Abstract
Coal serves as the primary energy source for China, with production anticipated to reach 4.76 billion tons in 2024. However, the mining process generates a significant amount of gangue, with approximately 800 million tons produced in 2023 alone. Currently, China faces substantial gangue stockpiles, characterized by a low comprehensive utilization rate that fails to meet the country’s ecological and environmental protection requirements. The environmental challenges posed by the treatment and disposal of gangue are becoming increasingly severe. This review employs bibliometric analysis and theoretical perspectives to examine the latest advancements in gangue utilization, specifically focusing on the application of computational chemistry to elucidate the structural features and interaction mechanisms of coal gangue, and to collate how these insights have been leveraged in the literature to inform its potential utilization routes. The aim is to promote the effective resource utilization of this material, and key topics discussed include evaluating the risks of spontaneous combustion associated with gangue, understanding the mechanisms governing heavy metal migration, and modifying coal byproducts to enhance both economic viability and environmental sustainability. The case studies presented in this article offer valuable insights into the gangue conversion process, contributing to the development of more efficient and eco-friendly methods. By proposing a theoretical framework, this review will support ongoing initiatives aimed at the sustainable management and utilization of coal gangue, emphasizing the critical need for continued research and development in this vital area. This review uniquely combines bibliometric analysis with computational chemistry to identify new trends and gaps in coal waste utilization, providing a roadmap for future research.
1. Introduction
The current low-carbon energy transition is at a pivotal moment within the framework of carbon peaking and carbon neutrality goals. Energy activities are the leading source of global CO2 emissions, with coal contributing a substantial portion [1]. Coal gangue, a byproduct of coal mining and washing, is generated in large quantities. Its effective resource utilization is essential for environmental protection and resource conservation as shown in Figure 1 [2]. The accumulation of coal gangue presents challenges such as secondary contamination and environmental degradation, which necessitate further research into its effects [3]. There is a growing interest in the recycling of coal gangue for various applications, including construction materials [4,5,6], energy generation [7,8], and soil improvement [9,10,11], all of which can help mitigate its environmental impact [12]. Despite its potential, the current utilization rate of coal gangue remains low, primarily due to considerable barriers to effective recycling, including the need for pre-treatment methods [13]. Furthermore, the management of coal gangue is complicated by its inherent properties, such as alkalinity and HM content, which pose risks during transportation and storage [14]. There is a notable lack of comprehensive strategies for the global management of coal gangue, highlighting the urgent need for innovative approaches and policies to address its disposal and utilization effectively.
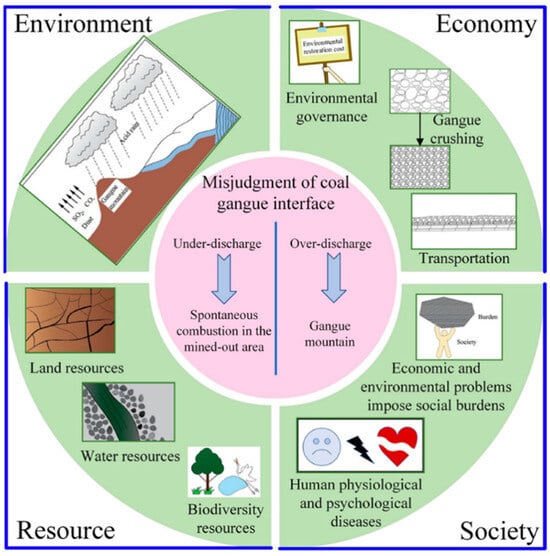
Figure 1.
Environmental hazards and social and economic impacts of coal gangue. Figure adapted with permission from Ref. [2] (Copyright 2023 ACS publication).
Studies have shown that coal gangue contains a certain amount of useful components, such as coal, kaolin, bauxite, etc. [15]. These components can be extracted through advanced separation and conversion technologies and used to produce a variety of high-value-added products. At present, a variety of ways to utilize coal gangue resources have been proposed, such as preparing calcined kaolin, power generation, ceramic microbeads, fiber materials, and silica aerogels, as well as innovative applications such as extracting alumina, white carbon black, producing fertilizers and soil conditioners [16,17,18]. Most recently, research on the fine separation and efficient conversion of abundant silicon, aluminum, iron, and rare metal elements in gangue has gradually become popular [19,20]. However, although these solutions held great potential for coal gangue utilization, they are often limited by problems such as difficulty in exploring reaction mechanisms, low technical maturity, and secondary environmental pollution. To substantially realize the multi-dimensional and high-value utilization of gangue, it is necessary to accurately explain the physical and chemical reactions and corresponding mechanisms in the process of gangue resource utilization.
Computational chemistry methods such as molecular simulations, density-functional theory (DFT), and ab initio approaches are crucial for analyzing the behavior of molecules during the reaction and the transformation of forms before and after the reaction, which is essential for addressing challenges related to coal utilization and environmental impact. Molecular simulations, including molecular dynamics and Monte Carlo simulations, are employed to model the behavior of gases like CO2 within coal gangue structures under varying temperature and pressure conditions [21]. In addition, molecular dynamics simulations have been employed to study the adsorption characteristics of water molecules in coal gasification fine slag, elucidating the impact of pore structures on flotation efficiency [22]. The combination of molecular dynamics simulations with flotation techniques has established a theoretical foundation for improving the separation processes and resource recovery from coal waste [20]. Quantum chemical calculations are known for their accuracy and efficiency, offering significant advantages in the establishment of molecular models, the prediction and analysis of chemical reactivity, the simulation of reaction pathways, and the forecasting of potential excited and transition states [23,24]. Quantum chemistry research makes a significant contribution to our understanding of complex coal systems and their chemical behaviors, especially in processes such as gasification and liquefaction. The application of quantum chemistry has uncovered fundamental attributes of coal, including its electronic properties and reactivity, which are crucial for optimizing resource utilization [16,25]. The advent of GPU-enabled reactive molecular dynamics (ReaxFF MD) has facilitated the simulation of large-scale coal models, yielding insights into coal pyrolysis mechanisms and product profiles. These approaches not only enhance our comprehension of coal chemistry but also pave the way for innovative solutions to optimize resource extraction and waste management within the coal industry.
This review employs bibliometric analysis to first map emergent research hotspots in coal gangue utilization and then couples these findings with computational chemistry perspectives, principally DFT, to dissect the key structural motifs and interaction mechanisms that dictate reactivity. We also address the challenges and opportunities associated with coal gangue utilization, highlighting the necessity of a synergistic approach that integrates theoretical predictions with experimental validation. The review concludes with recommendations for future research, emphasizing the development of sustainable and economically viable pathways for coal gangue utilization, informed by the insights derived from bibliometric analysis and computational chemistry. This integrated approach advances the science and technology of coal gangue resource utilization, contributing to a circular economy and furthering sustainable development goals.
2. Bibliometric Analysis of Coal Gangue
The methodology of bibliometric analysis applied to coal gangue research entails systematic data collection and analysis, which unveils trends in publication, research focus, and the environmental implications of coal gangue utilization. Lin et al. [26] provided a macroscopic trend map (country, journal, keyword) in the comprehensive utilization of coal gangue, while our work delved into the detailed mechanisms and linked several key hotspots with specific computational chemistry methods.
Data were sourced from the “Web of Science” Core Collection, covering the period from 2000 to 2024. A search for the term “coal gangue” resulted in 2998 documents, predominantly articles, which accounted for 82.09% of the total, followed by meeting papers at 13.81% and review articles at 2.43%. The combination of “coal gangue” and “utilization” yielded 579 documents, where articles made up the majority at 94.30%, with conference papers comprising 8.12% and review articles 5.87%. Other document types represented a negligible fraction. As illustrated in Figure 2, there has been a significant increase in the number of published articles on coal gangue. Notably, the annual publication volume related to coal gangue utilization reflects a sustained and growing interest in discovering innovative and fundamental solutions for the effective management and utilization of this waste material.
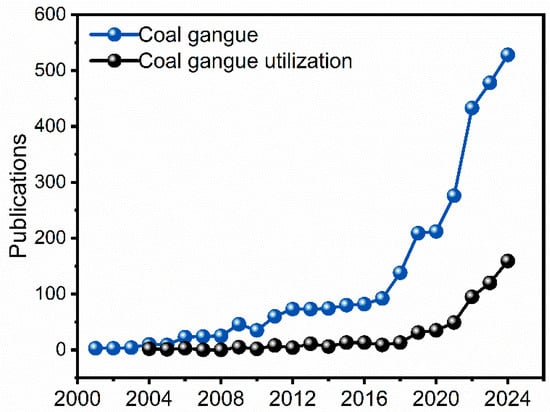
Figure 2.
Annual published articles on “Coal Gangue” and “Coal Gangue + Utilization” collected from the “Web of Science” Core Collection, spanning from 2000 to 2024.
CiteSpace was also employed for visual analysis and to map scientific knowledge. By clustering keywords, keywords with close relationships among the many keywords are grouped into one category for scholars to analyze and study. CiteSpace offers three algorithms: Latent Semantic Indexing (LSI), Log-Likelihood Ratio (LLR), and Mutual Information (MI). In this work, the LSI algorithm was utilized. Running CiteSpace resulted in keyword clustering; the modularity value Q (Modularity Q) is greater than 0.3, and the average silhouette value S (Weighted Mean Silhouette) is greater than 0.7 [27]. By analyzing these co-occurrence networks of key terms, researchers can gain insights into the knowledge structure and research frontiers of the coal gangue research field, identify the evolution of research hotspots across different years, and obtain the current research hotspots and development trends, as well as understand the interdisciplinary and cross-disciplinary integration in the field of coal gangue utilization.
As illustrated in Figure 3, Figures S1 and S2, the scientific knowledge maps illustrate the co-occurrence relationships and developmental trends of key terms in coal gangue research. The chart in Figure 3 employs color coding to distinctly identify 13 core research themes, each marked by a unique color and number, thereby facilitating easy recognition and analysis. The size of the nodes reflects the frequency of key terms in the literature, while the lines between nodes indicate co-occurrence relationships, with the thickness of the lines underscoring the strength of these connections. For instance, “coal gangue” as a central theme is represented by pink nodes that persist along the timeline. Furthermore, the chart reveals dynamic changes in research focus. Earlier research priorities such as “spontaneous combustion,” “heavy metals,” and “kaolinite” are depicted as larger nodes, indicating their enduring significance in the field [28]. The structural changes during calcination, such as dehydroxylation and sintering, significantly enhance the reactivity of coal gangue, making it suitable for use in construction materials [29,30]. Calcination desiliconization is conducted to synthesize molecular sieve products to realize high-value resource utilization of coal gangue [31].
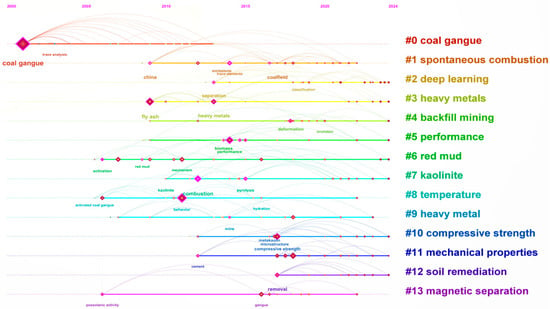
Figure 3.
Sequence diagram of international emerging terms in coal gangue.
As illustrated in Figure 4, the timeline on the map extends from 2000 to 2024, providing insights into the evolving research interests and advancements in the utilization of coal gangue. The terms range from “co-combustion” to “comprehensive utilization,” reflecting a multifaceted approach to managing and utilizing coal gangue. Notably, the inclusion of terms such as “mechanical properties” and “compressive strength” highlights the focus on the material characteristics of coal gangue and its potential applications in construction. The prominence of “ecological restoration” indicates a rising awareness of the environmental impacts associated with coal gangue, as well as the development of strategies to address these issues. Additionally, the mention of “solid waste” and “fly ash” situates coal gangue within the broader context of waste management, emphasizing its potential role in contributing to sustainable solutions.
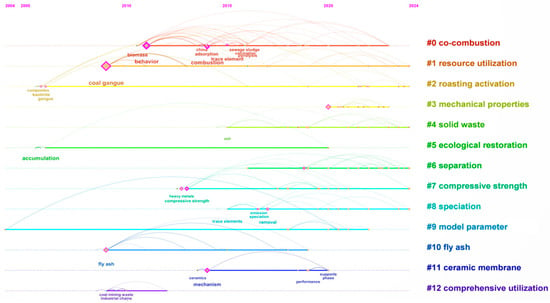
Figure 4.
Sequence diagram of international emerging terms in coal gangue utilization.
These trends highlight the innovative approaches to repurpose coal gangue and related byproducts for sustainable applications, addressing both environmental concerns and resource efficiency. Coal gangue is activated to highly active Si-Al species for the synthesis of fluidized catalytic cracking (FCC) catalysts [32]. Coal gangue is being utilized in the production of composite cementitious materials, enhancing waste utilization and providing thermal insulation properties [33,34]. Magnetic catalysts derived from coal gangue have exhibited remarkable catalytic properties for the removal of pollutants, highlighting their potential applications in environmental cleanup [35,36,37,38]. Coal gangue has been successfully synthesized into sodalite, which demonstrates effective adsorption capabilities for HM ions and dyes [39]. The adsorption processes of Cd2+ ions and methylene blue by sodalite follow the Langmuir model and pseudo-second-order kinetics, indicating a strong interaction between the adsorbate and adsorbent [40].
In particular, the process of “co-combustion” and “roasting activation” involves complex chemical reactions and pathways that are crucial for efficient coal resource utilization. The relationship between the chemical structure and the thermal reactivity is essential for predicting behaviors of “co-combustion” and “roasting activation”. For example, ReaxFF molecular dynamics simulations provide a powerful tool for investigating the kinetics of coal pyrolysis at the atomic level, allowing for the detailed analysis of reaction mechanisms and product formation [41]. Large-scale molecular models, such as those for Illinois no. 6 coal, have been utilized to study structural modifications and the effects of heteroatoms like sulfur on reaction kinetics during pyrolysis [42]. The integration of simulation results with experimental data enhances the understanding of coal thermal chemistry and aids in the development of more efficient coal utilization strategies [43]. In delving into the theoretical research of coal gangue utilization, the application of computational chemistry is particularly crucial. The insights from computational chemistry encompass an in-depth analysis of chemical structure and reactivity, while also addressing environmental concerns associated with coal gangue utilization, ensuring sustainable development in this field.
In the subsequent sections of this paper, we will elaborate on the close connection between coal gangue utilization and computational chemistry. By exploring the simulation of coal gangue’s spontaneous combustion risk, the mechanism of HM migration, and the modification of coal byproducts, we can comprehensively enhance the efficiency and safety of coal and its byproducts management. This collection of research themes not only enriches our theoretical understanding of coal gangue utilization but also provides a solid scientific foundation for achieving sustainable management of coal gangue.
3. Theoretical Insights into Key Hotspots Identified by Bibliometric Analysis
Guided by the bibliometric findings presented in Section 2, this review concentrates on three pivotal research hotspots: the spontaneous combustion mechanism of coal gangue, heavy metal migration and solid-phase control, and surface modification/activation for enhanced utilization. It critically examines molecular simulations and quantum chemical studies published in the past years, distilling key computational models, dominant reaction mechanisms, and their quantitative implications for resource-oriented pathways. The resulting theoretical analysis is intended to furnish a systematic reference for subsequent experimental validation and multi-scale technological integration.
3.1. Spontaneous Combustion Risk of Coal Gangue
The spontaneous combustion of coal gangue piles results in a compromised structure and diminished stability, consequently leading to significant disasters such as landslides and collapses. These events pose serious threats to both the surrounding environment and the safety of personnel. The phenomenon of spontaneous combustion in coal gangue has emerged as a prominent area of research due to its recurrent nature and the considerable hazards it entails. This process involves a complex interplay of physicochemical changes, influenced by a range of factors categorized as internal and external. Internal factors pertain to the physical properties and the inherent combustibility tendencies of the coal gangue. In contrast, the principal external factor is the permeability of the gangue piles, which governs the accessibility of oxygen to the coal gangue and plays a crucial role in facilitating spontaneous combustion [44,45,46].
Extensive studies were conducted on permeability and diffusion characteristics using quantum chemistry and molecular simulation methods [47]. According to the coal–oxygen complex principle [48], the spontaneous combustion of dispersed coal gangue is a process of oxygen adsorption by coal gangue. Coal gangue is a porous material with a large specific surface area and many active sites on its surface, which have a strong adsorption capacity for oxygen. As oxygen diffuses toward the surface particles of the gangue, it is continuously absorbed, leading to the release of heat through physicochemical reactions. This heat accumulation can ultimately trigger the spontaneous combustion of coal gangue piles [49]. To mitigate the risk of spontaneous combustion in coal gangue hills, Wang et al. [50] investigated the adsorption, diffusion, and seepage behaviors of oxygen within the slit structures of coal gangue. Molecular dynamics (MD), grand canonical Monte Carlo (GCMC) simulations, and finite-element analyses were employed to quantify the influence of temperature and pressure on O2 behavior at the pore scale (Figure 5). The findings reveal that increased pressure enhances oxygen adsorption and decreases the diffusion coefficient, while elevated temperatures have the opposite effect. At 10 MPa, the O2–gangue system exhibits the largest variation in total energy, underscoring pressure as the dominant thermodynamic driver for both adsorption and diffusion. Below 423.15 K, physisorption prevails; the concomitant exposure of kaolinite surfaces amplifies O2 uptake, thereby accelerating oxidation and increasing the probability of self-ignition. The oxygen percolation process was further resolved into four sequential stages governed by (i) slit-channel geometry, (ii) gas diffusivity, and (iii) the intrinsic oxygen consumption rate of the gangue. Molecular simulations using Materials Studio and COMSOL software further elucidate the adsorption–diffusion behavior and microscopic pore seepage characteristics of coal gangue. Collectively, the findings indicate that pressure regulation is more effective than temperature control in suppressing spontaneous combustion of coal gangue, thereby providing a theoretical basis for proactive prevention and management strategies.
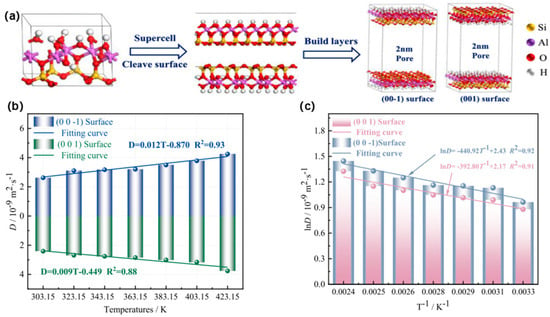
Figure 5.
Temperature- and pressure-dependent adsorption–diffusion–seepage behavior of O2 in coal gangue pores. (a) Unit-cell model of kaolinite, its exposed crystallographic surfaces, and the corresponding slit-pore geometry. (b) Temperature evolution of the self-diffusion coefficient (D) for different surfaces. (c) Arrhenius-type plots (ln D vs. T−1) for different surfaces, with linear regression fits. Figure adapted with permission from Ref. [50] (Copyright 2023 Elsevier BV).
GCMC and MD simulations are also widely used to study the adsorption and diffusion behavior of gases in coal. Huang et al. [51] focused on the adsorption and diffusion mechanisms of CO2 and CH4 during carbon dioxide-enhanced coalbed methane (CO2-ECBM) recovery in high-volatile bituminous coal, whereas Gao et al. [21] examined the kinetics of the same process within anthracite pores. Consistent with thermodynamic expectations, both studies reported a preferential adsorption of CO2 over CH4, indicating that CO2 exhibits a higher affinity for the coal matrix. Moreover, the diffusion coefficients of CO2 and CH4 decrease with the increase in pressure and increase with the rise in temperature. Wang et al. [52] conducted GCMC and MD simulations to investigate the CO2 and N2 adsorption behaviors of three bituminous coal molecular models—LL (C186H153NO6), PDS-1 (C207H165NO11), and XZ (C181H163NO6)—representing a systematic gradient in volatile matter content. The results show that the adsorption capacity and rate of CO2 by bituminous coal molecules are both greater than those of N2. The ultimate adsorption capacity of low-volatile bituminous coal is the largest and decreases with the increase in volatile matter. The adsorption heat of both gases is less than 40 kJ/mol, which means they are not sensitive to changes in pressure and temperature and are more likely to be adsorbed near oxygen-containing functional groups and benzene rings. The diffusion capacity of CO2 and N2 in coal is greater than that of CO2, as CO2 has a stronger adsorption capacity. The application of molecular simulation methods to explore the influence of different coals on the gas adsorption law has positive significance for injecting inert gas for fire prevention and extinguishing in coal mine goaf areas.
The differences in permeability of various gases are not only dependent on the diffusion properties of the gases themselves but also on the structure and type of the permeable material. Utilizing the Wuhai mining area as a reference point, Wang et al. [53] quantified the permeation behavior of five gas species in coal and coal gangue models under variable environmental conditions through grand canonical Monte Carlo (GCMC) simulations coupled with molecular dynamics (MD). The coal model featured a complex molecular structure of bituminous coal, incorporating cycloalkanes and methyl groups, while the kaolinite model represented the coal gangue. A 1nm slit model was created, and ten molecules each of SO2, NO2, O2, N2, and O3 were introduced into both the coal molecular model and the kaolinite model, thereby forming a material–gas system, as depicted in Figure 6. Adsorption simulations conducted under varying pressures using a gas permeation model demonstrated that, at 298K, an increase in pressure significantly influences gas permeation through the coal gangue slit model. Based on the calculations of continuous isodensity and potential energy of five gas molecules O2, N2, SO2, NO2, and O3 in the coal model and the coal gangue slit model at the temperature of 298 K, the blue areas in Figure 6 represent the low-energy regions of gas molecule adsorption, while the red areas represent the high-energy regions of gas adsorption. The larger the display area and the darker the blue zone, the more adsorption sites there are and the lower the energy required, making it easier for gas molecules to adsorb. Conversely, the smaller the displayed area and the darker the red zone, the less likely the gas molecules are to be adsorbed. By analyzing the isosteric heat of adsorption in conjunction with potential energy and the isodensity surface distribution area, it was observed that five types of gas molecules display a consistent adsorption capacity pattern in both coal and coal gangue, ranked as SO2 > O2 > O3 > N2 > NO2. The energy distribution at adsorption equilibrium further confirmed these findings, indicating that the adsorption capacity for SO2 surpasses that of the other gas molecules. Additionally, when examining the effect of temperature on the permeation coefficient, the diffusion coefficient, solubility coefficient, permeation coefficient, and isosteric heat of adsorption were assessed across different temperatures. The results revealed that while SO2 molecules exhibit the weakest diffusion capability, they possess the highest solubility, reinforcing the established pattern. Increasing the temperature aids in the permeation and diffusion of gases, with the rate of permeation being contingent upon the physicochemical properties of the model’s surface, and the adsorption of most gas molecules is primarily physical. In the study of the effects between gas molecules, the gas molecules in the coal model were divided into three groups for comparative study: O2 + N2 (ambient air), SO2 + NO2 + O3 (pollutant gases), and O2 + N2 + SO2 + NO2 + O3 (mixed gases). It was discovered that the permeation and diffusion of gas molecules in coal and coal gangue are influenced by their mixed components. In a mixed gas system, there is a synergistic effect on the diffusion of gas molecules and a hindering effect on adsorption. Moreover, the more components there are in the mixed gas, the stronger the permeation effect of each gas molecule.
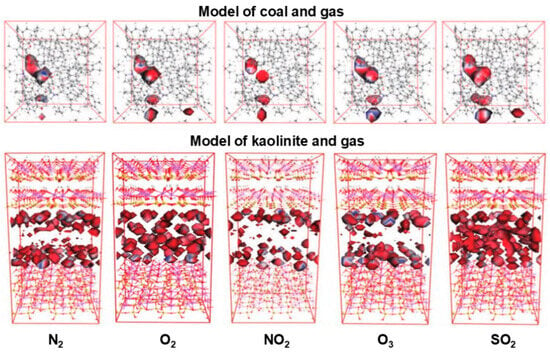
Figure 6.
Isopycnic distribution of gas molecules permeation diffusion in coal structure and gangue slit structure. Figure adapted with permission from Ref. [53] (Copyright 2023 Coal Science Group Shenyang Research Institute Co., LTD).
Current investigations of coal gangue are predominantly limited to macroscopic experiments or isolated simulations of kaolinite; consequently, the coupled influence of organic carbon and kaolinite on gas adsorption and spontaneous combustion remains insufficiently characterized. Zhang et al. [54] offer a comprehensive analysis of CO gas dynamics within coal gangue slits, highlighting the critical role of coal’s carbon content in gas adsorption and diffusion processes. As shown in Figure 7, a novel kaolinite–coal–kaolinite (KCK) model was constructed by integrating the Hongqingliang (HQL) coal macromolecular framework with the Bish kaolinite structure to accurately represent the fracture architecture of coal gangue. Molecular dynamics (MD), reactive force-field (ReaxFF), grand canonical Monte Carlo (GCMC), and conventional Monte Carlo (MC) simulations were integrated to probe gas adsorption at the atomic scale. The Freundlich isotherm was subsequently employed to quantify adsorptive capacity; the kaolinite–coal–kaolinite (KCK) model exhibited a CO adsorption capacity 110.4–132.9% higher than that of the reference tri-kaolinite (TriK) framework. The simulation results indicate that CO production is intricately linked to the decomposition of ether and phenol within the organic structure of coal, with reduced oxygen levels accelerating CO generation. The KCK model’s carbon layer provides additional adsorption sites, leading to a higher adsorption capacity and a more accurate reflection of gas dynamics within the gangue. The study also finds that the diffusion coefficient of CO in the KCK model is lower than in the TriK model, with temperature playing a significant role in influencing CO diffusion rates. By integrating these theoretical calculations, the research provides a robust framework for understanding the adsorption and diffusion of CO in coal gangue. The findings are pivotal for managing the risks associated with spontaneous combustion in coal waste piles and for developing strategies to enhance coal mine safety and waste management practices. The comprehensive approach of this study, combining theoretical models with practical implications, contributes to the field’s knowledge base and informs future research directions in coal gangue management. While DFT and MD studies excel in characterizing initial adsorption, a critical gap persists in modeling large-scale pile combustion dynamics. The integrated model is needed currently to bridge quantum-scale reactions to macro-scale heat/mass transfer, hindering risk prediction capabilities.
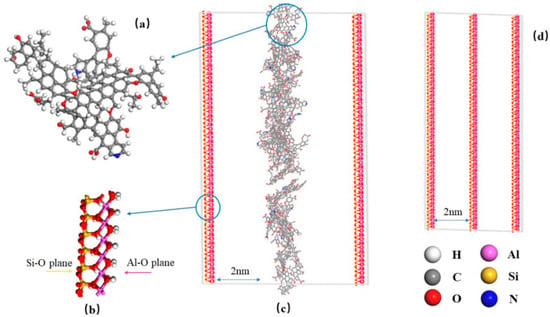
Figure 7.
Coal and gangue slit model used in simulation of CO production and adsorption. (a) Optimized model of HQL coal, (b) kaolinite crystal model, (c) KCK slit model, and (d) TriK slit model. Figure adapted with permission from Ref. [54] (Copyright 2024 Royal Society of Chemistry).
3.2. Mechanisms of HM Migration in Coal Gangue
Coal gangue is enriched with various HM ions. During prolonged stockpiling, these ions can be progressively mobilized and transported into adjacent soils and groundwater, leading to their accumulation in mining districts and subsequent exceedances in downstream surface waters [55,56]. The attendant risk of diffuse secondary contamination threatens local ecosystems and undermines prospects for the sustainable valorization of coal gangue. Consequently, elucidating the environmental fate and ecological impacts of HM release from coal gangue is imperative for its safe reuse.
Traditional extraction methods provide empirical measurements of HM speciation, without quantifying binding affinities, thereby constraining the reliability of long-term risk assessments. Dong et al. [23] investigated the release and migration behaviors of six HMs (Cd, As, Pb, Ni, Cu, and Cr) using a combined experimental and computational approach, as shown in Figure 8. It was found that they exhibited significant differences in speciation distribution and binding energy but underwent similar release behaviors. Coal gangue is predominantly composed of kaolinite, α-quartz, and anatase. Sequential extraction shows that heavy metals (HMs) are primarily sequestered within stable silicate phases and Fe–Mn oxides. Toxicity characteristic leaching procedure (TCLP) analysis classifies Cu, Cr, Ni, and Pb as highly toxic; consequently, continuous monitoring and containment are warranted. Meanwhile, the initial concentration of HMs in CG plays a dominant role in short-term release and risk, while the presence state also significantly impacts migration and long-term risk. To elucidate the migration pathways of heavy metals (HMs) in coal gangue, periodic density-functional theory (DFT) calculations were performed to quantify binding energies (E_b) and preferred adsorption sites on the principal mineral constituents—kaolinite, α-quartz, and anatase. Structural models of each mineral were constructed, and the six target HM cations were placed at distinct surface positions to evaluate E_b. Across all surfaces, Cr3+ and As3+ exhibited the highest E_b, followed by Cu2+ and Ni2+, whereas Pb2+ and Cd2+ displayed the weakest interaction. On the kaolinite (0 0 1) surface, coordination to surface hydroxyl groups dominates HM binding, whereas inner-surface hydroxyls contribute negligibly. For α-quartz (0 0 1), electrostatic attraction between HMs and the bridging oxygen atoms of SiO2 tetrahedra is the primary interaction. Adsorption on the anatase (0 0 1) surface is governed by a combination of van der Waals forces and electrostatic interactions. In addition, silicate frameworks can immobilize HMs through lattice incorporation and precipitation, further enhancing stability. Theoretical predictions corroborate sequential extraction data: HMs exhibiting the highest E_b (51.95, 46.36, 43.22, and 35.90 eV) display the lowest proportion of readily mobilized fractions (2%).
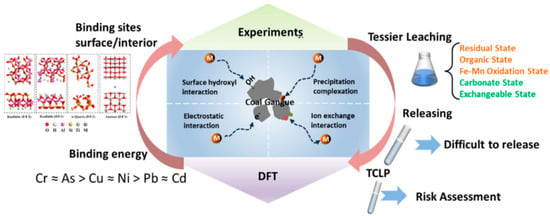
Figure 8.
Integrated experimental–computational workflow for elucidating the environmental fate and potential impacts of heavy metals in coal gangue. Figure adapted with permission from Ref. [23] (Copyright 2024 Elsevier B.V.).
Coal gangue is rich in kaolinite, and kaolinite, as a common mineral adsorbent, is effective in reducing the hazards of HM ions [57,58]. Zhao et al. [59] conducted a theoretical study on the adsorption of HMs Cd, Cu, Hg, and Ni(II) on the kaolinite (0 0 1) surface using DFT calculations. The adsorption of Cd(II), Cu(II), Ni(II), and Hg(II) on the kaolinite (0 0 1) surface was systematically examined across coverages θ = 0.11–1.0 ML (Figure 9). Cd(II) preferentially occupies two-fold bridge sites, followed by one-fold top sites; Cu(II) and Ni(II) favor the top sites, whereas Hg(II) adopts three-fold hollow positions. Adsorption energies (Eads) increase monotonically with θ for Cd, Cu, and Hg, indicating stabilizing lateral interactions and island formation. Conversely, Eads for Ni(II) decrease at higher θ, consistent with repulsive Ni–Ni interactions. The intrinsic affinity sequence is Ni(II) > Cu(II) > Cd(II) > Hg(II). Partial density of states (PDOS) analyses reveal pronounced charge redistribution, confirming the formation of both ionic and covalent bonds between the adsorbates and surface oxygen atoms. The findings provide a deeper understanding of the microscopic adsorption mechanisms of HM atoms on the kaolinite surface and contribute to the development and optimization of kaolinite as an adsorbent for environmental remediation applications.
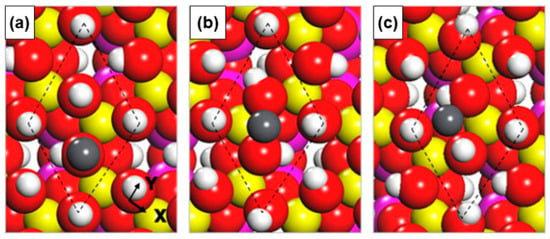
Figure 9.
Top views of divalent heavy metal adsorption on the kaolinite (0 0 1) surface: (a) Cd2+/Cu2+/Ni2+ at atop sites, (b) Cd2+/Hg2+ at bridge sites, and (c) Hg2+ at hollow sites. Adsorbed metal atoms are rendered in dim gray for clarity. Figure adapted with permission from Ref. [59] (Copyright 2014 Elsevier B.V.).
In the coal or coal gangue power generation, HM ions are prone to enrichment in fine particulate matter and escape from pollution control devices into the atmosphere, constituting a significant source of air pollution [60]. Therefore, it is essential to explore the migration issues of HM ions during the combustion process, and kaolinite is commonly added as an ion adsorbent. The computational chemistry strategies provide sustainable management solutions by employing quantum chemistry to explore the mechanisms of interaction between coal gangue and heavy metal species. Cheng et al. [61] employed an integrated experimental–theoretical approach to elucidate the adsorption behavior and mechanistic origins of Pb- and Cd-containing vapors on Si/Al-based sorbents. Adsorbate species—Pb, PbCl, PbCl2, Cd, CdCl, and CdCl2—were modeled on the metakaolinite (0 0 1) surface, and adsorption energies together with charge-density-difference analyses were evaluated. Chemisorption was identified as the dominant interaction mode, with Pb-bearing species exhibiting consistently higher adsorption energies than their Cd counterparts. Surface-exposed oxygen atoms and under-coordinated aluminum centers act as primary active sites: Pb/Cd atoms coordinate to surface O sites, while Cl atoms interact strongly with unsaturated Al atoms, thereby facilitating the retention of Pb and Cd chlorides by metakaolinite. Through the application of frontier orbital theory and DFT calculations, Li et al. [62] elucidated the adsorption characteristics and active sites involved in the capture of semivolatile heavy metal elements during coal combustion. The computational models revealed that metakaolin, a thermal transformation product of kaolinite, exhibits stronger adsorption activity than kaolinite itself, with PbO being more readily adsorbed than PbCl2 onto the kaolinite (DeOH 001) surface. The dehydroxylation process of kaolinite and the subsequent changes in aluminum coordination numbers have been shown to significantly influence the adsorption activity, highlighting the importance of understanding the thermal conversion processes of coal gangue minerals. Yang et al. [63] conducted a comprehensive study on the efficient capture and characteristics of HMs (Pb, Cd, Cr, and Zn) emanating from coal combustion using modified kaolinite as illustrated in Figure 10. They employed a combination of experimental methods and DFT simulations to explore the adsorption behaviors and mechanisms. Utilizing Frontier Molecular Orbital (FMO) analyses, they elucidated the adsorption of HM oxides and chlorides onto original kaolin (OKA), metakaolin (MKA), θ-Al2O3, and α-Al2O3. Competitive adsorption was observed between oxides and chlorides, with oxides showing a significant advantage. Specifically, for OKA and MKA, the Al-O-Si-H and Al-O-Si surfaces were identified as the most favorable for adsorption. However, it was noted that CdO and CdCl2 showed minimal adsorption on the Si-O surface of OKA, highlighting the selectivity of the adsorbent surfaces towards different HM species. These theoretical findings offer valuable guidance for the development of modified kaolinite additives designed to enhance the capture of HMs in high-temperature furnaces, thereby contributing to the sustainable management of coal gangue and the mitigation of environmental pollution from heavy metal emissions.
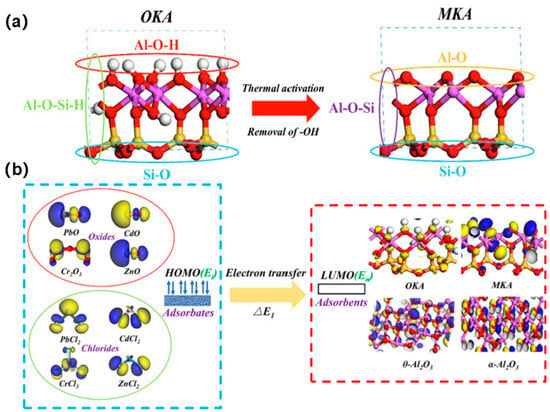
Figure 10.
Efficient capture of heavy metal (HM) vapors by modified kaolinite. (a) Three representative adsorption surfaces on the original kaolinite (OKA) and the modified kaolinite (MKA). (b) Frontier-orbital interactions: electron donation from the highest occupied molecular orbital (HOMO) of the HM species to the lowest unoccupied molecular orbital (LUMO) of the adsorbent. Figure adapted with permission from Ref. [63] (Copyright 2022 Elsevier B.V.).
Unburned carbon in fly ash from power generation is also considered a promising adsorbent [64], and theoretical insights from computational chemistry offer strategic advantages in the utilization of this byproduct from coal combustion. Yan et al. [65] conducted a comprehensive density-functional theory (DFT) study of Hg0 adsorption on defective carbonaceous surfaces to identify the active sites governing mercury capture. The study systematically modeled various defective structures of carbonaceous surfaces, including those with zigzag and armchair edges, to evaluate their adsorption potential. The DFT calculations revealed that defective carbonaceous surfaces exhibit enhanced chemisorption properties compared to non-defective ones, with significant improvements in adsorption energy observed at surface defects. As shown in Figure 11, the electron localization function (ELF) and electron density difference analyses elucidate the nature of interactions between Hg0 and the carbon surface, underscoring the role of vacancies as primary adsorption sites. Furthermore, the study delves into the influence of oxygen-containing functional groups on the adsorption process, finding that semiquinone groups on certain defective surfaces promote chemisorption of Hg0. This theoretical work not only advances the understanding of mercury capture mechanisms on carbon materials but also paves the way for the development of cost-effective and environmentally benign strategies for mercury removal from coal combustion flue gas, thereby contributing to the sustainable management of coal gangue.
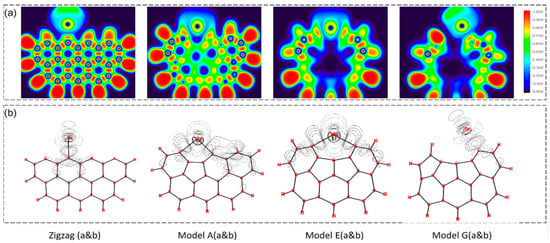
Figure 11.
Mercury adsorption sites on the carbonaceous surface. (a) Electron localization function (ELF) isosurfaces, and (b) electron-density-difference maps showing charge redistribution upon Hg0 adsorption. Figure adapted with permission from Ref. [65] (Copyright 2020 Elsevier B.V.).
Hua et al. [66] systematically studied the adsorption process of PbCl2 on the surface of unburned carbon with different defects and found that the defect adsorption site was the best site for PbCl2 adsorption. In the investigation of oxidized biochar (OBC) derived from weathered coal as a sorbent for Pb(II), Xu et al. [67] determined that the carboxyl (-COOH) group possesses the lowest adsorption energy for Pb(II) and forms markedly more stable chemical bonds than hydroxyl (-OH), carbonyl (-C=O), or ether (-C-O-C-) moieties. These oxygen-containing functional groups enhance Pb(II) uptake by simultaneously promoting electrostatic attraction and enabling the formation of covalent bonds. Xie et al. [68] probed the Pb(II)–biochar interaction at the molecular level. ZnCl2-activated biochar exhibited a polyaromatic scaffold (C47H23NO12) with an extensive microporous network and a high density of oxygenated surface groups. Adsorption preferentially occurs at electron-rich oxygen sites, where Pb2+ is stabilized by cooperative weak ionic bonding with aldehydic moieties, van der Waals interactions with aromatic rings, and cation–π effects.
Alkaline metals are ubiquitously present across various types of coal, with particularly high concentrations found in certain coals known as high-alkali coals [69,70]. High-alkali coals are commonly blended with Si/Al-rich coals to suppress ash-related fouling and slagging during combustion [71,72]. Kaolinite and its dehydroxylated analogue, metakaolin, have proven highly effective in scavenging alkali metal vapors [73,74,75]. Consequently, kaolinite faces simultaneous exposure to both alkali metals and heavy metals (HMs), mandating an improved mechanistic understanding of competitive adsorption and the rational design of strategies that favor selective HM capture. Luo et al. [76] quantified the influence of alkali metals (Na and Ca) on the adsorption of Cu, Zn, Pb, and Cd by kaolinite during coal combustion. Density-functional theory (DFT) calculations reveal that NaCl and Na2O suppress HM uptake on the metakaolin (0 0 1) surface, whereas the CaO-modified surface sustains high adsorption capacities. Charge-density analysis identifies Ca as the preferential anchoring site for oxygen atoms of oxidized HM species, thereby stabilizing the adsorbate.
These computational studies not only enhance our understanding of the complex interactions between alkaline metals and HMs on kaolinite surfaces but also provide a theoretical foundation for developing strategies to mitigate the environmental impact of HM emissions from coal combustion. The integration of experimental data with DFT simulations offers a robust approach to guide the sustainable management of coal gangue by elucidating structure–property relationships that inform the selection of conditions for HM adsorption and retention, thereby supporting the development of cleaner coal utilization technologies. With respect to heavy metal migration and solid-phase control, the literature reveals a pronounced pivot from single-metal adsorption studies toward multi-component competitive complexation models incorporating Al-(oxyhydr)oxide surface terminations. However, current DFT-derived complexation energies are overwhelmingly reported for idealized (hydr)oxide slabs, leaving the influence of nanoporous carbon matrices, ubiquitous in weathered gangue, largely unexplored. We pinpoint this omission as an opportunity for reliable risk assessment and stabilization design from computational chemistry.
3.3. Modified Coal Gangue with Enhanced Activity
To enhance the value of coal gangue and promote resource utilization, various modification methods have been extensively studied as shown in Figure 12, including mechanochemical modification [77], acid or alkali treatment [78], surface organic modification [79], calcination [80], hydrothermal modification [81], and composite modification. These methods aim to alter the physicochemical properties of coal gangue, thereby improving its reactivity and stability and expanding its potential applications across various fields. Computational chemistry offers theoretical support and predictive tools for the modification of coal gangue by simulating and calculating the electronic structure of molecules and materials. Furthermore, the application of computational chemistry is also reflected in the assessment of thermodynamic and kinetic stability during the coal gangue modification process, which is crucial for designing and developing novel modification methods.
Figure 12.
Typical modification methods of coal gangue.
Calcination modification refers to the process of transforming the low-surface-activity kaolinite in coal gangue into highly active metakaolin through high-temperature calcination. This process alters the porosity and crystal structure within the coal gangue. The extent of calcination modification is primarily influenced by the calcination temperature and duration. Variations in these two key factors lead to different phase transformations in the kaolinite within the coal gangue, resulting in performance disparities in the calcined coal gangue. High-temperature calcination can remove carbon components from the coal gangue and hydroxyl groups from kaolinite, leading to changes in the Si-O and Al-O tetrahedral structures, significantly enhancing the activation level of the coal gangue [82]. After reasonable treatment, it can be transformed into beneficial catalysts and electrode materials [83]. The various adsorption sites on the surface can participate in interfacial reactions to construct materials with high electrochemical activity [84,85]. Sun et al. [86] prepared a coal gangue-derived MoO2–coal gangue electrocatalyst, which serves as the cathode for lithium–oxygen batteries (LOBs) as shown in Figure 13. The LOB assembled with the MoO2–coal gangue cathode exhibits excellent rate performance and long-lasting cycling stability. The reason is that the activated coal gangue with an amorphous flake-layer structure acts as a protective layer, optimizing the structural stability of MoO2–coal gangue, providing ample space for product deposition, and uniformly loading N-doped MoO2 nanoparticles and coal gangue, which are conducive to optimizing catalytic performance and serving as substrates to ensure structural stability. To clarify the intrinsic properties of MoO2–coal gangue composites and to assess how N-doped MoO2 influences the formation pathway of LiO2 intermediates, periodic density-functional theory (DFT) calculations were conducted. Amorphous SiO2 present on the gangue surface exhibits semiconducting behavior with a computed band gap of 1.68 eV, whereas N–MoO2@SiO2 displays a metallic character and markedly enhanced electronic conductivity. The N-doped MoO2 nanoparticles accelerate charge transport and increase electrocatalytic activity. Adsorption of LiO2 is pivotal for Li2O2 formation/decomposition and overall Li–O2 battery (LOB) performance; N–MoO2@SiO2 affords an adsorption energy of −2.84 eV, substantially exceeding that of pristine SiO2 (−1.78 eV). This stronger LiO2 anchoring promotes LiO2 oxidation and improves both oxygen reduction (ORR) and evolution (OER) kinetics during cycling. The N–MoO2 nanoparticles thus enhance electronic conductivity and LiO2 adsorption, while the amorphous SiO2 substrate stabilizes the composite architecture. This work provides a new route for valorizing mining solid waste as electrocatalysts for energy storage systems and advances the sustainable utilization of coal gangue.
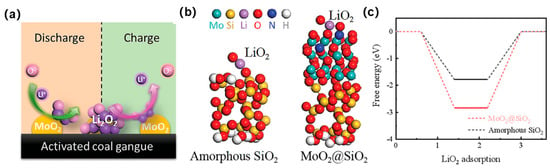
Figure 13.
(a) Mechanism diagram of MoO2@coal gangue-based LOBs during the first cycle. (b) Optimized structures of LiO2 adsorbed on amorphous SiO2 and MoO2@SiO2. (c) Adsorption energy diagrams. Figure adapted with permission from Ref. [86] (Copyright 2023 Wiley-VCH).
Coal gangue primarily consists of kaolinite, quartz, and other minor minerals such as illite and calcite, which affect its reactivity [87]. The presence of high alumina content in coal gangue is linked to the transformation of kaolinite into metakaolinite, which is crucial for enhancing chemical reactivity [88]. Different types of coal gangue exhibit varying mineral compositions, which can influence their physical properties and reactivity [89]. Kaolinite has attracted widespread attention due to its excellent surface properties, such as regular contours, abundant adsorption sites, and rich hydroxyl groups. Zhang et al. [90] explored the utilization of coal gangue for the activation of potassium monopersulfate (PMS) to degrade tetracycline hydrochloride (TC). The research underscores the pivotal role of CG’s surface-bonded and structural hydroxyl groups in facilitating the adsorption of PMS and the generation of active species, which are crucial for TC degradation. DFT results indicate that peroxymonosulfate (PMS) exhibits the highest adsorption affinity for coal gangue-derived carbon (CG) among the tested oxidants—sodium persulfate (PS), potassium persulfate (PDS), and hydrogen peroxide (H2O2). Fukui-function analyses further identify the preferential electrophilic, nucleophilic, and radical attack sites on tetracycline (TC) molecules. CG activates PMS, PS, PDS, and H2O2 for TC degradation without external energy input, underscoring the broad applicability of the CG/PMS system in pollutant remediation. In addition, Dong et al. [91] presented a comprehensive study on the photocatalytic potential of coal gangue (CG) for antibiotic degradation, in which theoretical insights gained from DFT and quantitative structure–activity relationship (QSAR) models highlight the significance of superoxide anion (⁎O2−) and electron–hole (e−-h+) pairs in TC degradation. The research further proposes that oxygen vacancies on CG’s surface and the presence of hydroxyl groups contribute to the enhanced production of ⁎O2− and facilitate electron–hole separation, respectively. These works contribute to the sustainable management of coal gangue by transforming a waste material into a catalyst for advanced oxidation processes (AOPs).
Surface functionalization of mineral-based composite catalysts derived from coal gangue offers a promising route for sustainable pollutant degradation. Li et al. [92] examined the interfacial coupling between TiO2 and kaolinite and the influence of surface-bound hydrogen, using a CH3COOH-functionalized TiO2/kaolinite model (Figure 14a). Both the pristine and CH3COOH-functionalized composites exhibit a direct band gap, which facilitates enhanced charge carrier mobility and accelerates reaction kinetics. The introduction of hydrogen altered the orbital constitution, increasing the proportion of d and f orbitals, which is favorable for electron transition or migration. Zhang et al. [93] focused on the hybrid catalysts (CN-CGs) derived from coal gangue and graphitic carbon nitride (g-C3N4), as shown in Figure 14b, which are designed to activate peroxymonosulfate (PMS) for the degradation of bisphenol A (BPA). The introduction of double-nitrogen defects, cyano groups, and nitrogen vacancies into the g-C3N4 framework, facilitated by the hydroxyl groups of CG, results in a narrowed band-gap energy and enhanced catalytic activity. By leveraging the properties of kaolinite and integrating it with FeOx/MnOy, this formed FeMn@kaolinite, a catalyst with enhanced activity for peroxymonosulfate (PMS) activation, as shown in Figure 14c [94]. Zhai et al. [95] employ DFT calculations to elucidate the mechanism behind PMS activation and the degradation pathways of polyvinyl alcohol (PVA), which reveals the role of oxygen vacancies in facilitating electron transfer and the interaction between PVA and the catalyst. The computational chemistry perspective provides insights into the sustainable utilization of coal gangue by transforming it into a functional material that can effectively degrade pollutants, thereby offering an environmentally friendly solution for waste management and contamination control.
Figure 14.
The models of composite catalysts based on natural minerals. (a) TiO2/kaolinite model. (b) the hybrid model (CN-CGs) derived from coal gangue and graphitic carbon nitride (g-C3N4). (c) FeMn@kaolinite model. Figure adapted with permission from Ref. [92] (Copyright Elsevier Inc.), Ref. [93] (Copyright 2020 Elsevier B.V.), and Ref. [94] (Copyright 2020 Elsevier B.V.).
Alkaline metal-catalyzed gasification has emerged as a novel approach for the clean and efficient utilization of coal gangue and biomass [96]. Theoretically, alkali activation is feasible for any material composed of silicon and aluminum [97]. Miao et al. [98] conducted a comprehensive study combining experimental and theoretical analyses to investigate the effects of Na2CO3 and Na2SO4 catalysts on the catalytic gasification reactivity and mineral structure of coal gangue. To precisely elucidate the differences between Na2SO4 and Na2CO3 on the structure of coal gangue, DFT calculations were employed to determine the bond lengths and Bader charges when kaolinite interacts with alkali metals. The interaction of coal gangue with Na2SO4 results in longer Al-O bond lengths of 1.97Å and 1.89Å compared to 1.76Å with Na2CO3, indicating that Na2SO4 promotes the depolymerization of the aluminum–oxygen framework, which facilitates aluminum extraction. This structural modification makes the gasification ash structure with added Na2SO4 more favorable for aluminum removal. Catalytic gasification theory posits that alkali metals, particularly Na, accelerate the process via the formation of C–O–Na ensembles at carbon edge sites. Electron density is transferred from the negatively charged edge carbon of the char to Na+, weakening C–C and C–O bonds and thereby lowering the activation energy for gasification. This insight highlights the role of Na2SO4 in enhancing gasification reactivity and aluminum extraction, underscoring its potential in the valorization of coal gangue. Activation strategies for value-added utilization have transitioned from simple alkaline roasting to mechanochemically driven defect engineering coupled with heteroatom doping. Meanwhile, there is the absence of validated structure–activity relationships that connect quantified defect densities (e.g., EPR-derived •O−/g) with catalytic or adsorptive performance, thereby preventing rational scale-up. Computational simulations elucidate the mechanisms by which selected modification agents alter the surface reactivity of coal gangue under defined process conditions, providing mechanistic insight to inform subsequent experimental work.
4. Conclusions and Prospects
Overall, this article provides a comprehensive review of the application of current theoretical calculation research on the utilization of coal gangue. Various computational methods and models, including DFT and MD, have been employed to investigate the properties and reactivity of coal gangue, providing a more nuanced understanding of the chemical transformations involved in its utilization, facilitating the development of more efficient and environmentally friendly processes. Future research should combine materials science, environmental science, and computational chemistry, leveraging advances in high-performance computing and machine learning to improve coal gangue modeling and utilization strategies. These insights will advance sustainable management practices, support cleaner coal technologies, and contribute to environmental protection and resource optimization in the coal industry. The theoretical insights gained from computational chemistry strategies are pivotal for advancing our understanding of coal gangue utilization and for devising sustainable management practices. The application of computational chemistry in coal gangue modification not only enhances its utilization value but also promotes the further development and refinement of coal gangue resource utilization technologies. With the continuous advancement of computational chemistry methods, their application in coal gangue catalytic activation holds broad prospects and is expected to provide new ideas and solutions for the high-value utilization of coal gangue. These efforts will clarify the underlying mechanisms for cleaner coal utilization and provide a mechanistic basis for environmental protection and resource recovery in the coal industry.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17157135/s1. Figure S1: Keyword co-occurrence cluster map for coal-gangue research; Figure S2: Top 15 keywords with the strongest citation bursts in coal-gangue research.
Author Contributions
X.W. and X.N. designed the review. X.M. and K.Z. supervised the project and oversaw all manuscript work. X.Z. helped with the grammar checking of the entire manuscript. All the authors contributed significantly to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 42177037 and 22373113), the Beijing Natural Science Foundation (No. 2232019), and the Fundamental Research Funds for the Central Universities (No. 2023ZKPYHH01).
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leclerc, H.O.; Erythropel, H.C.; Backhaus, A.; Lee, D.S.; Judd, D.R.; Paulsen, M.M.; Ishii, M.; Long, A.; Ratjen, L.; Bertho, G.G.; et al. The CO2 tree: The potential for carbon dioxide utilization pathways. ACS Sustain. Chem. Eng. 2021, 9, 10318–10325. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zeng, Q. Research on coal gangue recognition based on multi-source time–frequency domain feature fusion. ACS Omega 2023, 8, 25221–25235. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Song, W.; He, Z.W.; Li, J.R.; Ding, Z.W.; Li, S.Y. Research progress on the pathway and mechanism of high-value-added utilization of coalgangue in China. Mod. Chem. Ind. 2025, 1–8. [Google Scholar]
- Li, X.Y.; Qiao, Y.J.; Shao, J.H.; Bai, C.Y.; Li, H.Q.; Lu, S.; Zhang, X.H.; Yang, K.; Colombo, P. Sodium-based alkali-activated foams from self-ignition coal gangue by facile microwave foaming route. Ceram. Int. 2022, 48, 33914–33925. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, Z.; Li, Z.; Xu, J.; Peng, H.; Guan, J.; Zhou, F.; Huang, J.; Zhua, Y. Properties of coal gangue used in building materials in wulanmulun coalfield, inner mongolia, China. Adv. Mater. Eng. 2015, 108–114. [Google Scholar]
- Li, N.; Han, B.Q. Chinese research into utilisation of coal waste in ceramics, refractories and cements. Adv. Appl. Ceram. 2006, 105, 64–68. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wu, H.J.; Dong, Z.F.; Wang, Q.Q.; Chen, X.Y. Life cycle energy use efficiency and greenhouse gas emissions of circulating fluidized bed coal-fired plant with coal gangue and coal co-combustion. Environ. Dev. Sustain. 2023, 26, 20049–20071. [Google Scholar] [CrossRef]
- Peng, H.; Jia, X.L. Experimental study on heat energy recovery and utilization of coal gangue hill based on gravity heat pipe. Energy Rep. 2022, 8, 220–229. [Google Scholar] [CrossRef]
- Luo, C.; Li, S.H.; Ren, P.Y.; Yan, F.; Wang, L.; Guo, B.; Zhao, Y.M.; Yang, Y.; Sun, J.; Gao, P.C.; et al. Enhancing the carbon content of coal gangue for composting through sludge amendment: A feasibility study. Environ. Pollut. 2024, 348, 123439. [Google Scholar] [CrossRef]
- Zhao, G.W.; Wu, T.; Ren, G.Z.; Zhu, Z.; Gao, Y.; Shi, M.; Ding, S.J.; Fan, H.H. Reusing waste coal gangue to improve the dispersivity and mechanical properties of dispersive soil. J. Clean. Prod. 2023, 404, 136993. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Chen, L.Z.; Wu, S.; Liu, Y. Opportunities, challenges and modification methods of coal gangue as a sustainable soil conditioner—A review. Environ. Sci. Pollut. Res. 2024, 31, 58231. [Google Scholar] [CrossRef]
- Shen, L.; Liu, W.B.; Zhou, W.; Zhu, J.B.; Qiao, E.L. Recycling coal from coal gangue. Filtr. Sep. 2017, 54, 40–41. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, Y.; Liu, X.; Liu, M.; Liao, L.B.; Liu, G.C. Environmental hazards and comprehensive utilization of solid waste coal gangue. Prog. Nat. Sci. Mater. Int. 2024, 34, 223–239. [Google Scholar] [CrossRef]
- Keskin, T.; Yilmaz, E.; Kasap, T.; Sari, M.; Cao, S. Toward viable industrial solid residual waste recycling: A review of its innovative applications and future perspectives. Minerals 2024, 14, 943. [Google Scholar] [CrossRef]
- Ju, T.Y.; Han, S.Y.; Meng, Y.; Jiang, J.G. High-end reclamation of coal fly ash focusing on elemental extraction and synthesis of porous materials. ACS Sustain. Chem. Eng. 2021, 9, 6894–6911. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, L.; Dong, C.X.; Qi, Z.; Zhang, Z.R.; Li, J.S. Comprehensive study on coal gangue-based geopolymer activated by phosphoric acid: From macroscale properties to molecular simulation. Constr. Build. Mater. 2024, 438, 137271. [Google Scholar] [CrossRef]
- Huang, Z.C.; Cai, Y.T.; Fan, X.L.; Ning, K.; Yu, X.H.; Zheng, S.C.; Chen, H.S.; Xie, Y.L. Synthesis of 4A zeolite molecular sieves by modifying fly ash with water treatment residue to remove ammonia nitrogen from water. Sustainability 2024, 16, 5683. [Google Scholar] [CrossRef]
- Ma, X.; Ding, C.L.; Yang, H.S.; Zhu, X. Effects of a cellulose aerogel template on the preparation and adsorption properties of coal gangue-based multistage porous ZSM-5. Materials 2023, 16, 3896. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, H.; Liu, J.; Xia, Y.; Xu, M.; Xing, Y.; Li, J.; Gui, X. Improving flotation decarbonization efficiency of coal gasification fly ash by mechanically breaking pore: An experimental and molecular dynamics simulation study. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 663, 131074. [Google Scholar] [CrossRef]
- Gao, F.; Dong, L.P.; Xue, Z.H.; Cai, S.J.; Fan, M.Q.; Hao, B.; Fan, P.P.; Bao, W.R.; Wang, J.C. Dodecylamine enhanced coal gasification fine slag flotation and its molecular dynamics simulation. Miner. Eng. 2023, 203, 108322. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Chen, X. Adsorption and diffusion characteristics of CO2 and CH4 in anthracite pores: Molecular dynamics simulation. Processes 2024, 12, 1131. [Google Scholar] [CrossRef]
- Xue, Z.H.; Feng, Y.L.; Li, H.R.; Xu, C.L.; Ju, J.R.; Dong, L.P.; Bao, W.R.; Wang, J.C.; Fan, P.P.; Zhu, Z.L.; et al. Molecular simulation investigation of pore structure impact on the wettability and flotation efficiency of coal gasification fine slag. J. Mol. Liq. 2023, 386, 122452. [Google Scholar] [CrossRef]
- Dong, J.H.; Li, J.B.; Huang, Y.; Zhong, J.Y.; Dun, K.; Wu, M.; Zhang, L.J.; Chen, Q.; Pan, B. Understanding the release, migration, and risk of heavy metals in coal gangue: An approach by combining experimental and computational investigations. J. Hazard. Mater. 2024, 416, 132707. [Google Scholar] [CrossRef]
- Lunghi, A.; Sanvito, S. Computational design of magnetic molecules and their environment using quantum chemistry, machine learning and multiscale simulations. Nat. Rev. Chem. 2022, 6, 761–781. [Google Scholar] [CrossRef]
- Chen, L.Z.; Qi, X.Y.; Tang, J.; Xin, H.H.; Liang, Z.Q. Reaction pathways and cyclic chain model of free radicals during coal spontaneous combustion. Fuel 2021, 293, 120436. [Google Scholar] [CrossRef]
- Lin, Y.C.; Si, M.Y.; Han, H.J.; Jin, H.; Long, Y.L.; Deng, L.; Gong, Z.L.; Feng, W.; Nie, T.; Xu, X.Q.; et al. Advances in studies investigating the comprehensive utilization of coal gangue: A visualization analysis based on CiteSpace. Discov. Appl. Sci. 2025, 7, 38. [Google Scholar] [CrossRef]
- Chen, C.M. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Assoc. Inf. Sci. Technol. 2014, 57, 359–377. [Google Scholar] [CrossRef]
- Maruthupandian, S.; Chaliasou, A.; Kanellopoulos, A. Recycling mine tailings as precursors for cementitious binders - Methods, challenges and future outlook. Constr. Build. Mater. 2021, 312, 125333. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xu, L.; Seetharaman, S.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2015, 48, 2779–2793. [Google Scholar] [CrossRef]
- Xue, H.F.; Dong, X.S.; Fan, Y.P.; Ma, X.M.; Yao, S.L. Study of structural transformation and chemical reactivity of kaolinite-based high ash slime during calcination. Minerals 2023, 13, 466. [Google Scholar] [CrossRef]
- Liu, F.Q.; Xie, M.Z.; Yu, G.Q.; Ke, C.Y.; Zhao, H.L. Study on calcination catalysis and the desilication mechanism for coal gangue. ACS Sustain. Chem. Eng. 2021, 9, 10318–10325. [Google Scholar] [CrossRef]
- Wang, R.Y.; Song, Y.; Yang, X.; Zhou, J.H.; Jiang, Q.Q.; Wang, Z.B.; Wang, L.; Peng, B.; Song, H.T.; Lin, W. Self-combustion–Depolymerization approach to activate solid-waste coal gangue minerals for fluid catalytic cracking catalyst synthesis. ACS Sustain. Chem. Eng. 2022, 10, 11376–11386. [Google Scholar] [CrossRef]
- Ren, H.Y.; Mao, R.Y.; Wu, H.W.; Liang, X.; Zhou, J.R.; Zhang, Z.J. Preparation and properties of phosphogypsum-based calcined coal gangue composite cementitious materials. Case Stud. Constr. Mater. 2024, 21, e03963. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, H.Y.; Zhang, Z.Y.; Guo, X.L.; Du, H.B.; Han, W.; Zhuang, Y.X.; Xing, P.F. An improved process for the preparation of Si-Fe-Al-Ca alloy from coal gasification fine slag via three-phase plasma smelting. Process Saf. Environ. Prot. 2024, 192, 907–914. [Google Scholar] [CrossRef]
- Liang, C.; Wang, J.J.; Li, C.Q.; Han, W.; Song, Y.M.; Li, B.; Yin, S.J.; Sun, Z.M. Magnetic coal gangue-based catalysts for peroxymonosulfate activation and benzo[a]pyrene degradation: The construction of Fe and N dual active sites. J. Environ. Chem. Eng. 2024, 12, 114613. [Google Scholar] [CrossRef]
- Guo, C.B.; Song, Y.H.; Ye, M.Y.; Sun, Y.P.; Liang, S.; Zou, J.J. Synthesis of tobermorite using coal fly ash and its utilization in highly efficient CO2 adsorption. Sep. Purif. Technol. 2025, 358, 130382. [Google Scholar] [CrossRef]
- Farwa, M.; Muhammad, Z.; Ijaz, A.B.; Saqib, N.; Tajamal, H. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Luoyang, Y.X.; Wang, H.; Li, J.; Chen, B.; LI, X.; Zhang, G.T. Microstructural tuning and high-efficiency adsorption performance of carbonaceous porous adsorbents from coal gasification fine slag for methylene blue removal. Sep. Purif. Technol. 2025, 357, 130135. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zou, Y.; Jiang, J.J.; Liu, X.L.; Zhu, T.Y. Exploring the promotion effect of low MnCoOx doping for low-temperature NH3-SCR of 13X zeolite synthesized from coal fly ash. Sep. Purif. Technol. 2025, 357, 130064. [Google Scholar] [CrossRef]
- Wang, C.; Feng, K.; Wang, L.P.; Yu, Q.R.; Du, F.L.; Guo, X.L. Characterization of coal gangue and coal gangue-based sodalite and their adsorption properties for Cd2+ ion and methylene blue from aqueous solution. J. Mater. Cycles Waste Manag. 2023, 25, 1622–1634. [Google Scholar] [CrossRef]
- Liu, S.H.; Wei, L.H.; Zhou, Q.; Yang, T.H.; Li, S.B.; Zhou, Q. Simulation strategies for ReaxFF molecular dynamics in coal pyrolysis applications: A review. J. Anal. Appl. Pyrolysis 2023, 170, 105882. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; Russo, M.F.; Van Duin, A.C.T.; Mathews, J.P. Pyrolysis of a large-scale molecular model for Illinois no. 6 coal using the ReaxFF reactive force field. J. Anal. Appl. Pyrolysis 2014, 109, 79–89. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.X.; Bai, J.; Guo, L. Chemical structure effects on coal pyrolyzates and reactions by using large-scale reactive molecular dynamics. Fuel 2022, 327, 125089. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Yang, S.Q.; Zhou, B.Z.; Song, W.X.; Cai, J.W.; Xu, Q.; Yang, K. The variations of free radical and index gas CO in spontaneous combustion of coal gangue under different oxygen concentrations. Fire Mater. 2021, 46, 549–559. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Yang, S.Q.; Zhou, B.Z.; Cai, J.W. Study on spontaneous combustion characteristics of waste coal gangue hill. Combust. Sci. Technol. 2023, 195, 713–727. [Google Scholar] [CrossRef]
- Querol, X.; Zhuang, X.; Font, O.; Izquierdo, M.; Alastuey, A.; Castro, I.; Drooge, B.L.; Moreno, T.; Grimalt, J.O.; Elvira, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Hu, Y.N.; Devegowda, D.; Striolo, A.; Phan, A.T.V.; Ho, T.A.; Civan, F.; Sigal, R. Microscopic dynamics of water and hydrocarbon in shale-kerogen pores of potentially mixed wettability. SPE J. 2015, 20, 112–124. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, Y.B.; Li, Y.Q.; Shi, X.Q.; Zhang, Y.J. Heat effects and kinetics of coal spontaneous combustion at various oxygen contents. Energy 2021, 234, 121299. [Google Scholar] [CrossRef]
- Wang, Q.B.; Ao, L.X.; Zhang, K. Research progress on the key influencing factors of spontaneous combustion of coal gangue and control methods. Clean Coal Technol. 2024, 30, 228–238. [Google Scholar]
- Wang, W.C.; Cao, Z.; Cao, Y.D.; Wu, Z.K.; Su, B.S. Study on adsorption-diffusion-seepage behavior of oxygen in coal gangue under the coupling of temperature and pressure. Mater. Today Commun. 2023, 36, 106817. [Google Scholar] [CrossRef]
- Huang, M.L.; Kang, J.Q.; Li, X.; Liu, X.; Fu, X.H. Microscopic mechanisms of competitive gas adsorption and diffusion during high volatile bituminous coal CO2-ECBM process. Fuel 2026, 404, 136265. [Google Scholar] [CrossRef]
- Wang, H.; Si, J.H.; Wang, C.Y.; Cheng, G.Y.; Zhang, Q.; Tang, X.C. Study on Adsorption Characteristics of CO2/N2 in Bituminous Coal with Different Volatile Contents. J. Chem. Eng. Technol. 2023, 13, 189–198. [Google Scholar]
- Wang, W.C.; Wang, P.; Cao, Z.; Li, J.P. Simulation of gas molecules permeation diffusion in coal structure and gangue slit structure. Safety in Coal Mines 2023, 54, 33–41. [Google Scholar]
- Zhang, J.; Li, Z.; Li, X.P.; Ren, X.P.; Zhou, C.H.; Li, T.Y. Molecular simulation of CO production and adsorption in a coal–kaolinite composite gangue slit model. RSC Adv. 2024, 14, 19301–19311. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Kazi, T.G.; Baig, J.A.; Arain, M.B.; Afridi, H.I. Exposure of heavy metals in coal gangue soil, in and outside the mining area using BCR conventional and vortex assisted and single heavy metals step extraction methods. Chemosphere 2020, 255, 126960. [Google Scholar] [CrossRef]
- Wen, S.; Xu, R.P.; Li, X.J.; Min, X.Y.; Zhang, J.N.; Zhang, H.Z.; Hu, X.; Li, J.Y. Soil reconstruction and heavy metals pollution risk in reclaimed cultivated land with coal gangue filling in mining areas. Catena 2023, 228, 107147. [Google Scholar] [CrossRef]
- Zhou, C.C.; Liu, G.J.; Fang, T.; Wu, D.; Lam, P.K.S. Partitioning and transformation behavior of toxic elements during circulated fluidized bed combustion of coal gangue. Fuel 2014, 135, 1–8. [Google Scholar] [CrossRef]
- Zhou, C.C.; Liu, G.J.; Xu, Z.Y.; Sun, H.; Lam, P.K.S. Retention mechanisms of ash compositions on toxic elements (Sb, Se and Pb) during fluidized bed combustion. Fuel 2018, 213, 98–105. [Google Scholar] [CrossRef]
- Zhao, J.; He, M.C. Theoretical study of heavy metals Cd, Cu, Hg, and Ni(II) adsorption on the kaolinite(001) surface. Appl. Surf. Sci. 2014, 317, 718–723. [Google Scholar] [CrossRef]
- Zhao, S.L.; Duan, Y.F.; Li, Y.N.; Liu, M.; Lu, J.H.; Ding, Y.J.; Gu, X.B.; Tao, J.; Du, M.S. Emission characteristic and transformation mechanism of hazardous trace elements in a coal-fired power plant. Fuel 2018, 214, 597–606. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Huang, Y.J.; Zhu, Z.C.; Yu, M.Z.; Xu, W.T.; Li, Z.Y.; Xiao, Y.X. Experimental and theoretical studies on the adsorption characteristics of Si/Al-based adsorbents for lead and cadmium in incineration flue gas. Sci. Total Environ. 2023, 858, 159895. [Google Scholar] [CrossRef]
- Li, J.F.; Zhong, Z.P.; Ma, Y.Y.; Lai, X.D.; Li, Z.Y. Adsorption mechanism of PbO/PbCl2 on kaolinite surfaces during coal combustion based on frontier orbital theory. Energy Fuels 2020, 34, 11258–11269. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhong, Z.P.; Li, J.F.; Du, H.R.; Li, Q.; Zheng, X.; Qi, R.Z.; Zhang, S.; Li, Z.Y. Low-consumption with efficient capture and characteristics of heavy metals from coal combustion by modified kaolin: Experimental and simulation studies. Fuel 2022, 332, 126094. [Google Scholar] [CrossRef]
- Serre, S.D.; Silcox, G.D. Adsorption of elemental mercury on the residual carbon in coal fly ash. Ind. Eng. Chem. Res. 2000, 39, 1723–1730. [Google Scholar] [CrossRef]
- Yan, G.; Gao, Z.Y.; Zhao, M.L.; Yang, W.J.; Ding, X.L. A comprehensive exploration of mercury adsorption sites on the carbonaceous surface: A DFT study. Fuel 2020, 282, 118781. [Google Scholar] [CrossRef]
- Hua, Q.J.; Wu, G.X.; Xu, W.; Zhao, X.W.; Li, D.; Dong, R.X. Adsorption mechanism of PbCl2 on defective zigzag unburned carbon. J. Fuel Chem. Technol. 2022, 50, 1141–1146. [Google Scholar]
- Xu, Q.; Yan, Y.; Jiao, Y.; Wu, J.X.; Yan, X.L.; Su, X.T. Highly efficient adsorption of Pb(II) by functionalized humic acid: Molecular experiment and theoretical calculation. Materials 2023, 16, 7290. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.W.; Ao, H.; Xu, L.J.; Ao, S.L.; Zhang, T.L.; Li, W.; Yang, Y.H. Quantum chemical DFT-based adsorption mechanism of Pb(II) on a modified biochar. Biomass Convers. Biorefinery 2024, 14, 13547–13562. [Google Scholar] [CrossRef]
- Xue, Z.Y.; Dong, L.; Zhong, Z.P.; Lai, X.D.; Huang, Y.J. Capture effect of Pb, Zn, Cd and Cr by intercalation-exfoliation modified montmorillonite during coal combustion. Fuel 2021, 290, 119980. [Google Scholar] [CrossRef]
- Gao, Z.F.; Long, H.M.; Dai, B.; Gao, X.P. Investigation of reducing particulate matter (PM) and heavy metals pollutions by adding a novel additive from metallurgical dust (MD) during coal combustion. J. Hazard. Mater. 2019, 373, 335–346. [Google Scholar] [CrossRef]
- Tang, C.W.; Pan, W.G.; Zhang, J.K.; Wang, W.H.; Sun, X.L. A comprehensive review on efficient utilization methods of High-alkali coals combustion in boilers. Fuel 2022, 316, 123269. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Cheng, L.M.; Ji, J.Q.; Zhang, W.G. Ash deposition behavior in co-combusting high-alkali coal and bituminous coal in a circulating fluidized bed. Appl. Therm. Eng. 2019, 149, 520–527. [Google Scholar] [CrossRef]
- Li, M.Q.; Zhang, Z.X.; Wu, X.J.; Fan, J.J. Experiment and mechanism study on the effect of kaolin on melting characteristics of zhundong coal ash. Energy Fuels 2016, 30, 7763–7769. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhao, L.; Sun, R.J.; Hu, Y.Y.; Tang, G.T.; Chen, W.F.; Du, Y.B.; Che, D.F. Effects of silicon-aluminum additives on ash mineralogy, morphology, and transformation of sodium, calcium, and iron during oxy-fuel combustion of zhundong high-alkali coal. Int. J. Greenh. Gas Control 2019, 91, 102832. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Lyu, Q.G.; Zhu, Z.P.; Zhang, H.X. The impact of additives upon the slagging and fouling during Zhundong coal gasification. J. Energy Inst. 2020, 93, 1651–1665. [Google Scholar] [CrossRef]
- Luo, J.Z.; Yi, H.C.; Wang, J.Q.; Wang, Z.Z.; Shen, B.X.; Xu, J.; Liu, L.J.; Shi, Q.Q.; Huang, C. Effect of alkaline metals (Na, Ca) on heavy metals adsorption by kaolinite during coal combustion: Experimental and DFT studies. Fuel 2023, 348, 128503. [Google Scholar] [CrossRef]
- Yu, Y.M.; Chen, M.; Zhu, J.M.; Sun, Y.; Zhou, Y.Z.; Chen, X.Y. Effects of mechanochemical modification on adsorption properties of Cd, Cu and Pb speciation in intrinsic heavy metals of coal gangue. Coal Convers. 2025, 48, 116–127. [Google Scholar]
- Liu, C.L.; Xia, J.P.; Fan, H.; Zheng, G.Y.; Liang, Y.F.; Ma, G.; Bai, X.L. Research progress on microwave technology in resource utilization of coal gangue. Appl. Chem. Ind. 2019, 48, 2246–2250. [Google Scholar]
- Zhang, N.; Tang, B.; Liu, X. Cementitious activity of iron ore tailing and its utilization in cementitious materials, bricks and concrete. Constr. Build. Mater. 2021, 288, 123022. [Google Scholar] [CrossRef]
- Xiong, J.B.; Zang, L.; Zha, J.F.; Mahmood, Q.; He, Z.L. Phosphate removal from secondary effluents using coal gangue loaded with zirconium oxide. Sustainability 2019, 11, 2453. [Google Scholar] [CrossRef]
- Han, L.N.; Ren, W.G.; Wang, B.; He, X.X.; Ma, L.J.; Huo, Q.H.; Wang, J.C.; Bao, W.R.; Chang, L.P. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.N.; Yao, X.H.; Han, R.C.; Li, L.L.; Zhang, M. Using chinese coal gangue as an ecological aggregate and its modification: A Review. Materials 2022, 15, 4495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Z.; Liu, J.X.; Wang, J.X.; Liu, X.S.; Liu, Y.J. Research progress in environmental functional materials prepared from coal gangue. Chemistry 2022, 85, 1090–1095. [Google Scholar]
- Sun, Z.H.; Wang, Y.b.; Ma, S.X.; Li, M. Energy storage modification of coal gangue and its application in high-specific-energy batteries. Coal Sci. Technol. 2025, 53, 318–326. [Google Scholar]
- Ding, D.F.; Guo, L.; Mu, Y.D.; Ye, G.T.; Chen, L.G. Use of coal gangue to prepare refractory saggars with superior corrosion resistance and thermomechanical properties for the calcination of Li-Ion battery cathode materials. ACS Sustain. Chem. Eng. 2021, 9, 254–263. [Google Scholar] [CrossRef]
- Sun, Z.H.; Hu, Y.J.; Zeng, K.; Li, M.; Zhao, S.; Zhang, J.X. Turn “Waste” into wealth: MoO2@coal gangue electrocatalyst with amorphous/crystalline heterostructure for efficient Li–O2 batteries. Small 2023, 19, 2208145. [Google Scholar] [CrossRef]
- Yang, C.G.; Yin, J.Q.; Wu, L.Q.; Zeng, Q.Y.; Zhang, L.W. Research on the identification mechanism of coal gangue based on the differences of mineral components. ACS Omega 2023, 8, 48–55. [Google Scholar] [CrossRef]
- Wang, A.; Hao, F.; Liu, P.; Mo, L.; Liu, K.; Li, Y.; Cao, J.; Sun, D. Separation of calcined coal gangue and its influence on the performance of cement-based materials. J. Build. Eng. 2022, 51, 104293. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Hao, Z. The thermal activation process of coal gangue selected from Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Liu, Z.; Du, C. Natural coal gangue activated persulfate for tetracycline hydrochloride degradation: Mechanisms, theoretical calculations, and comparative study. J. Mol. Struct. 2023, 1291, 136097. [Google Scholar] [CrossRef]
- Dong, J.H.; Li, J.B.; Zheng, S.Y.; Chen, Q.; Wu, M.; Yi, P.; Huang, Y.; Pan, B. Understanding the potential of coal gangue as photocatalyst for antibiotic degradation: The role of abundant oxygen vacancies and electron-hole pairs. J. Water Process Eng. 2024, 68, 106382. [Google Scholar] [CrossRef]
- Li, C.Q.; Sun, Z.M.; Dong, X.B.; Zheng, S.L.; Dionysiou, D.D. Acetic acid functionalized TiO2/kaolinite composite photocatalysts with enhanced photocatalytic performance through regulating interfacial charge transfer. J. Catal. 2018, 367, 126–138. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Zhang, N.; Su, Y.; Liu, Z.; Gao, R.; Du, C. Insight to unprecedented catalytic activity of double-nitrogen defective metal-free catalyst: Key role of coal gangue. Appl. Catal. B Environ. 2020, 263, 118316. [Google Scholar] [CrossRef]
- Shuang, Z.; Chen, Y.W.; Zhu, Y.A.; Ge, M.Q. Peroxymonosulfate activated by FeOx/MnOy modified kaolinite for the degradation of polyvinyl alcohol: Catalytic performance, mechanism and DFT study. Appl. Surf. Sci. 2022, 605, 154723. [Google Scholar]
- Zhai, S.; Chen, Y.; Ge, M. Efficient polyvinyl alcohol degradation via peroxymonosulfate activated by natural illite supported CoNi3O4 nanosheets. Chem. Eng. J. 2023, 452, 139155. [Google Scholar] [CrossRef]
- Fermoso, J.; Corbet, T.; Ferrara, F.; Pettinau, A.; Maggio, E.; Sanna, A. Synergistic effects during the co-pyrolysis and co-gasification of high volatile bituminous coal with microalgae. Energy Convers. Manag. 2018, 164, 399–409. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Miao, H.Y.; Wang, Z.Q.; Wang, Z.F.; Sun, H.C.; Li, X.Y.; Liu, Z.Y.; Dong, L.B.; Zhao, J.T.; Huang, J.J.; Fang, Y.T. Effects of Na2CO3/Na2SO4 on catalytic gasification reactivity and mineral structure of coal gangue. Energy 2022, 255, 124498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).