Effect of Silk Fibroin as a Sustainable Solvent on the Extraction of Bixin from Annatto Seeds (Bixa orellana L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Plant Material: Collection and Identification
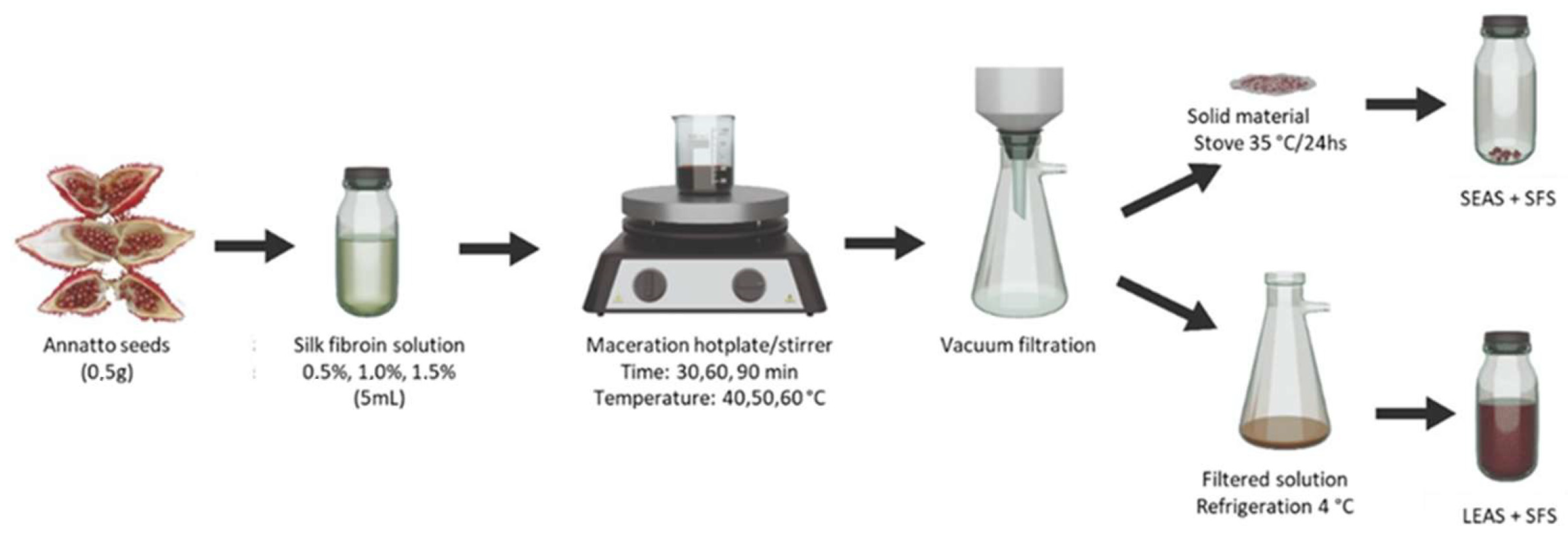
2.3. Preparation of Silk Fibroin Solution
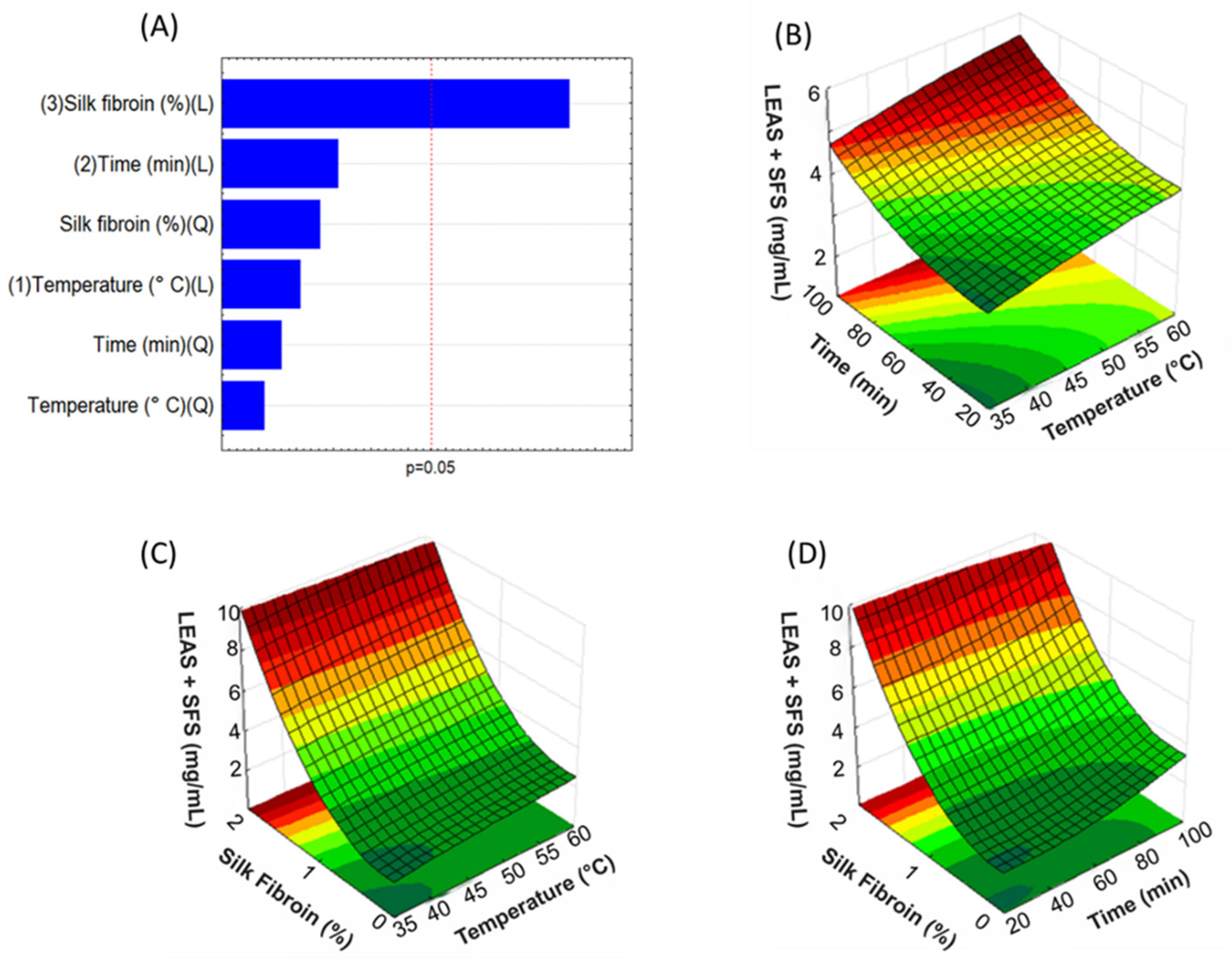
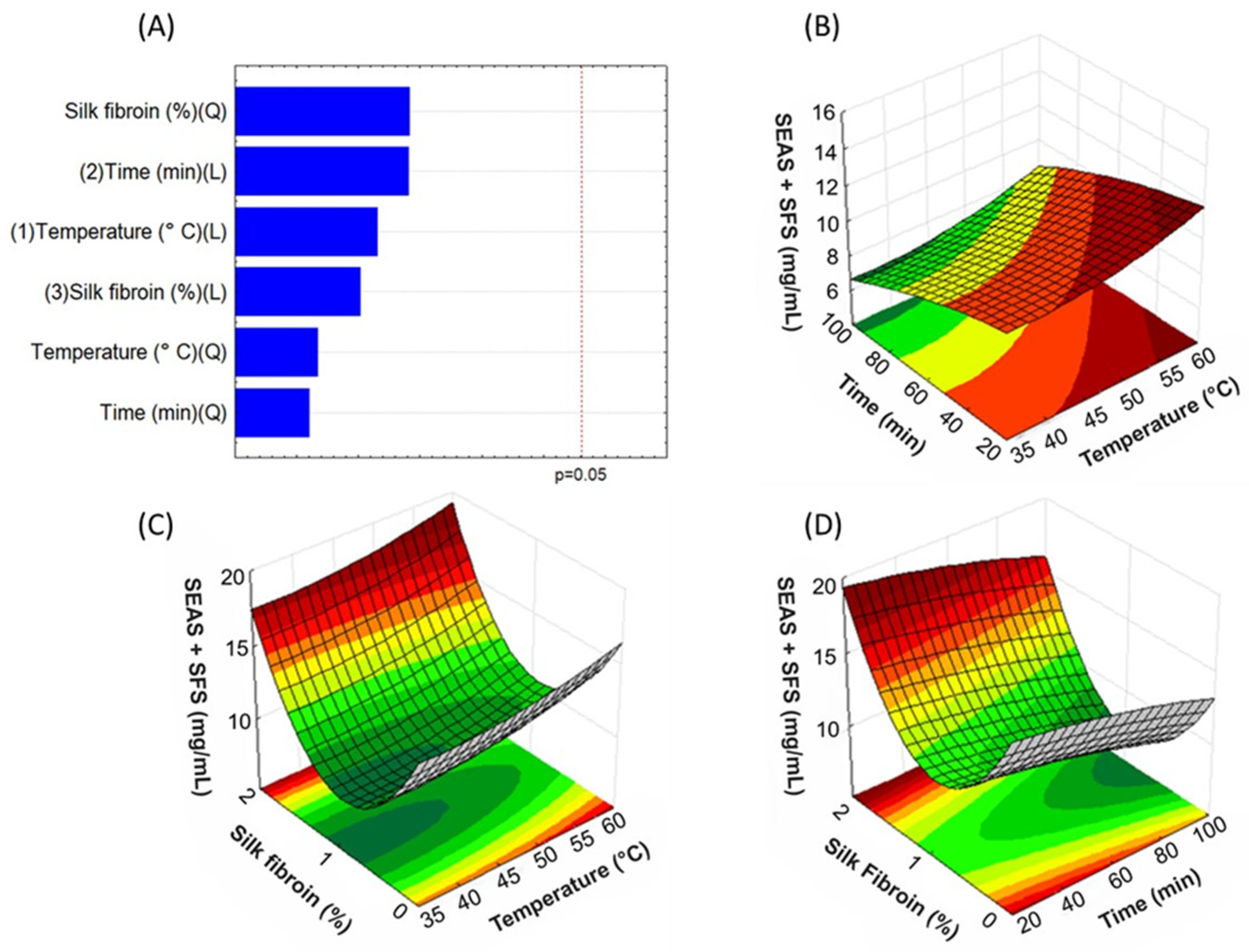
2.4. Experimental Design
2.5. Bixin Extraction Process with Silk Fibroin Solution
2.6. Purified Bixin
2.7. Chemical Characterization
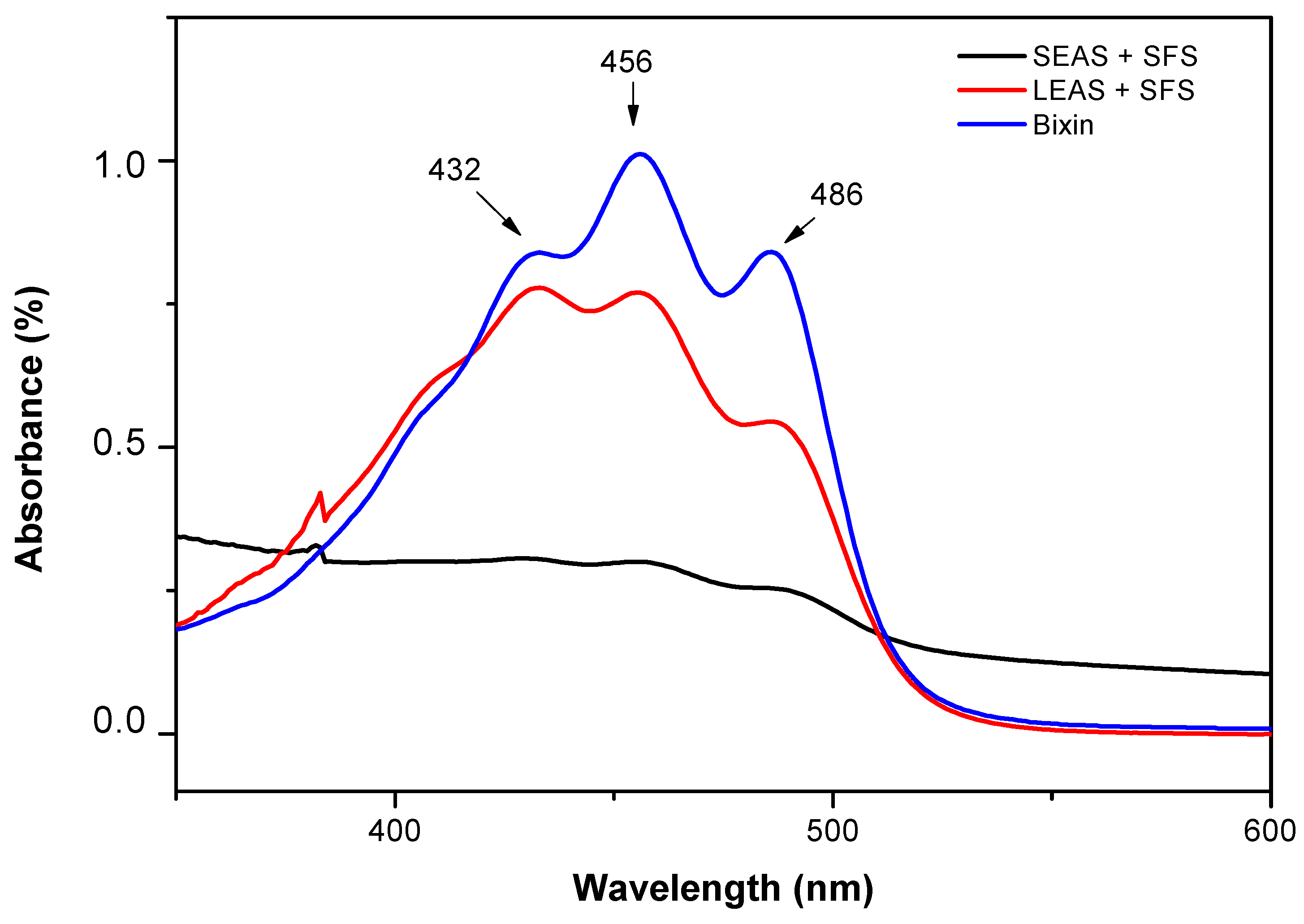
2.7.1. Quantification of Bixin Content by UV-Vis Spectroscopy
2.7.2. Fourier Transform Infrared (FTIR) Analysis
2.7.3. Analysis of Purified Bixin by 1H and 13C NMR
2.8. Cell Culture
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Bixin Content by UV-Vis Spectroscopy and Optimization of Extraction Process Parameters Using the Box-Behnken Design
3.2. Chemical Characterization
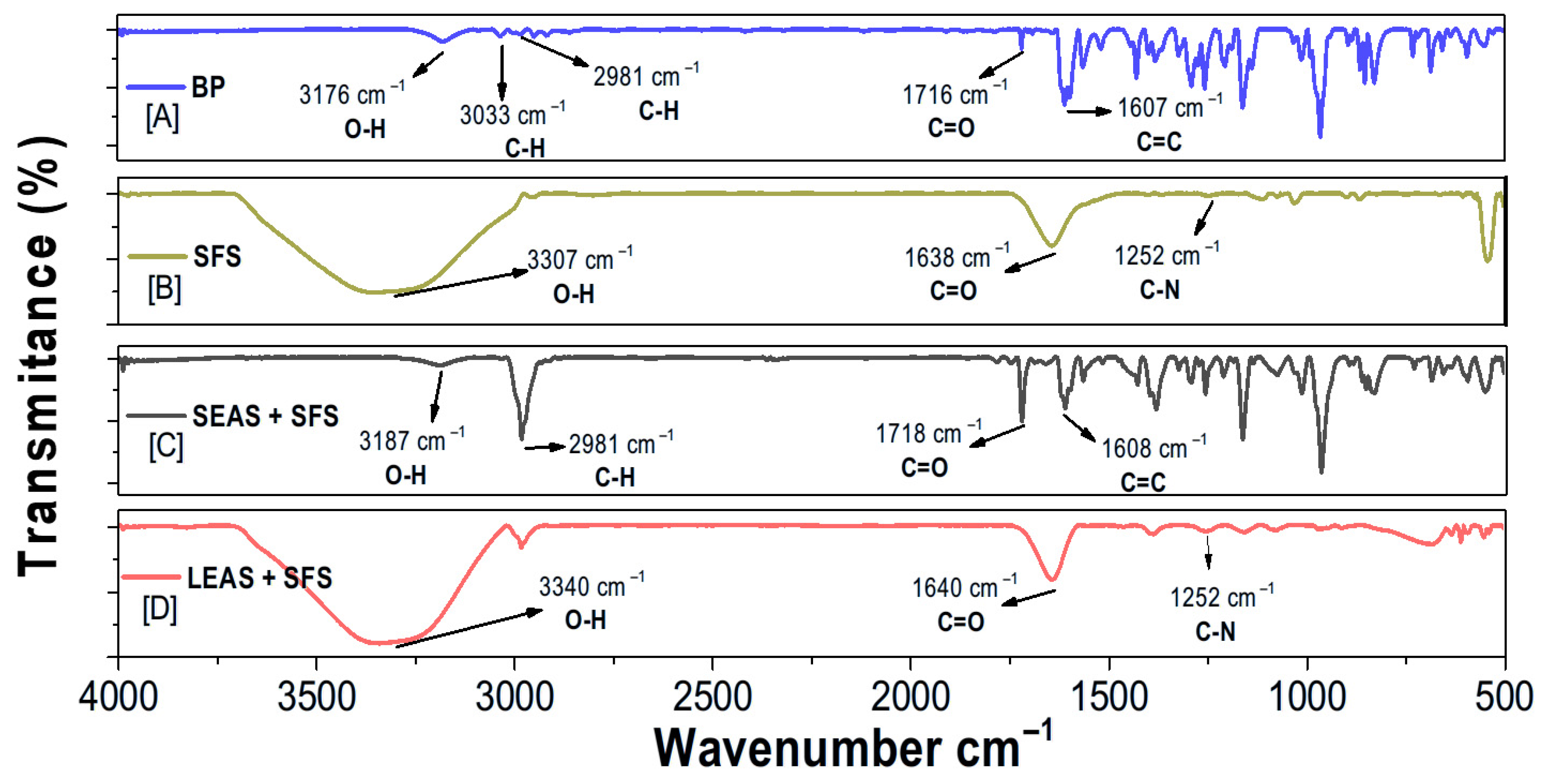
3.2.1. Fourier Transform Infrared (FTIR) Analysis
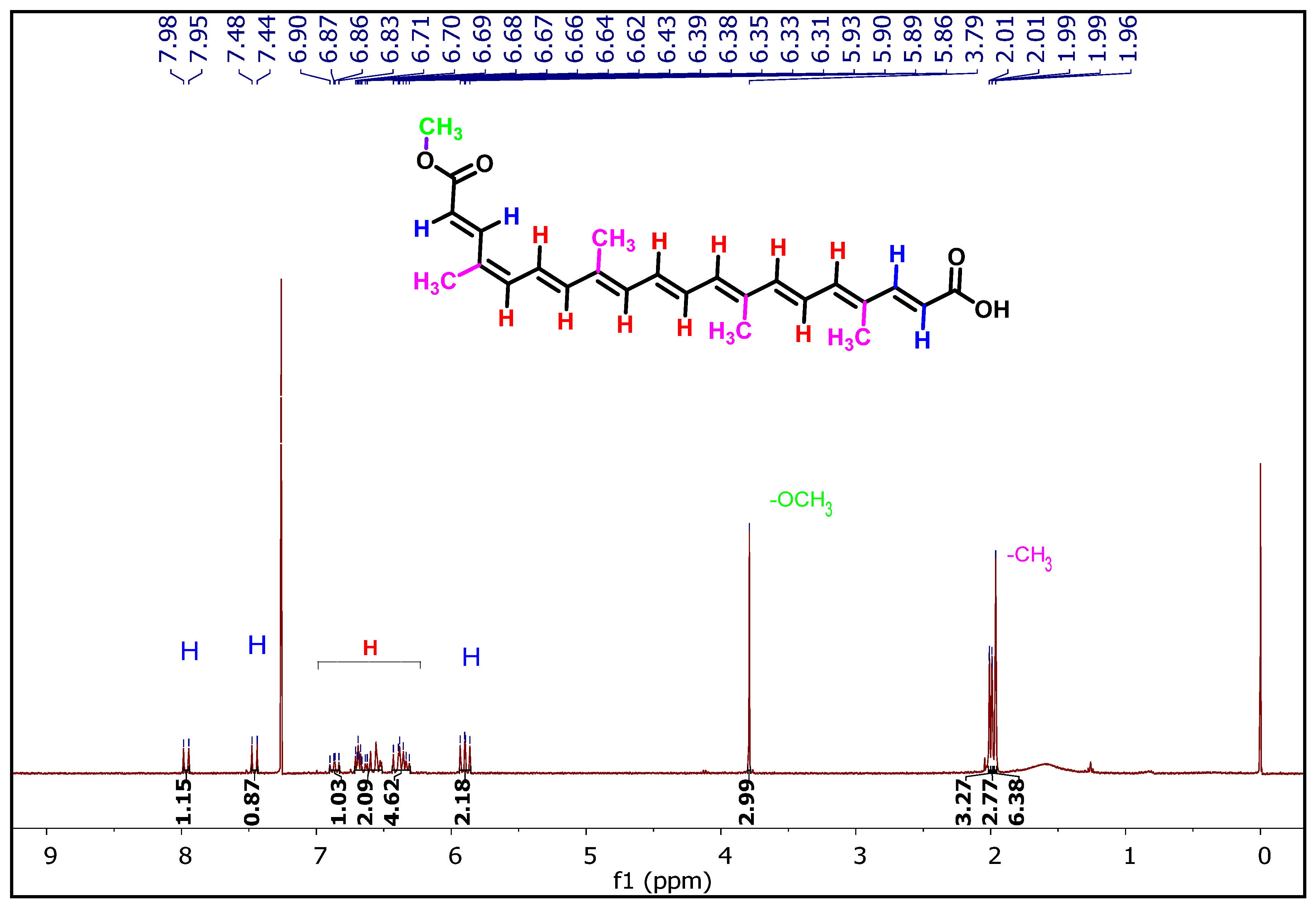
3.2.2. Analysis of Purified Bixin by 1H and 13C NMR
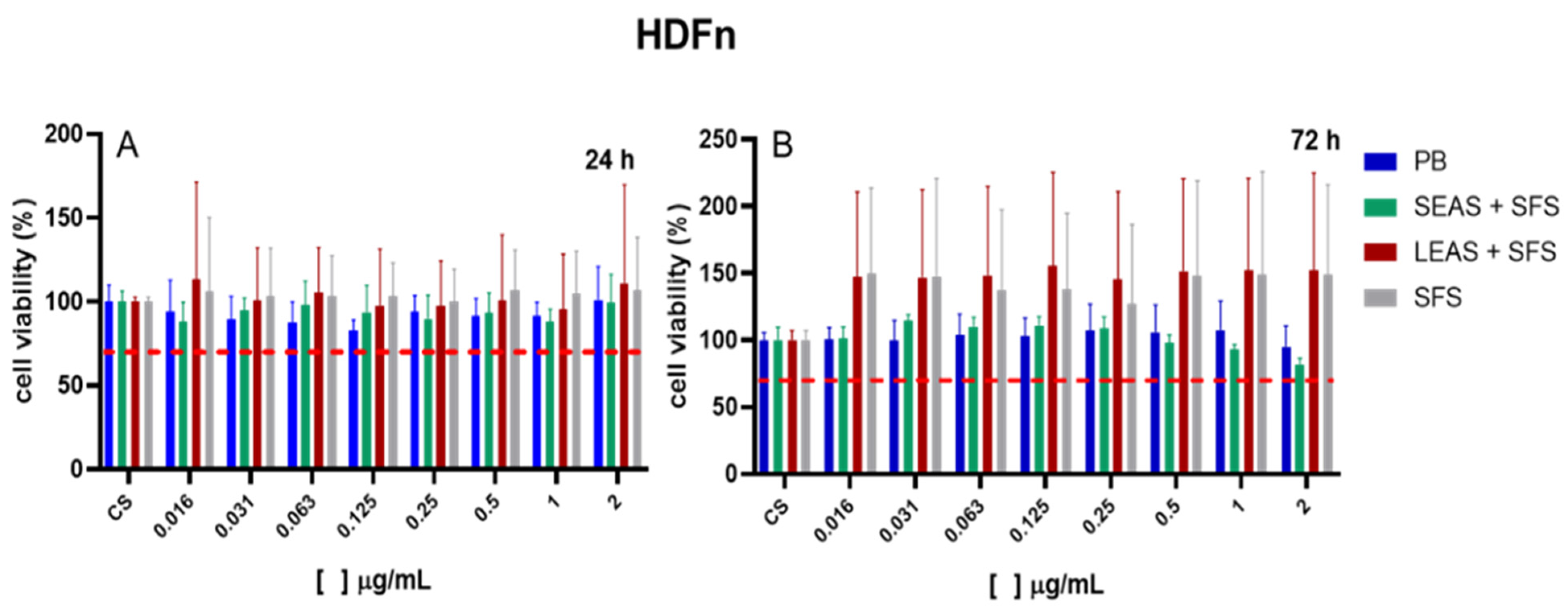
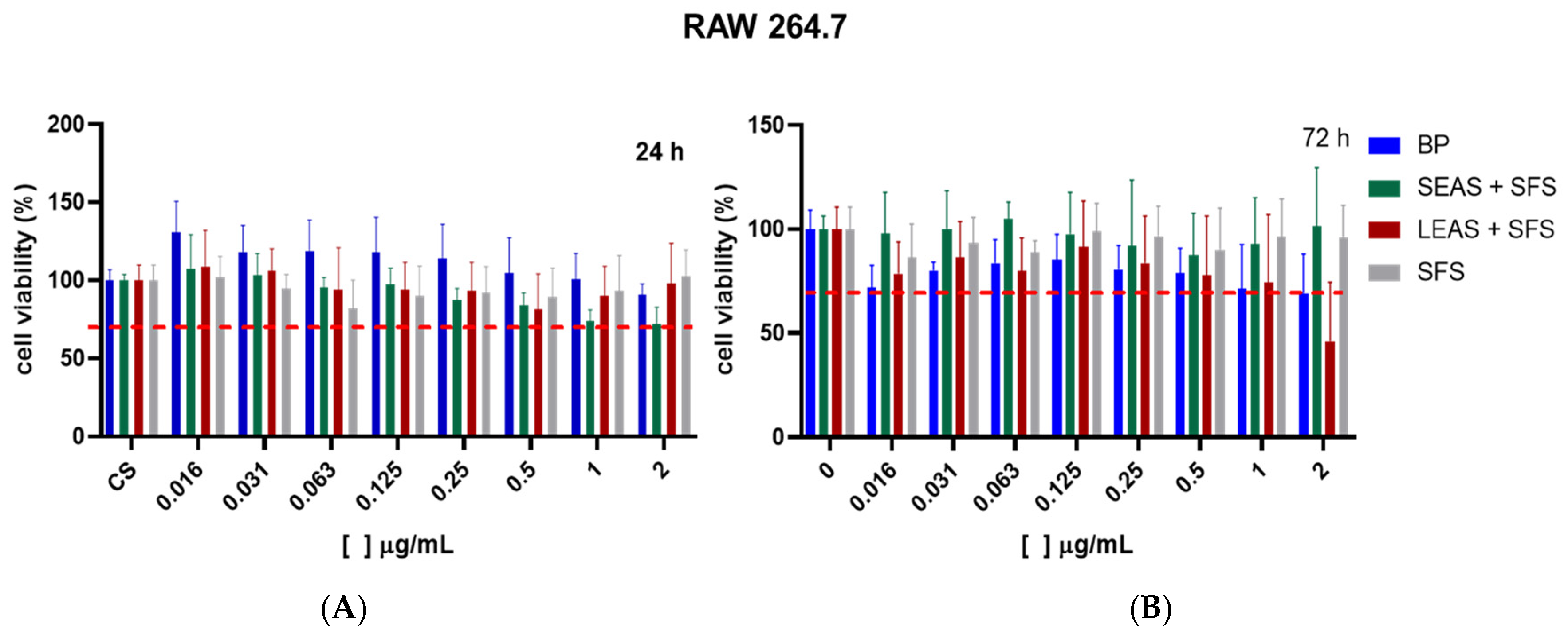
3.3. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PB | Purified bixin |
| SFS | Silk fibroin solution |
| BBD | Box-Behnken experimental design |
| FTIR | Fourier transform infrared |
| NMR | Nuclear magnetic resonance |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| LEAS + SFS | Liquid extract of annatto seeds from silk fibroin solution |
| SEAS + SFS | Solid extract of annatto seeds from silk fibroin solution |
| LOX | Lipoxygenase |
| HDFn | Human neonatal dermal fibroblasts |
| RAW 264.7 | Murine macrophages |
| DMEN | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide or dimethyl sulfoxide |
| Na2CO3 | Sodium carbonate |
| H2O: EtOH: CaCl2 | Water: Ethanol: Calcium chloride |
| KDa | Kilo Dalton |
| CO2 | Carbon dioxide |
| Gly | Glycine |
| Ala | Alanine |
| Ser | Serine |
| MHz | Megahertz |
| CDCl3 | Deuterated chloroform |
| CaCl2 | Calcium chloride |
References
- De Oliveira Júnior, R.G.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; da Silva Almeida, J.R.G.; Ferraz, C.A.A.; Picot, L. Bixin, an Apocarotenoid Isolated from Bixa orellana L., Sensitizes Human Melanoma Cells to Dacarbazine-Induced Apoptosis through ROS-Mediated Cytotoxicity. Food Chem. Toxicol. 2019, 125, 549–561. [Google Scholar] [CrossRef]
- Cruz Lima, R.J.; De Deus Moreno, A.J.; Loureiro De Castro, S.F.; Santos Gonçalves, J.D.R.; De Olivera, A.B.; Sasaki, J.M.; Cavalcante Freire, P.D.T. Taninos Hidrolisáveis Em Bixa orellana L. Quim. Nova 2006, 29, 507–509. [Google Scholar] [CrossRef]
- Medina-Flores, D.; Ulloa-Urizar, G.; Camere-Colarossi, R.; Caballero-García, S.; Mayta-Tovalino, F.; del Valle-Mendoza, J. Antibacterial Activity of Bixa orellana L. (Achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac. J. Trop. Biomed. 2016, 6, 400–403. [Google Scholar] [CrossRef]
- Keita, H.; dos Santos, C.B.R.; Ramos, M.M.; Padilha, E.C.; Serafim, R.B.; Castro, A.N.; Amado, J.R.R.; da Silva, G.M.; Ferreira, I.M.; Giuliatti, S.; et al. Assessment of the Hypoglycemic Effect of Bixin in Alloxan-Induced Diabetic Rats: In vivo and in silico Studies. J. Biomol. Struct. Dyn. 2021, 39, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Nathan, V.K.; Rani, M.E.; Rathinasamy, G.; Narayanan Dhiraviam, K. Antioxidant and Antimicrobial Potential of Natural Colouring Pigment Derived from Bixa orellana L. Seed Aril. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 137–143. [Google Scholar] [CrossRef]
- Oliveira, S.D.S.D.C.; Araújo, R.D.C.; da Silva, G.A.; Leitão, J.H.; da Silva Sousa, S.A.B.; Fonseca, L.P.; Carvalho, J.C.T.; Cantuária, P.; Hage-Melim, L.I.D.S.; Ferreira, I.M. Bixa orellana L. from Northern Brazil: Morphological Analysis, Phenolic Content, Antioxidant and Antibacterial Activities. Braz. J. Bot. 2022, 45, 883–896. [Google Scholar] [CrossRef]
- Gatsou Djibersou, D.; Rosnay Tietcheu Galani, B.; Dieudonne Djamen Chuisseu, P.; Yanou Njintang, N. Anti-Oxidant and Anti-Inflammatory Potential of Aqueous Extracts of Leaves, Barks and Roots of Bixa orellana L. (Bixaceae) on Acetaminophen-Induced Liver Damage in Mice. Avicenna J. Phytomed. 2020, 10, 428–439. [Google Scholar]
- Pacheco, S.D.G.; Gasparin, A.T.; Jesus, C.H.A.; Sotomaior, B.B.; Ventura, A.C.S.S.B.; Redivo, D.D.B.; Cabrini, D.D.A.; Gaspari Dias, J.D.F.; Miguel, M.D.; Miguel, O.G.; et al. Antinociceptive and Anti-Inflammatory Effects of Bixin, a Carotenoid Extracted from the Seeds of Bixa orellana. Planta Med. 2019, 85, 1216–1224. [Google Scholar] [CrossRef]
- Rivera-Madrid, R.; Aguilar-Espinosa, M.; Cárdenas-Conejo, Y.; Garza-Caligaris, L.E. Carotenoid Derivates in Achiote (Bixa orellana) Seeds: Synthesis and Health Promoting Properties. Front. Plant Sci. 2016, 7, 1406. [Google Scholar] [CrossRef]
- Kapoor, L.; Ramamoorthy, S. Strategies to Meet the Global Demand for Natural Food Colorant Bixin: A Multidisciplinary Approach. J. Biotechnol. 2021, 338, 40–51. [Google Scholar] [CrossRef]
- Tao, S.; Park, S.L.; De La Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Systemic Administration of the Apocarotenoid Bixin Protects Skin against Solar UV-Induced Damage through Activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.; Manoja, V.; Deepa, J.; Jayakumar, J.; Kishore, S.G.; Pandiselvam, R. Optimization of Microwave-Assisted Aqueous Extraction of Pigments from Annatto Seeds Using Box-Behnken Design. Biomass Convers. Biorefin 2023, 14, 18775–18788. [Google Scholar] [CrossRef]
- Jayakumar, J.; Sudha, P.; Rajkumar, P.; Pandiselvam, R.; Gurusamy, K.; Kumaran, K.; Subramanian, P. Comparative Study on the Effect of Solvents on Extraction of Bixin from Annatto Seed (Bixa orellana L.) and Optimization of Process Parameters Using Box–Behnken Design. Biomass Convers. Biorefin. 2023, 14, 19863–19874. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining Bixin from Semi-Defatted Annatto Seeds by a Mechanical Method and Solvent Extraction: Process Integration and Economic Evaluation. Food Res. Int. 2017, 99, 393–402. [Google Scholar] [CrossRef]
- David, S.L.J.; Julián, G.C.C.; Liliana, V.S.C.; Ayala-Aponte, A.A.; Liliana, S.C. Kinetics and Bixine Extraction Time from Achiote (Bixa orellana L.). J. Food Sci. Technol. 2022, 59, 1239–1246. [Google Scholar] [CrossRef]
- Hirko, B.; Getu, A. Bixa orellana (Annatto Bixa): A Review on Use, Structure, Extraction Methods and Analysis. J. Agron. Technol. Eng. Manag. 2022, 5, 687–696. [Google Scholar]
- Husa, N.N.; Hamzah, F.; Said, H.M. Characterization and Storage Stability Study of Bixin Extracted from Bixa orellana Using Organic Solvent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012035. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Torres, A.C.; Ramirez, L.M.; Garcia, M.S.; Gomez, G.C.; Rojas, J. Optimization of the Microwave-Assisted Extraction Process of Bioactive Compounds from Annatto Seeds (Bixa orellana L.). Antioxidants 2019, 8, 37. [Google Scholar] [CrossRef]
- Taham, T.; Silva, D.O.; Barrozo, M.A.S. Improvement of Bixin Extraction from Annatto Seeds Using a Screen-Topped Spouted Bed. Sep. Purif. Technol. 2016, 158, 313–321. [Google Scholar] [CrossRef]
- Yolmeh, M.; Habibi Najafi, M.B.; Farhoosh, R. Optimisation of Ultrasound-Assisted Extraction of Natural Pigment from Annatto Seeds by Response Surface Methodology (RSM). Food Chem. 2014, 155, 319–324. [Google Scholar] [CrossRef]
- Handayani, I.; Septiana, A.T.; Sustriawan, B. Propriedades de Pigmentos Naturais e Antioxidantes Do Extrato de Urucum Em Vários PHs de Solventes de Água Destilada e Tempos de Extração. Food Res. 2024, 8, 489–494. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.J. Increase the Yield of Bioactive Compounds from Elder Bark and Annatto Seeds Using Ultrasound and Microwave Assisted Extraction Technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Tabatabai, A.P.; Leisk, G.G.; Cebe, P.; Blair, D.L.; Kaplan, D.L. Silk Fibroin Degradation Related to Rheological and Mechanical Properties. Macromol. Biosci. 2016, 16, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.; Migliaresi, C.; Motta, A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J. Funct. Biomater. 2016, 7, 26. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Yoshioka, S.A.; Comasseto, J.V.; Porto, A.L.M. Immobilization of Amano Lipase from Pseudomonas Fluorescens on Silk Fibroin Spheres: An Alternative Protocol for the Enantioselective Synthesis of Halohydrins. RSC Adv. 2017, 7, 12650–12658. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef]
- Debari, M.K.; King, C.I.; Altgold, T.A.; Abbott, R.D. Silk Fibroin as a Green Material. ACS Biomater. Sci. Eng. 2021, 7, 3530–3544. [Google Scholar] [CrossRef]
- Marinho, V.H.S.; Neves, F.B.; Jimenez, D.E.Q.; Oliveira, F.R.; Santos, A.V.T.L.T.; Ferreira, R.M.A.; Souto, R.N.P.; Carvalho, J.C.T.; Yoshioka, S.A.; Ferreira, I.M. Development of an Environmentally Friendly Formulation of Silk Fibroin Combined with Fatty Acid from Astrocaryum murumuru Mart. Effective against Aedes aegypti Larvae. J. Drug Deliv. Sci. Technol. 2022, 75, 103626. [Google Scholar] [CrossRef]
- Sarquis, I.R.; Sarquis, R.S.F.R.; Marinho, V.H.S.; Neves, F.B.; Araújo, I.F.; Damasceno, L.F.; Ferreira, R.M.A.; Souto, R.N.P.; Carvalho, J.C.T.; Ferreira, I.M. Carapa guianensis Aubl. (Meliaceae) Oil Associated with Silk Fibroin, as Alternative to Traditional Surfactants, and Active against Larvae of the Vector Aedes Aegypti. Ind. Crops Prod. 2020, 157, 112931. [Google Scholar] [CrossRef]
- Marinho, V.H.S.; Holanda, F.H.; Araújo, I.F.; Jimenez, D.E.Q.; Pereira, R.R.; Porto, A.L.M.; Ferreira, A.M.; Carvalho, J.C.T.; Albuquerque de Freitas, A.C.G.; Fernandes, C.P.; et al. Nanoparticles from Silk Fibroin and Amazon Oils: Potential Larvicidal Activity and Oviposition Deterrence against Aedes aegypti. Ind. Crops Prod. 2023, 203, 117133. [Google Scholar] [CrossRef]
- Holanda, F.H.; Pereira, R.R.; Marinho, V.H.S.; Jimenez, D.E.Q.; Costa Ferreira, L.M.M.; Ribeiro-Costa, R.M.; de Sousa, F.F.O.; Ferreira, I.M. Development of Nanostructured Formulation from Naringenin and Silk Fibroin and Application for Inhibition of Lipoxygenase (LOX). RSC Adv. 2023, 13, 23063–23075. [Google Scholar] [CrossRef]
- de Castro Cantuária, P.; Medeiros, T.D.S.; Cantuária, M.F.; Soares, A.C.S.; e Silva, B.M.D.S.; Krahl, A.H.; Costa-Campos, C.E.; e Silva, R.B.L. Você Conhece a Nomenclatura Biológica? Aprenda a Forma Correta de Escrever Os Nomes Dos Organismos. Res. Soc. Dev. 2022, 11, e21711326378. [Google Scholar] [CrossRef]
- Maciel Ferreira, I.; Coutinho Rocha, L.; Akinobo Yoshioka, S.; Nitschke, M.; Haroldo Jeller, A.; Pizzuti, L.; Seleghim, M.H.R.; Porto, A.L.M. Chemoselective Reduction of Chalcones by Whole Hyphae of Marine Fungus Penicillium citrinum CBMAI 1186, Free and Immobilized on Biopolymers. Biocatal. Agric. Biotechnol. 2014, 3, 358–364. [Google Scholar] [CrossRef]
- de Oliveira Penido, C.A.F.; Pacheco, M.T.T.; Novotny, E.H.; Lednev, I.K.; Silveira, L. Quantification of Cocaine in Ternary Mixtures Using Partial Least Squares Regression Applied to Raman and Fourier Transform Infrared Spectroscopy. J. Raman Spectrosc. 2017, 48, 1732–1743. [Google Scholar] [CrossRef]
- Leite, C.M.; Araujo-Neto, J.H.; Guedes, A.P.M.; Costa, A.R.; Demidoff, F.C.; Netto, C.D.; Castellano, E.E.; Nascimento, O.R.; Batista, A.A. Copper(I)/Triphenylphosphine Complexes Containing Naphthoquinone Ligands as Potential Anticancer Agents. Inorganics 2023, 11, 367. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, H.; Wang, F.; Wei, D.; Wang, X. An Improved 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Reduction Assay for Evaluating the Viability of Escherichia Coli Cells. J. Microbiol. Methods 2010, 82, 330–333. [Google Scholar] [CrossRef] [PubMed]
- de Toledo, C.D.; Teixeira, D.A.; da Vitória Archanjo, E.; Pereira, F.F.; Siqueira, F.C.; Mininel, F.J.; de Lima, J.S.; da Mota Silveira, K. Revisitação Dos Métodos de Extração de Pigmentos Do Urucum (Review of Urucum Pigment Extraction Methods). Ciências Exatas e Tecnologia 2021, 16, 24–29. [Google Scholar] [CrossRef]
- Taham, T.; Cabral, F.A.; Barrozo, M.A.S. The Journal of Supercritical Fluids Extraction of Bixin from Annatto Seeds Using Combined Technologies. J. Supercrit. Fluids 2015, 100, 175–183. [Google Scholar] [CrossRef]
- Puri, S.; Mandal, S.K.; Jain, H.; Sharma, P.K.; Deepa, P.R. Comparative bioactivity assessment of bixin pigment and associated phytochemicals extracted from annatto seeds using conventional and green solvents. J. Food Drug Anal. 2024, 32, 168–183. [Google Scholar] [CrossRef]
- Miguel, K.B.; Cardoso, V.L.; Reis, M.H.M. Concentration of Pigments and Health-Promoting Bioactive Compounds from Annatto Seeds by Ultrafiltration. Waste Biomass Valorization 2024, 15, 127–137. [Google Scholar] [CrossRef]
- Witono, J.R.; Ramadhany, P.; Santoso, H.; Putri, A. The Potency of Norbixin as an Active Compound of Natural Dye in Textile Industry. Mater. Today Proc. 2022, 63, S248–S254. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Alcázar-Alay, S.C.; Petenate, A.J.; Meireles, M.A.A. Bixin Extraction from Defatted Annatto Seeds. Comptes Rendus Chim. 2014, 17, 268–283. [Google Scholar] [CrossRef]
- Peters, A.T.; Freeman, H.S. Physico-Chemical Principles of Color Chemistry, 1st ed.; Springer: Dordrecht, The Netherlands, 1996; 299p, ISBN 978-0-7514-0210-0. [Google Scholar]
- Satyanarayana, A.; Prabhakara Rao, P.G.; Rao, D.G. Chemistry, Processing and Toxicology of Annatto (Bixa orellana L.). J. Food Sci. Technol. 2003, 40, 131–141. [Google Scholar]
- Erdawati, E.; Allanas, E.; Wesnina, W. Extraction of Bixin from Annatto Seeds with Microwave. J. Phys. Conf. Ser. 2021, 1869, 012016. [Google Scholar] [CrossRef]
- Jin, X.; Streett, D.A.; Dunlap, C.A.; Lyn, M.E. Application of Hydrophilic–Lipophilic Balance (HLB) Number to Optimize a Compatible Non-Ionic Surfactant for Dried Aerial Conidia of Beauveria bassiana. Biol. Control 2008, 46, 226–233. [Google Scholar] [CrossRef]
- Weuts, I.; Kempen, D.; Verreck, G.; Decorte, A.; Heymans, K.; Peeters, J.; Brewster, M.; Mooter, G. Van den Study of the Physicochemical Properties and Stability of Solid Dispersions of Loperamide and PEG6000 Prepared by Spray Drying. Eur. J. Pharm. Biopharm. 2005, 59, 119–126. [Google Scholar] [CrossRef]
- Marelli, B.; Brenckle, M.A.; Kaplan, D.L.; Omenetto, F.G. Silk Fibroin as Edible Coating for Perishable Food Preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl Lactate as a Potential Green Solvent to Extract Hydrophilic (Polar) and Lipophilic (Non-Polar) Phytonutrients Simultaneously from Fruit and Vegetable by-Products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Bitencourt, A.P.R.; Duarte, J.L.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Carvalho, J.C.T.; Gomes, A.T.A.; Ferreira, I.M.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C.; Fernandes, C.P. Preparation of Aqueous Nanodispersions with Annatto (Bixa orellana L.) Extract Using an Organic Solvent-Free and Low Energy Method. Food Chem. 2018, 257, 196–205. [Google Scholar] [CrossRef]
- Patra, S.; Kar, S.; Bag, B.G. Isolation and Biological Activities of Naturally Occurring Apocarotenoid Bixin. Prayogik Rasayan 2021, 5, 112–119. [Google Scholar] [CrossRef]
- Mitra, I.; Mukherjee, S.; Reddy Venkata, P.B.; Dasgupta, S.; Jagadeesh Bose, C.K.; Mukherjee, S.; Linert, W.; Moi, S.C. Benzimidazole Based Pt(II) Complexes with Better Normal Cell Viability than Cisplatin: Synthesis, Substitution Behavior, Cytotoxicity, DNA Binding and DFT Study. RSC Adv. 2016, 6, 76600–76613. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Guo, P.; Du, P.; Zhao, P.; Chen, X.; Liu, C.; Du, Y.; Li, J.; Tang, X.; Yang, F.; Lv, G. Regulating the Mechanics of Silk Fi Broin Scaffolds Promotes Wound Vascularization. Biochem. Biophys. Res. Commun. 2021, 574, 78–84. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Yuan, X.; Bayat, A. Electrospun Silk Fibroin Fiber Diameter Influences In Vitro Dermal Fibroblast Behavior and Promotes Healing of Ex Vivo Wound Models. J. Tissue Eng. 2014, 5, 2041731414551661. [Google Scholar] [CrossRef] [PubMed]
- Millán-Rivero, J.E.; Martínez, C.M.; Romecín, P.A.; Aznar-cervantes, S.D.; Carpes-Ruiz, M.; Cenis, J.L.; Moraleda, J.M.; Atucha, N.M.; García-bernal, D. Silk Fibroin Scaffolds Seeded with Wharton’ s Jelly Mesenchymal Stem Cells Enhance Re-Epithelialization and Reduce Formation of Scar Tissue after Cutaneous Wound Healing. Stem Cell Res. Ther. 2019, 10, 126. [Google Scholar] [CrossRef]
- Woo, H.; Joo, O.; Min, J.; Mi, B.; Jung, H.; Ri, Y.; Chae, M.; Hyeon, S.; Ren, J.; Seok, C.; et al. Wound Healing Effect of Electrospun Silk Fibroin Nanomatrix In burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Sun, C.; Wang, Y. Polysaccharides from Polygonatum Sibiricum Delar. Ex Redoute Induce an Immune Response in the RAW264.7 Cell Line via an NF-ΚB/MAPK Pathway. RSC Adv. 2019, 9, 17988–17994. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Lee, O.J.; Kim, S.H.; Ju, H.W.; Park, C.H. Silk Fibroin in Wound Healing Process. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018; pp. 115–126. ISBN 978-981-13-0947-2. [Google Scholar]
- Capella, S.O.; Tillmann, M.T.; Félix, A.O.C.; Fontoura, E.G.; Fernandes, C.G.; Freitag, R.A.; Santos, M.A.Z.; Félix, S.R.; Nobre, M.O. Potencial Cicatricial Da Bixa orellana L. Em Feridas Cutâneas: Estudo Em Modelo Experimental. Arq. Bras. Med. Vet. Zootec. 2016, 68, 104–112. [Google Scholar] [CrossRef]
- Franklin, V.A.; Bach Hi, E.M.; Wadt, N.S.Y.; Bach, E.E. Aqueous Extract from Urucum (Bixa orellana L.): Antimicrobial, Antioxidant, and Healing Activity. Porto Biomed. J. 2023, 8, e183. [Google Scholar] [CrossRef] [PubMed]
- Pizoón-Garcia, A.D.; Cassini-vieira, P.; Ribeiro, C.C.; Eduardo, C.; Jensen, D.M.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient Cutaneous Wound Healing Using Bixin-Loaded PCL Nanofibers in Diabetic Mice. J. Biomed. Mat. Res. 2016, 105, 1938–1949. [Google Scholar] [CrossRef]
- Nikolaev, A.; Sokolov, N.N.; Kozlov, E.A.; Kutsman, M.E. Isolation and Properties of a Homogenous L Asparaginase Preparation from Pseudomonas Fluorescens AG. Biokhimiya 2009, 40, 984–989. (In Russian) [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Figueiredo-Junior, A.T.; Valença, S.S.; Finotelli, P.V.; Anjos, F.d.F.d.; de Brito-Gitirana, L.; Takiya, C.M.; Lanzetti, M. Treatment with Bixin-Loaded Polymeric Nanoparticles Prevents Cigarette Smoke-Induced Acute Lung Inflammation and Oxidative Stress in Mice. Antioxidants 2022, 11, 1293. [Google Scholar] [CrossRef]
- Somacal, S.; Schüler da Silva, L.C.; de Oliveira, J.; Emanuelli, T.; Fabro de Bem, A. Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways. Foods 2024, 13, 2002. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Sobral, C.S.; Blanes, L.; Ipolito, M.Z.; Horibe, E.K. Proliferation of Fibroblasts Cultured on a Hemi-Cellulose Dressing. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 865–869. [Google Scholar] [CrossRef]
- Gragnani, A.; Müller, B.R.; Silva, I.D.C.G.D.; Noronha, S.M.R.D.; Ferreira, L.M. Keratinocyte Growth Factor, Tumor Necrosis Factor-Alpha and Interleukin-1 Beta Gene Expression in Cultured Fibroblasts and Keratinocytes from Burned Patients. Acta Cir. Bras. 2013, 28, 551–558. [Google Scholar] [CrossRef]
- Keira, S.M.; Ferreira, L.M.; Gragnani, A.; Duarte, I.D.S.; Santos, I.A.N.D. Experimental Model for Fibroblast Culture. Acta Cir. Bras. 2004, 19, 11–16. [Google Scholar] [CrossRef]
- Campaner, A.; Filho, A.; Morgan, J.; Ferreira, L. Animal Model of Keloids Using Genetically Modified Fibroblasts. Rev. Bras. Cir. Plást. 2007, 22, 137–142. [Google Scholar]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Haque, A. Immunomodulatory effects of Tinospora crispa Extract and Its Major Compounds on the Immune Functions of RAW 264.7 Macrophages. Int. Immunopharmacol. 2018, 60, 141–151. [Google Scholar] [CrossRef]
- Kang, C.; Kim, J.; Kim, H.; Park, H.M.; Paek, N. Heat—Killed Lactic Acid Bacteria Inhibit Nitric Oxide Production via Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in RAW 264.7 cells. Probiotics Antimicrob. Proteins 2021, 13, 1530–1538. [Google Scholar] [CrossRef]
- Rivera, D.E.; Ocampo, Y.C.; Castro, J.P.; Barrios, L.; Diaz, F.; Franco, L.A. A Screening of Plants Used in Colombian Traditional Medicine Revealed the Anti-Inflammatory Potential of Physalis Angulata Calyces. Saudi J. Biol. Sci. 2019, 26, 1758–1766. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, J.; Oh, J.H.; Park, J.S.; Lee, D.; Kim, C.E.; Choi, H.-S.; Kim, S.-B.; Hwang, G.S.; Koo, B.A.; et al. Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages. Biomolecules 2020, 10, 424. [Google Scholar] [CrossRef]
- Eriksson, E.; Griffith, G.L.; Nuutila, K. Topical Drug Delivery in the Treatment of Skin Wounds and Ocular Trauma Using the Platform Wound Device. Pharmaceutics 2023, 15, 1060. [Google Scholar] [CrossRef]
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.-P.; Kotzbeck, P. Modelling the Complexity of Human Skin In Vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef]
- Gupta, R.; Polaka, S.; Rajpoot, K.; Tekade, M.; Sharma, M.C.; Tekade, R.K. Chapter 6—Importance of Toxicity Testing in Drug Discovery and Research. In Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 117–144. ISBN 978-0-323-98367-9. [Google Scholar]
| Factor | Variables | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| x1 | Temperature (°C) | 40 | 50 | 60 |
| x2 | Time (min) | 30 | 60 | 90 |
| x3 | SFS concentration (%) | 0.5 | 1.0 | 1.5 |
| Experiment (Run) | Temperature (°C) | Time (min) | Concentration SFS (%) | Bixin Content (LEAS + SFS) mg/mL | Bixin Content (SEAS + SFS) mg/g |
|---|---|---|---|---|---|
| 1 | 40 | 30 | 1. 0 | 6.44 | 72.48 |
| 2 | 60 | 60 | 1.5 | 10.87 | 150.72 |
| 3 | 50 | 30 | 0.5 | 2.89 | 106.68 |
| 4 | 60 | 30 | 1.0 | 3.54 | 216.36 |
| 5 | 50 | 60 | 1.0 | 4.38 | 103.32 |
| 6 | 40 | 90 | 1.0 | 5.12 | 70.56 |
| 7 | 50 | 60 | 1.0 | 3.49 | 95.52 |
| 8 | 40 | 60 | 0.5 | 2.16 | 175.44 |
| 9 | 50 | 90 | 0.5 | 4.94 | 134.52 |
| 10 | 50 | 60 | 1.0 | 6.94 | 136.56 |
| 11 | 60 | 90 | 1.0 | 5.52 | 90.6 |
| 12 | 50 | 90 | 1.5 | 9.20 | 130.56 |
| 13 | 50 | 30 | 1.5 | 6.73 | 150.24 |
| 14 | 40 | 60 | 1.5 | 6.48 | 137.4 |
| 15 | 60 | 60 | 0.5 | 2.96 | 84.72 |
 | ||
|---|---|---|
| Carbon Number * | δ 13C (ppm) | |
| Present Study | [53] | |
| 1 | 124.18 | 130.73 |
| 2 | 130.70 | 130.73 |
| 3 | 131.42 | 131.46 |
| 4 | 135.18 | 135.22 |
| 5 | 137.12 | 137.14 |
| 6 | 117.52 | 123.38 |
| 7 | 140.36 | 140.39 |
| 8 | 133.27 | 133.57 |
| 9 | 142.34 | 142.38 |
| 10 | 114.83 | 117.53 |
| 11 | 167.97 | 168.01 |
| 12 | 51.58 | 51.61 |
| 13 | 20.26 | 20.56 |
| 14 | 12.62 | 12.63 |
| 15 | 170.75 | 171.67 |
| 16 | 114.83 | 115.0 |
| 17 | 151.03 | 151.08 |
| 18 | 134.18 | 134.21 |
| 19 | 140.44 | 140.50 |
| 20 | 123.36 | 124.21 |
| 21 | 137.91 | 137.95 |
| 22 | 136.53 | 136.56 |
| 23 | 131.58 | 131.60 |
| 24 | 12.99 | 13.01 |
| 25 | 12.76 | 12.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, S.F.; Holanda, F.H.e.; De Maria, K.C.; Oliveira, S.d.S.d.C.; Jimenez, D.E.Q.; Leite, C.M.; Zucolotto, V.; Ferreira, I.M. Effect of Silk Fibroin as a Sustainable Solvent on the Extraction of Bixin from Annatto Seeds (Bixa orellana L.). Sustainability 2025, 17, 7518. https://doi.org/10.3390/su17167518
Borges SF, Holanda FHe, De Maria KC, Oliveira SdSdC, Jimenez DEQ, Leite CM, Zucolotto V, Ferreira IM. Effect of Silk Fibroin as a Sustainable Solvent on the Extraction of Bixin from Annatto Seeds (Bixa orellana L.). Sustainability. 2025; 17(16):7518. https://doi.org/10.3390/su17167518
Chicago/Turabian StyleBorges, Swanny Ferreira, Fabricio H. e Holanda, Kaio C. De Maria, Sônia do Socorro do C. Oliveira, David E. Q. Jimenez, Celisnolia Morais Leite, Valtencir Zucolotto, and Irlon M. Ferreira. 2025. "Effect of Silk Fibroin as a Sustainable Solvent on the Extraction of Bixin from Annatto Seeds (Bixa orellana L.)" Sustainability 17, no. 16: 7518. https://doi.org/10.3390/su17167518
APA StyleBorges, S. F., Holanda, F. H. e., De Maria, K. C., Oliveira, S. d. S. d. C., Jimenez, D. E. Q., Leite, C. M., Zucolotto, V., & Ferreira, I. M. (2025). Effect of Silk Fibroin as a Sustainable Solvent on the Extraction of Bixin from Annatto Seeds (Bixa orellana L.). Sustainability, 17(16), 7518. https://doi.org/10.3390/su17167518









