Quantifying the Environmental Performance of the Oyster (Crassostrea gigas) Supply Chain: A Life Cycle Assessment in Dalian, China
Abstract
1. Introduction
2. Materials and Methods
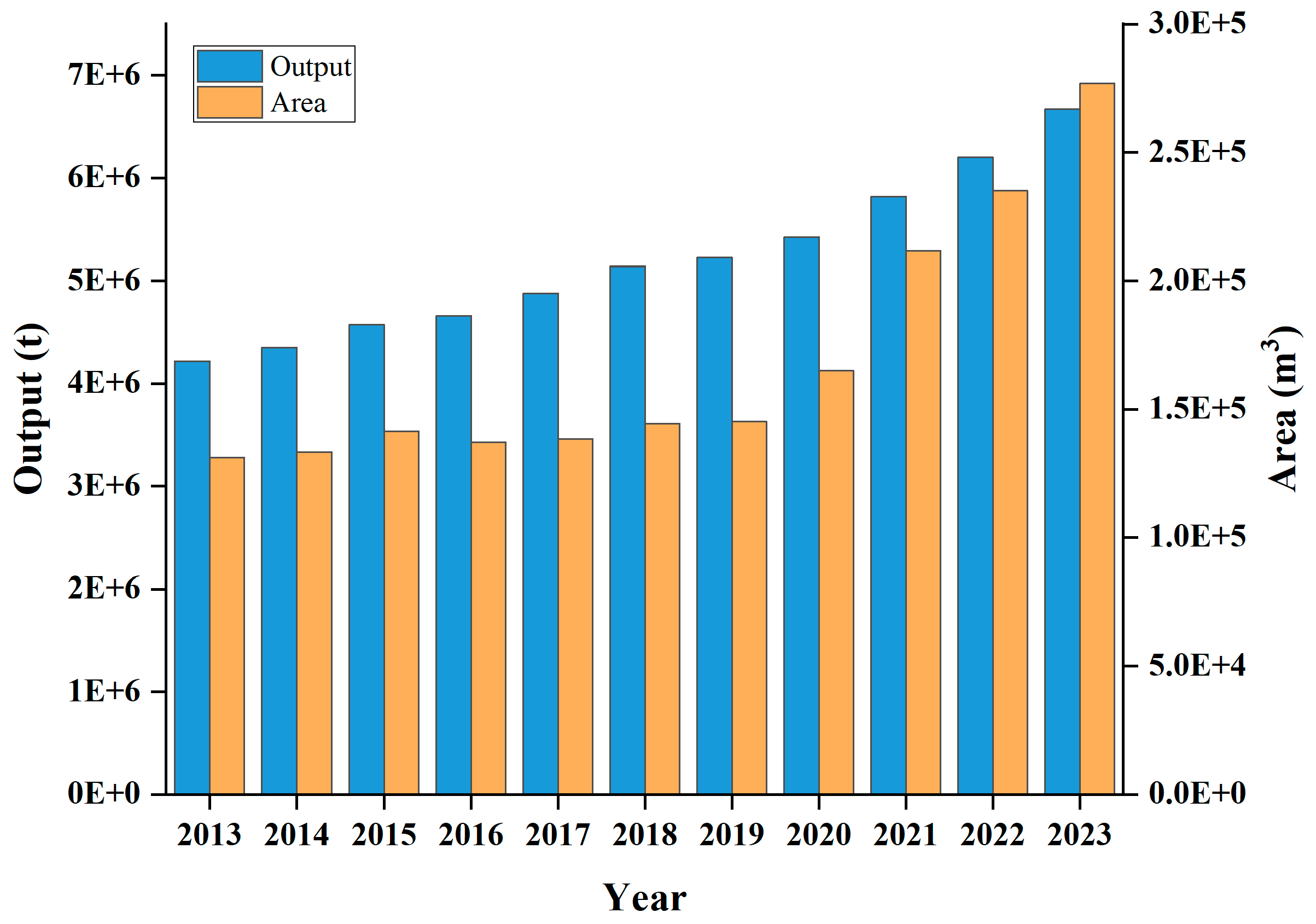
2.1. Description of Case Study
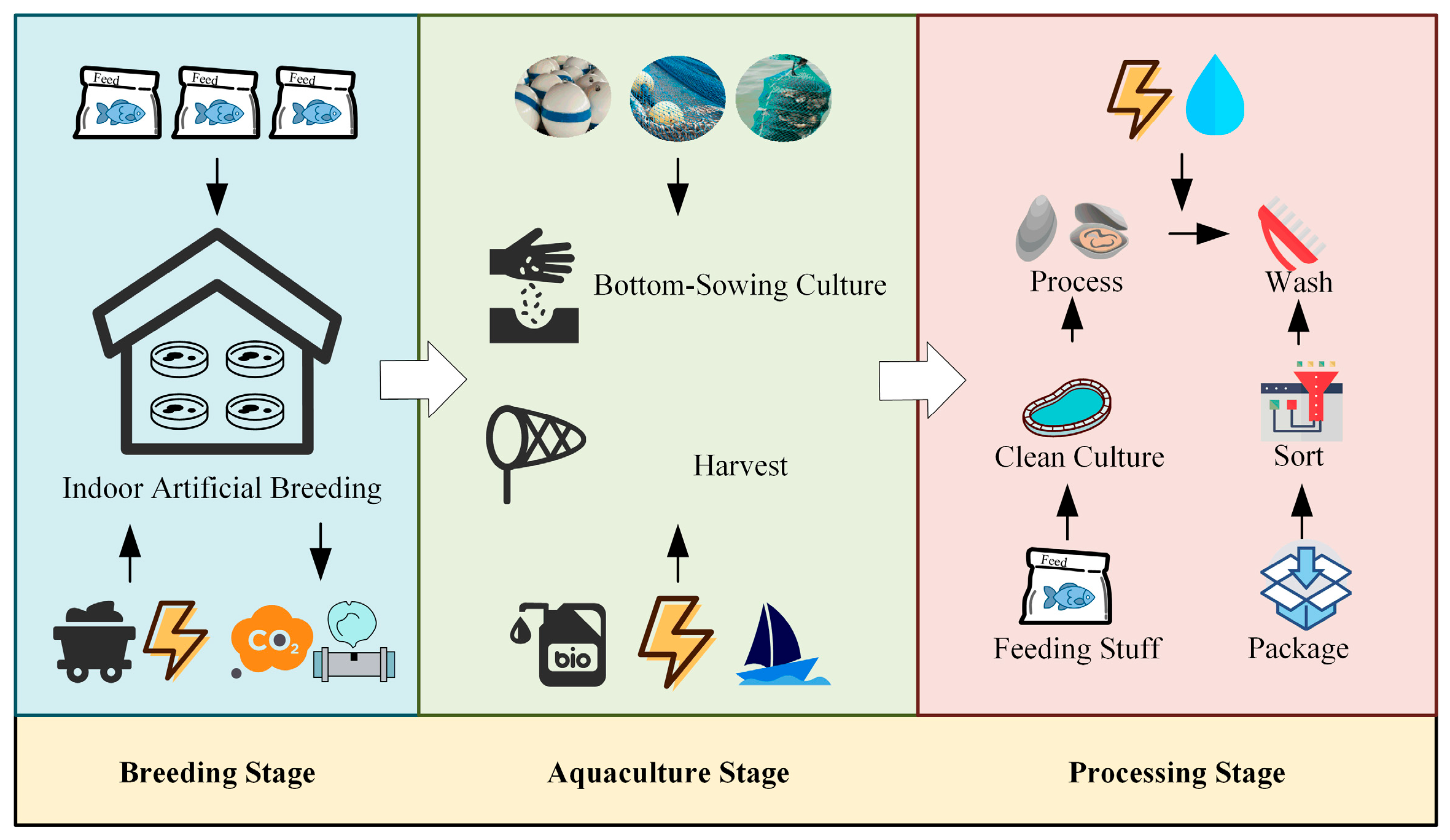
2.1.1. Breeding Stage (Stage 1)
2.1.2. Aquaculture Stage (Stage 2)
2.1.3. Processing Stage (Stage 3)
2.2. Life Cycle Assessment
2.2.1. Goal and Scope Definition
2.2.2. Inventory Analysis
2.2.3. Impact Assessment
2.2.4. Analysis of Uncertainty
3. Results
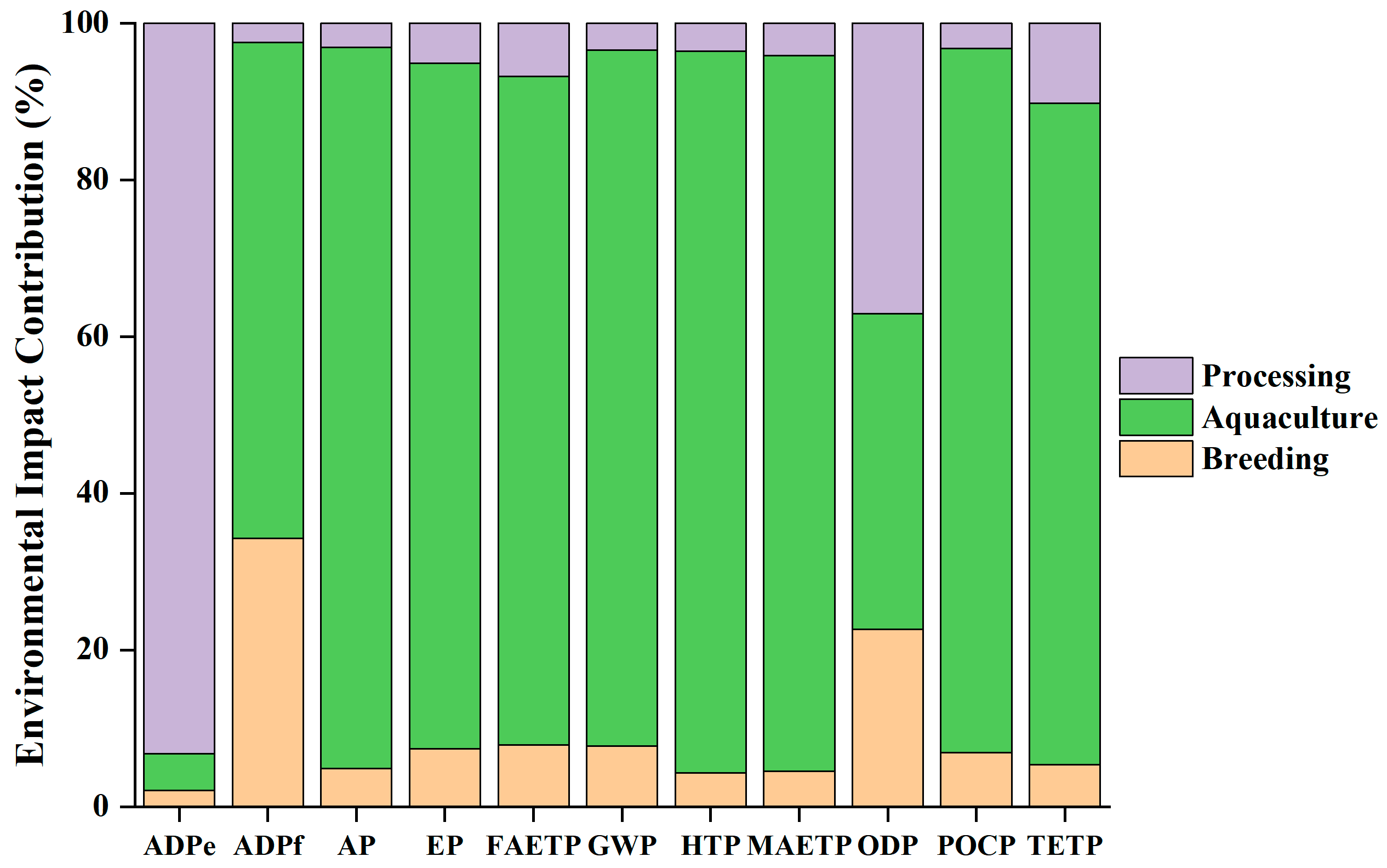
3.1. Characterization Results
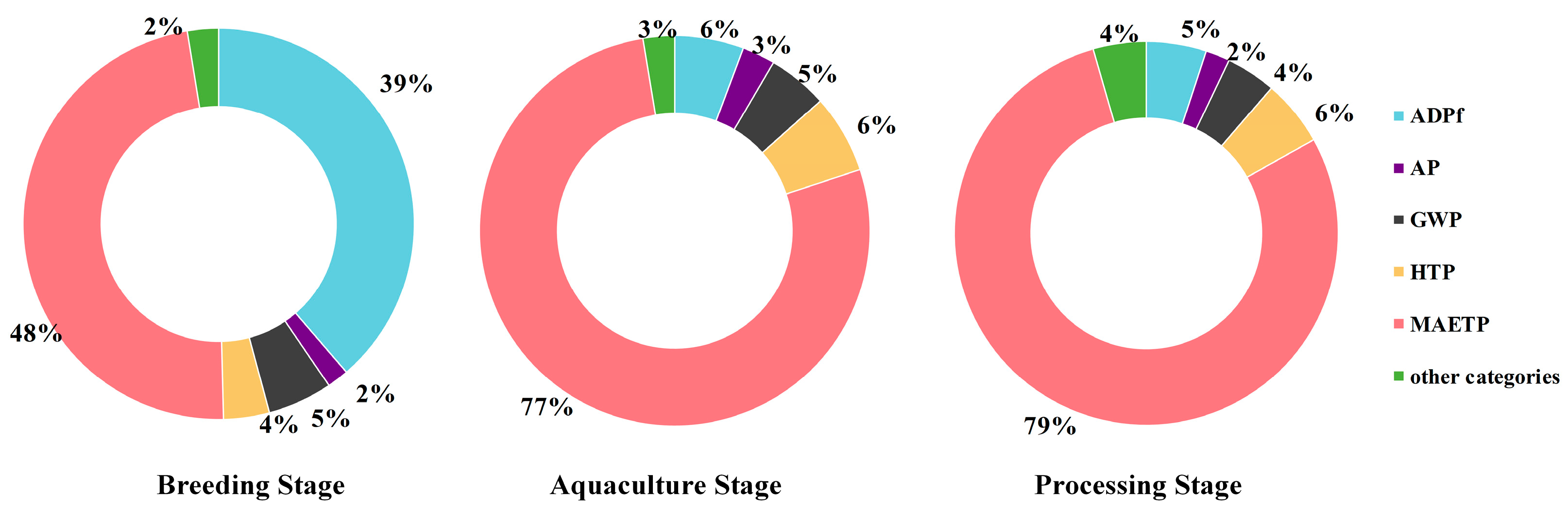
3.2. Normalization Results
3.3. Uncertainty Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024-Blue Transformation in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024; pp. 1, 41. Available online: https://openknowledge.fao.org/items/06690fd0-d133-424c-9673-1849e414543d (accessed on 20 June 2025).
- Norman, R.; Crumlish, M.; Stetkiewicz, S. The importance of fisheries and aquaculture production for nutrition and food security. Rev. Sci. Tech. Off. Int. Epizoot. 2019, 38, 395–407. [Google Scholar] [CrossRef]
- Lin, C.Y.; Dai, G.L.; Liu, Y.; Zhang, M.Q.; Liu, Y.; Jiang, W.; Fu, X.M.; Chen, H.X. Ecological value of mariculture shellfish resources in China: Assessment and management. Mar. Policy 2023, 148, 105406. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.X.; Chen, H.B.; Li, R.M.; Huang, C.X.; Lin, H.; Mu, J.L. Comparative Analysis of Carbon Footprint between Diploid and Triploid Oyster Products Based on Life Cycle Assessment. Fish. Res. 2025, 47, 270–279. [Google Scholar]
- Yin, P.; Xie, S.W.; Zhuang, Z.X.; He, X.S.; Tang, X.P.; Tian, L.X.; Liu, Y.J.; Niu, J. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Jiang, X.L.; Wang, X.Y.; Chen, N.S.; Li, S.L. Toxicological impacts of excessive lithium on largemouth bass (Micropterus salmoides): Body weight, hepatic lipid accumulation, antioxidant defense and inflammation response. Sci. Total Environ. 2022, 841, 156784. [Google Scholar] [CrossRef] [PubMed]
- Bergman, K.; Henriksson, P.; Hornborg, S.; Troell, M.; Borthwick, L.; Jonell, M.; Philis, G.; Ziegler, F. Recirculating Aquaculture Is Possible without Major Energy Tradeoff: Life Cycle Assessment of Warmwater Fish Farming in Sweden. Environ. Sci. Technol. 2020, 54, 16062–16070. [Google Scholar] [CrossRef]
- Hu, N.J.; Liu, C.H.; Chen, Q.; Zhu, L.Q. Life cycle environmental impact assessment of rice-crayfish integrated system: A case study. J. Clean. Prod. 2021, 280, 124440. [Google Scholar] [CrossRef]
- Hou, H.C.; Ren, A.Q.; Yu, L.X.B.; Ma, Z.; Zhang, Y.; Liu, Y. An Environmental Impact Assessment of Largemouth Bass (Micropterus salmoides) Aquaculture in Hangzhou, China. Sustainability 2023, 15, 12368. [Google Scholar] [CrossRef]
- Mcgrath, K.P.; Pelletier, N.L.; Tyedmers, P.H. Life Cycle Assessment of a Novel Closed-Containment Salmon Aquaculture Technology. Environ. Sci. Technol. 2015, 49, 5628–5636. [Google Scholar] [CrossRef]
- Cooney, R.; Tahar, A.; Kennedy, A.; Clifford, E. The dilemma of opportunity in developing a life cycle assessment of emerging aquaculture systems—A case study of a Eurasian perch (Perca fluviatilis) hatchery recirculating aquaculture system. Aquaculture 2021, 536, 736403. [Google Scholar] [CrossRef]
- Mungkung, R.; Aubin, J.; Prihadi, H.T.; Slembrouck, J.; Werf, D.V.G.M.H.; Legendre, M. Life Cycle Assessment for environmentally sustainable aquaculture management: A case study of combined aquaculture systems for carp and tilapia. J. Clean. Prod. 2013, 57, 249–256. [Google Scholar] [CrossRef]
- Tamburini, E.; Turolla, E.; Fano, A.E.; Castaldelli, G. Sustainability of Mussel (Mytilus galloprovincialis) Farming in the Po River Delta, Northern Italy, Based on a Life Cycle Assessment Approach. Sustainability 2020, 12, 3814. [Google Scholar] [CrossRef]
- Chary, K.; Aubin, J.; Sadoul, B.; Fiandrino, A.; Covès, D.; Callieret, D.M. Integrated multi-trophic aquaculture of red drum (Sciaenops ocellatus) and sea cucumber (Holothuria scabra): Assessing bioremediation and life-cycle impacts. Aquaculture 2020, 516, 734621. [Google Scholar] [CrossRef]
- Yu, S.; Mu, Y.T. Evaluation of green development in mariculture: The case of Chinese oyster aquaculture. Aquaculture 2023, 576, 739838. [Google Scholar] [CrossRef]
- Tamburini, E.; Fano, E.A.; Castaldelli, G.; Turolla, E. Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy. Resources 2019, 8, 170. [Google Scholar] [CrossRef]
- Summa, D.; Turolla, E.; Lanzoni, M.; Tamisari, E.; Castaldelli, G.; Tamburini, E. Life Cycle Assessment (LCA) of Two Different Oyster (Crassostrea gigas) Farming Strategies in the Sacca di Goro, Northern Adriatic Sea, Italy. Resources 2023, 12, 62. [Google Scholar] [CrossRef]
- Dias, A.C.; Almeida, C.; Pacheco da Costa, T.; Nunes, M.L.; Marques, A.; Quinteiro, P. Environmental assessment of oyster farming from a life cycle perspective. Sustain. Prod. Consum. 2025, 54, 102–114. [Google Scholar] [CrossRef]
- Ren, A.Q.; Yu, L.X.B.; Zhao, X.T.; Jia, F.; Han, F.F.; Hou, H.C.; Liu, Y. A multi-objective optimization approach for green supply chain network design for the sea cucumber (Apostichopus japonicus) industry. Sci. Total Environ. 2024, 927, 172050. [Google Scholar] [CrossRef] [PubMed]
- Ayer, N.; Martin, S.; Dwyer, L.R.; Gace, L.; Laurin, L. Environmental performance of copper-alloy Net-pens: Life cycle assessment of Atlantic salmon grow-out in copper-alloy and nylon net-pens. Aquaculture 2016, 453, 93–103. [Google Scholar] [CrossRef]
- Maiolo, S.; Forchino, A.A.; Faccenda, F.; Pastres, R. From feed to fork–Life Cycle Assessment on an Italian rainbow trout (Oncorhynchus mykiss) supply chain. J. Clean. Prod. 2021, 289, 125155. [Google Scholar] [CrossRef]
- Denham, C.F.; Howieson, R.J.; Solah, A.V.; Biswas, K.W. Environmental supply chain management in the seafood industry: Past, present and future approaches. J. Clean. Prod. 2015, 90, 82–90. [Google Scholar] [CrossRef]
- Martini, A.; Aguiari, L.; Capoccioni, F.; Martinoli, M.; Napolitano, R.; Pirlo, G.; Tonachella, N.; Pulcini, D. Is Manila Clam Farming Environmentally Sustainable? A Life Cycle Assessment (LCA) Approach Applied to an Italian Ruditapes philippinarum Hatchery. Sustainability 2023, 15, 3237. [Google Scholar] [CrossRef]
- Laso, J.; Margallo, M.; Serrano, M.; Vázquez-Rowe, I.; Avadí, A.; Fullana, P.; Bala, A.; Gazulla, C.; Irabien, Á.; Aldaco, R. Introducing the Green Protein Footprint method as an understandable measure of the environmental cost of anchovy consumption. Sci. Total Environ. 2018, 621, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.F.; Routroy, S.; Thakur, M. Evaluating sustainability of the surimi supply chain in India: A life cycle assessment approach. Int. J. Life Cycle Assess 2021, 26, 1319–1337. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management-Life Cycle Assessment-Principles and Framework. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management–Life Cycle Assessment–Requirements and Guide-Lines. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Hou, H.C.; Shao, S.; Zhang, Y.; Kang, H.; Qin, C.L.; Sun, X.Y.; Zhang, S.S. Life cycle assessment of sea cucumber production: A case study, China. J. Clean. Prod. 2019, 213, 158–164. [Google Scholar] [CrossRef]
- Hou, H.C.; Zhang, Y.; Ma, Z.; Wang, X.L.; Su, P.; Wang, H.H.; Liu, Y. Life cycle assessment of tiger puffer (Takifugu rubripes) farming: A case study in Dalian, China. Sci. Total Environ. 2022, 823, 153522. [Google Scholar] [CrossRef]
- Maurice, B.; Frischknecht, R.; Coelho-Schwirtz, V.; Hungerbuhler, K. Uncertainty analysis in life cycle inventory. Application to the production of electricity with French coal power plants. J. Clean. Prod. 2000, 8, 95–108. [Google Scholar] [CrossRef]
- Yu, M.M.; Liang, J.; Han, J.M.; Wang, L.; Wei, H.Y. Application of Solar Collector and Air-Source Heat Pump System in Aquaculture Seedling Cultivation. J. Aquac. 2022, 43, 66–68. [Google Scholar]
- Salemdeeb, R.; Saint, R.; Clark, W.; Lenaghan, M.; Pratt, K. A pragmatic and industry-oriented framework for data quality assessment of environmental footprint tools. Resour. Environ. Sustain. 2021, 3, 100019. [Google Scholar] [CrossRef]
- Hung, M.L.; Ma, H.W. Quantifying system uncertainty of life cycle assessment based on Monte Carlo simulation. Int. J. Life Cycle Assess 2009, 14, 19–27. [Google Scholar] [CrossRef]
- Jerbi, M.A.; Aubin, J.; Garnaoui, K.; Achour, L.; Kacem, A. Life cycle assessment (LCA) of two rearing techniques of sea bass (Dicentrarchus labrax). Aquacult. Eng. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Gaeta, H.F.; Parolini, M.; Bacenetti, J. Quantification of the environmental impact of lumpfish farming through a life cycle assessment. Aquaculture 2022, 549, 737781. [Google Scholar] [CrossRef]
- Liu, J.Y.; Gui, F.; Zhou, Q.; Cai, H.W.; Xu, K.D.; Zhao, S. Carbon Footprint of a Large Yellow Croaker Mariculture Models Based on Life-Cycle Assessment. Sustainability 2023, 15, 6658. [Google Scholar] [CrossRef]
- Huysveld, S.; Schaubroeck, T.; Meester, D.S.; Sorgeloos, P.; Langenhove, V.H.; Van linden, V.; Dewulf, J. Resource use analysis of Pangasius aquaculture in the Mekong Delta in Vietnam using Exergetic Life Cycle Assessment. J. Clean. Prod. 2013, 51, 225–233. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Hartleb, C.; Rabas, Z.; Hickset, A. Evaluation of environmental and economic implications of a cold-weather aquaponic food production system using life cycle assessment and economic analysis. J. Ind. Ecol. 2022, 26, 862–874. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, Y.; Pettersen, B.J.; Brandão, M.; Ma, X.N.; Røberg, S.; Frostellet, B. Life cycle assessment of recirculating aquaculture systems: A case of Atlantic salmon farming in China. J. Ind. Ecol. 2019, 23, 1077–1086. [Google Scholar] [CrossRef]
- Love, C.D.; Lane, M.R.; Kuehl, M.L.; Hudson, B.; Harding, J.; Clancy, K.; Fry, P.J. Performance and conduct of supply chains for United States farmed oysters. Aquaculture 2020, 515, 734569. [Google Scholar] [CrossRef]
- Chellapandi, P. Development of top-dressing automation technology for sustainable shrimp aquaculture in India. Discov. Sustain. 2021, 2, 26. [Google Scholar] [CrossRef]
- Liu, M.H.; Yan, Z.N.; Huang, C.W.; Lin, Z.H.; Peng, Z.L.; Zhao, C.X.; Zheng, X.F. Temporal dynamics of protist communities and environmental factors in the horizontal flow-polyculture pond aquaculture model of Sinonovacula constricta. Aquacult. Eng. 2024, 107, 102477. [Google Scholar] [CrossRef]
- Abualtaher, M.; Bar, S.E. Review of applying material flow analysis-based studies for a sustainable Norwegian Salmon aquaculture industry. J. Appl. Aquac. 2019, 32, 1–15. [Google Scholar] [CrossRef]
- Lu, X.N.; Cui, Y.L.; Chen, Y.T.; Xiao, Y.P.; Song, X.J.; Gao, F.Z.; Xiang, Y.; Hou, C.C.; Wang, J.; Gan, Q.H.; et al. Sustainable development of microalgal biotechnology in coastal zone for aquaculture and food. Sci. Total Environ. 2021, 780, 146369. [Google Scholar] [CrossRef]
- Shi, L.P.; Song, F.B.; Xing, S.Y.; Zhang, W.W.; Liang, Y.S.; Zhang, K.X.; Sun, J.L.; Luo, J. The muscle nutritional components analysis of golden pompano (Trachinotus blochii) in different mariculture area, growth stages, and genders. Front. Nutr. 2023, 10, 1148687. [Google Scholar] [CrossRef]
- Neofitou, N.; Syvri, R.; Tziantziou, L.; Mente, E.; Vafidis, D. The benthic environmental footprint of aquaculture in the Eastern Mediterranean: Organic vs conventional fish farming. Aquac. Res. 2020, 51, 2698–2710. [Google Scholar] [CrossRef]
- Lal, M.M.; Brown, T.K.; Chand, P.; Pickering, D.T. An assessment of the aquaculture potential of indigenous freshwater food fish of Fiji, Papua New Guinea, Vanuatu, Solomon Islands, Samoa and Tonga as alternatives to farming of tilapia. Rev. Aquac. 2022, 15, 625–644. [Google Scholar] [CrossRef]
- Vo, E.T.T.; Ko, H.; Huh, J.; Park, N. Overview of Solar Energy for Aquaculture: The Potential and Future Trends. Energies 2021, 14, 6923. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.; Li, H.; Tairab, M.A.; Jia, C.Y.; Li, N.; Zhang, Z.T. Design and analysis of the optimal spinning top-shaped buoy for wave energy harvesting in low energy density seas for sustainable marine aquaculture. Ocean Eng. 2022, 255, 111434. [Google Scholar] [CrossRef]
- Son, S.; Jeong, Y. An Automated Fish-Feeding System Based on CNN and GRU Neural Networks. Sustainability 2024, 16, 3675. [Google Scholar] [CrossRef]
- Medina, D.J.; Valencia-Arías, A.; Triana, M.J.; Giraldo, F.L.; Segura-Quijano, F.; Gonzalez-Mancera, A.; Zambrano, F.A.; Quimbayo, J.; Castilloet, E. Open-source low-cost design of a buoy for remote water quality monitoring in fish farming. PLoS ONE 2022, 17, e0270202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.S.; Huang, H.M.; Costello, J.M.; Chu, J.S. Climate change projections show shrinking deep-water ecosystems with implications for biodiversity and aquaculture in the Northwest Pacific. Sci. Total Environ. 2023, 861, 160505. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Asche, F.; Garlock, T. Economics of Aquaculture Policy and Regulation. Annu. Rev. Resour. Econ. 2019, 11, 101–123. [Google Scholar] [CrossRef]
- Chen, Y.H.; Han, M.Q.; Liu, X.H.; Cui, J.C.; Habuer; Gao, X.F. Assessing the environmental and economic impacts of the oyster life cycle under renewable energy expansion. J. Environ. Manag. 2025, 389, 126220. [Google Scholar] [CrossRef]
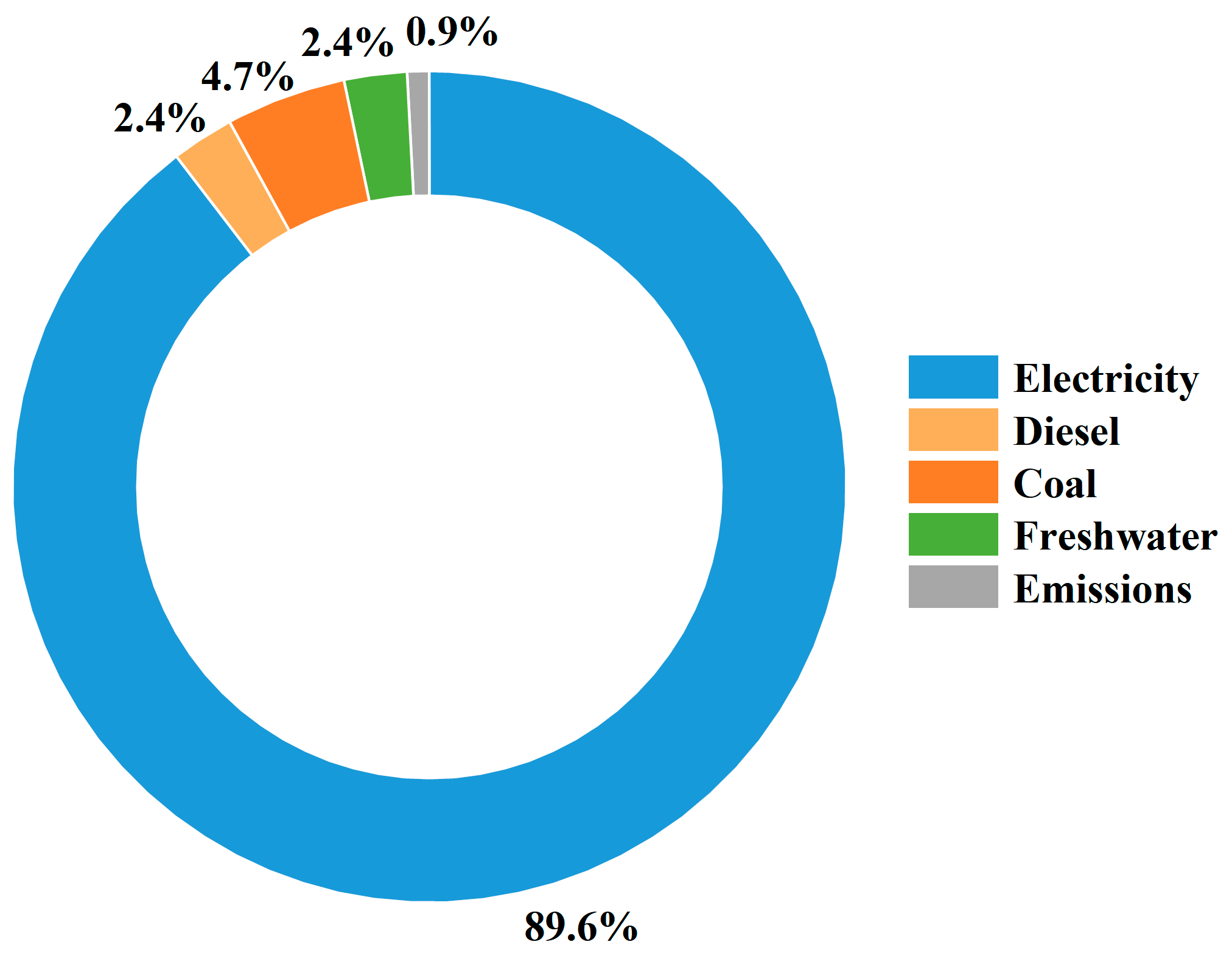
| Input/Output | Substance | Breeding | Unit |
| Input | Electricity | 23.8 | kWh |
| Coal | 200 | kg | |
| Seawater | 111.36 | m3 | |
| Output | CO2 | 0.52 | kg |
| SO2 | 0.0017 | kg | |
| NOX | 0.0015 | kg | |
| Total N | 0.5 | kg | |
| Total P | 0.05 | kg | |
| COD | 1 | kg | |
| Input/Output | Substance | Aquaculture | Unit |
| Input | Electricity | 810 | kWh |
| Diesel | 33 | L | |
| Output | CO2 | 75.9 | kg |
| SO2 | 0.2805 | kg | |
| NOX | 0.2442 | kg | |
| Input/Output | Substance | Processing | Unit |
| Input | Electricity | 15 | kWh |
| Fresh water | 11.13 | m3 |
| Category | Characterization Units | Abbreviation |
|---|---|---|
| Abiotic Depletion Potential (elements) | kg Sb-eq | ADPe |
| Abiotic Depletion Potential (fossil) | MJ | ADPf |
| Acidification Potential | kg SO2-eq | AP |
| Eutrophication Potential | kg Phosphate-eq | EP |
| Freshwater Aquatic Ecotoxicity Potential | kg DCB-eq | FAETP |
| Global Warming Potential | kg CO2-eq | GWP |
| Human Toxicity Potential | kg DCB-eq | HTP |
| Marine Aquatic Ecotoxicity Potential | kg DCB-eq | MAETP |
| Ozone Layer Depletion Potential | kg R11-eq | ODP |
| Photochemical Ozone Creation Potential | kg Ethene-eq | POCP |
| Terrestrial Ecotoxicity Potential | kg DCB-eq | TETP |
| Category | Breeding | Aquaculture | Processing |
|---|---|---|---|
| ADPe | 3.12 × 10−6 | 6.92 × 10−6 | 1.38 × 10−4 |
| ADPf | 5.63 × 103 | 1.04 × 104 | 4.09 × 102 |
| AP | 1.64 × 10−1 | 3.11 × 100 | 1.04 × 10−1 |
| EP | 2.31 × 10−2 | 2.72 × 10−1 | 1.58 × 10−2 |
| FAETP | 1.43 × 10−1 | 1.54 × 100 | 1.23 × 10−1 |
| GWP | 8.74 × 101 | 1.00 × 103 | 3.91 × 101 |
| HTP | 3.74 × 100 | 7.99 × 101 | 3.08 × 100 |
| MAETP | 3.57 × 103 | 7.22 × 104 | 3.27 × 103 |
| ODP | 5.96 × 10−11 | 1.06 × 10−10 | 9.75 × 10−11 |
| POCP | 2.68 × 10−2 | 3.46 × 10−1 | 1.24 × 10−2 |
| TETP | 4.76 × 10−2 | 7.46 × 10−1 | 9.02 × 10−2 |
| Category | Breeding | Aquaculture | Processing |
|---|---|---|---|
| ADPe | 8.64 × 10−15 | 1.92 × 10−14 | 3.82 × 10−13 |
| ADPf | 1.48 × 10−11 | 2.73 × 10−11 | 1.08 × 10−12 |
| AP | 6.85 × 10−13 | 1.30 × 10−11 | 4.36 × 10−13 |
| EP | 1.46 × 10−13 | 1.72 × 10−12 | 9.98 × 10−14 |
| FAETP | 6.06 × 10−14 | 6.53 × 10−13 | 5.20 × 10−14 |
| GWP | 2.05 × 10−12 | 2.37 × 10−11 | 9.00 × 10−13 |
| HTP | 1.45 × 10−12 | 3.10 × 10−11 | 1.19 × 10−12 |
| MAETP | 1.83 × 10−11 | 3.70 × 10−10 | 1.68 × 10−11 |
| ODP | 2.63 × 10−19 | 4.69 × 10−19 | 4.30 × 10−19 |
| POCP | 7.27 × 10−13 | 9.42 × 10−12 | 3.38 × 10−13 |
| TETP | 4.36 × 10−14 | 6.84 × 10−13 | 8.27 × 10−14 |
| Total | 3.83 × 10−11 | 4.78 × 10−10 | 2.13 × 10−11 |
| Stages | Normalization Results (yr) | Monte Carlo Simulation Results | ||
|---|---|---|---|---|
| Confidence Interval 95% (yr) | Mean (yr) | SD (yr) | ||
| Breeding | 3.83 × 10−11 | 3.70 × 10−11–3.95 × 10−11 | 3.83 × 10−11 | 7.77 × 10−13 |
| Aquaculture | 4.78 × 10−10 | 4.62 × 10−10–4.94 × 10−10 | 4.79 × 10−10 | 9.65 × 10−12 |
| Processing | 2.13 × 10−11 | 2.06 × 10−11–2.20 × 10−11 | 2.13 × 10−11 | 4.34 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Han, F.; Song, J.; Jia, F.; Bai, Y.; Ma, Z.; Huo, Z.; Liu, Y. Quantifying the Environmental Performance of the Oyster (Crassostrea gigas) Supply Chain: A Life Cycle Assessment in Dalian, China. Sustainability 2025, 17, 7392. https://doi.org/10.3390/su17167392
Hou H, Han F, Song J, Jia F, Bai Y, Ma Z, Huo Z, Liu Y. Quantifying the Environmental Performance of the Oyster (Crassostrea gigas) Supply Chain: A Life Cycle Assessment in Dalian, China. Sustainability. 2025; 17(16):7392. https://doi.org/10.3390/su17167392
Chicago/Turabian StyleHou, Haochen, Fengfan Han, Jie Song, Fei Jia, Yang Bai, Zhen Ma, Zhongming Huo, and Ying Liu. 2025. "Quantifying the Environmental Performance of the Oyster (Crassostrea gigas) Supply Chain: A Life Cycle Assessment in Dalian, China" Sustainability 17, no. 16: 7392. https://doi.org/10.3390/su17167392
APA StyleHou, H., Han, F., Song, J., Jia, F., Bai, Y., Ma, Z., Huo, Z., & Liu, Y. (2025). Quantifying the Environmental Performance of the Oyster (Crassostrea gigas) Supply Chain: A Life Cycle Assessment in Dalian, China. Sustainability, 17(16), 7392. https://doi.org/10.3390/su17167392






