In Vitro Culture Initiation and Micropropagation Optimization of Plantago Halophytes: A Sustainable Approach to Exploring Valuable Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Culture Initiation
2.3. Multiplication—Culture Condition
2.4. Physiological Conditions of Plants
2.4.1. Photosynthetic Pigments
- Chlorophyll a (abbreviated as Chl a) (μg cm−3) = 12.21 × A663 − 2.81 × A646
- Chlorophyll b (abbreviated as Chl b) (μg cm−3) = 20.13 × A646 − 5.03 × A663
- Total chlorophyll = Chl a + Chl b
- Total carotenoids (μg cm−3) = (1000 × A470 − 3.27 × [Chl a] − 104 × [Chl b])/229
2.4.2. Proline Determination
2.4.3. Total Phenolic Compounds Determination
2.5. Statystical Analysis
3. Results
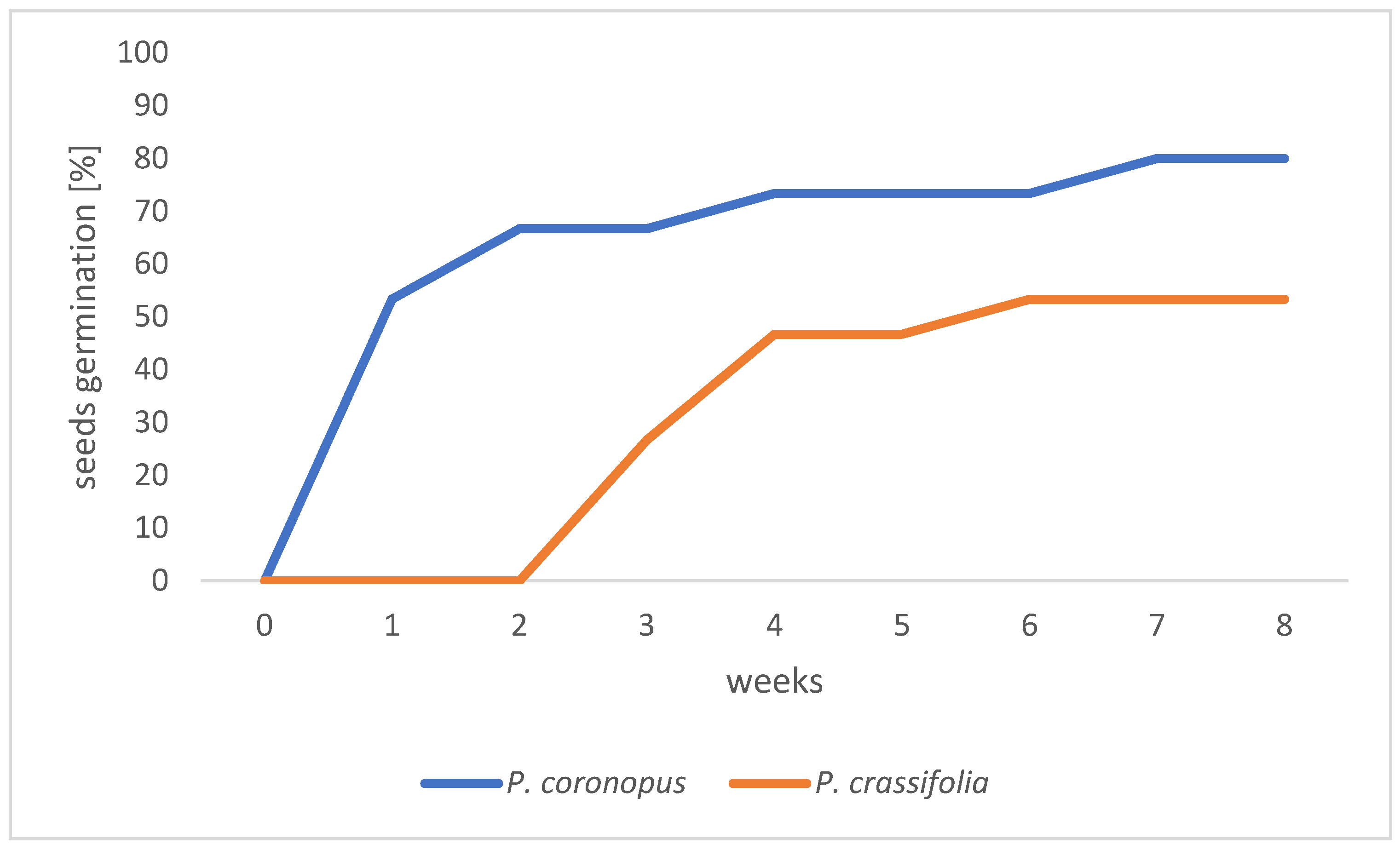
3.1. Germination Efficiency of Plantago Seeds
3.2. Growth Parameters of Plantago Plants
3.3. Physiological Analysis of Plant Material
3.3.1. Total Chlorophylls Content
3.3.2. Carotenoids Content
3.3.3. Proline Concentration
3.3.4. Total Phenolic Compounds Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henderson, L. Floral anatomy of several species of Plantago. Am. J. Bot. 1926, 13, 397–405. [Google Scholar] [CrossRef]
- Brown, M. A synopsis of the genus Plantago L. in Tasmania Pap. Proc. R. Soc. Tasman. 1991, 124, 65–74. [Google Scholar] [CrossRef]
- Höpke, J.; Mucina, L.; Albach, D. Phylogenetic and morphometric analysis of Plantago section Coronopus (Plantaginaceae). Taxon 2019, 68, 315–339. [Google Scholar] [CrossRef]
- Ltaeif, H.; Sakhraoui, A.; González-Orenga, S.; Faz, A.; Boscaiu, M.; Vicente, Ó.; Rouz, S. Responses to Salinity in Four Plantago Species from Tunisia. Plants 2021, 10, 1392. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Matysik-Woźniak, A.; Sulborska, A.; Rejdak, R. Commercially important properties of plants of the genus Plantago. Acta Agrobot. 2012, 65, 11–20. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Boscaiu, M.; Ballesteros, G.; Naranjo, M.A.; Vicente, O.; Boira, H. Responses of halophytes to salt stress. Bul. USAMV-CN 2007, 64, 13–18. [Google Scholar]
- Al Hassan, M.; Pacurar, A.; Gaspar, A.; Vicente, O.; Boscaiu, M. Growth and Reproductive Success under Saline Conditions of Three Plantago Species with Different Levels of Stress Tolerance. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 180–186. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of Salt Stress on Three Ecologically Distinct Plantago Species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef] [PubMed]
- Safarnejad, A.; Shoorvarzi, M.; Dalir, M. In vitro selection of Plantago ovata for NaCl tolerance. Iran. J. Rangel. For. Plant Breed. Genet. Res. 2017, 25, 275–287. [Google Scholar]
- Safarnejad, A.; Shoorvarzi, M.; Dalir, M. In vitro selection of Plantago psyllium L. for salt tolerance and Changes of Sodium, Calcium and Potassium levels at callus stage. Iran. J. Rangel. For. Plant Breed. Genet. Res. 2017, 24, 221–231. [Google Scholar]
- Norouzi, O.; Hesami, M.; Pepe, M.; Dutta, A.; Jones, A.M.P. In vitro plant tissue culture as the fifth generation of bioenergy. Sci. Rep. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Fons, F.; Gargadennec, A.; Rapior, S. Culture of Plantago species as bioactive components resources: A 20-year review and recent applications. Acta Bot. Gall. 2008, 155, 277–300. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mederos, S.; Martin, C.; Navarro, E.; Ayuso, M. Micropropagation of a medicinal plant, Plantago major L. Biol. Plant. 1997, 39, 465–468. [Google Scholar] [CrossRef]
- Andrzejewska-Golec, E.; Makowczyńska, J. Micropropagation of Plantago camtschatica Link. Acta Soc. Bot. Pol. 2008, 77, 269–273. [Google Scholar] [CrossRef][Green Version]
- Makowczyńska, J.; Andrzejewska-Golec, E. Micropropagation of Plantago asiatica L. through culture of shoot-tips. Acta Soc. Bot. Pol. 2003, 72, 191–194. [Google Scholar] [CrossRef]
- Makowczyńska, J.; Andrzejewska-Golec, E. Micropropagation of Plantago maritima L.—A vanishing species in Poland. Acta Soc. Bot. Pol. 2009, 78, 13–18. [Google Scholar] [CrossRef]
- Gastmann, J.; Klaus, M.V.V.; Winhelmann, M.C.; de Campos, S.S.; Hoehne, L.; de Freitas, E.M. In vitro germination and seedling formation of Plantago tomentosa Lam. (Plantaginaceae): Influence of concentrations of the MS medium. Ciência Nat. 2024, 46, e71895. [Google Scholar] [CrossRef]
- Rahamooz-Haghighi, S.; Bagheri, K.; Danafar, H.; Sharafi, A. Tissue culture, in vitro organogenesis and regeneration of Plantago lanceolata. J. Appl. Biotechnol. Rep. 2020, 7, 258–265. [Google Scholar] [CrossRef]
- Gonçalves, S.; Martins, N.; Romano, A. Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biol. Plant 2009, 53, 774–778. [Google Scholar] [CrossRef]
- Sharma, M.; Kumari, A.; Mahant, E. Micropropogation and analysis of phytochemical profile of tissue culture grown Plantago ovata Forsk. Asian J. Pharm. Clin. Res. 2017, 10, 202–206. [Google Scholar] [CrossRef]
- Custódio, L.; Charles, G.; Magné, C.; Barba-Espín, G.; Piqueras, A.; Hernández, J.; Hamed, K.; Castañeda-Loaiza, V.; Fernandes, E.; Rodrigues, M. Application of In Vitro Plant Tissue Culture Techniques to Halophyte Species: A Review. Plants 2022, 12, 126. [Google Scholar] [CrossRef]
- Pieracci, Y.; Vento, M.; Pistelli, L.; Lombardi, T.; Pistelli, L. Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants. Plants 2022, 11, 1081. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Palazzo de Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.; Meht, A. Optimization of in-vitro propagation protocol for Plantago ovata Forsk. J. Indian Bot. Soc. 2015, 94, 245–251. [Google Scholar]
- Mira, S.; Veiga-Barbosa, L.; González-Benito, M.; Pérez-García, F. Inter-population variation in germination characteristics of “Plantago lanceolata” seeds: Effects of temperature, osmotic stress and salinity. Appl. Bot. 2018, 39, 89–96. [Google Scholar] [CrossRef]
- Sarihan, E.; Ipek, A.; Mahmood, K.; Atak, M.; Gürbüz, B. Role of GA3 and KNO3 in improving the frequency of seed germination in Plantago lanceolata L. Pak. J. Bot. 2005, 37, 883–887. [Google Scholar]
- Waite, S.; Hutchings, M.J. The effects of sowing density, salinity and substrate upon the germination of seeds of Plantago coronopus L. New Phytol. 1978, 81, 341–348. [Google Scholar] [CrossRef]
- Zaady, E.; Gutterman, Y.; Boeken, B. The germination of mucilaginous seeds of Plantago coronopus, Reboudia pinnata, and Carrichtera annua on cyanobacterial soil crust from the Negev Desert. Plant Soil 1997, 190, 247–252. [Google Scholar] [CrossRef]
- Luciani, F.; Cristaudo, A.; Aricò, D. Germination ecology of three Plantago L. (Plantaginaceae) species living in a saline environment. Plant Biosyst. 2001, 135, 213–221. [Google Scholar] [CrossRef]
- Gutterman, Y.; Shem-Tov, S.; Gozlan, S. The effect of post-maturation temperatures and duration on seed germinability of Plantago coronopus occurring in natural populations in the Negev Desert highlands, Israel. J. Arid Environ. 1998, 38, 451–463. [Google Scholar] [CrossRef]
- Pramanik, S.; Raychaudhuri, S.; Chakraborty, S. Changes in esterase and superoxide dismutase isozymes during in vitro morphogenesis in Plantago ovata Forssk. Plant Cell Tissue Organ Cult. 1996, 44, 123–127. [Google Scholar] [CrossRef]
- Budzianowska, A.; Kikowska, M.; Budzianowski, J. Phenylethanoid glycosides accumulation and antiradical activity of fractionated extracts of Plantago ovata Forssk. callus cultures lines. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 54. [Google Scholar] [CrossRef]
- Gaspar, T.; Xhaufflaire, A. Effect of kinetin on growth, auxin catabolism, peroxidase and catalase activities. Planta 1966, 72, 252–257. [Google Scholar] [CrossRef]
- Hartmans, K.; Es, A. The influence of growth regulators GA3, ABA, kinetin and IAA on sprout and root growth and plant development using excised potato buds. Potato Res. 1979, 22, 319–332. [Google Scholar] [CrossRef]
- Lee, S.; Cho, W.; Jang, H.; Chandra, R.; Lee, S.; Kang, H. Effect of Plant Growth Regulators in In Vitro Culture of Hippophae rhamnoides. J. For. Environ. Sci. 2021, 37, 148–153. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Ahmad, A.; Anis, M. Meta-topolin Improves In Vitro Morphogenesis, Rhizogen-esis and Biochemical Analysis in Pterocarpus marsupium Roxb.: A Potential Drug-Yielding Tree. J. Plant Growth Regul. 2019, 38, 1007–1016. [Google Scholar] [CrossRef]
- Amoo, S.O.; Van Staden, J. Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micropropagated Huernia hystrix. Plant Cell Tissue Organ Cult. 2013, 112, 249–256. [Google Scholar] [CrossRef]
- Amoo, S.O.; Aremu, A.O.; Moyo, M.; Sunmonu, T.O.; Plíhalová, L.; Doležal, K.; Van Staden, J. Physiological and biochemical effects of a tetrahydropyranyl-substituted meta-topolin in micropropagated Merwilla plumbea. Plant Cell Tissue Organ Cult. 2015, 121, 579–590. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Novák, O.; Plačková, L.; Zatloukal, M.; Doležal, K.; Finnie, J.F.; Strnad, M.; Van Staden, J. Physiological responses and endogenous cytokinin profiles of tissue-cultured ‘Williams’ bananas in relation to roscovitine and an inhibitor of cytokinin oxidase/dehydrogenase (INCYDE) treatments. Planta 2012, 236, 1775–1790. [Google Scholar] [CrossRef]
- Mutui, T.M.; Mibus, H.; Serek, M. The influence of plant growth regulators and storage on root induction and growth in Pelargonium zonale cuttings. Plant Growth Regul. 2010, 61, 185–193. [Google Scholar] [CrossRef]
- Gentile, A.; Frattarelli, A.; Nota, P.; Condello, E.; Caboni, E. The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell Tissue Organ Cult. 2017, 128, 693–703. [Google Scholar] [CrossRef]
- Kulpa, D.; Wrobel, M.; Bednarek, M. Type of Explant Affects In Vitro Development and Multiplication Success of the Rare Halophyte Plant Honckenya peploides L. Ehrh. Plants 2020, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seliskar, D.M.; Gallagher, J.L. Tissue Culture and Plant Regeneration of the Salt Marsh Monocots Juncus roemerianus and Juncus gerardi. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 274–280. [Google Scholar] [CrossRef]
- Adem, M.; Sharma, L.; Shekhawat, G.; Šafranek, M.; Jásik, J. Auxin Signaling Transportation and Regulation during Adventitious Root Formation. Curr. Plant Biol. 2024, 40, 100385. [Google Scholar] [CrossRef]
- Baldwin, I.; Meza-Canales, I.; Schäfer, M.; Vaňková, R.; Grosskinsky, D.; Brütting, C.; Meldau, S. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Meng, Z.; Chen, M.; Zhang, M. Research Progress on the Roles of Cytokinin in Plant Response to Stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef]
- Al-Qudah, T.S.; Shibli, R.A.; Zatimeh, A.; Tahtamouni, R.W.; Al-Zyoud, F. A Sustainable Approach to In Vitro Propagation and Conservation of Salvia dominica L.: A Wild Medicinal Plant from Jordan. Sustainability 2023, 15, 14218. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability 2023, 15, 4025. [Google Scholar] [CrossRef]
- Dobránszki, J.; Mendler-Drienyovszki, N. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. J. Plant Physiol. 2014, 171, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Paiva, R.; Nogueira, R.; Pereira, F.; De Oliveira, L.; Santana, J. Effect of cytokinins on in vitro development of autotrophism and acclimatization of Annona glabra L. Vitr. Cell. Dev. Biol. Plant 2008, 44, 128–135. [Google Scholar] [CrossRef]
- Andreev, I.; Lystvan, K.; Konvalyuk, I.; Kunakh, V.; Twardovska, M. The content of phenolic compounds and flavonoids in in vitro plants and tissue culture of Deschampsia antarctica E. Desv. Fakt. Eksperimental Noi Evol. Org. 2020, 26, 276–281. [Google Scholar] [CrossRef]
- Cruz-Sosa, F.; Franco-Vásquez, A.; Arreguín-Espinosa, R.; Nieto-Camacho, A.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Motolinía-Alcántara, E. Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity. Plants 2023, 12, 1107. [Google Scholar] [CrossRef]
- Jurado-Mañogil, C.; Díaz-Vivancos, P.; Hernández, J.; Piqueras, A.; Barba-Espín, G. Efficient In Vitro Platform for Multiplication, Acclimatization, and Deliver of High-NaCl-Tolerant Clones of the Halophyte Arthrocaulon macrostachyum. J. Plant Growth Regul. 2024, 43, 1631–1641. [Google Scholar] [CrossRef]

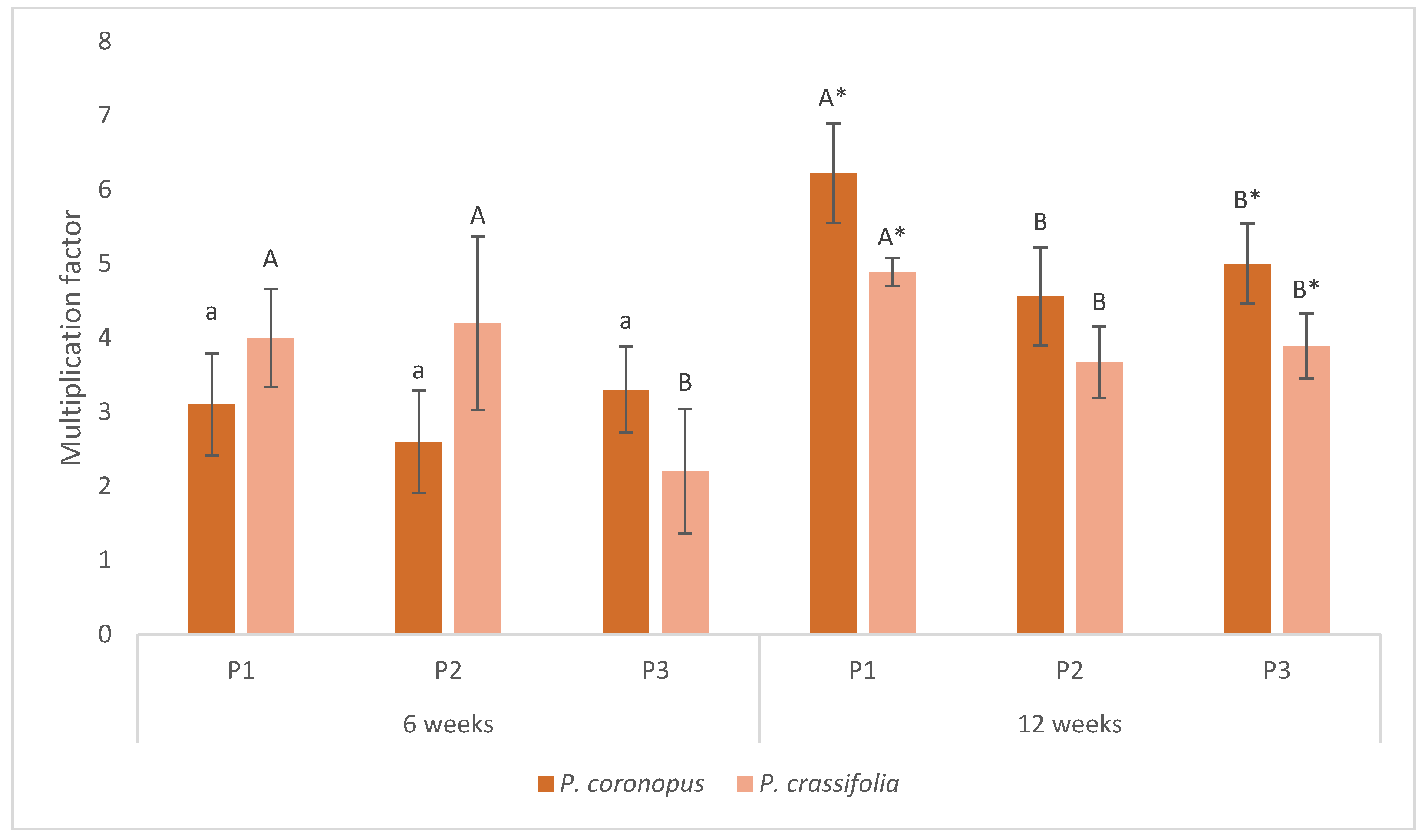

| Medium 1 (P1) | Medium 2 (P2) | Medium 3 (P3) |
|---|---|---|
| MS macronutrients and micronutrients 20 cm3 dm−3 iron (Fe) | ||
| 2 mg dm−3 glycine 0.1 mg dm−3 thiamine 0.5 mg dm−3 pyridoxine 0.5 mg dm−3 nicotinic acid | ||
| 0.5 mg dm−3 kinetin 0.5 mg dm−3 IAA | 1 mg dm−3 BAP 0.5 mg dm−3 IAA | 0.5 mg dm−3 meta-topolin 0.5 mg dm−3 IAA |
| 0.5 g dm−3 mesoinositol 0.65 g dm−3 calcium gluconate 20 g dm−3 saccharose 0.6 g dm−3 activated carbon pH 5.8 7.5 g dm−3 agar | ||
| Plant Species | Time [Weeks] | Culture Medium | Shoots Height [cm] | Roots Length [cm] | Number of Roots | Number of Leaves | Water Content (WC) [%] |
|---|---|---|---|---|---|---|---|
| P. coronopus | 6 | P1 | 11.86 ± 0.87 a | 9.10 ± 1.08 b | 13.22 ± 0.95 b | 22.64 ± 1.99 a | 93.70 ± 0.94 a |
| P2 | 9.72 ± 0.90 b | 10.42 ± 0.85 b | 13.14 ± 1.02 b | 17.67 ± 1.47 b | 95.78 ± 1.02 a | ||
| P3 | 10.23 ± 0.31 b | 11.27 ± 0.48 a | 15.98 ± 0.82 a | 21.5 ± 1.67 a | 95.48 ± 1.34 a | ||
| 12 | P1 | 11.81 ± 0.81 A | 7.67 ± 0.60 C | 9.36 ± 0.56 B | 31.00 ± 2.08 A* | 93.91 ± 0.75 A | |
| P2 | 8.78 ± 0.77 B | 10.5 ± 0.67 B | 11.28 ± 1.24 B | 24.20 ± 3.27 B* | 94.70 ± 0.98 A | ||
| P3 | 10.64 ± 1.37 A | 12.24 ± 0.94 A | 17.44 ± 1.42 A | 22.14 ± 2.38 B | 93.65 ± 1.14 A | ||
| P. crassifolia | 6 | P1 | 12.50 ± 1.70 b | 11.43 ± 1.20 a* | 15.46 ± 2.06 b | 24.40 ± 3.29 a* | 95.26 ± 1.52 a |
| P2 | 8.79 ± 1.23 c | 10.92 ± 0.57 a | 20.08 ± 1.94 a* | 16.14 ± 0.83 b | 96.04 ± 1.68 a | ||
| P3 | 16.73 ± 0.44 a* | 11.21 ± 1.04 a | 17.14 ± 1.78 ab | 20.42 ± 2.10 a | 93.68 ± 0.98 a | ||
| 12 | P1 | 13.93 ± 0.89 B* | 15.17 ± 1.22 B* | 16.32 ± 1.14 B* | 18.00 ± 2.30 BA | 93.55 ± 1.18 A | |
| P2 | 12.81 ± 1.09 B* | 13.57 ± 2.04 B* | 24.5 ± 2.22 A* | 16.11 ± 1.84 B | 93.82 ± 2.02 A | ||
| P3 | 19.53 ± 2.92 A* | 19.14 ± 1.54 A* | 21.62 ± 2.04 A* | 23.75 ± 1.79 A | 92.26 ± 1.44 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koźmińska, A.; Kocot, D.; Kaleta, K. In Vitro Culture Initiation and Micropropagation Optimization of Plantago Halophytes: A Sustainable Approach to Exploring Valuable Plant Species. Sustainability 2025, 17, 7471. https://doi.org/10.3390/su17167471
Koźmińska A, Kocot D, Kaleta K. In Vitro Culture Initiation and Micropropagation Optimization of Plantago Halophytes: A Sustainable Approach to Exploring Valuable Plant Species. Sustainability. 2025; 17(16):7471. https://doi.org/10.3390/su17167471
Chicago/Turabian StyleKoźmińska, Aleksandra, Dawid Kocot, and Karolina Kaleta. 2025. "In Vitro Culture Initiation and Micropropagation Optimization of Plantago Halophytes: A Sustainable Approach to Exploring Valuable Plant Species" Sustainability 17, no. 16: 7471. https://doi.org/10.3390/su17167471
APA StyleKoźmińska, A., Kocot, D., & Kaleta, K. (2025). In Vitro Culture Initiation and Micropropagation Optimization of Plantago Halophytes: A Sustainable Approach to Exploring Valuable Plant Species. Sustainability, 17(16), 7471. https://doi.org/10.3390/su17167471







