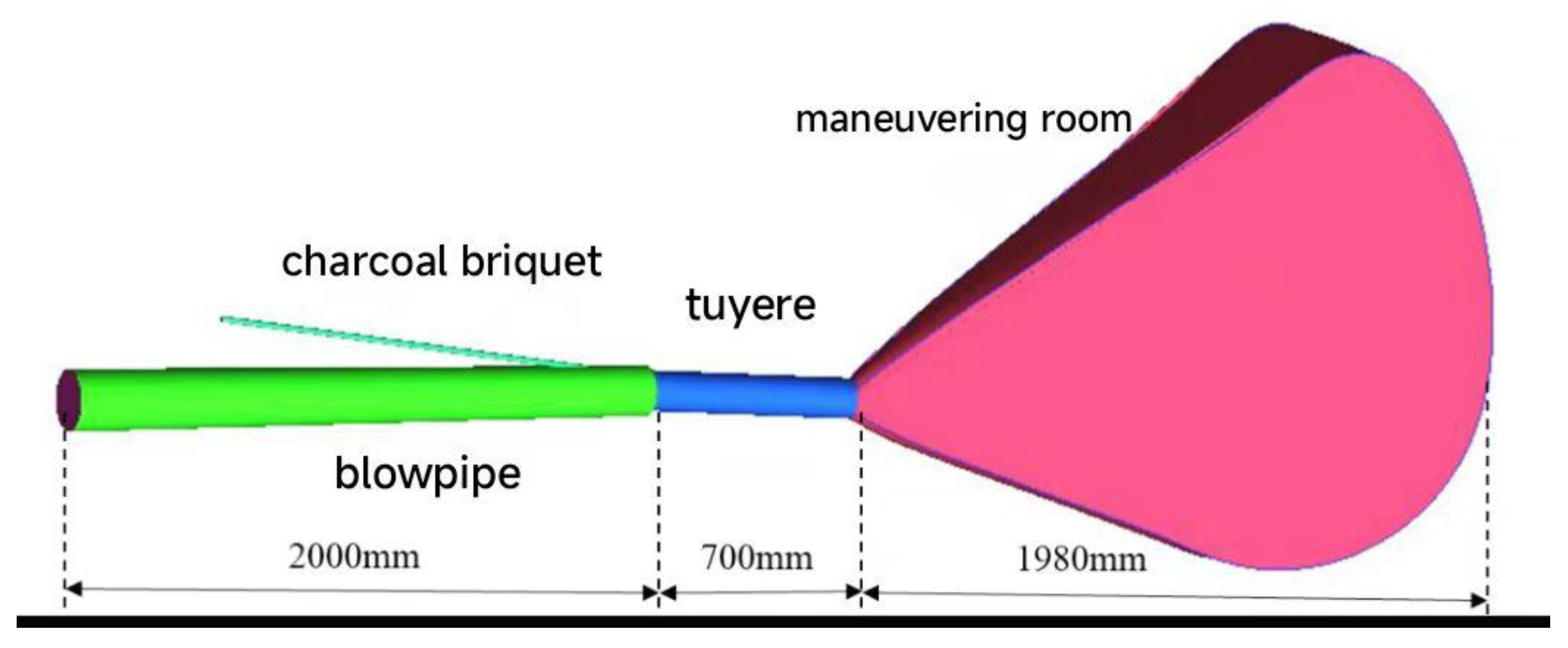
3.1. Analytical Results for Different Oxygen Enrichment Rates
The combustion process when pulverized coal is co-injected with hydrochar was simulated for oxygen enrichment rates of 5%, 7.2%, 9%, and 11%, respectively.
Figure 2 shows the temperature cloud on the symmetric surface at the center of the model and the temperature variation curve at the centerline of the coal plume. Analyzing the temperature field in the figure, it can be found that with the increase in oxygen concentration, the difference in the temperature field distribution near the exit of the coal lance is not particularly large. This is due to the fact that even if the oxygen concentration of the blast is increased, the heat transfer coefficient between the blast and the pulverized coal will not be changed, and thus cannot promote the heat absorption and combustion of the pulverized coal. In addition, although the increase in oxygen concentration can improve the mass transfer coefficient between the pulverized coal particles and O
2, due to the process of momentum exchange with the high-speed hot air, the pulverized coal flow rate is very fast, subject to the constraints of the combustion time and space, which restricts the improvement of the mass transfer rate. It can be seen that due to the increase in oxygen concentration in the blast air makes the coal dust and hydrochar can be more fully combusted. Therefore, with the increase in oxygen concentration in the blast air, the high temperature region within the gyratory zone expands. After entering the gyratory zone at the wind mouth, it can be seen from the temperature curve that the temperature increases rapidly, and the highest temperatures on the centerline of the four coal plumes are 2464 K, 2533 K, 2581 K, and 2632 K. The temperature changes are more obvious with the increase in oxygen enrichment rate.
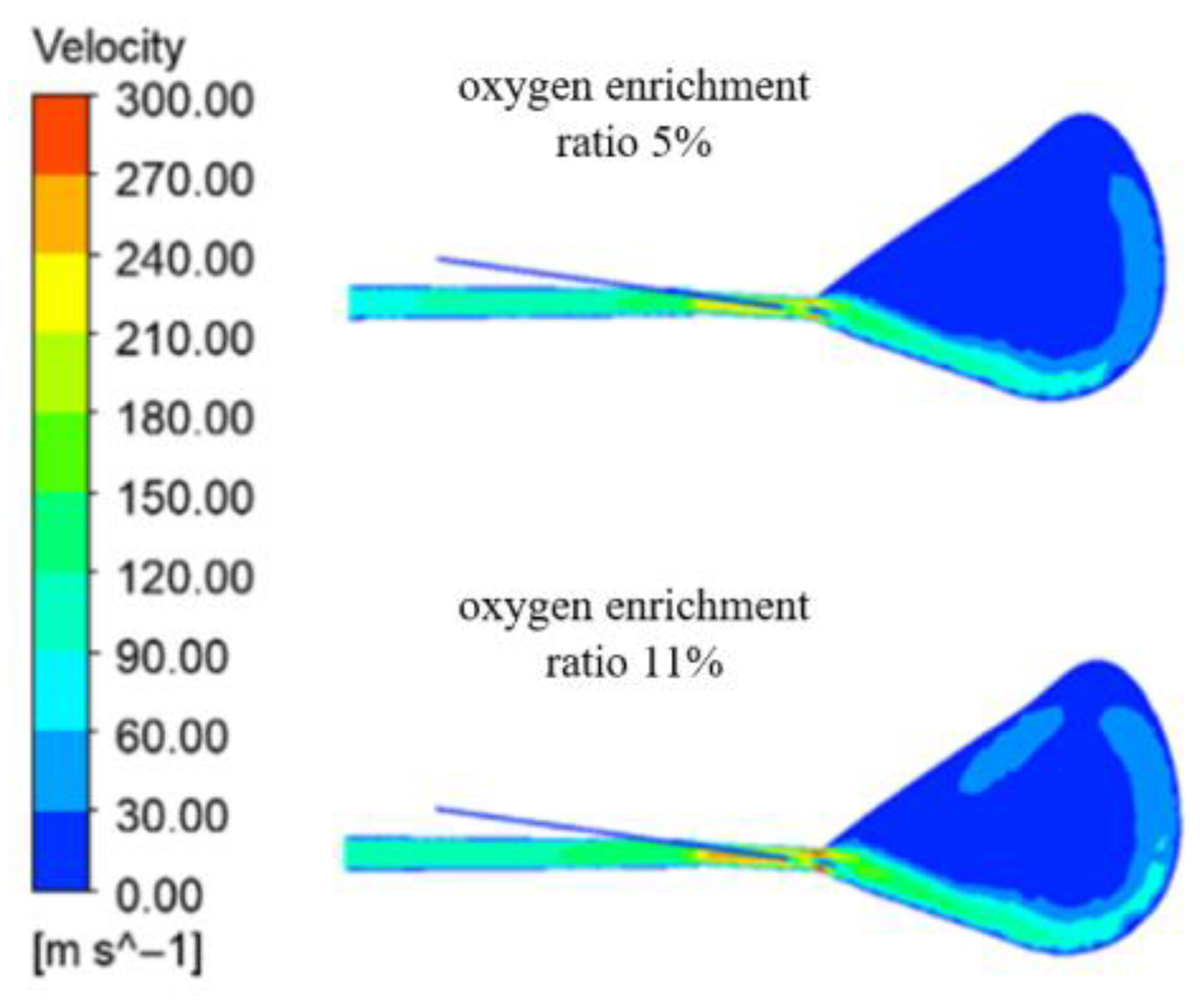
The velocity field in the gyratory region under different oxygen concentrations is shown in
Figure 3. Comparing the velocity field cloud diagrams, it can be seen that under the condition of high-speed blast, the gas with very high velocity moves along the centerline of the wind outlet at high speed and moves upward rapidly in the center region of the blast furnace, so as to form a main stream gas velocity formed by the high-speed gas in front of the wind outlet and upward movement of the gas in the center of the furnace chamber. The maximum velocity of the gas under four kinds of oxygen-rich rate appeared at the outlet of the wind mouth, respectively, as 312.5 m/s, 315.7 m/s, 320.4 m/s, and 324.4 m/s. It can be seen that the movement of pulverized coal particles increases in the process of the movement of the gas velocity in the wind mouth, below the gyratory area, with the increase in the oxygen-rich rate. This may be due to the increase in the concentration of oxygen resulting in higher temperatures, volatile reaction, and faster reaction of residual carbon to generate increases in gas volume. This may be the result of the increase in oxygen concentration which leads to the increase in temperature, while the volume of gas generated after the reaction of the volatile matter and residual carbon increases quickly.
Figure 4 shows the distribution cloud of the main gas content under different oxygen enrichment rates, and
Figure 5 shows the change curve of the main gas content in the centerline of the coal plume under different oxygen enrichment rates. Overall, the higher the oxygen concentration, the more CO
2 and CO are generated in the cyclone zone, which is easy to understand. After the blended coal enters the cyclone area, i.e., around 0.2–0.4 m, a large amount of CO
2 is generated, the oxygen is consumed drastically, and less CO is generated at this time. After 0.4 m, CO is generated. On the one hand, it is the lack of oxygen that leads to the generation of CO, on the other hand, a large amount of CO
2 reacts with the residual carbon to generate CO. Due to the fact that the amount of oxygen is small in comparison with that of the blowing pulverized coal, the amount of oxygen is always insufficient, and the four types of gas content are not enough to be generated after leaving the blowhole after 0.4 m. After 0.4 m, the vast majority of O
2 in the four cases has been consumed and the mass fraction is no longer different.
For carbon dioxide, the carbon dioxide molar fractions at different oxygen enrichment rates increase rapidly at high oxygen content and reach the maximum value around 0.43 m. The carbon dioxide molar fractions at different oxygen enrichment rates increase rapidly at high oxygen content and reach the maximum value around 0.43 m. Before this stage, the oxygen concentration decreases very quickly, and when the oxygen content is low, the generation of CO begins, after which the carbon dioxide content gradually decreases and finally tends to zero due to the solvation reaction of the residual carbon, at this time, the carbon dioxide mole fraction corresponding to an oxygen enrichment rate of 11% is about 3.5% higher than that corresponding to an oxygen enrichment rate of 7.2%. The difference of the highest CO2 mole fraction is mainly influenced by the oxygen content, high oxygen concentration will make the unit volume of pulverized coal burn more fully, and then generate more CO2.
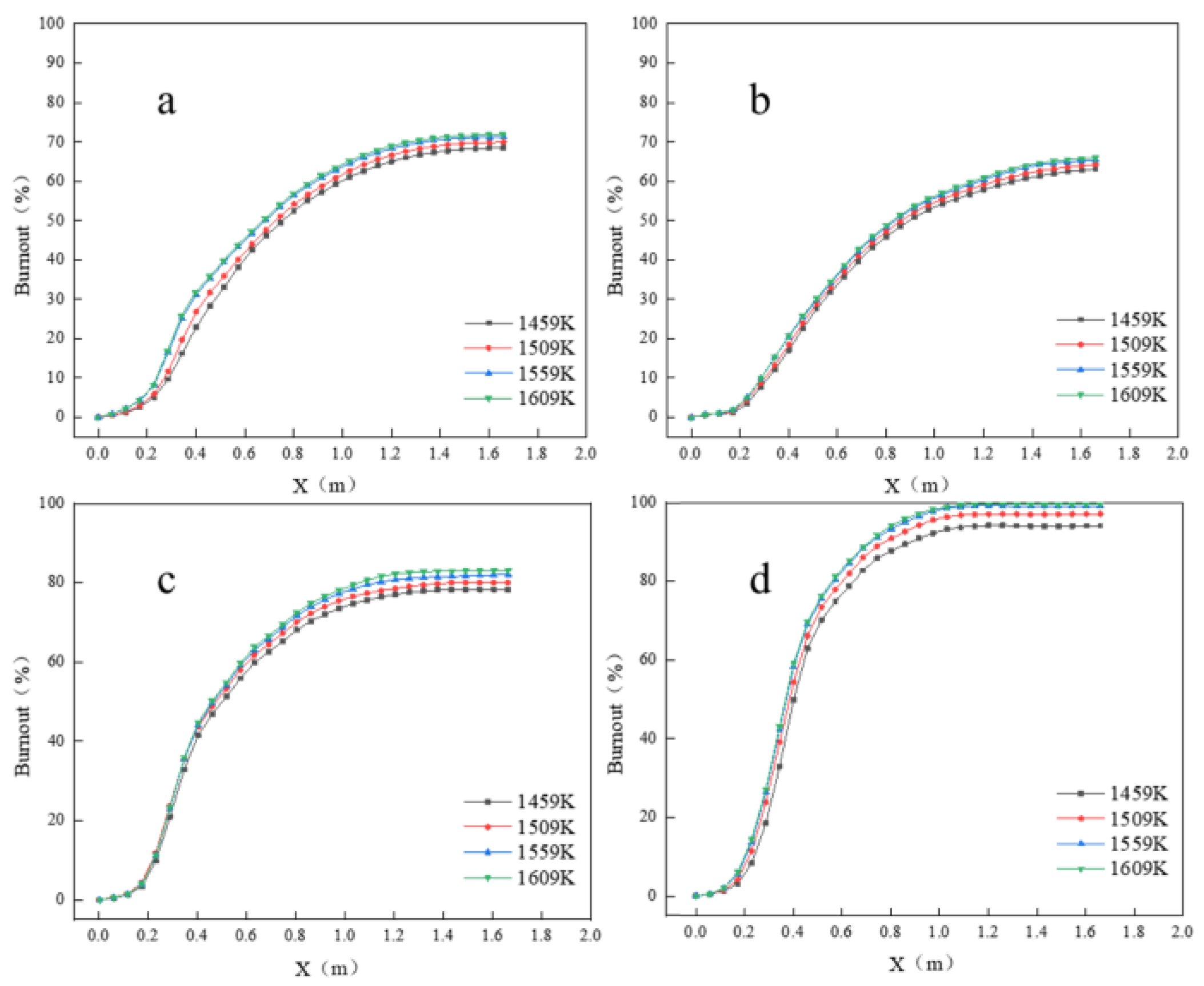
Figure 6 shows the blended coal burnout rate and the burnout rate of each coal type under different oxygen enrichment rates.
Figure 6a shows the variation of blended coal burnout rate, and it can be seen that there is almost no difference in the blended coal burnout rate under different oxygen enrichment rates in the preheating stage. After that, due to the difference in oxygen enrichment rate, the burnout rate of pulverized coal is higher in the case of high oxygen enrichment rate. In the case of high oxygen enrichment rate, the remaining residual carbon after volatile matter volatilization can be reacted with the excess of oxygen, and more CO
2 can be generated after the oxygen is consumed to react with more residual carbon to make the burnout rate of pulverized coal is higher. Jingtang blast furnace coal is relatively high, resulting in a lower amount of relative oxygen, so the change in the oxygen concentration has a greater impact on the burnout rate of blended coal. Four kinds of oxygen-rich rates of blended coal had burnout rates of 67.08%, 69.96%, 72.25%, and 73.8%, respectively.
Figure 6b–d show the burnout rates of anthracite, bituminous coal, and hydrochar, respectively. As can be seen from the figures, hydrochar has the highest burnout rate, even close to complete burnout under high oxygen enrichment, and anthracite has the lowest burnout rate, which is least affected by the oxygen enrichment rate. Due to the high content of volatile matter in hydrochar, the combustion exhaustion rate in the pre-combustion period can reach about 60%, and the combustion exhaustion rate can reach 99.91% in the case of 11% oxygen enrichment after 1 m of spraying out of the coal lance. As the residual carbon content of hydrochar is less, after the volatile matter is completely volatilized, the dissolution reaction of residual carbon is also carried out more thoroughly. On the other hand, anthracite is different, the volatile fraction of anthracite is only 11.12%, while the fixed carbon can reach 78.54%, the burnout rate of anthracite is mainly expressed in the dissolution reaction of residual carbon. Relative to bituminous coal, the burnout rate of anthracite is less affected, the oxygen enrichment rate increases from 5% to 11%, the burnout rate of anthracite increases from 61.7% to 67.82%, an increase of 6.12%. Bituminous coal, on the other hand, increased from 76.4% to 86.4%, an increase of 10%. The enhanced burnout rate with higher oxygen enrichment not only improves energy efficiency but also reduces unburned carbon waste, aligning with sustainable development’s emphasis on resource optimization. Hydrochar’s high burnout rate (up to 99.91% under high oxygen enrichment) further amplifies this effect. As a biomass-derived fuel, its carbon cycle is near-neutral, reducing net carbon emissions compared to fossil coal and supporting the industry’s low-carbon transition.
3.2. Analysis Results for Different Blast Temperatures
Keeping the oxygen enrichment rate at 7.2%, the comparative analysis of different temperatures was carried out by changing the blast temperature. The simulated blast temperatures were set to 1459 K, 1509 K, 1559 K, and 1609 K.
Figure 7 shows the temperature change of the centerline of the coal plume and the temperature distribution on the symmetric surface at the center of the model. In
Figure 7a, the coal plume centerline temperature in the pre-combustion difference is more obvious, and higher hot air temperature, the distance to reach the maximum temperature is shorter; the temperature increase of 100 K, to reach the maximum temperature from the exit of the coal lance of the distance is shortened by about 0.007 m. And the overall temperature of the coal plume centerline with the increase in the temperature of the blower air, with a maximum temperature of 2516 K, 2531 K, 2541 K, and 2552 K, respectively, 2541 K and 2552 K; the higher the temperature, the smaller the temperature difference of the highest temperature in the centerline of the coal plume.
Figure 7b shows that there is no obvious difference in the temperature distribution within the cyclone area below the hot air temperature of 1559 K, and when the hot air temperature reaches 1609 K, the high temperature area is slightly expanded, but the change is small. From the above temperature cloud and curve, with the increase in the blast temperature, the location of the high-temperature zone appeared to shift forward and the high-temperature zone area becomes larger and the highest temperature increases. That is, the main combustion reaction area is closer to the mouth of the wind and put out more heat. There are two main reasons: first, high wind temperature into the cyclone area of the heat increased; second, the unit mass of pulverized coal around the temperature rises, so that the volatile components precipitated combustion process occurs faster and earlier. Combustion of released heat promoted the gas-phase temperature, the gas-phase high temperature and, in turn, promoted the continued combustion of residual carbon. The interaction between the two aspects and mutual influence leads to the location of the high temperature zone to advance and expand.
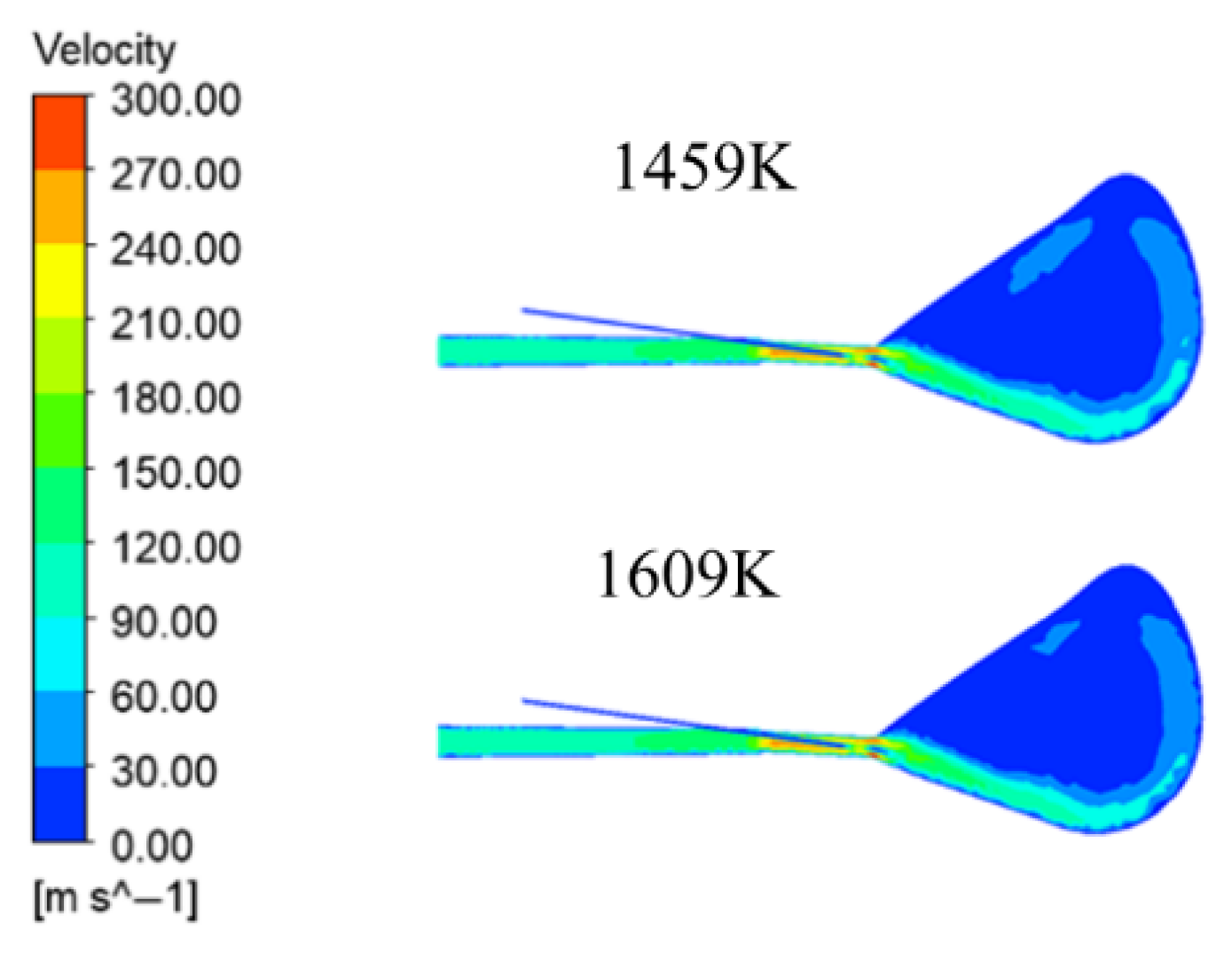
The high temperature air passes through the outlet of the coal lance and the velocity value increases due to the extrusion of the coal lance, after which it exchanges momentum with the coal dust and the velocity value decreases. At the end of the air outlet, the diameter decreases and the velocity increases, and, after entering the gyratory zone, due to the sudden increase in space, the airflow velocity gradually decreases according to the continuity equation. In addition, due to the buoyancy effect, the high temperature air appears to flow diagonally upward, and a part of the gas flows out from the top, while the other part of the gas flows out at a high speed after cycling for a period of time in the cyclotron zone. Among the four blast temperatures in
Figure 8, the maximum velocity occurs at the air opening. The higher the wind temperature, the greater the gas velocity; the maximum velocity was 313.5 m/s, 315.7 m/s, 316.8 m/s, and 318.6 m/s. This is due to the increase in the temperature of the blower, where the gas volume slightly increased, and higher temperatures made the volatile components burn faster to make the gas volume increase, prompting an increase in gas velocity. And above the cyclone area, the gas shows a trend of shrinking in the high velocity area after cyclone. The reason is due to the high blast temperature before this coal powder reached the highest combustion exhaustion, so that the gas volume no longer increases; but at a lower blast temperature, coal powder will have a small solvation reaction after slightly increasing the gas volume, slightly larger than the high velocity area of high temperature working conditions.
Figure 9 shows the distribution of the mass fraction of the main gases in the cloud and
Figure 10 shows the mass fraction change curve of the centerline of the coal plume. When the blast temperature is different, the trend of the same gas cloud is basically the same; after the coal dust leaves the gun and enters the air outlet, it mixes with the high temperature gas and is preheated by the hot air, and the volatile compounds in the preheated coal dust are precipitated and combusted and their combustion process consumes a large amount of oxygen, so the content of oxygen decreases rapidly. The combustion of volatile compounds generates a large amount of CO
2, which leads to the rapid increase in CO
2 in the first distance. In the early stage of pulverized coal combustion, the oxygen content is more sufficient, resulting in slow CO generation. In the later stage, the higher temperature of the blast wind and the more heat brought in make the dissolution reaction of CO
2 faster, so the CO
2 consumption is faster.
For CO2, the CO2 mass fraction peaked at 0.44 m under the blast temperature of 1609 K, which was 0.02 m earlier than the stabilization at 0.47 m under the blast temperature of 1459 K. The figure shows that after the large amount of oxygen consumption, accompanied by the emergence of CO2 peak, and the higher the temperature, the lower the CO2 peak. This indicates that the residual carbon has been carried out at this time the solvation reaction and incomplete combustion. The higher the temperature, the faster the progress of the solvation reaction. CO2 consumption is slightly larger, so when the volatile components of the combustion are completed, most of the residual carbon is only solvation reaction, and the CO2 peak is the degree of solvation reaction.
Figure 11 shows the variation of combustion exhaustion rate of blended coal and each coal type at different blast temperatures.
Figure 11a shows that in the pre-combustion period when the volatile components are burned in large quantities, higher blast temperatures give higher burnout results. After the oxygen is consumed, the combustion exhaustion rate of all four blast temperatures slows down and increases from 1459 K to 1559 K, and the combustion exhaustion rate increases from 68.52% to 71.3%, which is a more obvious change. And from 1559 K to 1609 K, the burnout rate only increased by 0.65%. The optimal blast temperature of 1559 K, balancing burnout efficiency and cost, reflects sustainable development’s focus on economic feasibility alongside environmental benefits. Avoiding excessive temperature increases beyond this point prevents unnecessary energy consumption, minimizing resource waste, and aligning with the principles of efficient energy use in sustainable industrial systems.
Figure 11b shows the burnout rate curve of anthracite. Due to the lower volatile matter of anthracite, after spraying, the lance in the pre-combustion burnout rate is lower, and then due to the higher residual carbon, the dissolution reaction is not as deep as the other two types of coals. So, the overall view of the burnout rate of anthracite by the temperature of the smaller effect. In
Figure 11c, the bituminous coal at 0.4 m combustion rate difference is obvious. This is due to the wind temperature bringing different heat, resulting in higher wind temperature conditions in the bituminous coal in the volatile components of the more intense combustion, this phenomenon is more obvious in the
Figure 11d hydrochar combustion rate curve.
Figure 11c,d shows that the burning rate of coal (hydrochar) reaches a plateau more quickly at higher wind temperatures compared to
Figure 11a.
3.3. Analysis Results of Different Coal Dust Particle Sizes
Particle size mainly affects the ignition characteristics of particles. The ignition characteristics refer to the baseline characteristics of pulverized coal at the combustion stage, when the critical conditions for pulverized coal combustion are reached. Although the combustibility requirements of pulverized coal are generally the same, the ignition time varies, which leads to a change in the combustion efficiency of pulverized coal. In the comparison of pulverized coal samples with different particle sizes, the ignition times of pulverized coal with small particle size is shorter than that of pulverized coal with large particle size. This is due to the fact that the contact surface area of pulverized coal increases after refinement, which helps to improve the combustion speed. And the overall reduction of particle size will make the pulverized coal combustion characteristics enhanced. Therefore, in conjunction with the basic operating parameters and blast conditions of the Jingtang No. 1 blast furnace, simulations were carried out for the blended coal containing 75% anthracite, 15% bituminous coal, and 10% hydrochar with three different particle size distributions (90%, 70%, and 50% of particle size less than 80 um, respectively).
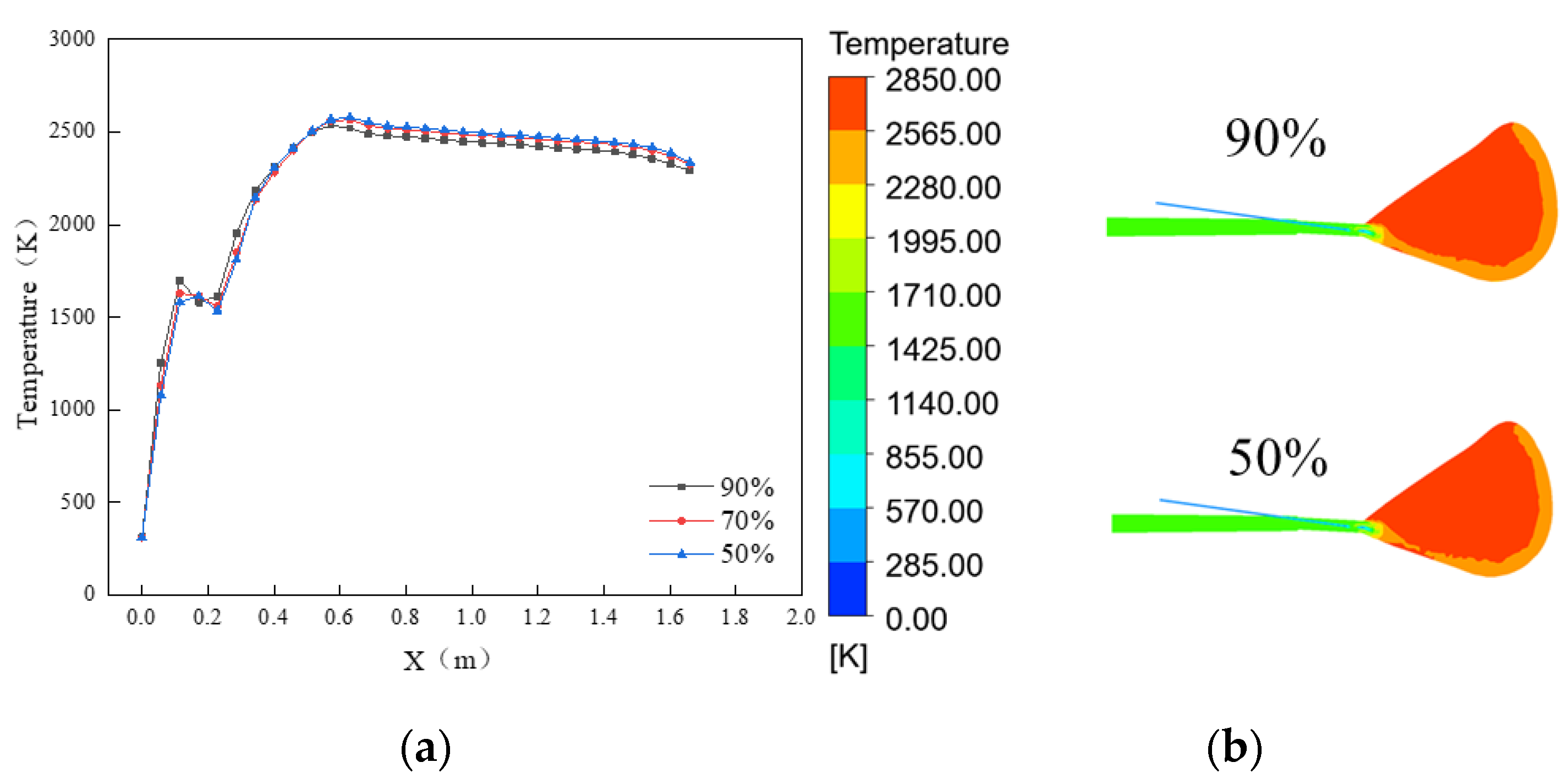
Figure 12 shows the temperature performance of the symmetric surface at the center of the model for different particle sizes.
Figure 12a shows the temperature variation curves at the center of the coal plume, and the analysis shows that at the same location, where the blended coal with smaller average particle sizes obtained higher temperatures and reached the maximum temperature more quickly. The maximum temperatures that can be reached by the three particle sizes are 2531 K, 2569 K, and 2581 K, respectively, and with the increase in the average particle size, the maximum temperature increases gradually. This is because after the volatile fraction is burned, most of the oxygen is consumed, and the little remaining oxygen continues to react with the residual carbon. At this time, the residual carbon and CO
2 react and absorb heat to generate CO. Due to dissolution, reaction of particles with larger particle sizes happens less than that of particles with smaller sizes, so its temperature continues to increase, resulting in a higher maximum temperature than that of particles with smaller particle sizes.
Figure 12b shows the temperature cloud of the center section, and the analysis shows that the larger the average particle size, the higher temperature area inside the cyclotron zone increases slightly, and the temperature difference is less than 100 K in general.
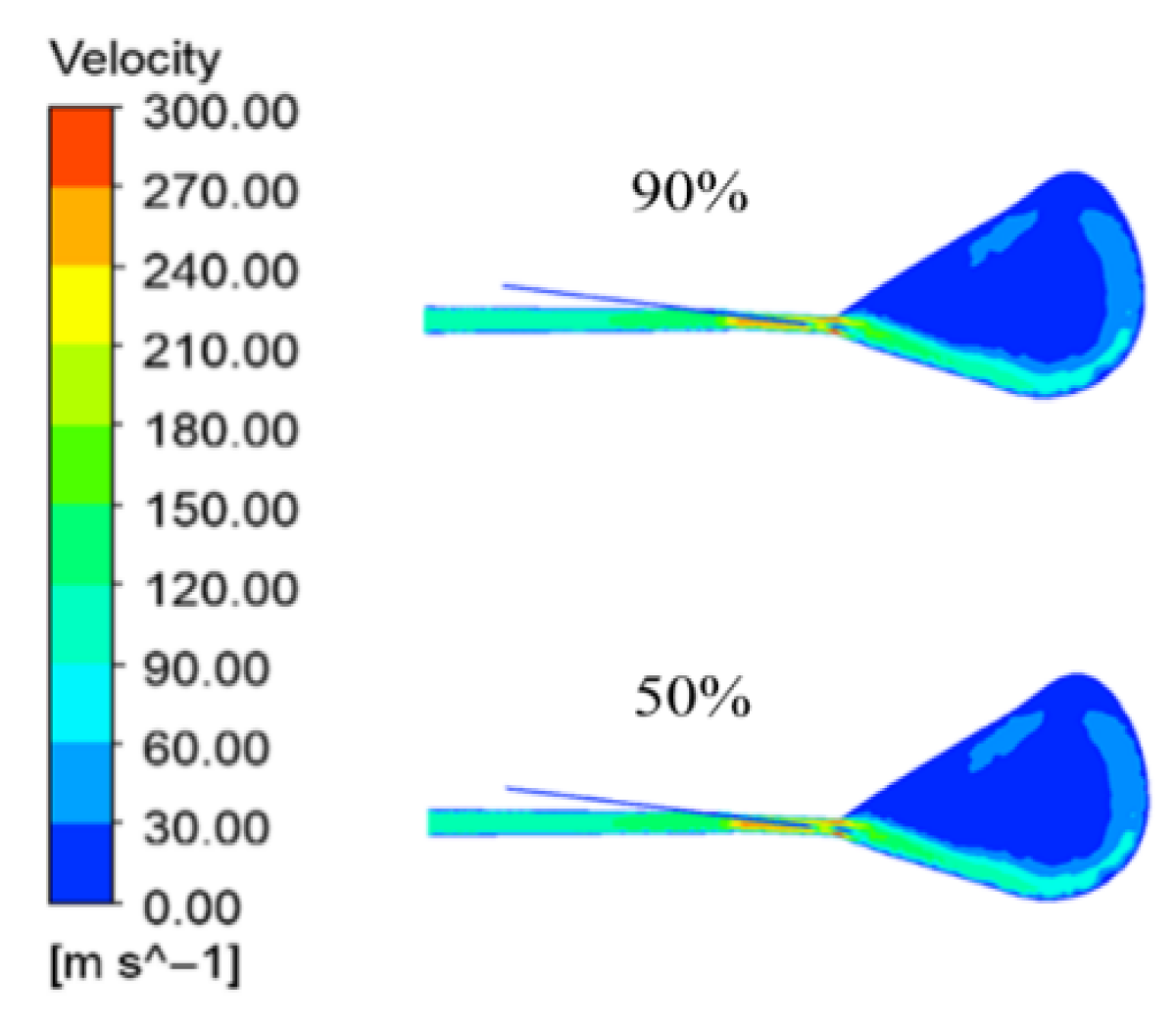
Figure 13 shows the velocity clouds for three different average particle sizes. The maximum velocities just out of the air outlet are 315.7 m/s, 310.4 m/s, and 306.5 m/s. The smaller the average particle size, the greater the maximum velocity in the pre-combustion phase, because the rapid combustion of volatile matter leads to an increase in temperature, which in turn leads to the expansion of the gas (the same reason for the reaction of the gas generation), resulting in a slightly greater velocity of the gas when the gas is spewed out of the air outlet. In the late cyclone area above, the high-speed area slightly expanded, the same reason and the influence of the blast temperature, the late average particle size of coal dust residual carbon is higher than the small particle size of coal dust, dissolution reaction expands the gas volume slightly, resulting in the upper part of the high-speed area being slightly larger.
Figure 14 shows the distribution cloud diagrams of CO, CO
2, and O
2 on the sym-metric surface at the center of different particle sizes, while
Figure 15 illustrates the variation curves of major gas species along the coal plume centerline under varying particle diameters. Analysis of the cloud diagrams show that larger coal particle sizes correspond to higher CO
2 mass fractions at the initial exit of the blowpipe. However, as coal particles combust within the swirl zone, the CO
2 content in the coal plume at the swirl zone periphery decreases with increasing particle size, indicating that more CO
2 undergoes reaction with residual carbon to generate CO. Correspondingly, the CO content of the coal plume with the smaller particle size at the edge of the gyratory zone is higher in the CO plots. Similarly, in the inner part of the gyratory zone, the oxygen is basically consumed, and the larger the particle size of the residual carbon of the coal plume, after consuming the oxygen, causes the dissolution reaction to proceed more slowly and to a lower extent compared with that of the coal plume with a smaller particle size. As shown in the cloud diagram, the larger the particle size of the pulverized coal combustion, the more CO
2 content inside the cyclone area, the lower the CO content. When the temperature is high, the rate of reaction is mainly controlled by diffusion; when the temperature is low, the rate of reaction is mainly controlled by temperature.
Figure 16 shows the combustion rate of blended coal and each coal type with different particle sizes. The combustion exhaustion rates of the blended coal with three different particle size distributions are 69.96%, 68.8%, and 67.6%, respectively. It can be seen that as the proportion of small particle sizes decreases, the burnout rate gradually decreases. As the fuel particles are refined, the increase in specific surface area and the decrease in apparent activation energy are the main reasons for the increase in the burnout rate. This contributes to cleaner production. The low ash content of hydrochar further reduces pollutant emissions, supporting environmental sustainability.
Figure 16a shows that in the pre-combustion period of blended coal, especially in the volatile fraction of intense combustion, the difference between the burnout rates of blended coal with different particle sizes is large. This is mainly due to the faster combustion of finer hydrochar. It can also be seen in
Figure 16d, in the 0.2–0.4 m, that the difference between the burnout rates of hydrochar is large. However, the differences in their final combustion rates are not large, 97.3%, 96.28%, and 95.13%, respectively, which is because in the later dissolution reaction, the change in the particle size within a certain degree does not have much effect on the reaction of hydrochar. The overall difference between the burnup rates of anthracite and bituminous coal in
Figure 16b,c is not significant, which is due to the relatively low volatile matter and the fact that the later residual carbon dissolution reaction mainly relies on the heat released before.