Mitigating Soil Acidity: Impact of Aglime (CaCO3) Particle Size and Application Rate on Exchangeable Aluminium and Base Cations Dynamics
Abstract
1. Introduction
2. Materials and Methods
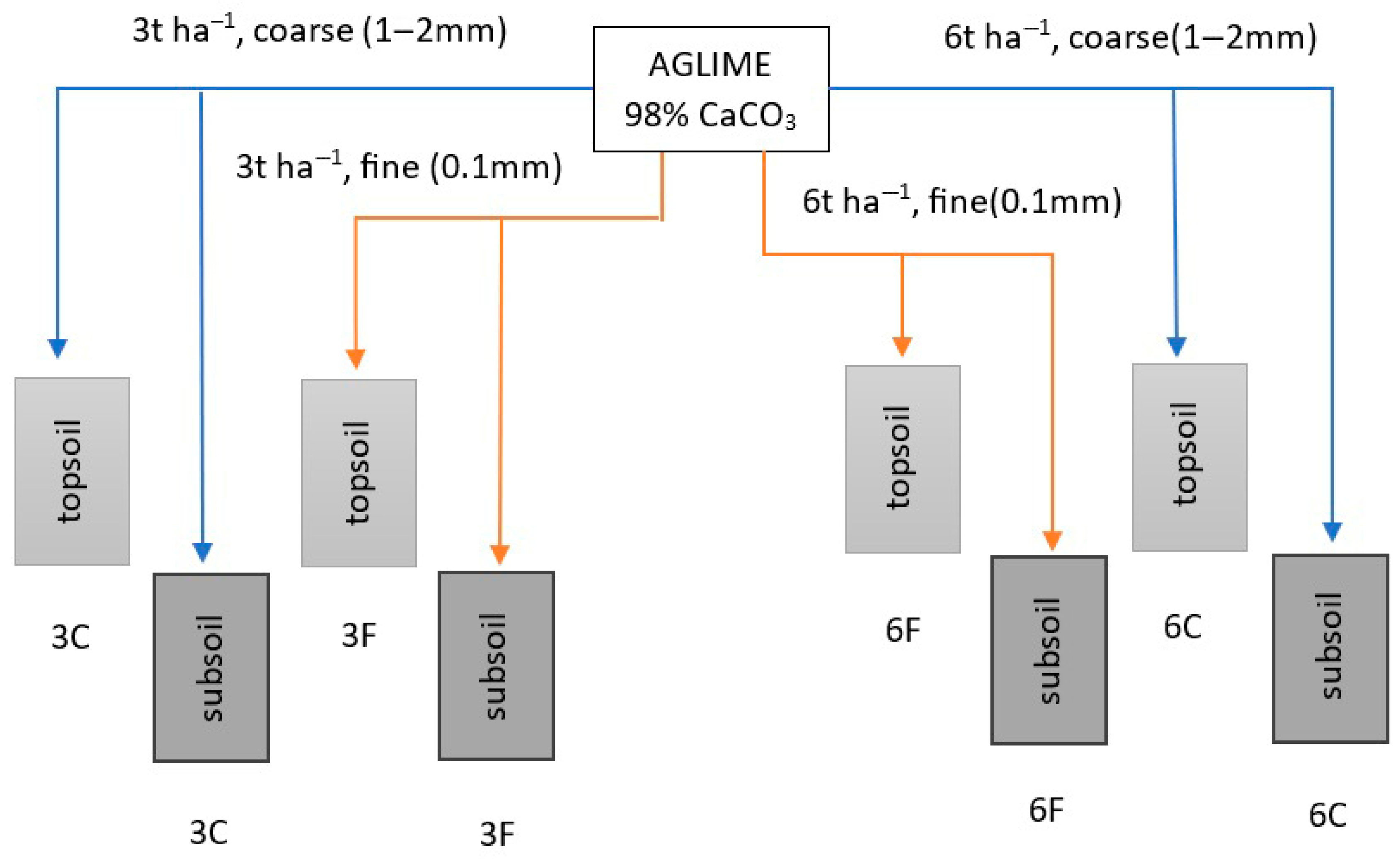
2.1. Experimental Site
2.2. Soil Sampling
2.2.1. pH Determination
2.2.2. Exchangeable Ca (Caexch), Mg (Mgexch), and K (Kexch) Determination
2.2.3. Exchangeable Al (Alexch) Determination
2.2.4. CEC Determination
2.3. Data Analysis
3. Results
3.1. Modification in Soil pH Values
3.2. CEC Values After Lime Application
3.3. Modification of Exchangeable Ca, Mg and K Values
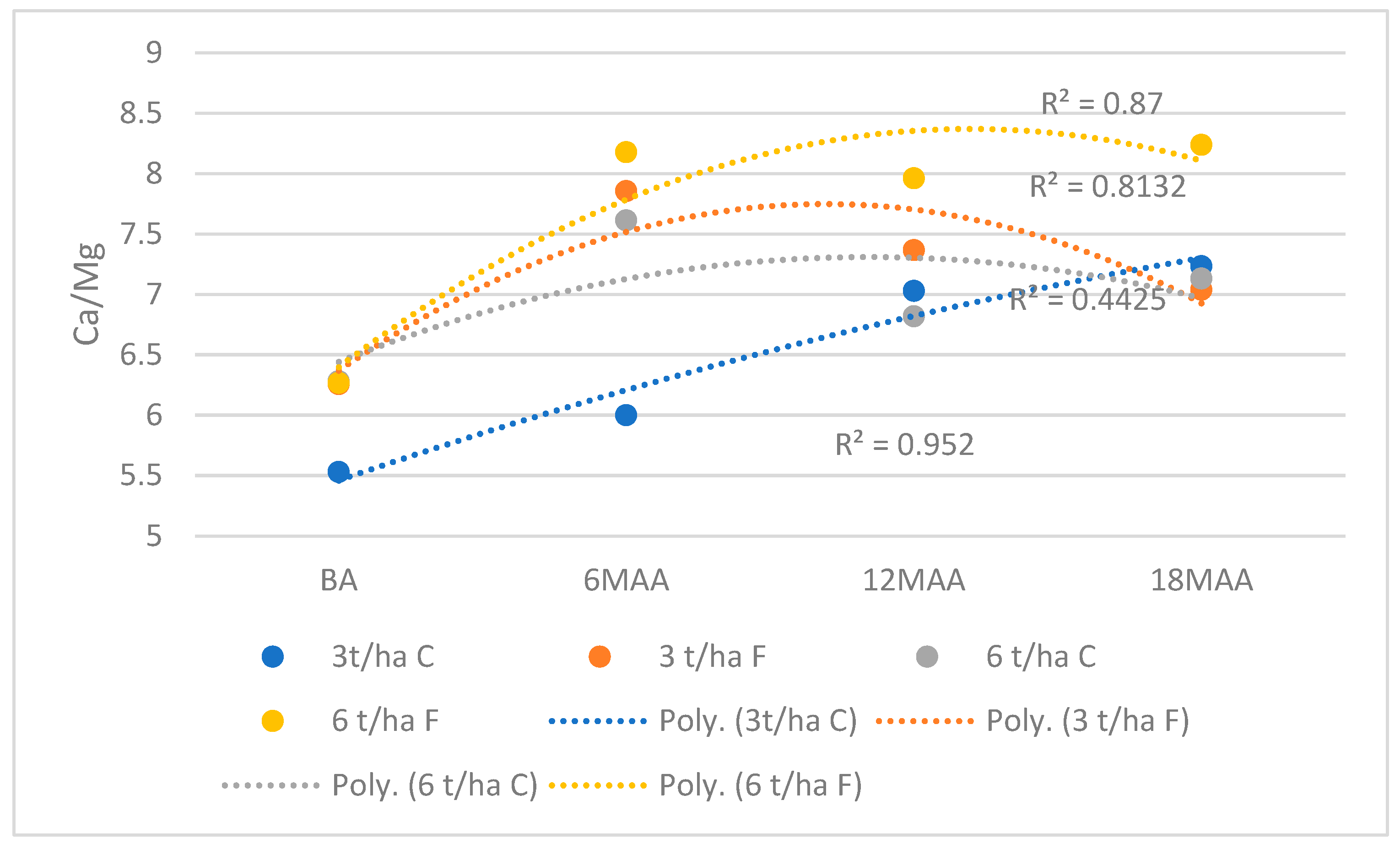
3.4. Exchangeable Aluminum
3.5. Pearson Correlations
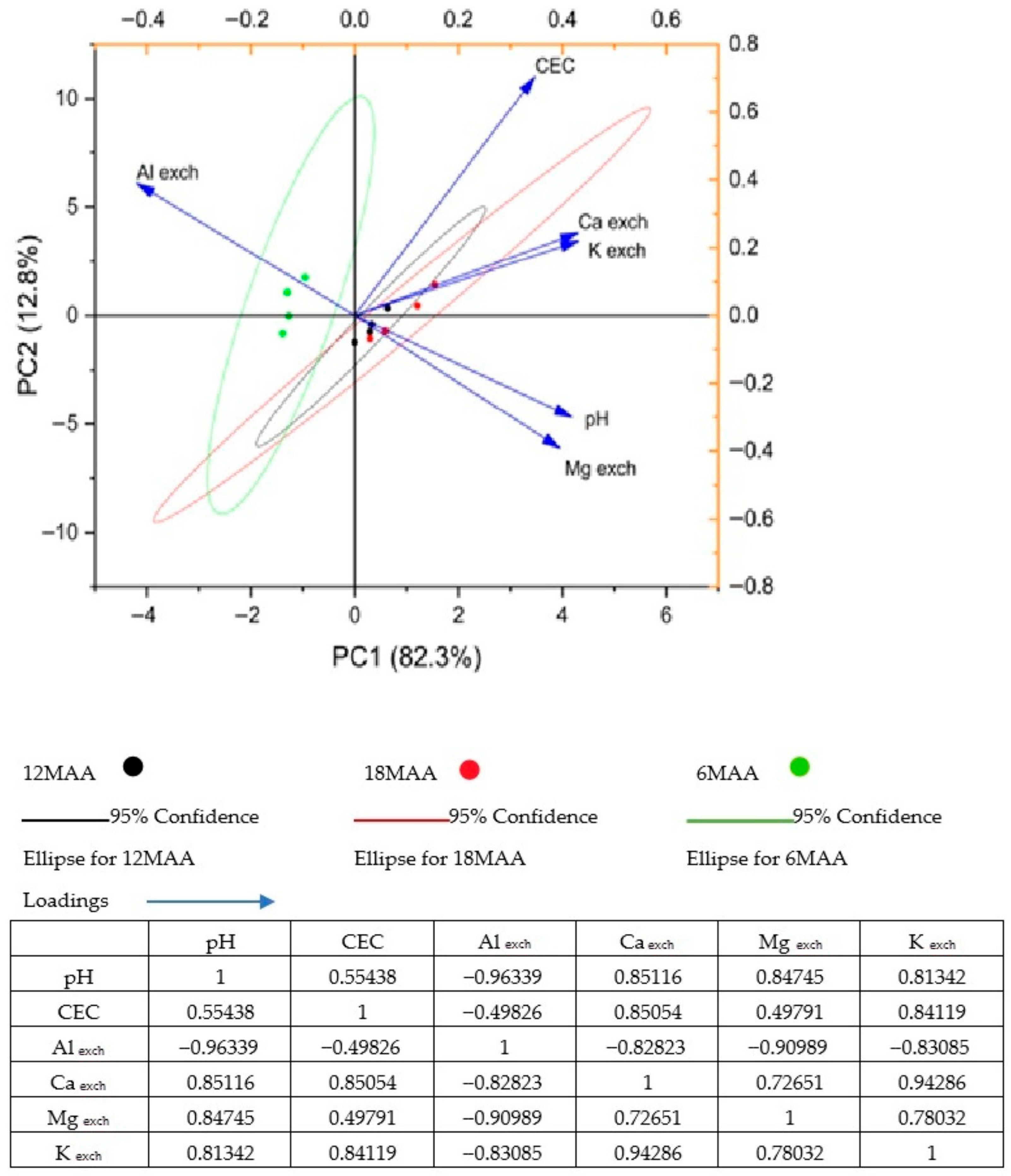
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aglime | Agricultural lime (CaCO3) |
| Kg a.s./ha | Kg active substance/ha |
References
- Ji, F.N.; Osumanu, H.A.; Mohamadu, B.J.; Latifah, O.; Yee, M.K.; Adiza, A.M.; Ken, H.P. Soil nutrient retention and pH buffering capacity are enhanced by calciprill and sodium silicate. Agronomy 2022, 12, 219. [Google Scholar] [CrossRef]
- Slessarev, E.; Lin, Y.; Bingham, N.J.; Johnson, E.; Dai, Y.; Schimel, J.P. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Nagasinghe, I.U.; Saito, T.; Takemura, T.; Kawamoto, K.; Komatsu, T.; Watanabe, N.; Kawabe, Y. Applicability of alkaline waste and by-products as low cost alternative neutralizers for acidic soils. ISIJ Int. 2022, 63, 228–234. [Google Scholar] [CrossRef]
- Sparks, D.L.; Singh, B.; Siebecker, M.G. Environmental Soil Chemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 351–360. [Google Scholar]
- Toth, G.; Montanarella, L.; Stolbovoy, V.; Mate, F.; Bodis, K.; Jones, A.; Panagos, P.; van Liedekerke, M. Soils of the European Union; Office for Official Publications of the European Communities: Luxembourg, 2008; pp. 28–32. ISBN 978-92-79-09530-6. [Google Scholar]
- Enesi, R.O.; Dyck, M.; Chang, S.; Thilakarathna, M.S.; Fan, X.; Strelkov, S.; Gorim, L.Y. Liming remediates soil acidity and im-proves crop yield and profitability—A meta-analysis. Front. Agron. 2023, 5, 1194896. [Google Scholar] [CrossRef]
- Holland, J.E.; White, P.J.; Glendining, M.J.; Goulding, K.W.T.; McGrath, S.P. Yield responses of arable crops to liming–An evaluation of relationships between yields and soil pH from a long-term liming experiment. Eur. J. Agron. 2019, 105, 176–188. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Corbett, D.; Wall, D.P.; Lynch, M.B.; Tuohy, P. The influence of lime application on the chemical and physical characteristics of acidic grassland soils with impeded drainage. J. Agric. Sci. 2021, 159, 206–215. [Google Scholar] [CrossRef]
- Dashuan, T.; Shuli, N. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Popescu, A.; Dinu, T.A.; Stoian, E.; Şerban, V. The use of chemical fertilizers in Romania’s agriculture. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 469–476. [Google Scholar]
- Vicar, N.; Lațo, A.; Lațo, I.; Crista, F.; Berbecea, A.; Radulov, I. Effect of Urease and Nitrification Inhibitors on Heavy Metal Mobility in an Intensively Cultivated Soil. Agronomy 2025, 15, 49. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Kobierski, M. Effect of liming on the change of some agrochemical soil properties in a long-term fertilization experiment. Plant Soil Environ. 2014, 60, 146–150. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: A global meta-analysis. J. Soils Sediments 2019, 19, 1393–1406. [Google Scholar]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Brown, T.T.; Koenig, R.T.; Huggins, D.R.; Harsh, J.B.; Rossi, R.E. Lime effects on soil acidity, crop yield, and aluminum chemistry in direct-seeded cropping systems. Soil Sci. Soc. Am. J. 2008, 72, 634–640. [Google Scholar]
- Daba, N.A.; Li, D.; Huang, J.; Han, T.; Zhang, L.; Ali, S.; Khan, M.N.; Du, J.; Liu, S.; Legesse, T.G. Long-term fertilization and lime-induced soil pH changes affect nitrogen use efficiency and grain yields in acidic soil under wheat-maize rotation. Agronomy 2021, 11, 2069. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Dijkstra, F.A. Ameliorating soil acidity with calcium carbonate and calcium hydroxide: Effects on carbon, nitrogen, and phosphorus dynamics. J. Soil. Sci. Plant. Nutr. 2023, 23, 5270–5278. [Google Scholar] [CrossRef]
- Herbei, M.V.; Sala, F. Evaluation of urban areas by remote sensing methods in relation to climatic conditions: Case study city of Timisoara. Carpathian J. Earth Environ. Sci. 2020, 15, 327–337. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; First Update 2007; World Soil Resources Reports No.103; FAO: Rome, Italy, 2007. [Google Scholar]
- Simansky, V. Can soil properties of Fluvisols be influenced by river flow gradient? Acta Fytotech. Zootech. 2018, 21, 63–76. [Google Scholar] [CrossRef]
- Abraham, Y. Evaluation of micro-dosing lime application on selected soil chemical properties and barley crop performance at Gedeo zone, Southern Ethiopia. Am. J. Chem. Eng. 2024, 12, 117–122. [Google Scholar] [CrossRef]
- Henderson, W.H.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 173–178. [Google Scholar]
- FAO. Standard Operating Procedure for Soil pH Determination; FAO: Rome, Italy, 2021. [Google Scholar]
- Jönsson, U.; Rosengren, U.; Bengt, N.; Thelin, G. A comparative study of two methods for determination of pH, exchangeable base cations, and aluminum. Commun. Soil Sci. Plant Anal. 2002, 33, 3809–3824. [Google Scholar] [CrossRef]
- Ross, D.S.; Ketterings, Q. Recommended methods for determining, soil cation exchange capacity. In Recommended Soil Testing Procedures for the Northeastern United States; University of Delaware: Newark, DE, USA, 2011. [Google Scholar]
- Ćirić, V.P.; Šeremešić, N.; Vojnov, S.; Pejic, B.; Radovanović, B.; Marinkovic, D. The Implication of Cation Exchange Capacity (CEC) Assessment for Soil Quality Management and Improvement. J. Agric. Forest. 2023, 69, 113–133. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Hang, H.; Chen, S.; Liu, H.; Su, J.; Lv, H.; Jia, H.; Zhao, G. Effects of Balancing Exchangeable Cations Ca, Mg, and K on the Growth of Tomato Seedlings (Solanum lycopersicum L.) Based on Increased Soil Cation Exchange Capacity. Agronomy 2024, 14, 629. [Google Scholar] [CrossRef]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Education Inc.: London, UK, 2017. [Google Scholar]
- Jones, J.D.; Mallarino, A.P. Influence of source and particle size on agricultural limestone efficiency at increasing soil pH. In Proceedings of the North Central Extension-Industry Soil Fertility Conference, Des Moines, IA, USA, 17–18 November 2016. [Google Scholar]
- Oliver, Y.M.; Gazey, C.; Fisher, J.; Robertson, M. Dissection of the contributing factors to the variable response of crop yield to surface applied lime in Australia. Agronomy 2021, 11, 829. [Google Scholar] [CrossRef]
- Tutivén, J.C.B.; Suarez, H.O.E.; Montúfar, G.H.V. Buffer capacity as a method to estimate the dose of liming in acid. Agro Product. 2022, 15, 105–112. [Google Scholar] [CrossRef]
- Caires, E.F.; Haliski, A.; Bini, A.R.; Scharr, D.A. Surface liming and nitrogen fertilization for crop grain production under no-till management in Brazil. Eur. J. Agron. 2015, 66, 41–53. [Google Scholar] [CrossRef]
- Kibet, P.K.; Mugwe, J.N.; Korir, N.K.; Mucheru-Muna, M.W.; Ngetich, F.K. Granular and powdered lime improves soil properties and maize (Zea mays L.) performance in humic Nitisols of central highlands in Kenya. Heliyon 2023, 9, e17286. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; Conyers, M.K.; Fisher, R.; Lill, W. Particle size determines the efficiency of calcitic limestone in amending acidic soil. Aust. J. Agric. Res. 1992, 43, 1175–1185. [Google Scholar] [CrossRef]
- du Toit, D.J.J.; Swanepoel, P.A.; Hardie, A.G. Effect of Lime Source, Fineness and Granulation on Soil Permeation with Contrasting Textures under Simulated Mediterranean Climate Rainfall Conditions. Commun. Soil Sci. Plant Anal. 2024, 55, 3011–3024. [Google Scholar] [CrossRef]
- Moir, J.L.; Moot, D.J. Soil pH, exchangeable aluminium and lucerne yield responses to lime in a South Island high country soil. In Proceedings of the New Zealand Grassland Association, Lincoln, NE, USA, 5 October 2010; Volume 72, pp. 191–196. [Google Scholar]
- Ianos, G.; Pusca, I.; Goian, M. Banat Soils—Part II—Natural Conditions and Fertility; Mirton: Timisoara, Romania, 1997; pp. 118–130. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Education Inc.: London, UK, 2008; pp. 423–444. [Google Scholar]
- Wang, X.; Tang, C.; Baldock, J.A.; Butterly, C.R.; Gazey, C. Long-term effect of lime application on the chemical composition of soil organic carbon in acid soils varying in texture and liming history. Biol. Fertil. Soils 2016, 52, 295–306. [Google Scholar]
- Olego, M.Á.; Quiroga, M.J.; Mendaña-Cuervo, C.; Cara-Jiménez, J.; López, R.; Garzón-Jimeno, E. Long-term effects of calcium-based liming materials on soil fertility sustainability and rye production as soil quality indicators on a Typic Palexerult. Processes 2021, 9, 1181. [Google Scholar] [CrossRef]
- Workineh, E.; Yihenew, G.S.; Eyasu, E.; Eyayu, M. Effect of lime rates and method of application on soil properties of acidic Luvisols and wheat (Triticum aestivum, L.) yields in northwest Ethiopia. Heliyon 2023, 9, e13988. [Google Scholar] [CrossRef]
- Miyazawa, M.; Pavan, M.A.; Ziglio, C.O.; Franchini, J.C. Reduction of Exchangeable Calcium and Magnesium in Soil with Increasing pH. Braz. Arch. Biol. Technol. 2001, 44, 149–153. [Google Scholar] [CrossRef][Green Version]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Munyaneza, V.; Zhang, W.; Haider, S. Strategies for alleviating aluminum toxicity in soils and plants. Plant Soil 2024, 504, 167–190. [Google Scholar] [CrossRef]
- Whitley, A.E.; Moir, J.L.; Almond, P.C.; Moot, D.J. Soil pH and exchangeable aluminium in contrasting New Zealand high and hill country soils, Hill Country. Grassl. Res. Pract. Ser. 2016, 16, 169–172. [Google Scholar] [CrossRef]
- Caires, E.F.; Churka, S.; Barth, G.; Garbuio, F.J. Different limestone particle sizes for soil acidity correction, Ca and Mg supply and corn yield. Rev. Bras. Cienc. Solo 2006, 30, 275–286. [Google Scholar]


| Characteristic | Horizon Ap/A (0–20 cm) | Horizon Eg (20–40 cm) |
|---|---|---|
| Texture | Loamy sand | Clay loam |
| Structure | Granular | Lamellar/weakly structured |
| Humus content | 2.82 ± 0.02% | 2.16 ± 0.03% |
| pH (H2O) | 5.07 ± 0.03 | 5.17 ± 0.04 |
| CEC (cation exchange capacity) | 9.38 ± 0.06 cmol kg−1 | 9.17 ± 0.02 cmol kg−1 |
| Caexch (exchangeable Ca) | 4.68 ± 0.03 cmol kg−1 | 4.64 ± 0.01 cmol kg−1 |
| Mgexch (exchangeable Mg) | 0.77 ± 0.05 cmol kg−1 | 0.87 ± 0.05 cmol kg−1 |
| Kexch (exchangeable K) | 0.145 ± 0.004 cmol kg−1 | 0.120 ± 0.007 cmol kg−1 |
| Alexch (exchangeable Al) | 3.11 ± 0.21 cmol kg−1 | 2.62 ± 0.18 cmol kg−1 |
| Date | Dose And | pH | CEC (cmol kg−1) | Alexch (cmol kg−1) | |||
|---|---|---|---|---|---|---|---|
| Granulation | TS | SS | TS | SS | TS | SS | |
| BA | 3C | 5.08 ± 0.10 | 5.15 ± 0.04 | 9.4 ± 0.18 | 9.31 ± 0.12 | 3.10 ± 0.310 | 2.79 ± 0.470 |
| 3F | 5.12r ± 0.02 | 5.24 ± 0.05 | 9.27 ± 0.03 | 9.24 ± 0.16 | 2.78 ± 0.250 | 2.590 ± 0.140 | |
| 6C | 5.03 ± 0.05 | 5.13 ± 0.03 | 9.42 ± 0.11 | 9.3 ± 0.25 | 3.300 ± 0.590 | 2.330 ± 0.540 | |
| 6F | 5.06 ± 0.12 | 5.17 ± 0.03 | 9.41 ± 0.19 | 9.26 ± 0.06 | 3.290 ± 0.360 | 2.790 ± 0.630 | |
| 6MAA | 3C | 5.62 ± 0.06 ab | 5.39 ± 0.02 cde | 9.71 ± 0.05 c | 9.62 ± 0.04 c | 1.98 ± 0.050 | 2.43 ± 0.460 |
| 3F | 5.80 ± 0.06 a | 5.6 ± 0.12 abc | 9.92 ± 0.08 c | 9.93 ± 0.03 c | 1.74 ± 0.290 | 2.29 ± 0.440 | |
| 6C | 5.49 ± 0.04 abc | 5.21 ± 0.1 cde | 10.61 ± 0.06 a | 10.47 ± 0.24 a | 2.24 ± 0.540 | 2.23 ± 0.190 | |
| 6F | 5.62 ± 0.08 ab | 5.31 ± 0.08 de | 11.22 ± 0.20 b | 11.05 ± 0.16 b | 2.19 ± 0.480 | 2.61 ± 0.370 | |
| 12MAA | 3C | 6.47 ± 0.05 a | 5.43 ± 0.08 d | 10.21 ± 0.10 d | 10.12 ± 0.24 d | 0.43 ± 0.080 b | 1.98 ± 0.250 a |
| 3F | 6.55 ± 0.03 a | 5.72 ± 0.04 c | 10.79 ± 0.27 c | 11.1 ± 0.15 abc | 0.31 ± 0.030 b | 1.84 ± 0.230 a | |
| 6C | 6.28 ± 0.04 b | 5.39 ± 0.06 d | 11.04 ± 0.15 a | 10.75 ± 0.08 abc | 0.44 ± 0.210 b | 1.94 ± 0.300 a | |
| 6F | 6.57 ± 0.03 a | 5.45 ± 0.07 d | 11.55 ± 0.13 bc | 11.44 ± 0.17 | 0.35 ± 0.130 b | 2.1 ± 0.210 a | |
| 18MAA | 3C | 6.39 ± 0.04 b | 5.64 ± 0.05 d | 10.88 ± 0.14 b | 10.05 ± 0.16 c | 0.08 ± 0.060 b | 1.71 ± 0.530 a |
| 3F | 6.33 ± 0.05 b | 5.85 ± 0.06 c | 10.37 ± 0.08 c | 10.19 ± 0.19 c | 0.05 ± 0.010 b | 1.61 ± 0.260 a | |
| 6C | 6.6 ± 0.05 a | 5.36 ± 0.05 a | 12.43 ± 0.17 a | 12.21 ± 0.19 a | - | 1.7 ± 0.400 a | |
| 6F | 6.63 ± 0.08 a | 5.6 ± 0.14 d | 12.09 ± 0.19 a | 12.13 ± 0.15 a | - | 1.86 ± 0.460 a | |
| Date | Dose And | Caexch (cmol kg−1) | Mgexch (cmol kg−1) | Kexch (cmol kg−1) | |||
|---|---|---|---|---|---|---|---|
| Granulation | TS | SS | TS | SS | TS | SS | |
| BA | 3C | 4.70 ± 0.32 | 4.65 ± 0.39 | 0.85 ± 0.085 | 0.740 ± 0.123 | 0.151 ± 0.022 | 0.122 ± 0.013 |
| 3F | 4.63 ± 0.50 | 4.62 ± 0.39 | 0.740 ± 0.028 | 0.830 ± 0.046 | 0.140 ± 0.035 | 0.110 ± 0.019 | |
| 6C | 4.71 ± 0.31 | 4.65 ± 0.37 | 0.750 ± 0.051 | 0.840 ± 0.062 | 0.150 ± 0.023 | 0.129 ± 0.024 | |
| 6F | 4.70 ± 0.30 | 4.65 ± 0.37 | 0.75 ± 0.073 | 0.840 ± 0.113 | 0.142 ± 0.013 | 0.120 ± 0.011 | |
| 6MAA | 3C | 5.82 ± 0.79 ab | 5.69 ± 0.44 ab | 0.97 ± 0.174 | 0.77 ± 0.147 | 0.212 ± 0.017 | 0.159 ± 0.024 |
| 3F | 6.45 ± 0.56 ab | 5.45 ± 0.46 b | 0.82 ± 0.060 | 0.89 ± 0.102 | 0.225 ± 0.05 | 0.172 ± 0.021 | |
| 6C | 6.47 ± 0.56 ab | 5.28 ± 0.51 b | 0.85 ± 0.016 | 0.92 ± 0.198 | 0.231 ± 0.042 | 0.18 ± 0.095 | |
| 6F | 6.95 ± 0.26 a | 5.96 ± 0.31 ab | 0.85 ± 0.051 | 0.9 ± 0.150 | 0.246 ± 0.048 | 0.21 ± 0.069 | |
| 12MAA | 3C | 7.25 ± 0.56 ab | 6.18 ± 0.71 b | 1.03 ± 0.069 | 0.82 ± 0.182 | 0.264 ± 0.038 | 0.191 ± 0.017 |
| 3F | 7.66 ± 0.54 ab | 6.54 ± 0.28 b | 1.04 ± 0.06 | 0.93 ± 0.115 | 0.267 ± 0.027 | 0.213 ± 0.014 | |
| 6C | 7.50 ± 0.49 ab | 6.20 ± 0.80 b | 1.1 ± 0.101 | 0.99 ± 0.091 | 0.283 ± 0.054 | 0.22 ± 0.065 | |
| 6F | 8.20 ± 0.38 a | 7.00 ± 0.52 b | 1.03 ± 0.126 | 0.88 ± 0.122 | 0.289 ± 0.063 | 0.243 ± 0.046 | |
| 18MAA | 3C | 8.10 ± 0.30 ab | 7.03 ± 0.30 bc | 1.12 ± 0.188 | 0.91 ± 0.058 | 0.286 ± 0.082 abc | 0.209 ± 0.023 c |
| 3F | 7.46 ± 0.47 bc | 6.82 ± 0.20 c | 1.06 ± 0.064 | 1.03 ± 0.047 | 0.297 ± 0.029 abc | 0.215 ± 0.016 bc | |
| 6C | 8.20 ± 0.75 ab | 7.65 ± 0.43 bc | 1.15 ± 0.068 | 1.02 ± 0.127 | 0.334 ± 0.044 ab | 0.254 ± 0.04 abc | |
| 6F | 9.06 ± 0.26 a | 7.85 ± 0.43 bc | 1.1 ± 0.202 | 1.01 ± 0.016 | 0.36 ± 0.041 a | 0.274 ± 0.028 abc | |
| Date | TS (Topsoil) | SS (Subsoil) |
|---|---|---|
| 6MAA (6months after application) | Positive moderate correlations: Ca-CEC (R = 0.521); CEC-Al (R = 0.501) | Positive moderate and strong correlations: Ca-K (R = 0.614), Mg-K (R = 0.888) |
| Negative moderate and strong correlations: pH-CEC (R = −0.442), pH-Al (R = −0.734) | Negative moderate correlations: Al-Ca (R = −0.434), Al-Mg (R = −0.446) pH-CEC (R = −0.537), | |
| 12MAA (12 months after application) | Positive moderate correlation: pH-Ca (R = 0.440), Ca-K (R = 0.569), CEC-Ca (R = 0.569) | Positive moderate and strong correlations: pH-Ca (R = 0.415), CEC-K (R = 0.452), CEC-Ca (R = 0.479), Ca-Mg (R = 0.840) |
| Negative moderate and strong correlations: pH-Al (R = −0.552), Ca-Al (R = −0.650), K-Al (R = −0.804) | Negative moderate and strong correlations: pH-Al (R = −0.586), Ca-Al (R = −0.609), Mg-Al (R = −0.823) | |
| 18MAA (18 months after application) | Positive moderate and strong correlations: Ca-Mg (R = 0.453), CEC-K (R = 0.487), Ca-K (R = 0.655), pH-ca (R = 0.657), pH-K (R = 0.662), CEC-Ca (R = 0.766), pH-CEC (R = 0.909) | Positive moderate and strong correlations: Ca-Mg (R = 0.518), K-Mg (R = 0.699), CEC-K (R = 0.766), CEC-Ca (R = 0.813), Ca-K (R = 0.967) |
| Negative moderate correlations: Ca-Al (R = −0.468), CEC-Al (R = −0.622), Al-K (R = −0.658), pH-Al (R = −0.663) | Negative moderate correlations: pH-Al (R = −0.401), K-Al (R = −0.445), Al-Mg (R = −0.621), pH-CEC (R = −0.669) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lațo, A.; Berbecea, A.; Lațo, I.; Crista, F.; Crista, L.; Sala, F.; Radulov, I. Mitigating Soil Acidity: Impact of Aglime (CaCO3) Particle Size and Application Rate on Exchangeable Aluminium and Base Cations Dynamics. Sustainability 2025, 17, 8135. https://doi.org/10.3390/su17188135
Lațo A, Berbecea A, Lațo I, Crista F, Crista L, Sala F, Radulov I. Mitigating Soil Acidity: Impact of Aglime (CaCO3) Particle Size and Application Rate on Exchangeable Aluminium and Base Cations Dynamics. Sustainability. 2025; 17(18):8135. https://doi.org/10.3390/su17188135
Chicago/Turabian StyleLațo, Alina, Adina Berbecea, Iaroslav Lațo, Florin Crista, Laura Crista, Florin Sala, and Isidora Radulov. 2025. "Mitigating Soil Acidity: Impact of Aglime (CaCO3) Particle Size and Application Rate on Exchangeable Aluminium and Base Cations Dynamics" Sustainability 17, no. 18: 8135. https://doi.org/10.3390/su17188135
APA StyleLațo, A., Berbecea, A., Lațo, I., Crista, F., Crista, L., Sala, F., & Radulov, I. (2025). Mitigating Soil Acidity: Impact of Aglime (CaCO3) Particle Size and Application Rate on Exchangeable Aluminium and Base Cations Dynamics. Sustainability, 17(18), 8135. https://doi.org/10.3390/su17188135






