Phosphorus Mobilization from Lake Sediments Driven by Silver Carp Fecal Inputs: A Microcosm Study
Abstract
1. Introduction
2. Materials and Methods
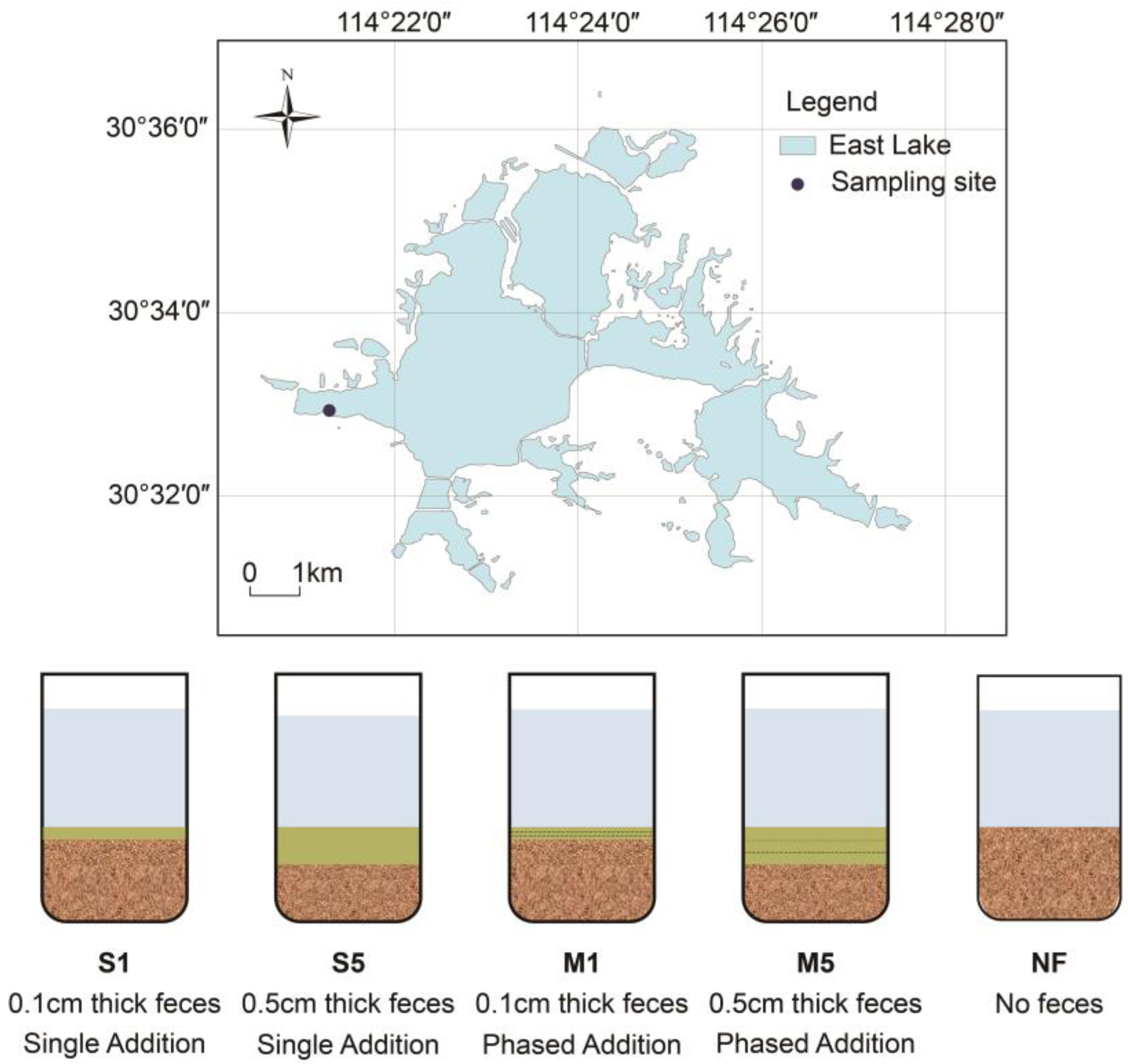
2.1. Collection of Fish Feces
2.2. Experimental Setup
2.3. Sampling and Analysis
2.4. High-Throughput Sequencing
2.5. Data Analyses
2.5.1. P Diffusion Flux on SWI
2.5.2. Statistical Analyses
3. Results
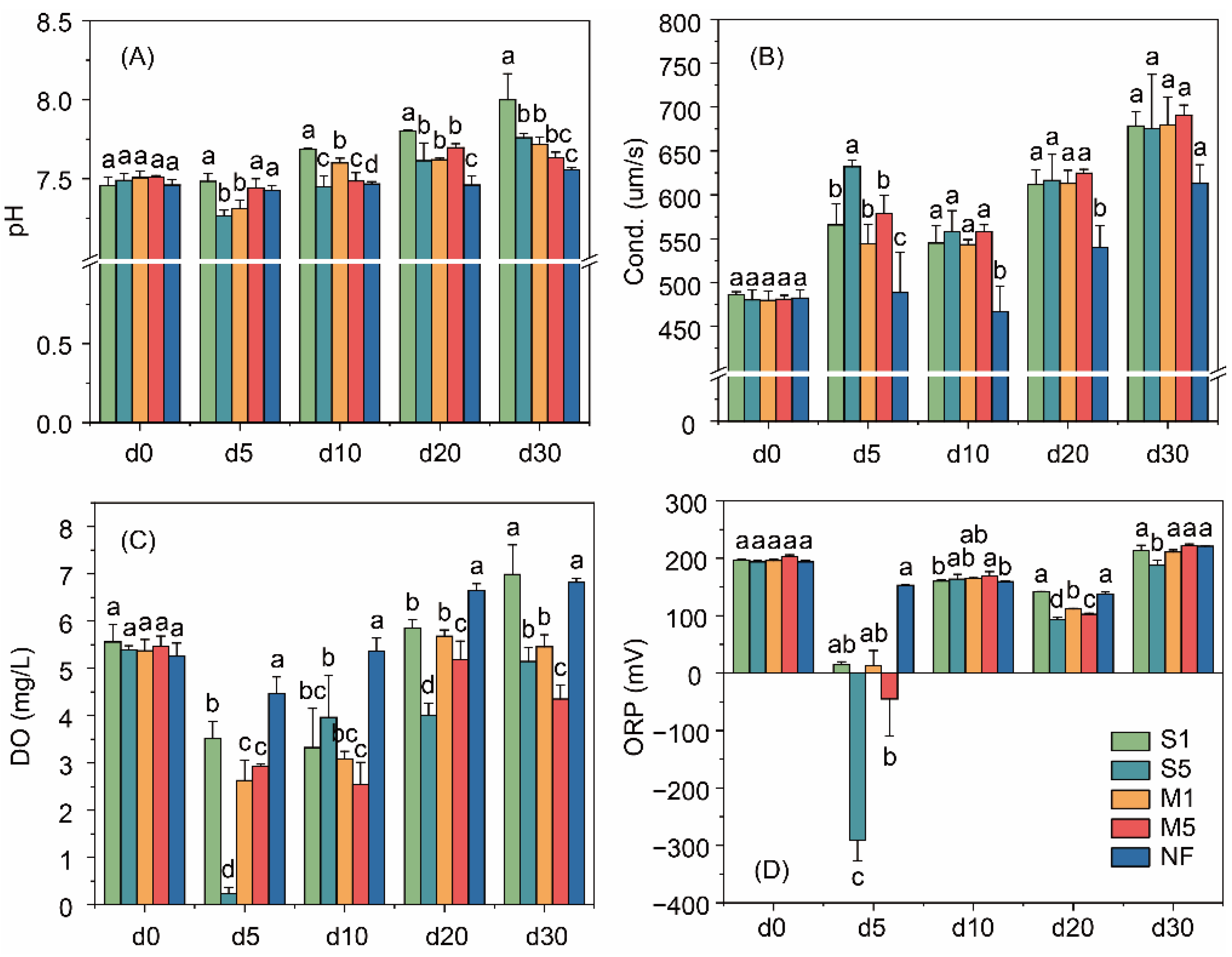
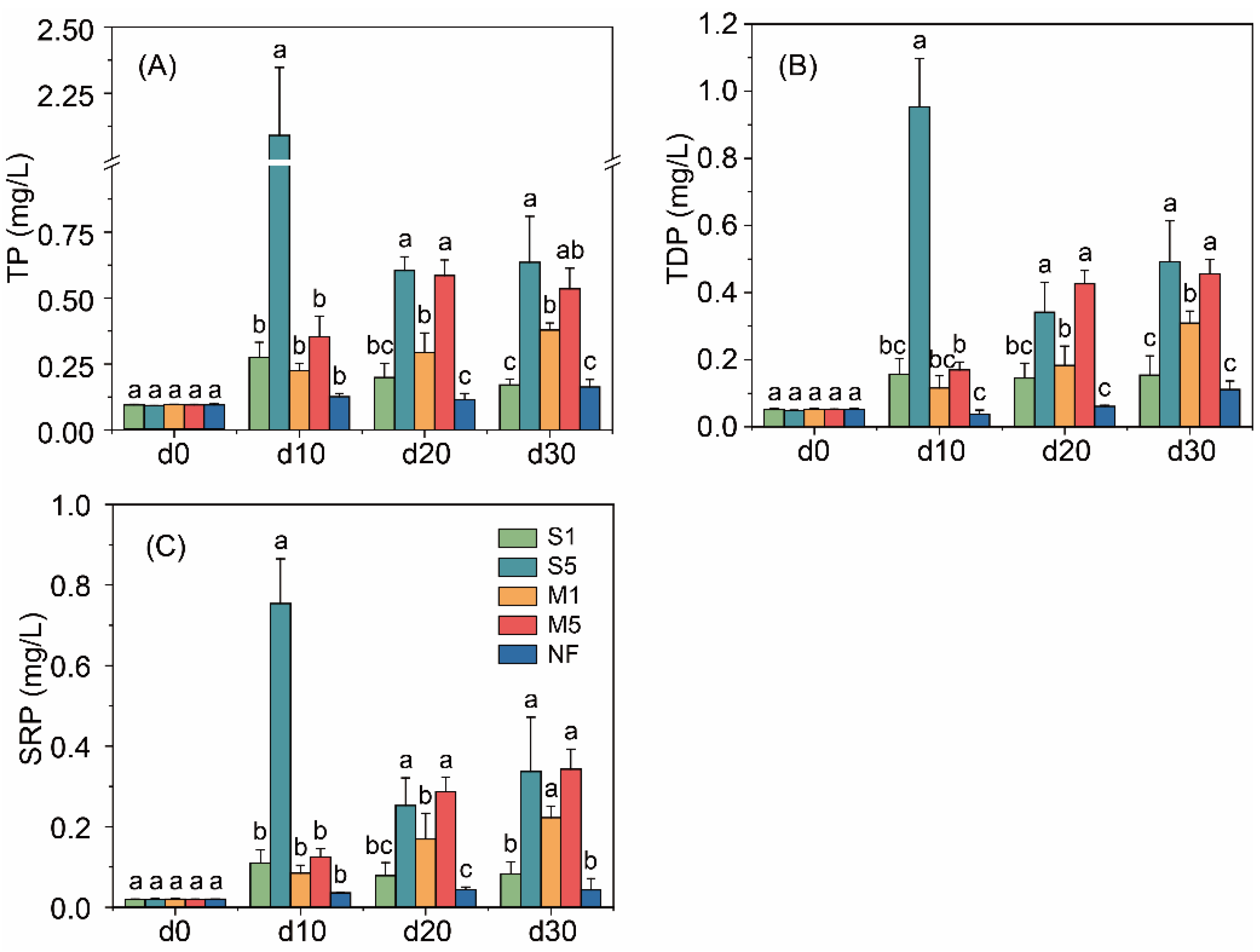
3.1. Changes of the Overlying Water Properties
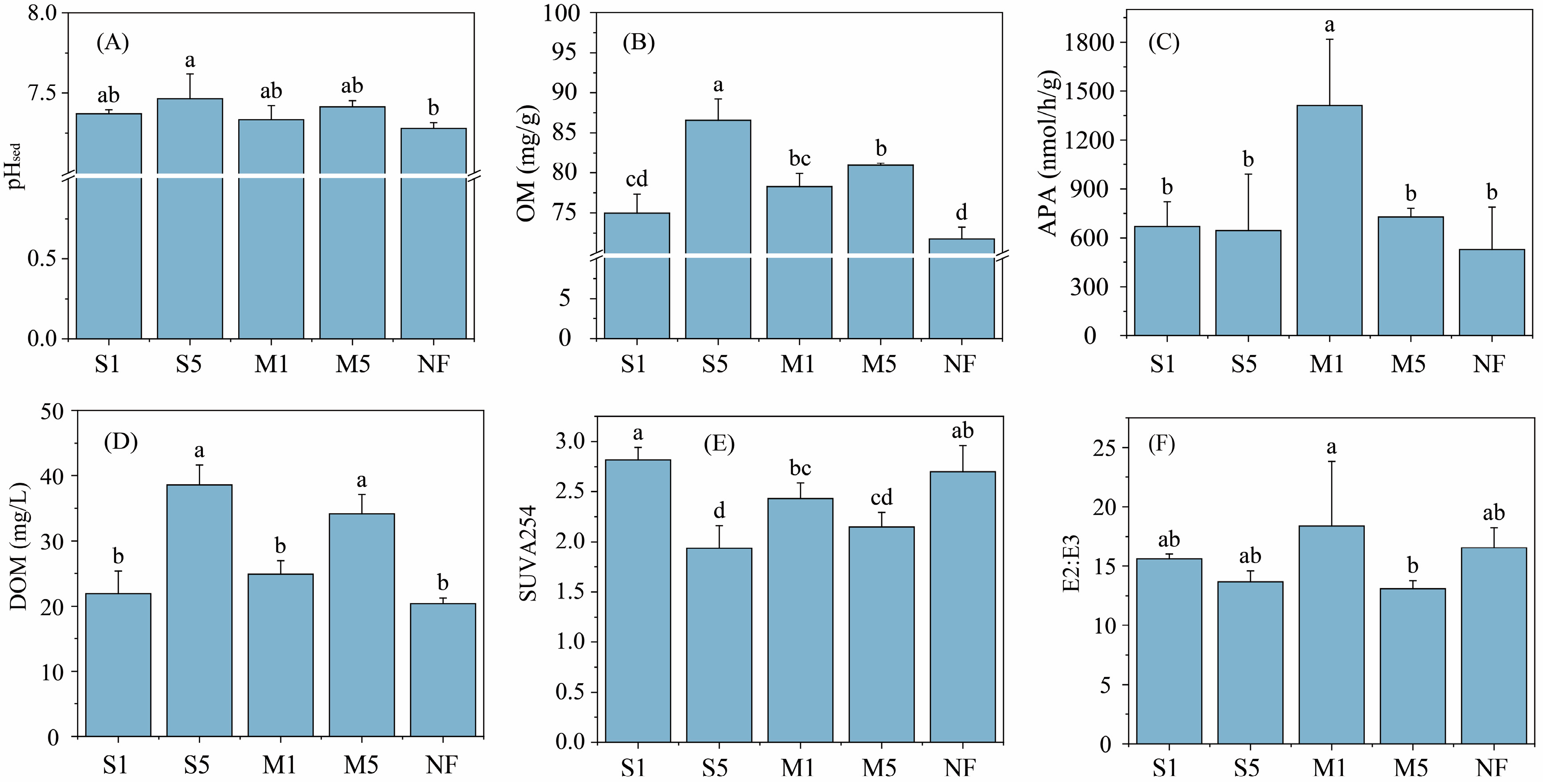
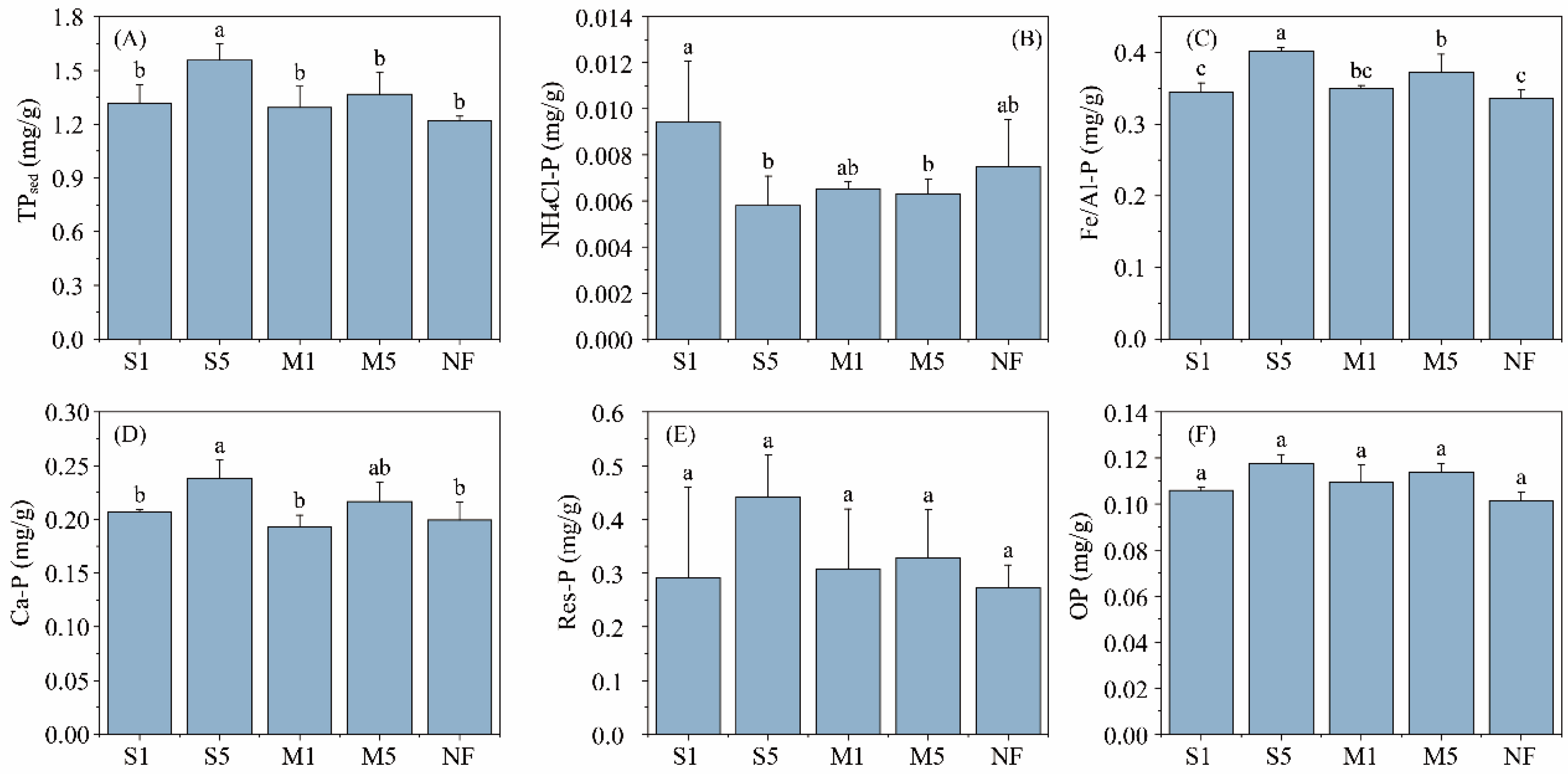
3.2. Changes of the Sediment Properties
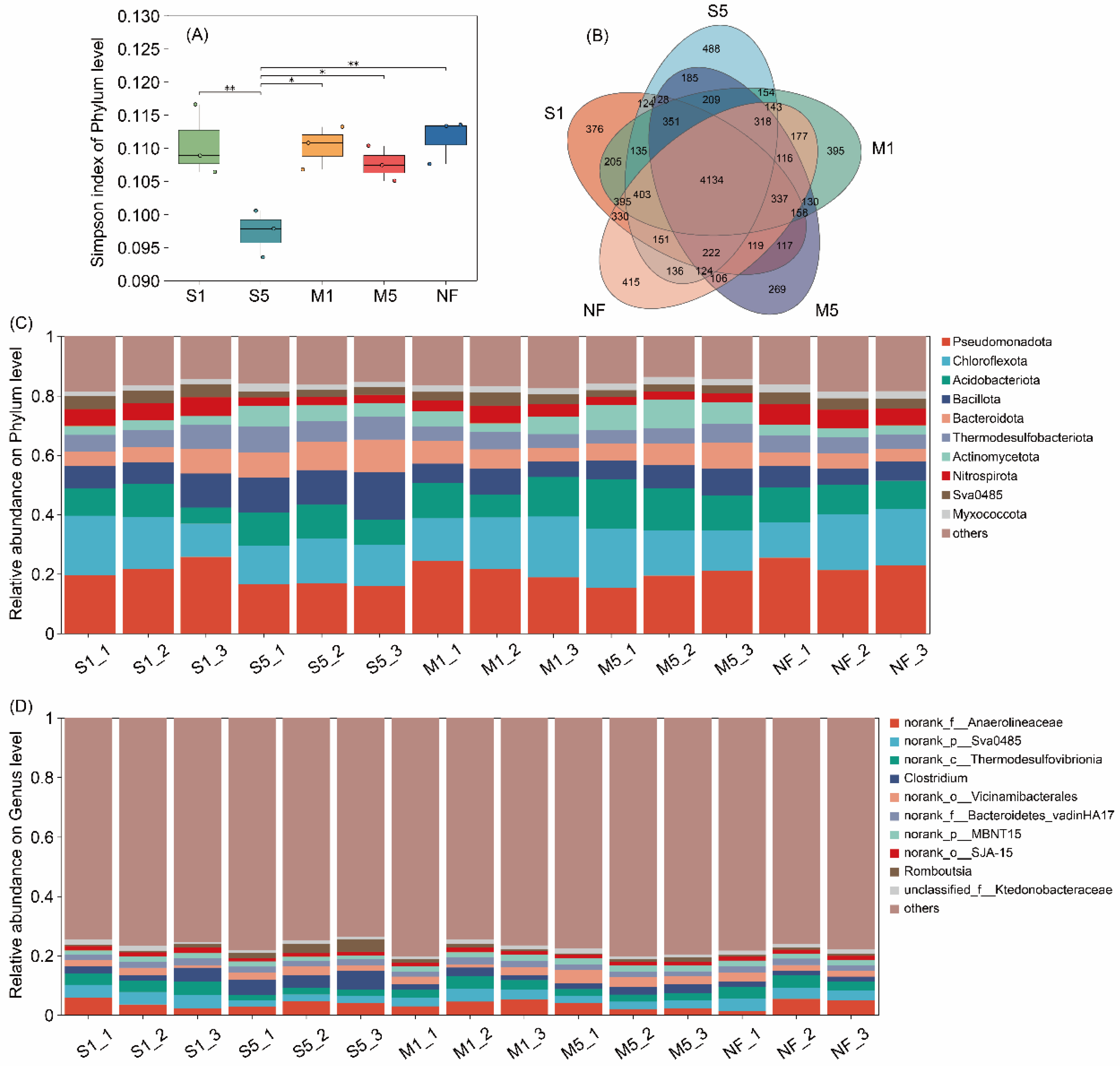
3.3. Sediment Bacterial Communities
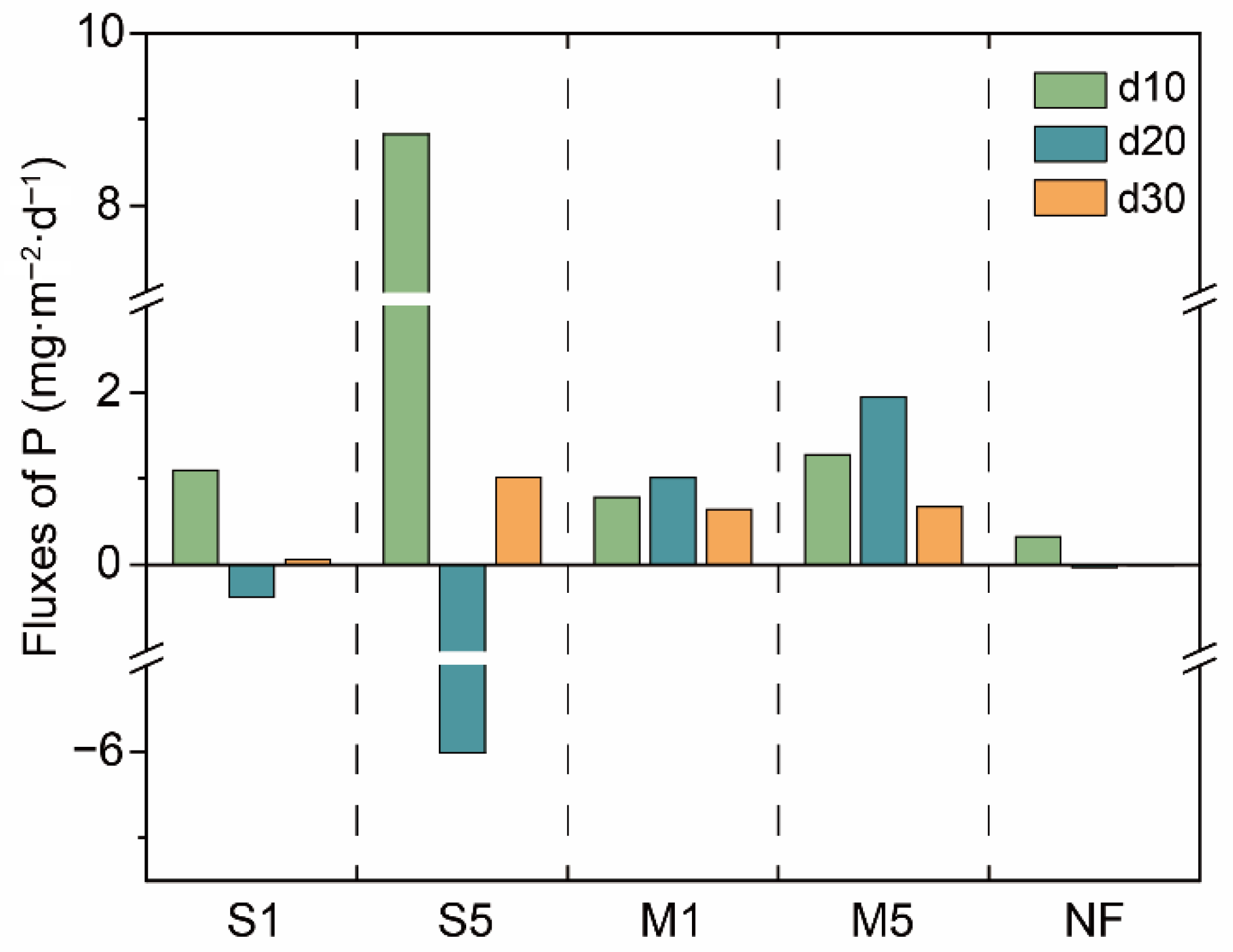
3.4. P Release Flux from the Sediment
4. Discussion
4.1. Effects of Fecal Input on Overlying Water
4.2. Effects of Fecal Input on Sediment Properties and P Fractions
4.3. Effects on Sediment Microbial Communities
4.4. Mechanism of Sediment P Release
4.5. Implications for Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Massey, I.Y.; Al Osman, M.; Yang, F. An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Rev. 2022, 41, 326–346. [Google Scholar] [CrossRef]
- Yi, C.; Guo, L.; Ni, L.; Yin, C.; Wan, J.; Yuan, C. Biomanipulation in mesocosms using silver carp in two Chinese lakes with distinct trophic states. Aquaculture 2016, 452, 233–238. [Google Scholar] [CrossRef]
- Jančula, D.; Míkovcová, M.; Adámek, Z.; Maršálek, B. Changes in the photosynthetic activity of Microcystis colonies after gut passage through Nile tilapia (Oreochromis niloticus) and silver carp (Hypophthalmichthys molitrix). Aquac. Res. 2008, 39, 311–314. [Google Scholar] [CrossRef]
- Wang, J.; Beusen, A.H.W.; Liu, X.; Bouwman, A.F. Aquaculture Production is a Large, Spatially Concentrated Source of Nutrients in Chinese Freshwater and Coastal Seas. Environ. Sci. Technol. 2020, 54, 1464–1474. [Google Scholar] [CrossRef]
- Saba, G.K.; Burd, A.B.; Dunne, J.P.; Hernández-León, S.; Martin, A.H.; Rose, K.A.; Salisbury, J.; Steinberg, D.K.; Trueman, C.N.; Wilson, R.W.; et al. Toward a better understanding of fish-based contribution to ocean carbon flux. Limnol. Oceanogr. 2021, 66, 1639–1664. [Google Scholar] [CrossRef]
- Li, H.; Song, C.-L.; Cao, X.-Y.; Zhou, Y.-Y. The phosphorus release pathways and their mechanisms driven by organic carbon and nitrogen in sediments of eutrophic shallow lakes. Sci. Total Environ. 2016, 572, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hu, X.; Jiang, J.; Hu, J.; Zhu, C.; Zhou, S. Phosphorus sorption—Desorption behaviors in the sediments cultured with Hydrilla verticillata and Scripus triqueter as revealed by phosphorus fraction and dissolved organic matter. Chemosphere 2021, 271, 129549. [Google Scholar] [CrossRef]
- Kristensen, E. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 2000, 426, 1–24. [Google Scholar] [CrossRef]
- Kibria, G.; Nugegoda, D.; Fairclough, R.; Lam, P. The nutrient content and the release of nutrients from fish food and faeces. Hydrobiologia 1997, 357, 165–171. [Google Scholar] [CrossRef]
- Montanhini Neto, R.; Ostrensky, A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac. Res. 2015, 46, 1309–1322. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.; Geng, Y.; Zhu, Y.; Wu, Y.; Wu, X.; Li, G. Changes of vertical distribution of Microcystis colonies driven by short-term rainfall: Disappearance and reformation of surface bloom. J. Environ. Manag. 2024, 369, 122295. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Ribeiro, D.C.; Martins, G.; Nogueira, R.; Cruz, J.V.; Brito, A.G. Phosphorus fractionation in volcanic lake sediments (Azores—Portugal). Chemosphere 2008, 70, 1256–1263. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, S.; Cui, L.; He, J.; Fang, Y.; Premarathne, C.; Li, L.; Wu, Y.; Huang, X.; Kumar, M. Sand supplementation favors tropical seagrass Thalassia hemprichii in eutrophic bay: Implications for seagrass restoration and management. BMC Plant Biol. 2022, 22, 296. [Google Scholar] [CrossRef]
- Dean, W.E. Determination of carbonate and organic-matter in calcareous sediments and sedimentary-rocks by loss on ignition comparison with other methods. J. Sediment. Petrol. 1974, 44, 242–248. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; He, Z.; Bing, X.; Wang, K.; Ma, H.; Liu, F.; Ding, J.; Wei, J. Multiple spectral comparison of dissolved organic matter in the drainage basin of a reservoir in Northeast China: Implication for the interaction among organic matter, iron, and phosphorus. Heliyon 2023, 9, e14797. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, X.; Huang, D.; Liu, W.; Liu, X.; Zhang, J. Phosphorus characteristics and microbial community in the sediment-water-algal system during algal growth. Environ. Sci. Pollut. Res. 2019, 26, 31414–31421. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K.W. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Li, F.; Feng, J.; Zhou, X.; Xu, C.; Haissam Jijakli, M.; Zhang, W.; Fang, F. Impact of rice-fish/shrimp co-culture on the N2O emission and NH3 volatilization in intensive aquaculture ponds. Sci. Total Environ. 2019, 655, 284–291. [Google Scholar] [CrossRef]
- Alvariño, L.; Castañeda, L.; Panduro, G.; da Silva Acioly, T.M.; Viana, D.C.; Iannacone, J. Use of multispecies (Nannochloropsis oceanica, Artemia franciscana, and Arbacia nigra) approach to assess the quality of marine water from Callao Bay, Peru. Sci. Rep. 2025, 15, 1189. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, H.Z.; Liang, X.M.; Wu, S.K. Total phosphorus thresholds for regime shifts are nearly equal in subtropical and temperate shallow lakes with moderate depths and areas. Freshw. Biol. 2014, 59, 1659–1671. [Google Scholar] [CrossRef]
- Ruan, Y. Phosphorus excretion of three species of fish and its role in the recycling of phosphorus in freshwater microcosms. Acta Hydrobiol. Sin. 2005, 29, 55–60. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qi, L.; Xu, M.; Yang, C.; Wang, X. Impact of the migration behavior of mesopelagic fishes on the compositions of dissolved and particulate organic carbon on the northern slope of the South China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 167, 46–54. [Google Scholar] [CrossRef]
- Boers, P.C.M. The influence of pH on phosphate release from lake-sediments. Water Res. 1991, 25, 309–311. [Google Scholar] [CrossRef]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Kuenzler, E.J.; Perras, J.P. Phosphatases of marine algae. Biol. Bull. 1965, 128, 271–284. [Google Scholar] [CrossRef]
- Robison, B.H.; Bailey, T.G. Sinking rates and dissolution of midwater fish fecal matter. Mar. Biol. 1981, 65, 135–142. [Google Scholar] [CrossRef]
- Nowicki, M.; Devries, T.; Siegel, D.A. Quantifying the Carbon Export and Sequestration Pathways of the Ocean’s Biological Carbon Pump. Glob. Biogeochem. Cycles 2022, 36, e2021GB007083. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Fang, F.-T.; Hossen, S.; Zhao, Y.; Chen, Z.-Z.; Zeng, C.; Zhou, M. Impact of wild fish faeces to deep sea dissolved organic carbon as revealed by laboratory incubations. Deep Sea Res. Part I Oceanogr. Res. Pap. 2023, 200, 104148. [Google Scholar] [CrossRef]
- Deng, Y.; Weng, L.; Li, Y.; Ma, J.; Chen, Y. Understanding major NOM properties controlling its interactions with phosphorus and arsenic at goethite-water interface. Water Res. 2019, 157, 372–380. [Google Scholar] [CrossRef]
- Yao, Y.; Li, D.; Chen, Y.; Han, X.; Wang, G.; Han, R. High-resolution characteristics and mechanisms of endogenous phosphorus migration and transformation impacted by algal blooms decomposition. Sci. Total Environ. 2022, 820, 152907. [Google Scholar] [CrossRef]
- Gomez, E.; Durillon, C.; Rofes, G.; Picot, B. Phosphate adsorption and release from sediments of brackish lagoons: pH, O2 and loading influence. Water Res. 1999, 33, 2437–2447. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Li, X.; Zhang, Y.; Zhang, J.; Wang, J.; Mu, J.; Yu, X.; Hui, R. Seasonal and anthropogenic influences on bacterioplankton communities: Ecological impacts in the coastal waters of Qinhuangdao, Northern China. Front. Microbiol. 2024, 15, 1431548. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-F.; Zhou, X.-P. Characterization of phosphorus-releasing bacteria in a small eutrophic shallow lake, Eastern China. Water Res. 2005, 39, 4623–4632. [Google Scholar] [CrossRef]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Liang, B.; Wang, L.-Y.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. AMB Express 2015, 5, 37. [Google Scholar] [CrossRef]
- Begmatov, S.; Beletsky, A.V.; Dedysh, S.N.; Mardanov, A.V.; Ravin, N.V. Genome analysis of the candidate phylum MBNT15 bacterium from a boreal peatland predicted its respiratory versatility and dissimilatory iron metabolism. Front. Microbiol. 2022, 13, 951761. [Google Scholar] [CrossRef]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.-Q.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F.; et al. Genome-Resolved Metagenomics Reveals Distinct Phosphorus Acquisition Strategies between Soil Microbiomes. mSystems 2022, 7, e01107-21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, D.; Liu, S.; Zhang, Y.; Yan, X.; Song, X.; Li, Z.; Li, B.; Shan, S.; Zhu, Y.; et al. Long-term effects of dead algal deposition on sediment surfaces: Behavior of endogenous phosphorus release in sediments. Water Res. 2025, 268, 122742. [Google Scholar] [CrossRef]
- Vuillemin, A.; Horn, F.; Friese, A.; Winkel, M.; Alawi, M.; Wagner, D.; Henny, C.; Orsi, W.D.; Crowe, S.A.; Kallmeyer, J. Metabolic potential of microbial communities from ferruginous sediments. Environ. Microbiol. 2018, 20, 4297–4313. [Google Scholar] [CrossRef]
- Lehtoranta, J.; Ekholm, P.; Pitkänen, H. Coastal Eutrophication Thresholds: A Matter of Sediment Microbial Processes. AMBIO J. Hum. Environ. 2009, 38, 303–308. [Google Scholar] [CrossRef]
- Yao, Y.; Li, D.; Chen, Y.; Liu, H.; Wang, G.; Han, R. High-resolution distribution of internal phosphorus release by the influence of harmful algal blooms (HABs) in Lake Taihu. Environ. Res. 2021, 201, 111525. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Zhang, Y.; Zhu, Y.; Kong, M.; Xu, X.; Wang, Y.; Huang, T. Effects of Plagiogathops micrloepis Bleeker, Hypophthalmichthys molitrix and Aristichthys nobilis polyculture on water environment and nitrogen migration and transformation. China Environ. Sci. 2019, 39, 1181–1188. [Google Scholar]
- Guo, Y.; Gao, Y.; Zhang, Y.; Sun, L.; He, D.; Wu, D. Effect of Xenocypris microlepis on feces and microcystis activity from microcystis-dietary silver carp and bighead carp. China Environ. Sci. 2016, 36, 3784–3792. [Google Scholar]
- Saba, G.K.; Steinberg, D.K. Abundance, Composition and Sinking Rates of Fish Fecal Pellets in the Santa Barbara Channel. Sci. Rep. 2012, 2, 716. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.A.; Alperin, M.J. The efficacy of Phoslock® in reducing internal phosphate loading varies with bottom water oxygenation. Water Res. X 2021, 11, 100095. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Chen, X.; Cheng, H.; Jia, J.; Li, X.; Hu, S.; Chen, X.; Wu, C. Phosphorus Mobilization from Lake Sediments Driven by Silver Carp Fecal Inputs: A Microcosm Study. Sustainability 2025, 17, 7468. https://doi.org/10.3390/su17167468
Lu S, Chen X, Cheng H, Jia J, Li X, Hu S, Chen X, Wu C. Phosphorus Mobilization from Lake Sediments Driven by Silver Carp Fecal Inputs: A Microcosm Study. Sustainability. 2025; 17(16):7468. https://doi.org/10.3390/su17167468
Chicago/Turabian StyleLu, Shenghong, Xin Chen, Huaqiang Cheng, Jia Jia, Xin Li, Shenghua Hu, Xiaofei Chen, and Chenxi Wu. 2025. "Phosphorus Mobilization from Lake Sediments Driven by Silver Carp Fecal Inputs: A Microcosm Study" Sustainability 17, no. 16: 7468. https://doi.org/10.3390/su17167468
APA StyleLu, S., Chen, X., Cheng, H., Jia, J., Li, X., Hu, S., Chen, X., & Wu, C. (2025). Phosphorus Mobilization from Lake Sediments Driven by Silver Carp Fecal Inputs: A Microcosm Study. Sustainability, 17(16), 7468. https://doi.org/10.3390/su17167468





