Carbon Dioxide Fertilization Effects Offset the Vegetation GPP Losses of Woodland Ecosystems Due to Surface Ozone Damage in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Description
2.2. Data Sources
2.2.1. Gridded Meteorological Data
2.2.2. China-Wide LAI Map
2.2.3. Land Cover Map
2.2.4. Site Verification
2.3. Simulation Protocol
2.4. Causality Analysis—SURD
3. Result
3.1. Site Verification of the GPP Simulated by the BEPS_O3 Model
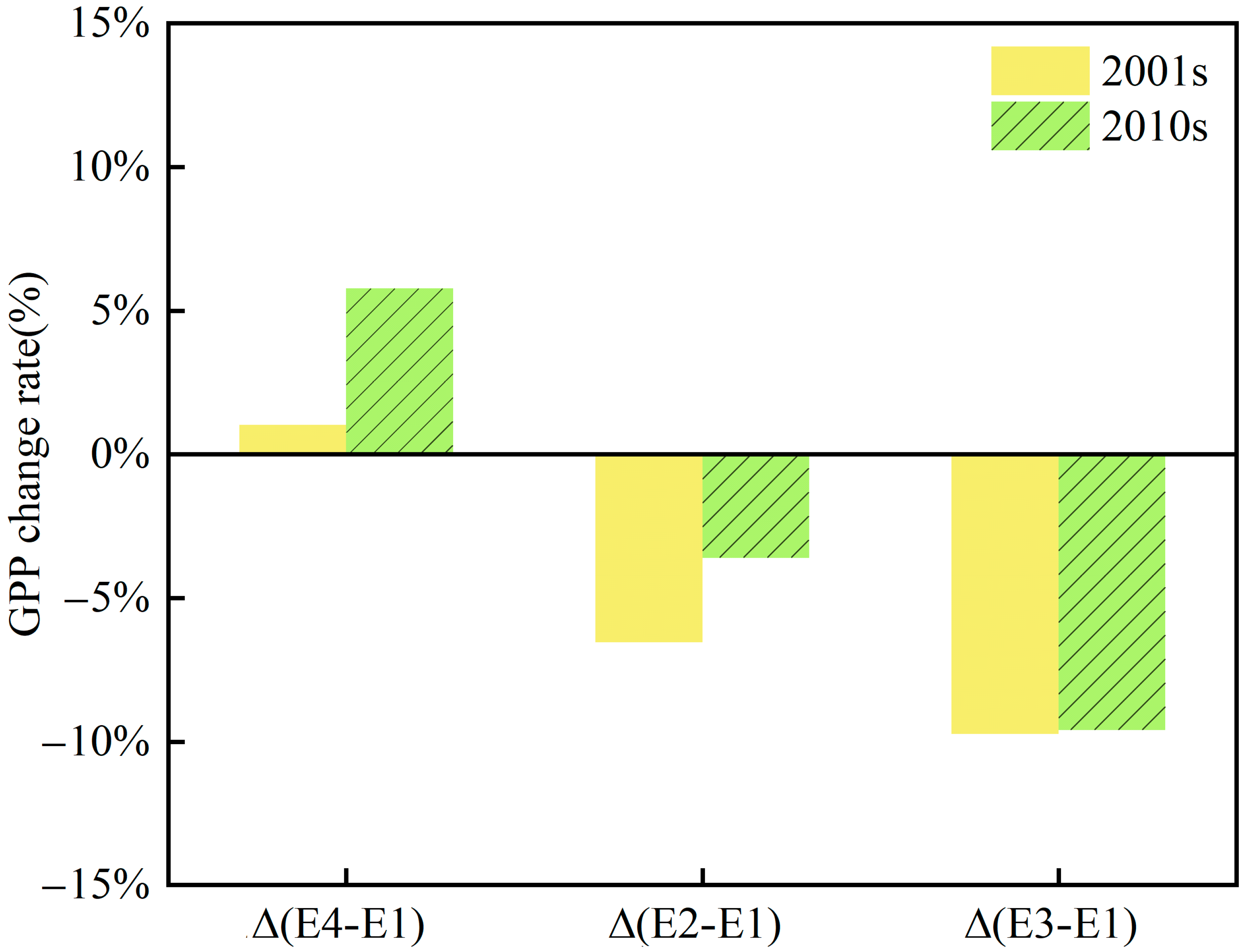
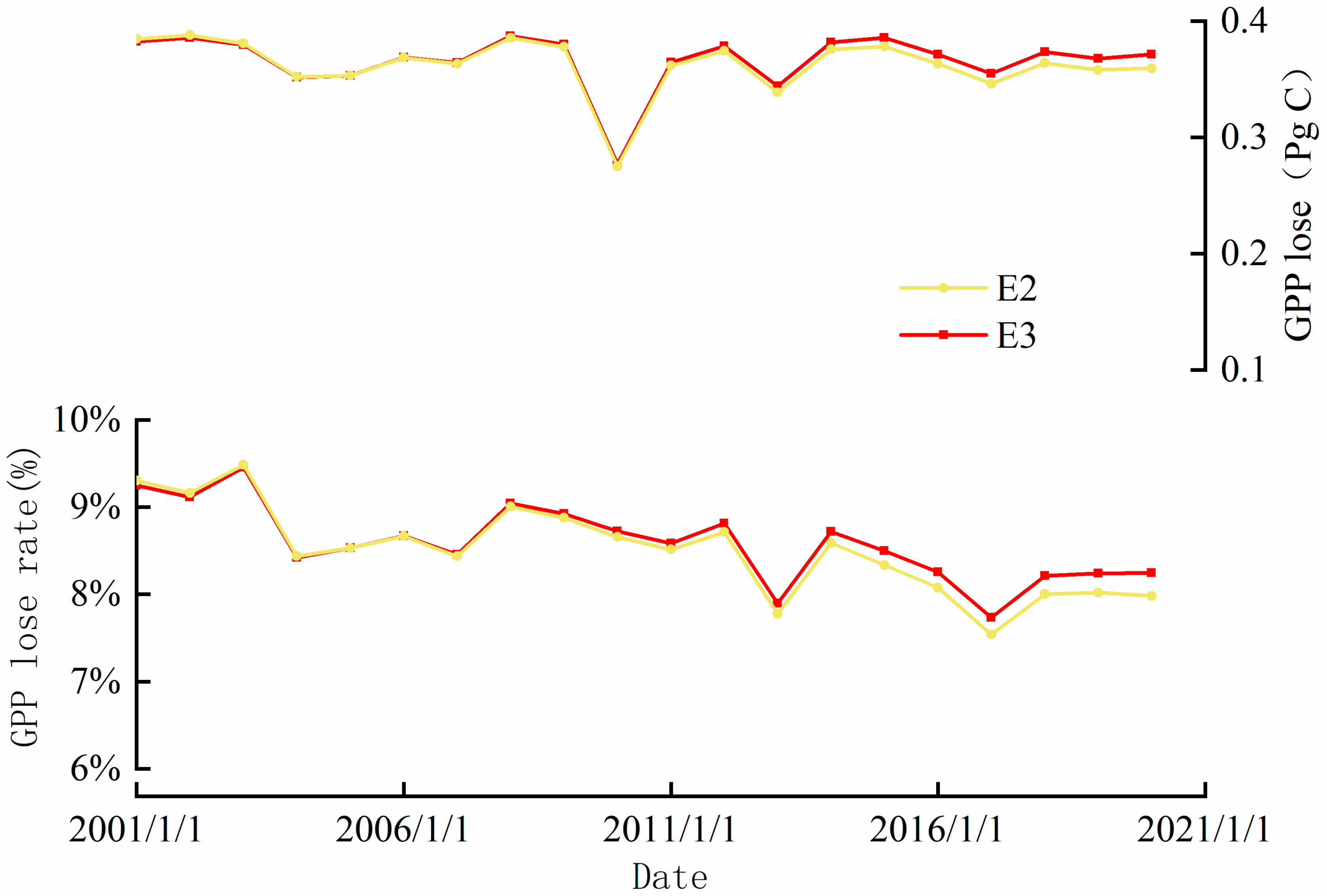
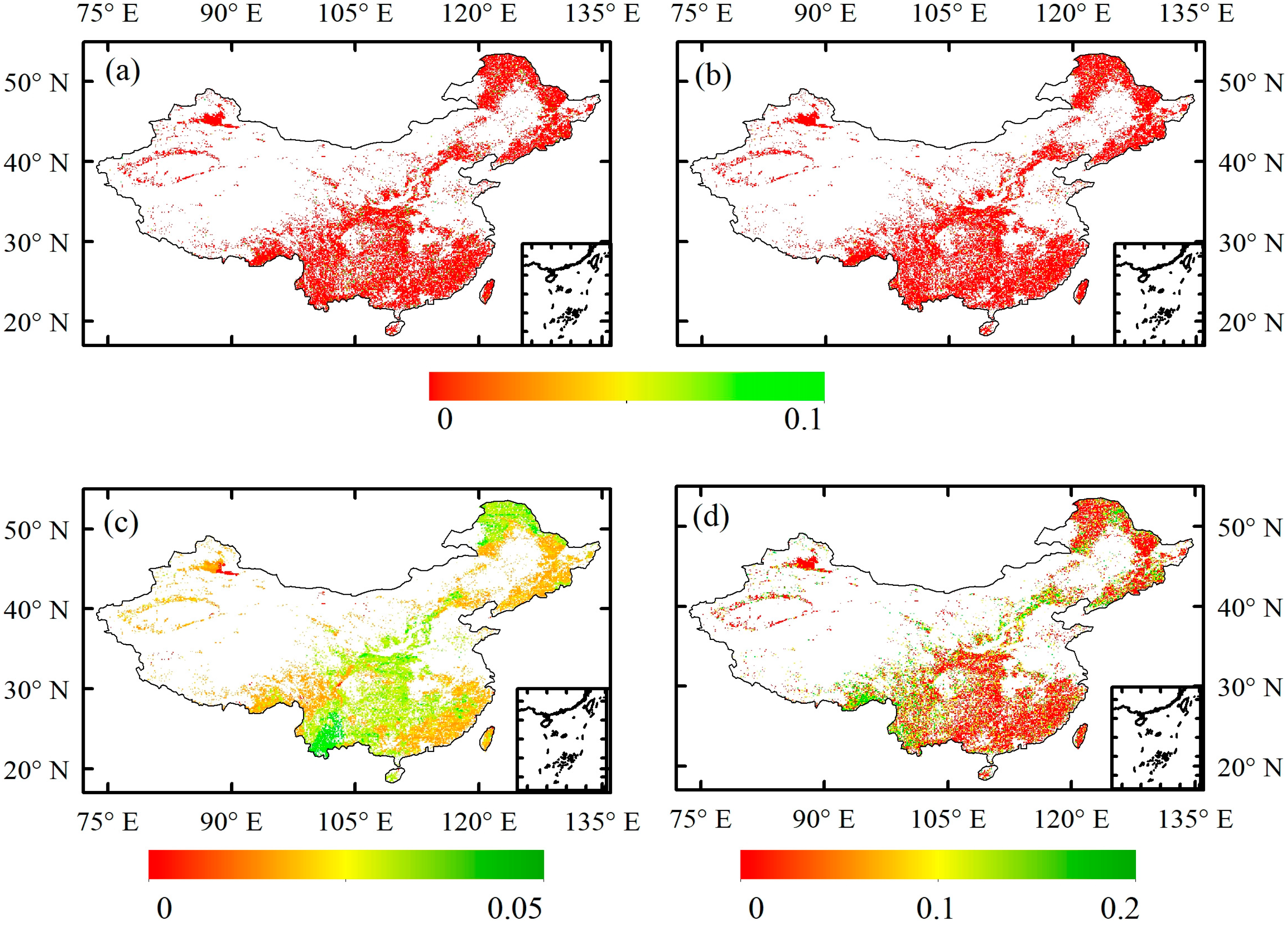
3.2. Individual and Synergistic Effects of Climate Change, CO2 and O3
4. Discussion
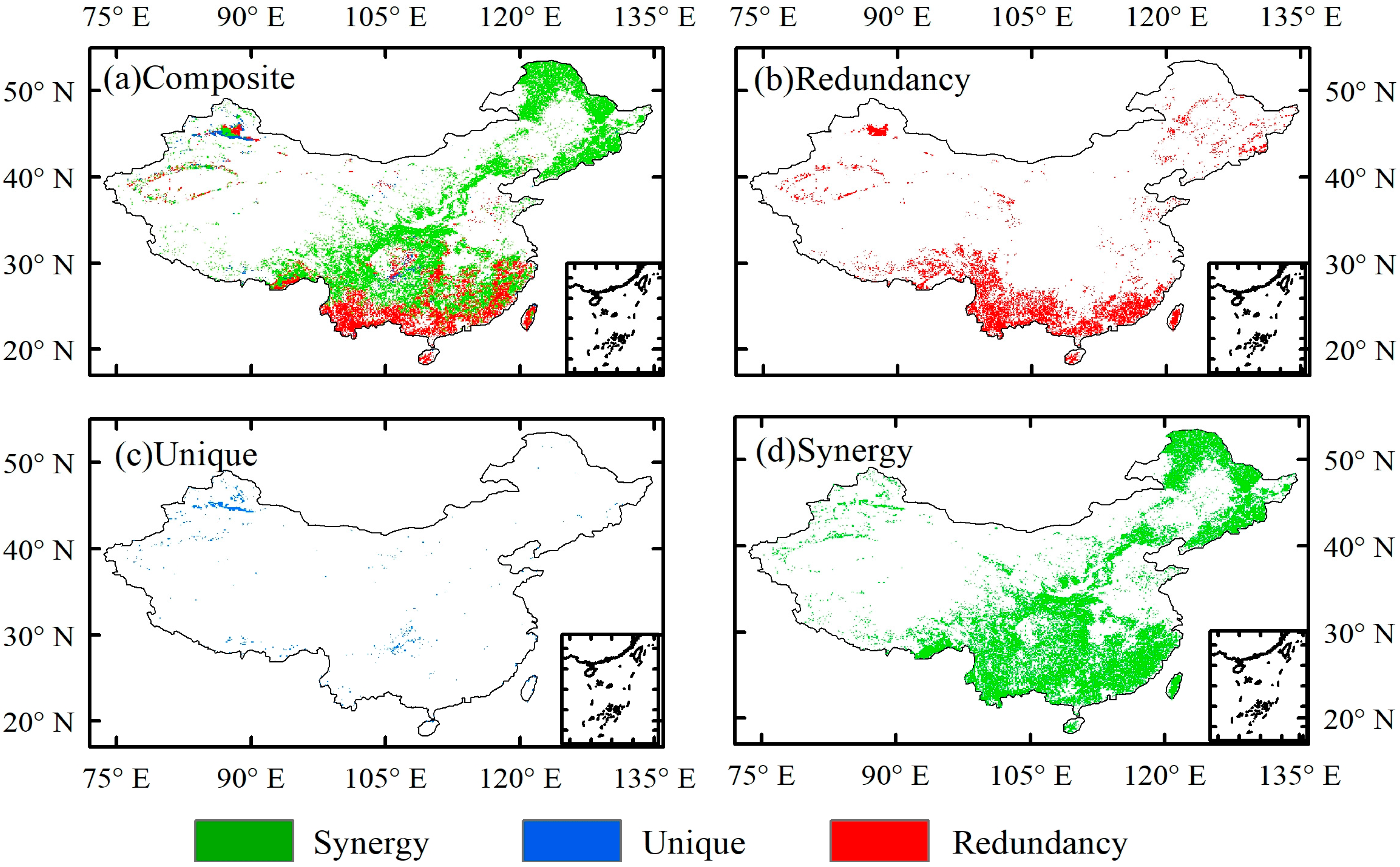
4.1. The Synergistic Effect of O3 and CO2
4.2. Discussion on the Synergistic Mechanism of O3 and CO2
4.3. Thoughts on Sustainable Development Resulting from Emission Reduction and Pollution Control
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakob, M.; Steckel, J.C. Implications of climate change mitigation for sustainable development. Environ. Res. Lett. 2016, 11, 104010. [Google Scholar] [CrossRef]
- Fujimori, S.; Hasegawa, T.; Takahashi, K.; Dai, H.; Liu, J.; Ohashi, H.; Xie, Y.; Zhang, Y.; Matsui, T.; Hijioka, Y. Measuring the sustainable development implications of climate change mitigation. Environ. Res. Lett. 2020, 15, 85004. [Google Scholar] [CrossRef]
- Hong, C.; Zhong, R.; Xu, M.; He, P.; Mo, H.; Qin, Y.; Shi, D.; Chen, X.; He, K.; Zhang, Q. Interactions Among Food Systems, Climate Change, and Air Pollution: A Review. Engineering 2025, 44, 215–233. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Pandita, P.; Bhutto, J.K.; Alreshidi, M.A.; Ravindran, B.; Yaseen, Z.M.; Osman, S.M.; Cabral-Pinto, M.M.S. Review on biofuel production: Sustainable development scenario, environment, and climate change perspectives A sustainable approach. J. Environ. Chem. Eng. 2024, 12, 111996. [Google Scholar] [CrossRef]
- Fares, S.; Weber, R.; Park, J.; Gentner, D.; Karlik, J.; Goldstein, A.H. Ozone deposition to an orange orchard: Partitioning between stomatal and non-stomatal sinks. Environ. Pollut. 2012, 169, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wedow, J.M.; Ainsworth, E.A.; Li, S. Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem. Sci. 2021, 46, 992–1002. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2019, 22, 12–37. [Google Scholar] [CrossRef]
- Li, P.; Calatayud, V.; Gao, F.; Uddling, J.; Feng, Z. Differences in ozone sensitivity among woody species are related to leaf morphology and antioxidant levels. Tree Physiol. 2016, 36, 1105–1116. [Google Scholar] [CrossRef]
- Chen, X.; Mo, X.; Hu, S.; Liu, S. Contributions of climate change and human activities to ET and GPP trends over North China Plain from 2000 to 2014. J. Geogr. Sci. 2017, 27, 661–680. [Google Scholar] [CrossRef]
- Hu, L.; Fan, W.; Yuan, W.; Ren, H.; Cui, Y. Spatiotemporal Variation of Vegetation Productivity and Its Feedback to Climate Change in Northeast China over the Last 30 Years. Remote Sens. 2021, 13, 951. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Chen, Y.; Gang, C.; Shen, Y. Quantifying the contributions of climate change and human activities to vegetation dynamic in China based on multiple indices. Sci. Total Environ. 2022, 838, 156553. [Google Scholar] [CrossRef]
- Xie, S.; Mo, X.; Hu, S.; Liu, S. Contributions of climate change, elevated atmospheric CO2 and human activities to ET and GPP trends in the Three-North Region of China. Agr. For. Meteorol. 2020, 295, 108183. [Google Scholar] [CrossRef]
- Hao, M.; Li, Y.; Zhuang, W. Crustal movement and strain distribution in East Asia revealed by GPS observations. Sci. Rep. 2019, 9, 16797. [Google Scholar] [CrossRef]
- Jarvis, P.G. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos. Trans. R. Soc. London B Biol. Sci. 1976, 273, 593–610. [Google Scholar]
- Schimel, D.; Stephens, B.B.; Fisher, J.B. Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Haworth, M.; Marino, G.; Materassi, A.; Raschi, A.; Scutt, C.P.; Centritto, M. The functional significance of the stomatal size to density relationship: Interaction with atmospheric [CO2] and role in plant physiological behaviour. Sci. Total Environ. 2023, 863, 160908. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.S.L.; El-Soda, M.; Pagel, E.; Meyer, R.C.; Tõldsepp, K.; Nilsson, A.K.; Brosché, M.; Kollist, H.; Uddling, J.; Andersson, M.X. Genetic controls of short- and long-term stomatal CO2 responses in Arabidopsis thaliana. Ann. Bot. 2020, 126, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Azoulay Shemer, T.; Palomares, A.; Bagheri, A.; Israelsson Nordstrom, M.; Engineer, C.B.; Bargmann, B.O.R.; Stephan, A.B.; Schroeder, J.I. Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2-and ABA-induced stomatal closing. Plant J. 2015, 83, 567–581. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.; Iba, K.; Schroeder, J.I. CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Allen, S.; Bindoff, N.; Breon, F.; Church, J.; Cubasch, U.; Emori, S.; Forster, P.; Friedlingstein, P.; Gillett, N.; et al. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I (WGI) to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef]
- Saxena, P.; Srivastava, A.; Tyagi, M.; Kaur, S. Impacts of Tropospheric Ozone on Plant Metabolism—A Review. Pollut. Res. 2019, 38, 175–180. [Google Scholar]
- Zapletal, M.; Juran, S.; Krpeš, V.; Michna, K.; Edwards-Jonášová, M.; Cudlín, P. Effect of Ozone Flux on Selected Structural and Antioxidant Characteristics of a Mountain Norway Spruce Forest. Balt. For. 2018, 24, 261–267. [Google Scholar]
- Calatayud, V.; Cerveró, J.; Calvo, E.; García-Breijo, F.; Reig-Armiñana, J.; Sanz, M.J. Responses of evergreen and deciduous Quercus species to enhanced ozone levels. Environ. Pollut. 2011, 159, 55–63. [Google Scholar] [CrossRef]
- Paoletti, E.; Grulke, N. Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ. Pollut. 2005, 137, 483–493. [Google Scholar] [CrossRef]
- Feng, Z.; Pang, J.; Kobayashi, K.; Zhu, J.; Ort, D.R. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Glob. Change Biol. 2011, 17, 580–591. [Google Scholar] [CrossRef]
- Fusaro, L.; Palma, A.; Salvatori, E.; Basile, A.; Maresca, V.; Asadi Karam, E.; Manes, F. Functional indicators of response mechanisms to nitrogen deposition, ozone, and their interaction in two Mediterranean tree species. PLoS ONE 2017, 12, e185836. [Google Scholar] [CrossRef] [PubMed]
- Hayes, F.; Harmens, H.; Mills, G.; Bender, J.; Grünhage, L. Ozone critical levels for (semi-)natural vegetation dominated by perennial grassland species. Environ. Sci. Pollut. Res. 2021, 28, 15090–15098. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.; Pang, J.Y.S.; Tai, A.P.K.; Lam, T.; Tao, D.K.C.; Sharps, K. Evidence of Ozone-Induced Visible Foliar Injury in Hong Kong Using Phaseolus Vulgaris as a Bioindicator. Atmosphere 2020, 11, 266. [Google Scholar] [CrossRef]
- Li, D.; Shindell, D.; Ding, D.; Lu, X.; Zhang, L.; Zhang, Y. Surface ozone impacts on major crop production in China from 2010 to 2017. Atmos. Chem. Phys. 2022, 22, 2625–2638. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Wang, X.; Wang, W.; Leung, F.; Liu, X.; Wang, C. Growth reduction and alteration of nonstructural carbohydrate (NSC) allocation in a sympodial bamboo (Indocalamus decorus) under atmospheric O3 enrichment. Sci. Total Environ. 2022, 826, 154096. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Change 2014, 4, 817–821. [Google Scholar] [CrossRef]
- Seltzer, K.M.; Shindell, D.T.; Kasibhatla, P.; Malley, C.S. Magnitude, trends, and impacts of ambient long-term ozone exposure in the United States from 2000 to 2015. Atmos. Chem. Phys. 2020, 20, 1757–1775. [Google Scholar] [CrossRef]
- Tao, F.; Feng, Z.; Tang, H.; Chen, Y.; Kobayashi, K. Effects of climate change, CO2 and O3 on wheat productivity in Eastern China, singly and in combination. Atmos. Environ. 2017, 153, 182–193. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Sadiq, M.; Pang, J.Y.S.; Yung, D.H.Y.; Feng, Z. Impacts of Surface Ozone Pollution on Global Crop Yields: Comparing Different Ozone Exposure Metrics and Incorporating Co-effects of CO2. Front. Sustain. Food Syst. 2021, 5, 534616. [Google Scholar] [CrossRef]
- Mo, X.; Liu, S.; Chen, X.; Hu, S. Variability, tendencies, and climate controls of terrestrial evapotranspiration and gross primary productivity in the recent decade over China. Ecohydrology 2018, 11, e1951. [Google Scholar] [CrossRef]
- Feng, X.; Liu, G.; Chen, J.M.; Chen, M.; Liu, J.; Ju, W.M.; Sun, R.; Zhou, W. Net primary productivity of China’s terrestrial ecosystems from a process model driven by remote sensing. J. Environ. Manag. 2007, 85, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Tian, H.; Liu, M.; Zhang, C.; Chen, G.; Pan, S.; Felzer, B.; Xu, X. Effects of tropospheric ozone pollution on net primary productivity and carbon storage in terrestrial ecosystems of China. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Tian, H.; Melillo, J.; Lu, C.; Kicklighter, D.; Liu, M.; Ren, W.; Xu, X.; Chen, G.; Zhang, C.; Pan, S.; et al. China’s terrestrial carbon balance: Contributions from multiple global change factors. Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Chen, G.; Liu, M.; Zhang, C.; Chappelka, A.H.; Pan, S. Influence of ozone pollution and climate variability on net primary productivity and carbon storage in China’s grassland ecosystems from 1961 to 2000. Environ. Pollut. 2007, 149, 327–335. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Tao, B.; Chappelka, A.; Sun, G.; Lu, C.; Liu, M.; Chen, G.; Xu, X. Impacts of tropospheric ozone and climate change on net primary productivity and net carbon exchange of China’s forest ecosystems. Glob. Ecol. Biogeogr. 2011, 20, 391–406. [Google Scholar] [CrossRef]
- Chen, J.M.; Liu, J.; Cihlar, J.; Goulden, M.L. Daily canopy photosynthesis model through temporal and spatial scaling for remote sensing applications. Ecol. Model. 1999, 124, 99–119. [Google Scholar] [CrossRef]
- Chen, J.M.; Mo, G.; Pisek, J.; Liu, J.; Deng, F.; Ishizawa, M.; Chan, D. Effects of foliage clumping on the estimation of global terrestrial gross primary productivity. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.M.; Baldocchi, D.D.; Liu, Y.; Wang, S.; Zheng, T.; Black, T.A.; Croft, H. Including soil water stress in process-based ecosystem models by scaling down maximum carboxylation rate using accumulated soil water deficit. Agric. For. Meteorol. 2019, 276–277, 107649. [Google Scholar] [CrossRef]
- Farquhar, G.; O’Leary, M.H.; Berry, J. On the Relationship Between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Aust. J. Plant Physiol. 1982, 13, 281–292. [Google Scholar] [CrossRef]
- Anav, A.; Liu, Q.; De Marco, A.; Proietti, C.; Savi, F.; Paoletti, E.; Piao, S. The role of plant phenology in stomatal ozone flux modeling. Glob. Change Biol. 2018, 24, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Anav, A.; Menut, L.; Khvorostyanov, D.; Viovy, N. Impact of tropospheric ozone on the Euro-Mediterranean vegetation. Glob. Change Biol. 2011, 17, 2342–2359. [Google Scholar] [CrossRef]
- Sitch, S.; Cox, P.M.; Collins, W.J.; Huntingford, C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.J.; Mercado, L.M.; Sitch, S.; Simpson, D.; Medlyn, B.E.; Lin, Y.; Folberth, G.A. Large but decreasing effect of ozone on the European carbon sink. Biogeosciences 2018, 15, 4245–4269. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Bell, B.; Hersbach, H.; Simmons, A.; Berrisford, P.; Dahlgren, P.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis: Preliminary extension to 1950. Q. J. R. Meteorol. Soc. 2021, 147, 4186–4227. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Chen, J.M. Retrospective retrieval of long-term consistent global leaf area index (1981–2011) from combined AVHRR and MODIS data. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Xing, X.; Wu, M.; Zhang, W.; Ju, W.; Tagesson, T.; He, W.; Wang, S.; Wang, J.; Hu, L.; Yuan, S.; et al. Modeling China’s terrestrial ecosystem gross primary productivity with BEPS model: Parameter sensitivity analysis and model calibration. Agr. For. Meteorol. 2023, 343, 109789. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, P.; Nie, A.; Yang, A.; Yao, Y.; Lü, W.; Zhang, H.; Liu, Q. Land cover mapping using time series HJ-1/CCD data. Sci. China Earth Sci. 2014, 57, 1790–1799. [Google Scholar] [CrossRef]
- Martínez-Sánchez, Á.; Arranz, G.; Lozano-Durán, A. Decomposing causality into its synergistic, unique, and redundant components. Nat. Commun. 2024, 15, 9296. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, M.; Wang, S.; Chen, B.; Liu, Z.; Wang, Z.; Chen, S.; Li, H.; Zhu, T.; Li, D.; et al. Evaluation of the impacts of ozone on the vegetation productivity of woodland and grassland ecosystems in China. Ecol. Model. 2023, 483, 110426. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.M.; Ju, W. Remote sensing-based ecosystem–atmosphere simulation scheme (EASS)—Model formulation and test with multiple-year data. Ecol. Model. 2007, 209, 277–300. [Google Scholar] [CrossRef]
- Li, Q.; Lu, X.; Wang, Y.; Huang, X.; Cox, P.M.; Luo, Y. Leaf area index identified as a major source of variability in modeled CO2 fertilization. Biogeosciences 2018, 15, 6909–6925. [Google Scholar] [CrossRef]
- Launiainen, S.; Katul, G.G.; Leppä, K.; Kolari, P.; Aslan, T.; Grönholm, T.; Korhonen, L.; Mammarella, I.; Vesala, T. Does growing atmospheric CO2 explain increasing carbon sink in a boreal coniferous forest? Glob. Change Biol. 2022, 28, 2910–2929. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Sitch, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Wang, X.; Ahlström, A.; Anav, A.; Canadell, J.G.; Cong, N.; et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Change Biol. 2013, 19, 2117–2132. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Tani, H.; Guo, E.; Yin, S.; Zhang, T. Evaluating and comparing remote sensing terrestrial GPP models for their response to climate variability and CO2 trends. Sci. Total Environ. 2019, 668, 696–713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhuang, Q.; Ciais, P.; Welp, L.; Li, W.; Xin, Q. Elevated atmospheric CO2 negatively impacts photosynthesis through radiative forcing and physiology-mediated climate feedback. Geophys. Res. Lett. 2017, 44, 1956–1963. [Google Scholar] [CrossRef]
- Ueyama, M.; Ichii, K.; Kobayashi, H.; Kumagai, T.; Beringer, J.; Merbold, L.; Euskirchen, E.S.; Hirano, T.; Marchesini, L.B.; Baldocchi, D.; et al. Inferring CO2 fertilization effect based on global monitoring land-atmosphere exchange with a theoretical model. Environ. Res. Lett. 2020, 15, 084009. [Google Scholar] [CrossRef]
- Otu-Larbi, F.; Conte, A.; Fares, S.; Wild, O.; Ashworth, K. Current and future impacts of drought and ozone stress on Northern Hemisphere forests. Glob. Change Biol. 2020, 26, 6218–6234. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Tcherkez, G.G.B.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Bernacchi, C.J.; Davey, P.A.; Morgan, P.B.; Hymus, G.J.; Leakey, A.D.B.; Osborne, C.P. Long-Term Responses of Photosynthesis and Stomata to Elevated [CO2] in Managed Systems. In Managed Ecosystems and CO2: Case Studies, Processes, and Perspectives; Nösberger, J., Long, S.P., Norby, R.J., Stitt, M., Hendrey, G.R., Blum, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 253–270. [Google Scholar]
- Portis, A.R. Rubisco activase–Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Von Caemmerer, S. C4 photosynthesis in a single C3 cell is theoretically inefficient but may ameliorate internal CO2 diffusion limitations of C3 leaves. Plant Cell Environ. 2003, 26, 1191–1197. [Google Scholar] [CrossRef]
- Long, S.P.; Drake, B.G. Chapter 4—Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In Crop Photosynthesis; Baker, N.R., Thomas, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 69–103. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising Atmospheric Carbon Dioxide: Plants Face the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Goumenaki, E.; Taybi, T.; Borland, A.; Barnes, J. Mechanisms underlying the impacts of ozone on photosynthetic performance. Environ. Exp. Bot. 2010, 69, 259–266. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Yang, X.L.; Xu, Y.S.; Feng, Z.Z. Response of key parameters of leaf photosynthetic models to increased ozone concentration in four common trees. Chin. J. Plant Ecol. 2022, 46, 321–329. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyerc, E.; Ferrio, J.P.; Gago, J.; et al. Corrigendum to ‘Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis’ [Plant Sci. 193–194 (2012) 70–84]. Plant Sci. 2012, 196, 31. [Google Scholar] [CrossRef]
- Knauer, J.; Zaehle, S.; De Kauwe, M.G.; Bahar, N.H.A.; Evans, J.R.; Medlyn, B.E.; Reichstein, M.; Werner, C. Effects of mesophyll conductance on vegetation responses to elevated CO2 concentrations in a land surface model. Glob. Change Biol. 2019, 25, 1820–1838. [Google Scholar] [CrossRef] [PubMed]
- Anav, A.; Menut, L.; Khvorostyanov, D.; Viovy, N. A comparison of two canopy conductance parameterizations to quantify the interactions between surface ozone and vegetation over Europe. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Wan, M.; Bo, Y.; He, K.; Liu, R. Evolution of surface ozone pollution in Yangtze Rriver Delta between 2019 and 2022: Influences of meteorology and emissions. Atmos. Environ. 2025, 358, 121339. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- Nam, K.; Waugh, C.J.; Paltsev, S.; Reilly, J.M.; Karplus, V.J. Carbon co-benefits of tighter SO2 and NOx regulations in China. Glob. Environ. Change 2013, 23, 1648–1661. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Env. 2022, 3, 141–155. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhao, Y. Collaborative Governance Mechanism of Climate Change and Air Pollution: Evidence from China. Sustainability 2021, 13, 6785. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y. Evaluating the impact of new energy demonstration city construction on urban air quality: Evidence from a DID analysis of Chinese cities. Energy 2025, 332, 137108. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, M.; Zhou, N.; Ma, Z.; Yan, R.; Ma, X. China’s plug-in hybrid electric vehicle transition: An operational carbon perspective. Energ. Convers. Manag. 2024, 320, 119011. [Google Scholar] [CrossRef]
- Guo, D.; Yan, W.; Gao, X.; Hao, Y.; Xu, Y.; E, W.; Tan, X.; Zhang, T. Forecast of passenger car market structure and environmental impact analysis in China. Sci. Total Environ. 2021, 772, 144950. [Google Scholar] [CrossRef]
- Gai, Y.; Sun, L.; Fu, S.; Zhu, C.; Zhu, C.; Li, R.; Liu, Z.; Wang, B.; Wang, C.; Yang, N.; et al. Impact of greening trends on biogenic volatile organic compound emissions in China from 1985 to 2022: Contributions of afforestation projects. Sci. Total Environ. 2024, 929, 172551. [Google Scholar] [CrossRef]
- Cao, J.; Situ, S.; Hao, Y.; Xie, S.; Li, L. Enhanced summertime ozone and SOA from biogenic volatile organic compound (BVOC) emissions due to vegetation biomass variability during 1981–2018 in China. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Huang, M.; Wang, Z.S.; Wang, S.Q.; Li, Y.L. Impact of ozone pollution on productivity and biomass of subtropical forests: A case study in Dinghushan. Acta Ecol. Sin. 2023, 43, 1832–1842. [Google Scholar] [CrossRef]
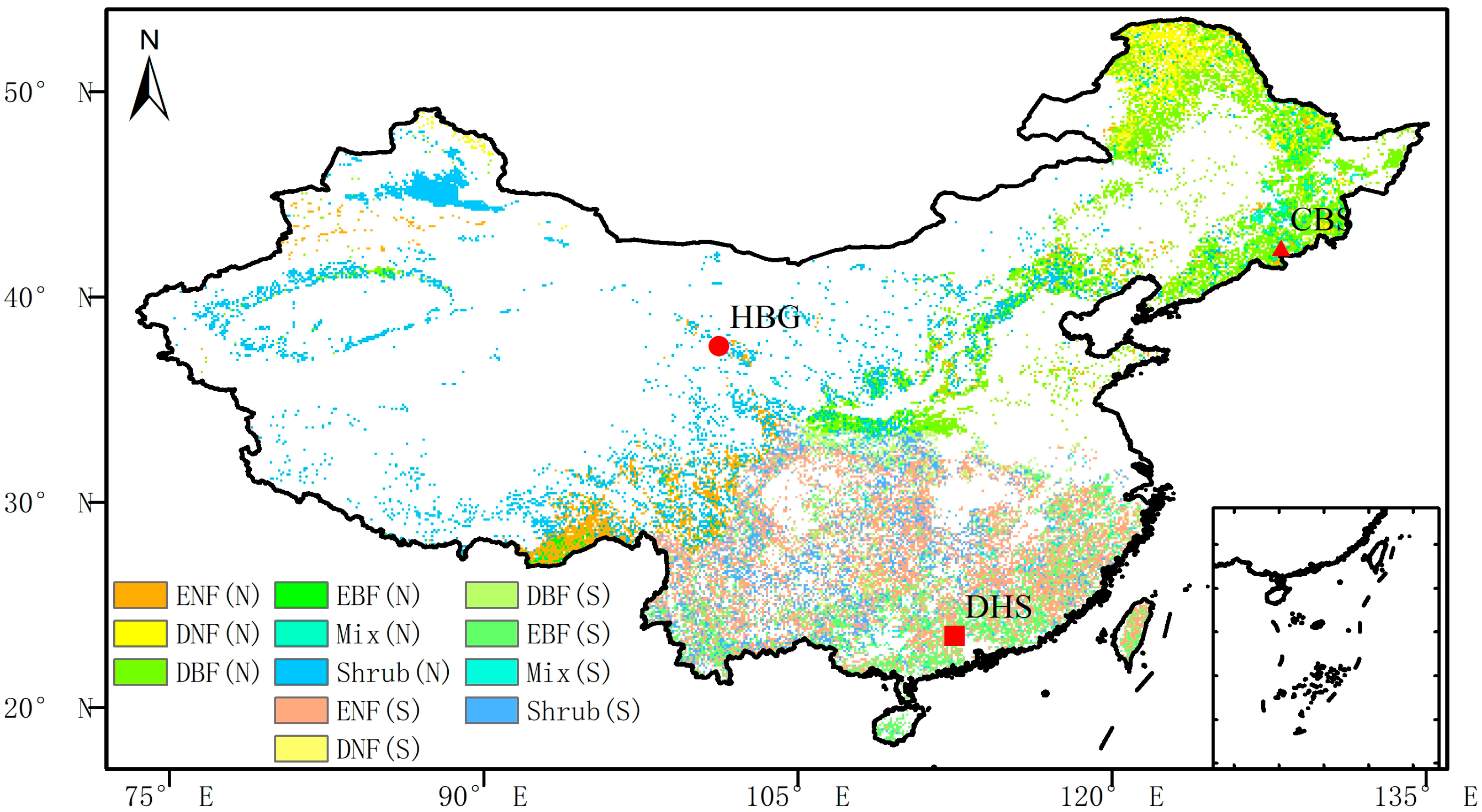

| Site Name | Site Coordinates | Vegetation Type | Year of Observation GPP |
|---|---|---|---|
| HaiBei | 101.25 N, 37.6 E | Shrub | 2009–2010 |
| Changbaishan | 128.1 N, 42.4 E | Conifer | 2009–2010 |
| Dinghushan | 112.5 N, 23.5 E | Hardleaf | 2016, 2010 |
| Experiment No. | Climate | History_O3 | History_CO2 |
|---|---|---|---|
| E1 | ✓ | ||
| E2 | ✓ | ✓ | ✓ |
| E3 | ✓ | ✓ | |
| E4 | ✓ | ✓ |
| Causal Relationships | Association of Variable |
|---|---|
| R (Redundant) | (1,2), (1,3), (2,3), (1,2,3) |
| U (Unique) | (1), (2), (3) |
| S (Synergistic) | (1,2), (1,3), (2,3), (1,2,3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Sun, L.; Wang, S.; Chen, B.; Liu, Z.; Chen, S.; Li, T.; Li, Y.; Huang, M. Carbon Dioxide Fertilization Effects Offset the Vegetation GPP Losses of Woodland Ecosystems Due to Surface Ozone Damage in China. Sustainability 2025, 17, 7198. https://doi.org/10.3390/su17167198
Wang Q, Sun L, Wang S, Chen B, Liu Z, Chen S, Li T, Li Y, Huang M. Carbon Dioxide Fertilization Effects Offset the Vegetation GPP Losses of Woodland Ecosystems Due to Surface Ozone Damage in China. Sustainability. 2025; 17(16):7198. https://doi.org/10.3390/su17167198
Chicago/Turabian StyleWang, Qinyi, Leigang Sun, Shaoqiang Wang, Bin Chen, Zhenhai Liu, Shiliang Chen, Tingyu Li, Yuelin Li, and Mei Huang. 2025. "Carbon Dioxide Fertilization Effects Offset the Vegetation GPP Losses of Woodland Ecosystems Due to Surface Ozone Damage in China" Sustainability 17, no. 16: 7198. https://doi.org/10.3390/su17167198
APA StyleWang, Q., Sun, L., Wang, S., Chen, B., Liu, Z., Chen, S., Li, T., Li, Y., & Huang, M. (2025). Carbon Dioxide Fertilization Effects Offset the Vegetation GPP Losses of Woodland Ecosystems Due to Surface Ozone Damage in China. Sustainability, 17(16), 7198. https://doi.org/10.3390/su17167198






