 122–129. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; Fernandes, B.; Viana, A.D.; Marques, G.R. Evaluation of spray drying conditions on properties of microencapsulated oregano essential oil. Int. J. Food Sci. Technol. 2012, 47, 2289–2296. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Role of Powder Particle Size on the Encapsulation Efficiency of Oils during Spray Drying. Dry. Technol. 2007, 25, 1081–1089. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J. Microencapsul. 2005, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Sheu, T. Microencapsulation of volatiles by spray-drying in whey protein-based wall systems. Int. Dairy J. 1996, 6, 273–284. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Effect of Maltodextrin Addition during Spray Drying of Tomato Pulp in Dehumidified Air: II. Powder Properties. Dry. Technol. 2008, 26, 726–737. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Roccia, P.; Martínez, M.L.; Llabot, J.M.; Ribotta, P.D. Influence of spray-drying operating conditions on sunflower oil powder qualities. Powder Technol. 2014, 254, 307–313. [Google Scholar] [CrossRef]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of rosemary essential oil. LWT Food Sci. Technol. 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of basil essential oil: Utilization of gum arabic/whey protein isolate/maltodextrin combinations for encapsulation efficiency and in vitro release. J. Food Meas. Charact. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Selim, K.A.; Alharthi, S.S.; Abu El-Hassan, A.M.; Elneairy, N.A.; Rabee, L.A.; Abdel-Razek, A.G. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules 2021, 26, 6109. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Bilia, A.R. Nanostructured Lipid Carriers Loaded with Cannabidiol Enhance Its Bioaccessibility to the Small Intestine. Nutraceuticals 2023, 3, 210–221. [Google Scholar] [CrossRef]
- Jia, C.; Huang, S.; Liu, R.; You, J.; Xiong, S.; Zhang, B.; Rong, J. Storage stability and in-vitro release behavior of microcapsules incorporating fish oil by spray drying. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127234. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; Tirado-Delgado, M.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Influence of emulsifier type and encapsulating agent on the in vitro digestion of fish oil-loaded microcapsules produced by spray-drying. Food Chem. 2022, 392, 133257. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. In-vitro evaluation of hydrocolloid–based encapsulated fish oil. Food Hydrocoll. 2009, 23, 1413–1419. [Google Scholar] [CrossRef]
- Lim, W.; Nyam, K. Characteristics and controlled release behaviour of microencapsulated kenaf seed oil during in-vitro digestion. J. Food Eng. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Azad, A.K.; Abdullah, S.M.; Chatterjee, B.; Wan Sulaiman, W.M.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Zhao, M.; Chen, W.; Ma, G.; Ma, Y.; Xianqing, H. Fabrication, characterization and simulated gastrointestinal digestion of sea buckthorn pulp oil microcapsule: Effect of wall material and interfacial bilayer stabilization. J. Sci. Food Agric. 2025, 105, 1737–1744. [Google Scholar] [CrossRef] [PubMed]


| Formula | In Emulsions | ||||
| Oil (%) | Coffee Pectin (%) | Maltodextrin (%) | Total Solids (%) | Core: Wall Ratio | |
| 1 | 3 | 4 | 13 | 20 | 3:17 |
| 2 | 4 | 4 | 12 | 20 | 1:4 |
| 3 | 5 | 4 | 11 | 20 | 1:3 |
| 4 | 6 | 4 | 10 | 20 | 3:7 |
| Formula | In Encapsulated Powder | ||||
| Oil (%) | Coffee Pectin (%) | Maltodextrin (%) | Total Solids (%) | ||
| 1 | 15 | 20 | 65 | 100 | |
| 2 | 20 | 20 | 60 | 100 | |
| 3 | 25 | 20 | 55 | 100 | |
| 4 | 30 | 20 | 50 | 100 | |
| Formula | Viscosity (cP) | Electrical Conductivity (mS/cm) | Zeta Potential (mV) | Particle Size (µm) |
|---|---|---|---|---|
| 1 | 12.50 ± 0.20 c | 5.77 ± 0.04 c | −6.00 ± 0.07 a | 8.98 ± 0.06 c |
| 2 | 12.89 ± 0.17 c | 5.88 ± 0.04 c | −6.15 ± 0.08 a | 9.11 ± 0.06 c |
| 3 | 13.20 ± 0.20 b | 6.14 ± 0.02 b | −6.90 ± 0.10 b | 10.22 ± 0.03 b |
| 4 | 14.89 ± 0.01 a | 6.51 ± 0.04 a | −7.17 ± 0.21 c | 11.32 ± 0.03 a |
| Formula | L* (-) | a* (-) | b* (-) |
|---|---|---|---|
| 1 | 18.31 ± 0.02 d | 0.09 ± 0.006 a | −0.24 ± 0.01 d |
| 2 | 20.57 ± 0.01 c | 0.07 ± 0.002 b | −0.19 ± 0.00 c |
| 3 | 28.04 ± 0.21 b | 0.02 ± 0.010 c | −0.11 ± 0.02 b |
| 4 | 32.16 ± 0.02 a | 0.02 ± 0.010 c | −0.07 ± 0.02 a |
| Formula 1 | Formula 2 | Formula 3 | Formula 4 | |
|---|---|---|---|---|
| MC (%) | 2.97 ± 0.02 a | 2.81 ± 0.03 c | 2.89 ± 0.02 b | 2.75 ± 0.03 c |
| aw | 0.07 ± 0.001 b | 0.08 ± 0.001 a | 0.08 ± 0.002 ab | 0.07 ± 0.002 ab |
| Particle size | 5.00 ± 0.02 c | 5.92 ± 0.13 c | 7.65 ± 0.50 b | 9.43 ± 0.01 a |
| Wettability (s) | 355.7 ± 4.04 d | 383.33 ± 0.6 c | 401.7 ± 2.10 b | 426.33 ± 0.60 a |
| Solubility (%) | 89.57 ± 0.21 a | 87.20 ± 0.26 b | 88.67 ± 0.21 c | 88.13 ± 0.15 c |
| Bulk density (g/mL) | 0.21 ± 0.02 c | 0.24 ± 0.02 c | 0.29 ± 0.01 b | 0.31 ± 0.02 a |
| Tapped density (g/mL) | 0.43 ± 0.01 c | 0.48 ± 0.02 c | 0.59 ± 0.02 b | 0.64 ± 0.04 a |
| Formula | Encapsulation Efficiency (%) | Peroxide Value (meq O2/kg Oil) |
|---|---|---|
| 1 | 70.80 ± 0.25 a | 10.70 ± 0.03 d |
| 2 | 68.50 ± 0.12 b | 11.00 ± 0.01 c |
| 3 | 65.80 ± 0.70 c | 11.23 ± 0.03 b |
| 4 | 63.30 ± 0.12 d | 11.40 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, O.; Petersen, G.E.; Fields, C. Valorization of Coffee Pulp: Spray-Dried Hemp Oil Microcapsules Stabilized with Coffee Pectin and Maltodextrin. Sustainability 2025, 17, 8152. https://doi.org/10.3390/su17188152
Kahraman O, Petersen GE, Fields C. Valorization of Coffee Pulp: Spray-Dried Hemp Oil Microcapsules Stabilized with Coffee Pectin and Maltodextrin. Sustainability. 2025; 17(18):8152. https://doi.org/10.3390/su17188152
Chicago/Turabian StyleKahraman, Ozan, Greg E. Petersen, and Christine Fields. 2025. "Valorization of Coffee Pulp: Spray-Dried Hemp Oil Microcapsules Stabilized with Coffee Pectin and Maltodextrin" Sustainability 17, no. 18: 8152. https://doi.org/10.3390/su17188152
APA StyleKahraman, O., Petersen, G. E., & Fields, C. (2025). Valorization of Coffee Pulp: Spray-Dried Hemp Oil Microcapsules Stabilized with Coffee Pectin and Maltodextrin. Sustainability, 17(18), 8152. https://doi.org/10.3390/su17188152






