Sustainable Management Approaches to Heavy Metal Pollution in Arid Soils Using Soil Amendments and Plant-Based Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Soil Collection
2.2. Soil Properties
2.2.1. Greenhouse Trial
2.2.2. Column Leaching Experiment
2.3. Calculation of the Bioaccumulation (BF) and Translocation Factors (TF)
- (a)
- Bioaccumulation factor (BF):
- (b)
- Translocation factor (TF):
2.4. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Characteristics
3.2. Some of the Essential Chemical Properties of OMWW
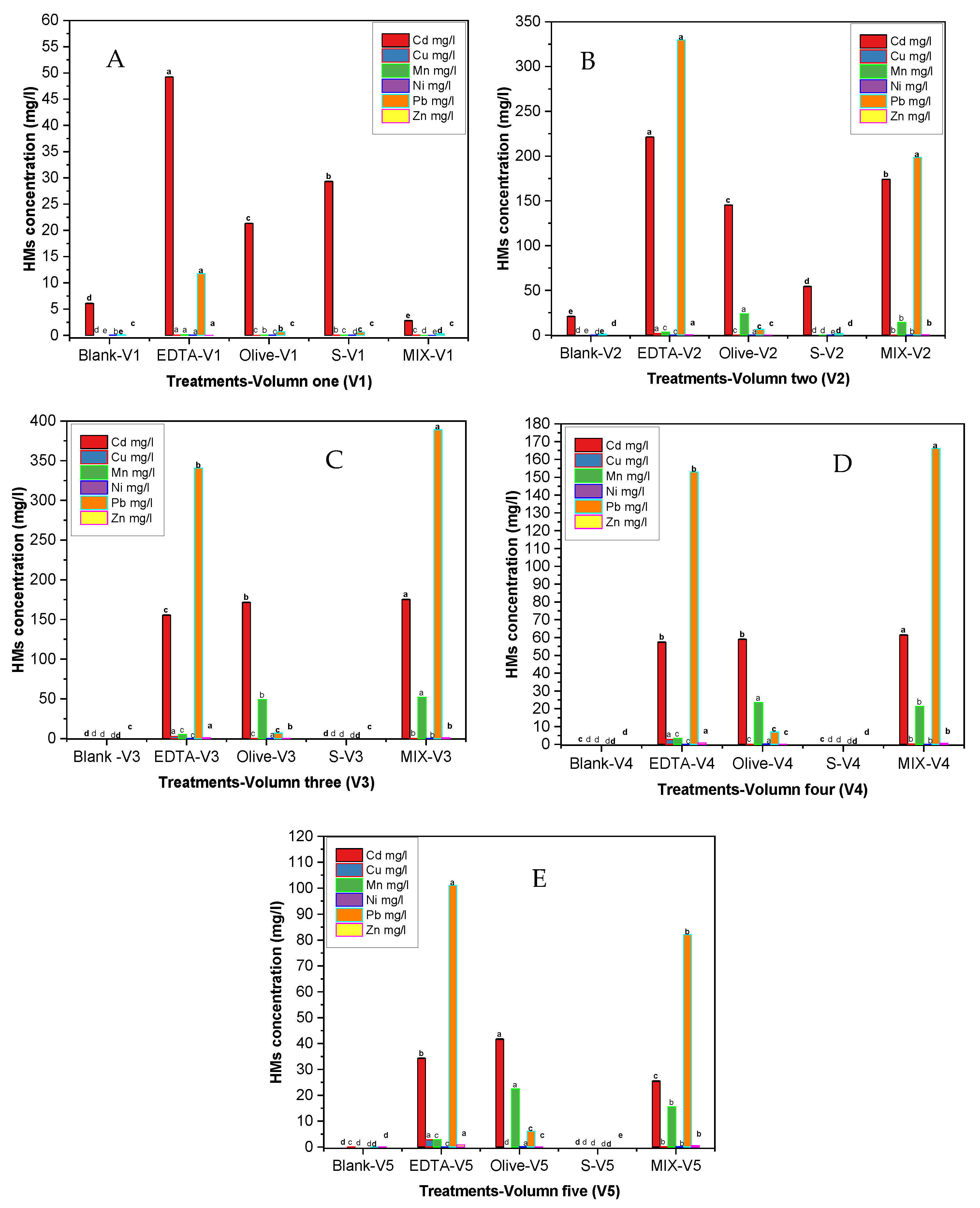
3.3. Heavy Metal Leaching Across Pour Volumes
3.4. pH and Electrical Conductivity (EC)
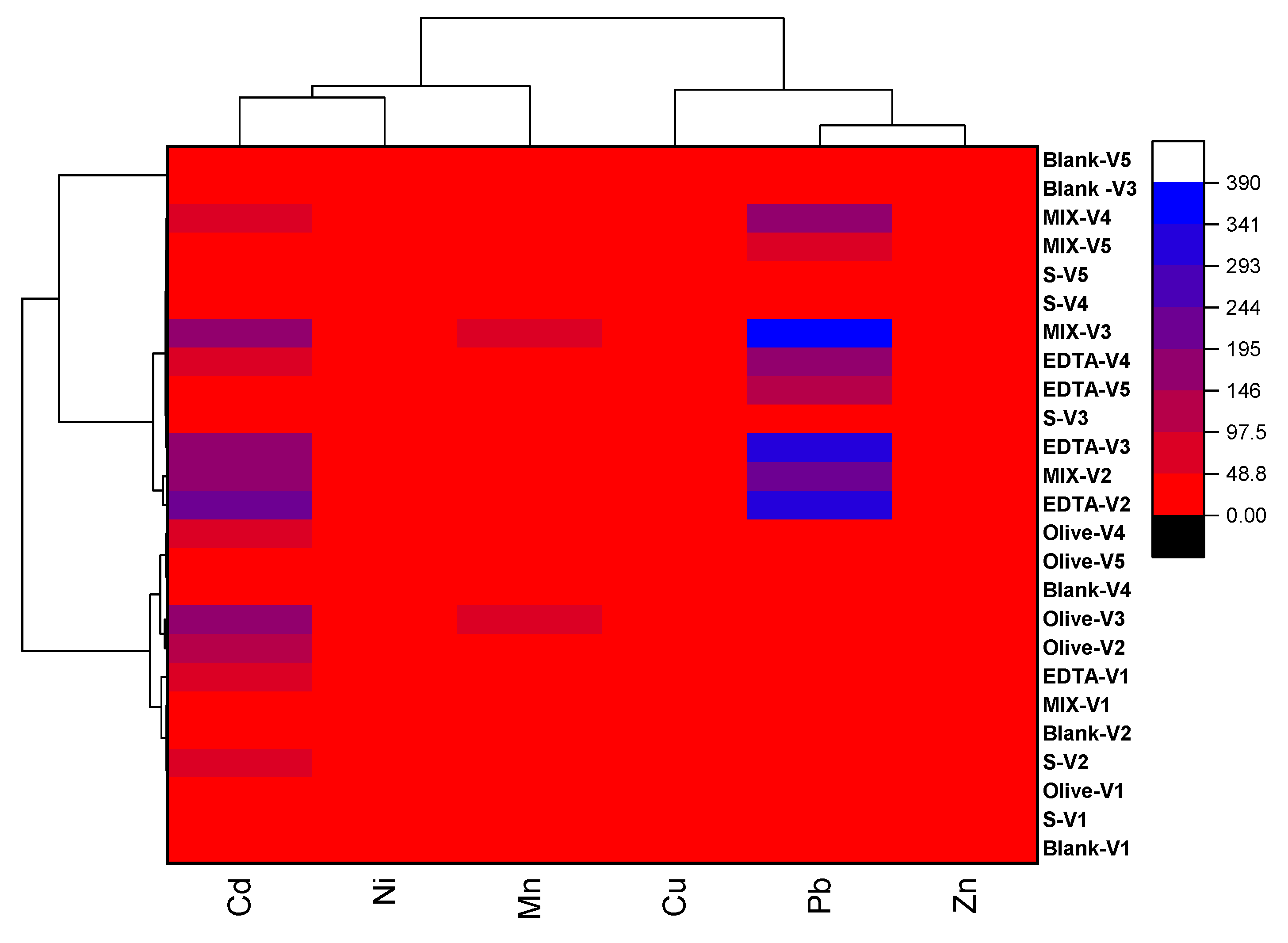
3.5. Metal Distribution Patterns and Leaching Trends Across Sequential Pour Volumes in Amended Soil Columns
4. Plant Response to Phytoremediation
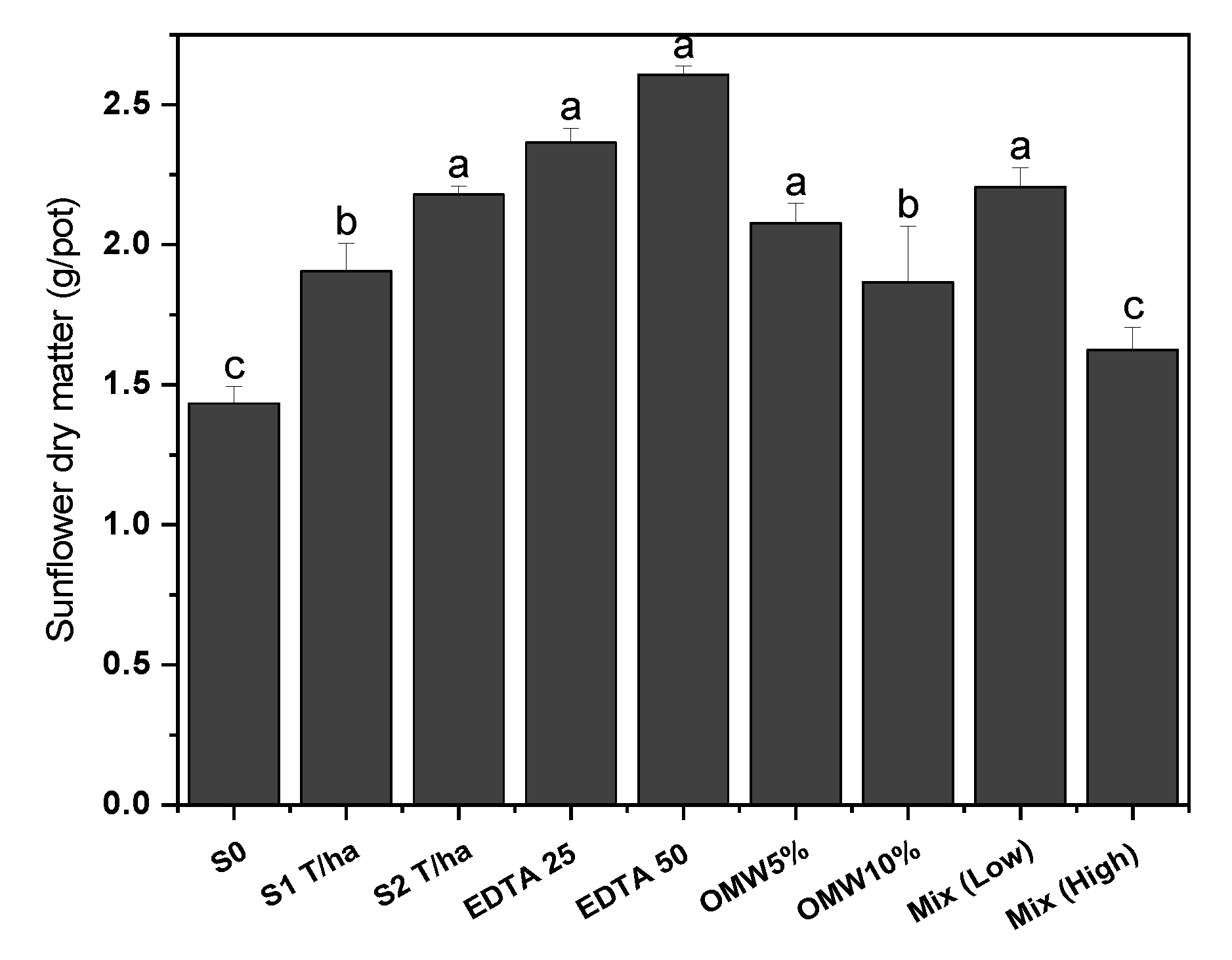
4.1. Dry Matter
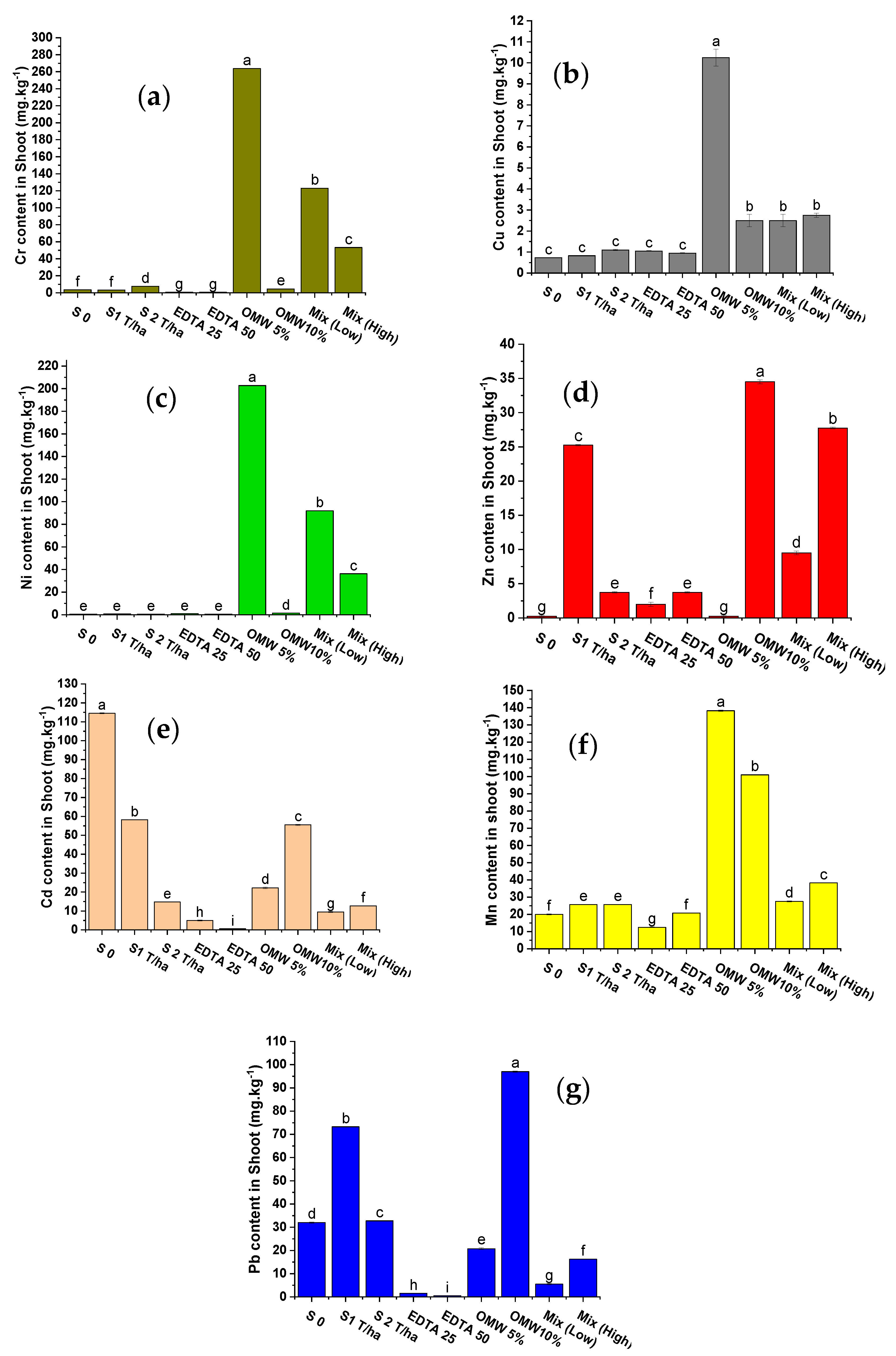
4.2. Total Heavy Metals (HMSs) Content in Sunflower Shoots
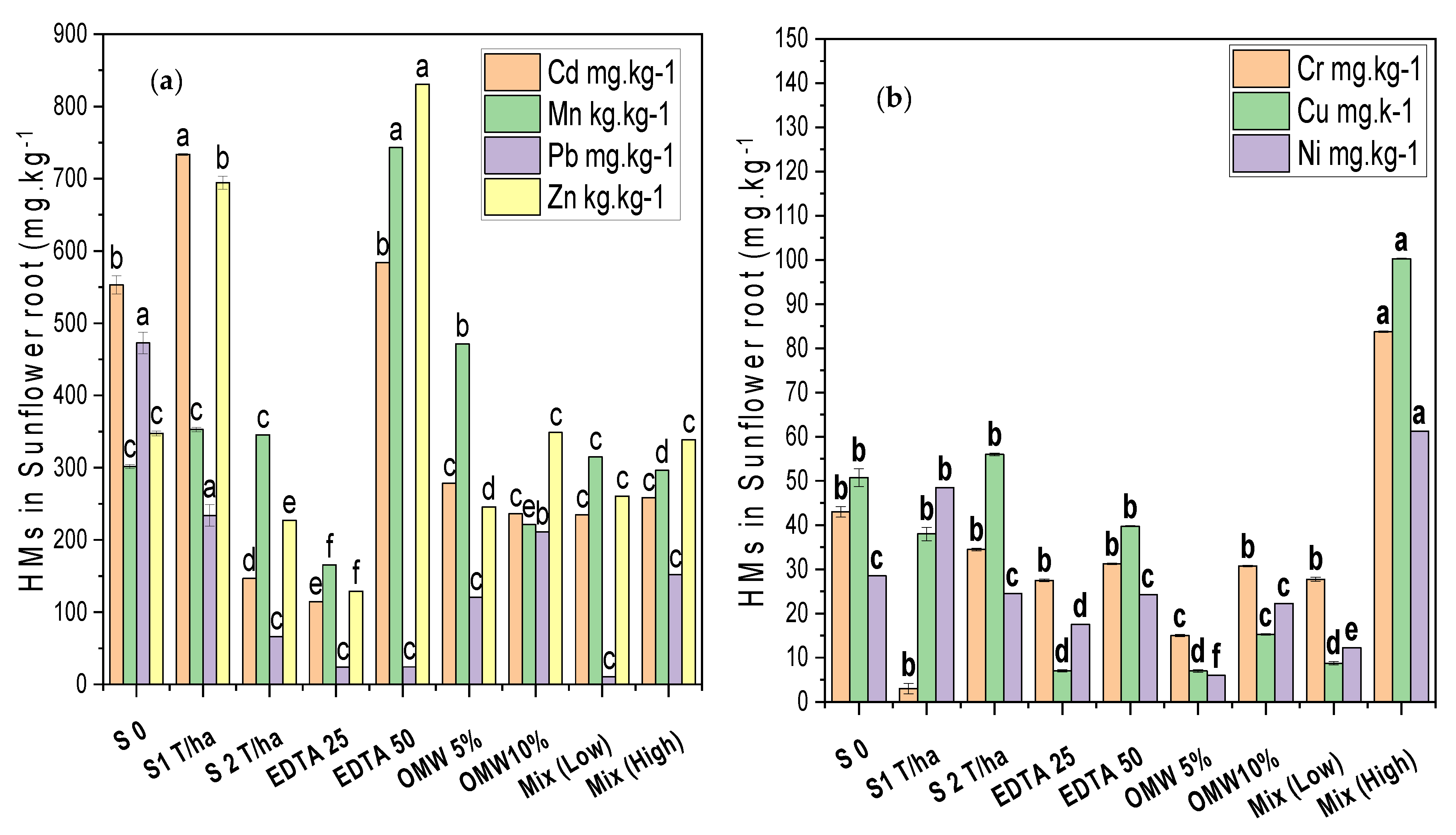
4.3. Total Heavy Metals (HMSs) in Sunflower Roots
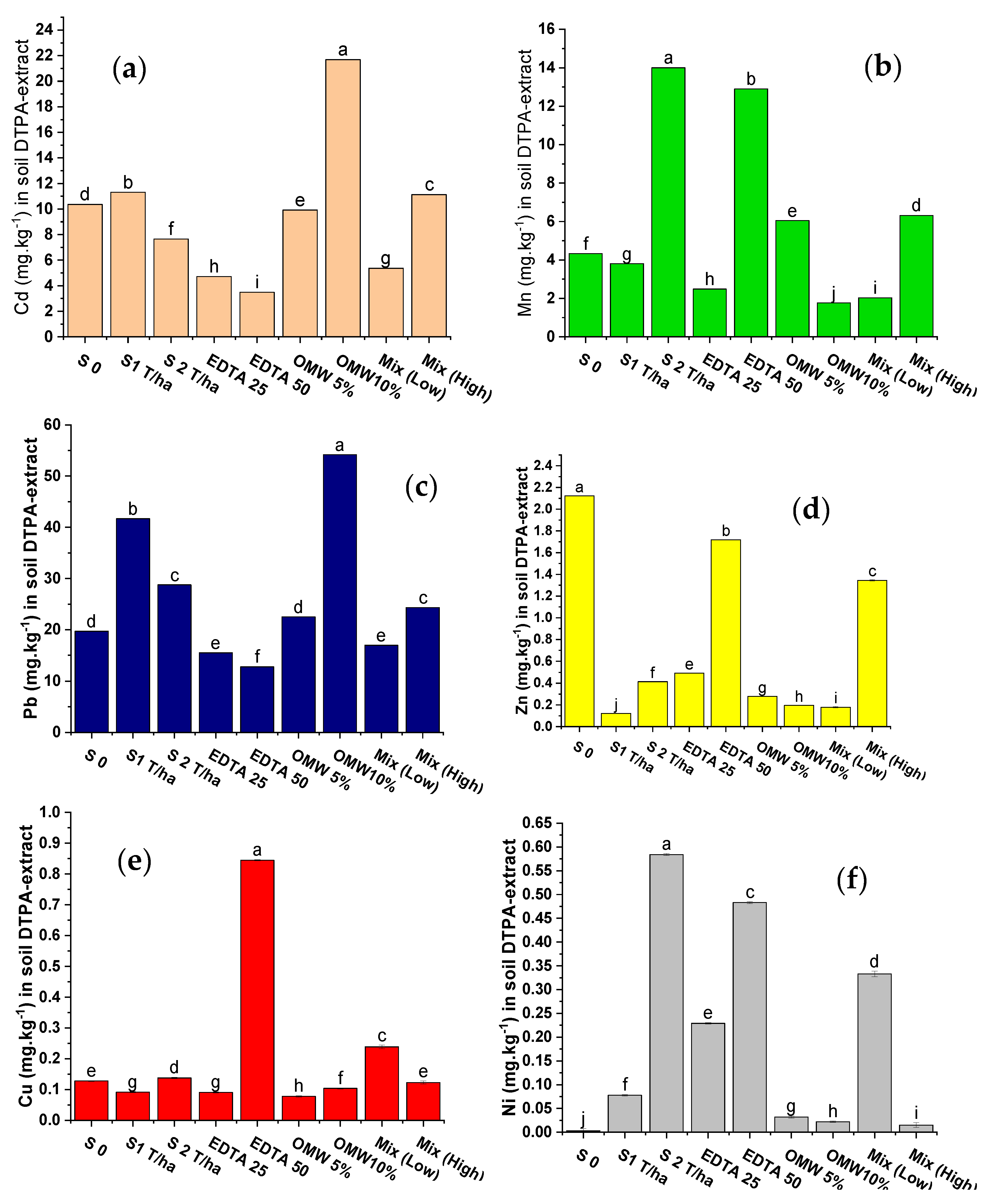
4.4. Available Heavy Metals (HMSs) as Extracted by DTPA Following Sunflower Growth
4.5. Effects on TF and BF
4.5.1. TF Calculation
4.5.2. BF Calculation
5. Discussion
5.1. Column Experiment
5.2. Greenhouse Experiment
5.2.1. Dry Matter of Maize, Mustard, and Sunflower Plants
5.2.2. Total Heavy Metals in Sunflower Shoots
5.2.3. Total Heavy Metals in Sunflower Roots
5.2.4. Available Heavy Metals (DTPA-Extract) in Soils Following Plant Growth
5.2.5. Changes in TF and BF
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelkrim, S.; Jebara, S.H.; Saadani, O.; Chiboub, M.; Abid, G.; Mannai, K.; Jebara, M. Heavy metal accumulation in Lathyrus sativus growing in contaminated soils and identification of symbiotic resistant bacteria. Arch. Microbiol. 2019, 201, 107–121. [Google Scholar] [CrossRef]
- Sonker, S.; Fulke, A.; Monga, A. Recent trends on bioremediation of heavy metals; an insight with reference to the potential of marine microbes. Int. J. Environ. Sci. Technol. 2024, 21, 9763–9774. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.; Ong, H.C.; Mofijur, M. Heavy metal toxicity, sources, and remediation techniques for contaminated water and soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Yang, W.; Liao, Q.; Gusiatin, M.Z. Remediation and health risks of heavy metal contaminated soils. Front. Environ. Sci. 2024, 12, 1501443. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Liu, H. Phytoremediation of heavy metal contaminated soil: Technology, mechanism, and implementation. Front. Plant Sci. 2024, 14, 1347564. [Google Scholar] [CrossRef]
- Mishra, S.; De, A. Various Sources of Heavy Metals Contamination, Including Industrial Activities, Mining, Agriculture, and Urbanization. In Heavy Metal Contamination in the Environment; CRC Press: Boca Raton, FL, USA, 2024; pp. 71–94. [Google Scholar]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal-Contaminated Soils; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2011; pp. 1–28. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2012; pp. 11–50. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy metals in agricultural soils: Sources, influencing factors, and remediation strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Gonzalez Henao, S.; Ghneim-Herrera, T. Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front. Environ. Sci. 2021, 9, 604216. [Google Scholar] [CrossRef]
- Hong, Y.K.; Kim, J.W.; Lee, S.P.; Yang, J.E.; Kim, S.C. Heavy metal remediation in soil with chemical amendments and its impact on activity of antioxidant enzymes in Lettuce (Lactuca sativa) and soil enzymes. Appl. Biol. Chem. 2020, 63, 42. [Google Scholar] [CrossRef]
- Popoola, O.J.; Ogundele, O.D.; Ladapo, E.A.; Senbore, S. The Impact of Heavy Metal Contamination in Soils on Soil Microbial Communities and Its Potential Health Risks for Humans. In Soil Microbiome in Green Technology Sustainability; Springer: Cham, Switzerland, 2024; pp. 351–375. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Agrelli, D.; Caporale, A.G.; Adamo, P. Assessment of the bioavailability and speciation of heavy metal (loid) s and hydrocarbons for risk-based soil remediation. Agronomy 2020, 10, 1440. [Google Scholar] [CrossRef]
- Violante, A.; Bollag, J.-M.; Gianfreda, L.; Huang, P. Dynamics, Mobility and Transformation of Pollutants and Nutrients; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Nie, X.; Huang, X.; Li, M.; Lu, Z.; Ling, X. Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics 2024, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M. Phytoremediation strategies for mitigating environmental toxicants. Heliyon 2024, 10, e38683. [Google Scholar] [CrossRef] [PubMed]
- Kalyvas, G.; Tsitselis, G.; Gasparatos, D.; Massas, I. Efficacy of EDTA and olive mill wastewater to enhance As, Pb, and Zn phytoextraction by Pteris vittata L. from a soil heavily polluted by mining activities. Sustainability 2018, 10, 1962. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy metal stabilization remediation in polluted soils with stabilizing materials: A review. Environ. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef]
- Pardo, T.; Bernal, P.; Clemente, R. The use of olive mill waste to promote phytoremediation. Olive Mill Waste 2017, 183–204. [Google Scholar] [CrossRef]
- Kavvadias, V.; Doula, M.; Theocharopoulos, S. Long-term effects on soil of the disposal of olive mill waste waters (OMW). Environ. Forensics 2014, 15, 37–51. [Google Scholar] [CrossRef]
- Almeaiweed, N.H.; Aloud, S.S.; Alotaibi, K.D.; Alotaibi, F.; Alshebel, B. Enhancing Phytoremediation of Heavy Metal-Contaminated Aridic Soil Using Olive Mill Wastewater, Sulfur, and Chelating Agents. Sustainability 2025, 17, 3745. [Google Scholar] [CrossRef]
- Akkam, Y.; Zaitoun, M.; Aljarrah, I.; Jaradat, A.; Hmedat, A.; Alhmoud, H.; Rababah, T.; Almajwal, A.; Al-Rayyan, N. Effective Detoxification of Olive Mill Wastewater Using Multi-Step Surfactant-Based Treatment: Assessment of Environmental and Health Impact. Molecules 2024, 29, 4284. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Hossner, L.R. Dissolution for total elemental analysis. In Methods of Soil Analysis; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; Volume 5, pp. 49–64. [Google Scholar] [CrossRef]
- Page, A.; Miller, R.; Kenney, D.J.P. Method of Soil Analysis, 2nd ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Kim, I.S.; Kang, K.H.; Johnson-Green, P.; Lee, E.J. Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environ. Pollut. 2003, 126, 235–243. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, T.; Wu, Y.; Tan, Z. A Review of the Electrochemical Coupled Leaching Technique: An Effective Solution for Remediation of Heavy Metal Contaminated Soils. Rev. Environ. Contam. Toxicol. 2024, 262, 17. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Albalasmeh, A.A.; Malkawi, H.I. Treated olive mill wastewater effects on soil properties and plant growth. Water Air Soil Pollut. 2016, 227, 135. [Google Scholar] [CrossRef]
- Hong, Y.; Li, D.; Xie, C.; Zheng, X.; Yin, J.; Li, Z.; Zhang, K.; Jiao, Y.; Wang, B.; Hu, Y. Combined apatite, biochar, and organic fertilizer application for heavy metal co-contaminated soil remediation reduces heavy metal transport and alters soil microbial community structure. Sci. Total Environ. 2022, 851, 158033. [Google Scholar] [CrossRef]
- Liu, H.; Luo, L.; Jiang, G.; Li, G.; Zhu, C.; Meng, W.; Zhang, J.; Jiao, Q.; Du, P.; Li, X. Sulfur enhances cadmium bioaccumulation in Cichorium intybus by altering soil properties, heavy metal availability and microbial community in contaminated alkaline soil. Sci. Total Environ. 2022, 837, 155879. [Google Scholar] [CrossRef]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic amendments for in situ immobilization of heavy metals in soil: A review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, L.; Wang, K.; Liu, C.; Zhang, J. Enhanced mobilization of arsenic from tailing soil by four types of low molecular weight organic acids with different functional groups. J Soils Sediments 2021, 21, 3834–3844. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Billah, M.; Akhtar, M.S.; Rukh, S.; Alam, S.; Munir, A.; Mahmood Aulakh, A.; Rahim, M.; Qaisrani, M.M. Organic amendments and elemental sulfur stimulate microbial biomass and sulfur oxidation in alkaline subtropical soils. Agronomy 2021, 11, 2514. [Google Scholar] [CrossRef]
- Wang, H.-q.; Lu, S.-j.; Li, H.; Yao, Z.-h. EDTA-enhanced phytoremediation of lead contaminated soil by Bidens maximowicziana. J. Environ. Sci. 2007, 19, 1496–1499. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.; Van Slycken, S.; Ruttens, A.; Du Laing, G.; Vangronsveld, J.; Verloo, M.G. Chemically assisted phytoextraction: A review of potential soil amendments for increasing plant uptake of heavy metals. Int. J. Phytoremediat. 2008, 10, 390–414. [Google Scholar] [CrossRef]
- Nyiramigisha, P. Harmful impacts of heavy metal contamination in the soil and crops grown around dumpsites. Rev. Agric. Sci. 2021, 9, 271–282. [Google Scholar] [CrossRef]
- Regni, L.; Bartucca, M.L.; Pannacci, E.; Tei, F.; Del Buono, D.; Proietti, P. Phytodepuration of nitrate-contaminated water using four different tree species. Plants 2021, 10, 515. [Google Scholar] [CrossRef]
- Zhang, C.; He, F.; Chen, L. Phytoremediation of cadmium-trichlorfon co-contaminated water by Indian mustard (Brassica juncea): Growth and physiological responses. Int. J. Phytoremediat. 2024, 26, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Leštan, D.; Luo, C.-L.; Li, X.-D. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
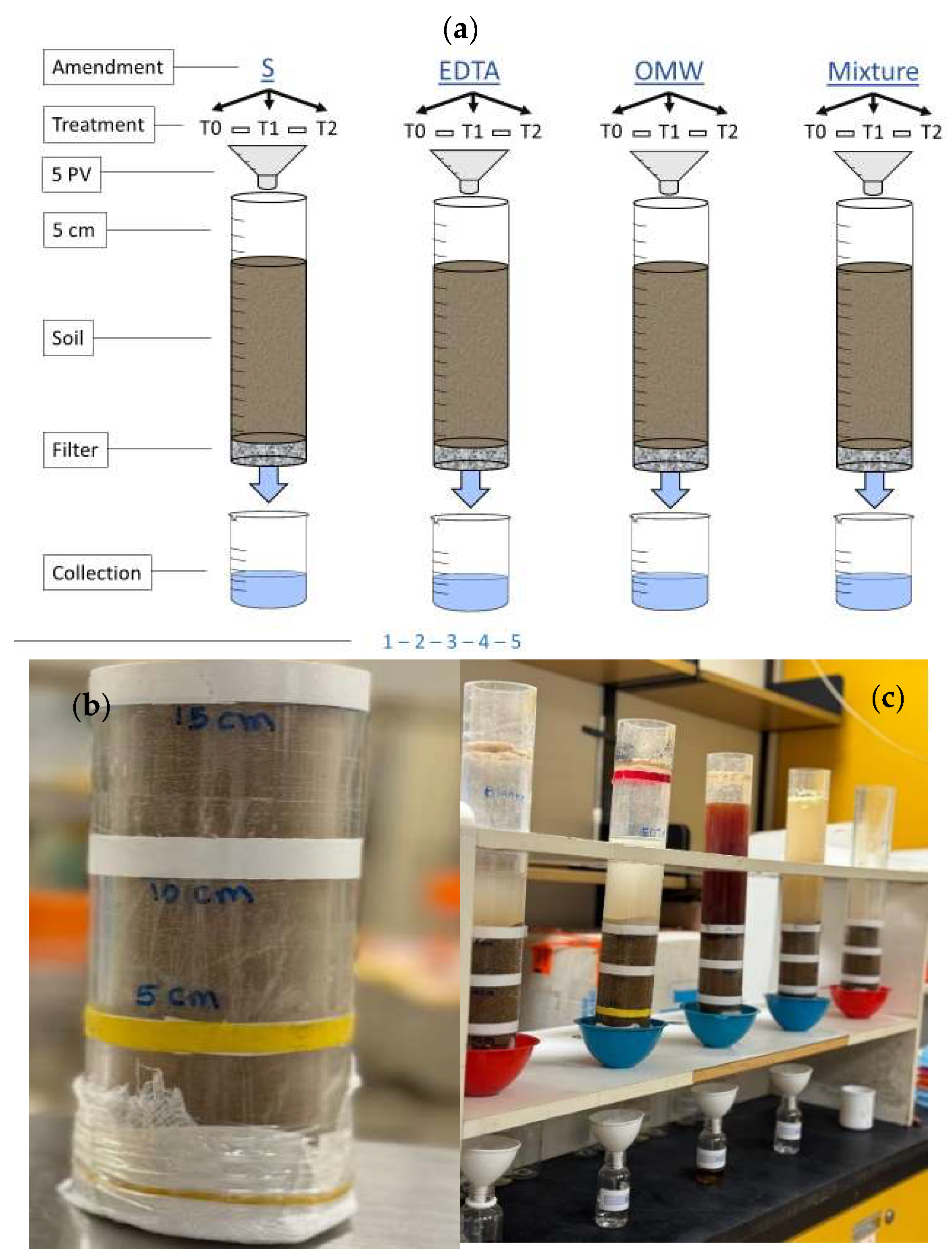
| Parameter | Mean |
|---|---|
| pH | 8.4 ± 0.31 |
| Ec Ms/cm | 83 ± 4.2 |
| Silt % | 20 |
| Clay % | 10 |
| Sand % | 70 |
| Caco3 | 5.5 ± 0.07 |
| TN % | 0.06 ± 0.003 |
| TP % | 0.064 ± 0.001 |
| TK % | 0.9 ± 0.003 |
| Cd mg g−1 | 53.6 ± 0.9 |
| Cr mg kg−1 | 94.5 ± 0.3 |
| Cu mg kg−1 | 47.1 ± 0.53 |
| Ni mg kg−1 | 53.1 ± 0.36 |
| Pb mg kg−1 | 84.9 ± 0.08 |
| Zn mg kg−1 | 106.4 ± 0.04 |
| Mn mg kg−1 | 1168 ± 0.11 |
| Characteristics | Value |
|---|---|
| pH | 4.47 ± 0.06 |
| EC dS/m | 14.2 ± 0.10 |
| Phenols mg/L | 5370 ± 3.21 |
| Total N mg/L | 845 ± 0.05 |
| NH4-N mg/L | 32.5 ± 0.04 |
| Nitrate-N mg/L | <1.13 |
| Total P mg/L | 515 ± 0.07 |
| K mg/L | 3690 ± 2.56 |
| Treatment | pH | EC (ms/cm) | ||||
|---|---|---|---|---|---|---|
| 5 cm | 10 cm | 15 cm | 5 cm | 10 cm | 15 cm | |
| Untreated soil | 7.6 | 7.8 | 7.6 | 140 | 107 | 111 |
| EDTA | 7.7 | 7 | 5.3 | 190 | 320 | 1763 |
| Olive | 7 | 6.8 | 6.9 | 850 | 672 | 935 |
| Sulfur | 7.6 | 7.7 | 7.7 | 138 | 120 | 146 |
| Mixture | 7.4 | 7.3 | 7.6 | 390 | 402 | 554 |
| TRT | Cd | Cr | Mn | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| SO | 0.21 ± 0.03 b | 0.08 ± 0.002 fg | 0.07 ± 0.001 f | 0.01 ± 0.001 e | 0.02 ± 0.005 d | 0.07 ± 0.008 g | 0 ± 0 c |
| S1 T/ha | 0.08 ± 0.005 d | 1.06 ± 0.02 c | 0.07 ± 0.002 ef | 0.02 ± 0.008 e | 0.01 ± 0.003 | 0.31 ± 0.003 d | 0.04 ± 0.009 bc |
| S2 T/ha | 0.1 ± 0.011 c | 0.22 ± 0.003 e | 0.07 ± 0.008 f | 0.02 ± 0 e | 0.02 ± 0.003 e | 0.5 ± 0.006 b | 0.02 ± 0.006 c |
| EDTA 25 | 0.04 ± 0.008 e | 0.03 ± 0.003 g | 0.08 ± 0.005 ef | 0.15 ± 0.005 c | 0.05 ± 0.001 e | 0.06 ± 0.006 g | 0.02 ± 0.05 a |
| EDTA 50 | 0 ± 0 f | 0.02 ± 0.008 g | 0.03 ± 0.001 g | 0.02 ± 0.001 e | 0.02 ± 0.008 e | 0.02 ± 0.008 h | 0 ± 0 c |
| OMW 5% | 0.24 ± 0.001 d | 17.58 ± 0.08 a | 0.29 ± 0.003 b | 1.46 ± 0.01 a | 33.79 ± 0.06 a | 0.17 ± 0.003 e | 0 ± 0 c |
| OMW 10% | 0.08 ± 0.001 a | 0.15 ± 0.003 ef | 0.46 ± 0.003 a | 0.16 ± 0.005 c | 0.07 ± 0.001 e | 0.46 ± 0.003 c | 0.1 ± 0.008 a |
| Mix (Low) | 0.04 ± 0.006 e | 4.43 ± 0.008 b | 0.09 ± 0.006 d | 0.29 ± 0.002 b | 7.51 ± 0.06 b | 0.51 ± 0.009 a | 0.04 ± 0.009 bc |
| Mix (High) | 0.05 ± 0.005 e | 0.64 ± 0.003 d | 0.13 ± 0.003 c | 0.03 ± 0.002 d | 0.59 ± 0.003 c | 0.11 ± 0.006 f | 0.001 ± 0.001 ab |
| LSD | 0.01 | 0.08 | 0.06 | 0.01 | 0.07 | 0.01 | 0.04 |
| TRT | Cd | Cr | Mn | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| SO | 2.14 ± 0.008 a | 0.04 ± 0.005 de | 0.02 ± 0.002 d | 0.02 ± 0.003 e | 0.01 ± 0 d | 0.38 ± 0.006 c | 0 ± 0 f |
| S1 T/ha | 1.55 ± 0.04 b | 0.03 ± 0.008 d | 0.02 ± 0.009 d | 0.02 ± 0.001 e | 0.01 ± 0.005 d | 0.57 ± 0.009 b | 0.23 ± 0.009 c |
| S2 T/ha | 1.43 ± 0.009 g | 0.09 ± 0.001 de | 0.03 ± 0.003 c | 0.03 ± 0.005 cd | 0.01 ± 0 d | 0.41 ± 0.006 c | 0.03 ± 0.003 e |
| EDTA 25 | 0.65 ± 0.01 e | 0.01 ± 0.003 e | 0.01 ± 0.003 e | 0.03 ± 0.003 de | 0.02 ± 0.003 d | 0.02 ± 0.003 f | 0 ± 0 e |
| EDTA 50 | 0.02 ± 0.003 h | 0.01 ± 0.001 e | 0.02 ± 0.005 d | 0.02 ± 0.001 e | 0.01 ± 0.001 d | 0.01 ± 0 f | 0.02 ± 0.001 e |
| OMW 5% | 0.7 ± 0.001 h | 2.6 ± 0.008 a | 0.11 ± 0.009 a | 0.19 ± 0.006 a | 3.95 ± 0.03 a | 0.32 ± 0.006 d | 0 ± 0 e |
| OMW 10% | 1.15 ± 0.001 c | 0.05 ± 0.003 de | 0.09 ± 0.003 b | 0.06 ± 0.006 bc | 0.03 ± 0.006 d | 0.73 ± 0.006 a | 0.39 ± 0.06 a |
| Mix (Low) | 0.83 ± 0.008 d | 1.3 ± 0.006 b | 0.03 ± 0.001 c | 0.06 ± 0.003 bc | 1.67 ± 0.009 b | 0.11 ± 0.001 e | 0.01 ± 0.003 d |
| Mix (High) | 1.49 ± 0.003 f | 0.59 ± 0.01 c | 0.03 ± 0 c | 0.05 ± 0.003 bc | 0.74 ± 0.01 c | 0.34 ± 0.01 d | 0.25 ± 0.01 b |
| LSD | 0.02 | 0.14 | 0.008 | 0.01 | 0.03 | 0.02 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeaiweed, N.H.; Aloud, S.S.; Alotaibi, K.D.; Al Watban, M.A.; Alrobaish, W.S.; Alorf, M.S. Sustainable Management Approaches to Heavy Metal Pollution in Arid Soils Using Soil Amendments and Plant-Based Remediation. Sustainability 2025, 17, 7558. https://doi.org/10.3390/su17167558
Almeaiweed NH, Aloud SS, Alotaibi KD, Al Watban MA, Alrobaish WS, Alorf MS. Sustainable Management Approaches to Heavy Metal Pollution in Arid Soils Using Soil Amendments and Plant-Based Remediation. Sustainability. 2025; 17(16):7558. https://doi.org/10.3390/su17167558
Chicago/Turabian StyleAlmeaiweed, Nasser H., Saud S. Aloud, Khaled D. Alotaibi, Mohannad A. Al Watban, Waeel S. Alrobaish, and Majed S. Alorf. 2025. "Sustainable Management Approaches to Heavy Metal Pollution in Arid Soils Using Soil Amendments and Plant-Based Remediation" Sustainability 17, no. 16: 7558. https://doi.org/10.3390/su17167558
APA StyleAlmeaiweed, N. H., Aloud, S. S., Alotaibi, K. D., Al Watban, M. A., Alrobaish, W. S., & Alorf, M. S. (2025). Sustainable Management Approaches to Heavy Metal Pollution in Arid Soils Using Soil Amendments and Plant-Based Remediation. Sustainability, 17(16), 7558. https://doi.org/10.3390/su17167558






