Abstract
The prevention of biofilm formation and algal biodeterioration on building materials, particularly on cultural heritage sites, is a growing concern. Due to regulatory restrictions on conventional algicidal biocides in Europe, natural alternatives such as essential oils are gaining interest for their potential use in heritage conservation. This study evaluates the anti-algal activity of Salvia officinalis and Equisetum arvense (essential oils, hydrolates, and extracts) against a mixed culture of five green algae species (Bracteacoccus minor, Stichococcus bacillaris, Klebsormidium nitens, Chloroidium saccharophilum, and Diplosphaera chodatii). The plant materials were processed using hydrodistillation and solvent extraction, followed by chemical characterization through gas chromatography–mass spectrometry (GC-MS). Biological efficacy was assessed by measuring algal growth inhibition, changes in biomass colour, chlorophyll a concentration, and fluorescence. S. officinalis yielded higher extract quantities (extraction yield: 23%) than E. arvense and contained bioactive compounds such as thujone, camphor, and cineole, which correlated with its strong anti-algal effects. The essential oil of S. officinalis demonstrated the highest efficacy, significantly inhibiting biofilm formation (zones of inhibition: 15–94 mm) and photosynthetic activity at 0.5% concentration (reduction in chlorophyll a concentration 90–100%), without causing visible discolouration of treated surfaces (∆E < 2). These findings highlight the potential of S. officinalis essential oil as a natural, effective, and material-safe algicidal biocide for the sustainable protection of cultural heritage sites.
1. Introduction
Aerophytic algae, also known as terrestrial or aerial algae, thrive in low-moisture environments and are common in terrestrial habitats [1]. They have developed unique metabolic pathways that allow them to survive and reproduce in environments with low water and dissolved minerals, intense sunlight and extreme temperature fluctuations [2,3,4]. They are ecological pioneers, colonizing areas before heterotrophs, and include mainly green algae (Chlorophyta, Streptophyta), with common genera such as Klebsormidium, Bracteacoccus, Apatococcus, Chlorella and Chlorococcum [4,5,6,7,8,9,10].
Green algae form photosynthetic biofilms on a variety of surfaces, including buildings, fences, monuments and other works of art. These biofilms affect the esthetics and structural integrity of the material, and the degree of damage depends on the species, type of material, and environmental conditions [9,11]. Due to the biosynthesis of pigments, most notably chlorophyll a, chlorophyll b and carotenoids, algal colonization is often associated with direct substrate discolouration or the formation of hard to remove colourful patinas and encrustations [10,12,13,14]. Beyond visual changes, affected surfaces absorb more solar radiation and are susceptible to temperature increases [15,16]. With the entrapment of dust particles within the biofilm, staining might progress, reaching darker hues [17]. Conversely, extensively hydrated EPS-rich biofilms formed on floor tiles or pavements might be hazardous, with increased slipperiness. As primary photoautotrophic colonizers capable of atmospheric CO2 fixation, algae function as pioneer organisms on inorganic substrates, establishing favourable microenvironments that facilitate subsequent heterotrophic microbial succession [10,18]. Photosynthetic carbon dioxide sequestration may also induce calcium carbonate precipitation, contributing to biogeophysical substrate modification [19]. Other direct changes include surface peeling, biofilm water absorption, retention and hygrothermic expansion, formation of cracks, microfissure propagation, leeching of elements, cation transfer and oxidation [10,14,18,20,21,22]. Although usually attributed to heterotrophs, some studies also point to the excretion of organic acids by microalgae, affecting the colonized surfaces directly through chemical dissolution or by localized pH modification [20,23,24]. The influence of aerophytic green biofilms on the discolouration, increased water absorption and pH decrease in inorganic building materials was also confirmed in more recent, experimental studies [9,25].
To protect heritage buildings and monuments, conservation methods are essential to maintain structures and surfaces. Prevention of algal growth includes biofilm removal, the use of cleaners, algicidal biocides (algicides) and protective treatments [10]. Some of the most popular physical methods are UV-C [26], heat shock treatment [27], IR irradiation [28] and mechanical cleaning—e.g., dry brushing [29] and scrubbing and washing [30]; chemical methods such as the use of nanoparticles and nanocomposites (NPs) [31], quaternary ammonium compounds (QACs) [32], plant based extracts and isolates [33] and microbial metabolites [34] are also used. Unfortunately, many of the abovementioned techniques are still limited. Basic mechanical treatments such as scrubbing or brushing require specialized care or pose the risk of secondary damage. On the other hand, although usually cost-effective, the efficiency of some biocides depends strongly on the environmental conditions in which they are applied. Moreover some biocides might increase substrate bioreceptivity, especially during repeated treatments [10]. Compounds of natural origin usually show low isolation efficiencies; are less tested than their synthetic, commercially available counterparts; and regularly require immobilization to increase their application potential. Authors have identified algicidal biocides, hydrophobic coatings and antifouling surfaces (e.g., superhydrophobic materials based on silicone and siloxane) as the most effective solutions [35,36,37].
In recent years, the use of algicidal biocides has declined due to their ecotoxic nature. In accordance with Article 16 (2) of Directive 98/8/EC [38], the European Chemicals Agency is carrying out a programme for the systematic examination and withdrawal of active compounds that adversely affect human health and the environment; examples of algicides recently withdrawn from the market are 2-octyl-2H-isothiazol-3-one, isoproturon, carbendazim, pyrithione zinc, and terbutryn. In addition, commercial biocides, e.g., quaternary ammonium compounds, can increase microbial resistance [39]. Moreover, some traditional algicidal biocides, such as hydrogen peroxide, chlorine-based substances, and quaternary ammonium salts, can damage the stone substrate and lead to higher bioadditivity, and they can also oxidize metal ions, causing corrosion [40].
Due to these limitations, new alternatives are currently being explored among compounds of natural origin, such as essential oils (EOs), which exhibit antimicrobial and anti-algae properties [40,41]. EOs, isolated through distillation, contain oxygenated and non-oxygenated terpene hydrocarbons with antimicrobial properties [42]. Hydrolates, by-products of essential oil distillation, include trace levels of water-soluble aromatic compounds from Eos (usually less than 1 g/L), and plant extracts obtained through solvent extraction also exhibit bioactive potential [43]. The chemical composition of EOs varies across different plant species due to factors such as their place of origin, the method of hydro distillation or extraction, and the yield of the obtained oil [44].
Studies indicate that EOs and their components from plants such as Foeniculum vulgare, Lavandula spp., Melaleuca alternifolia, Origanum vulgare L., Rosmarinus officinalis L., Syzygium aromaticum (L.) Merr. & L.M. Perry and Thymus vulgaris L. show promising algicidal activity against genera: Chlorococcum, Chlorella, Apatococcus and Cosmarium [40]. Ostric acid extracted from eelgrass has been identified as an antifouling agent to prevent overgrowth by algae [45]. Studies confirm that EO-based treatments inhibit algal biofilms efficiently at low doses, with rapid effects seen within 24 h [46]. Results of studies on natural algicidal biocides, including EOs, suggest minimal effects on the colour and integrity of the material when applied in limited lower amounts (up to three layers) [47].
Although algal colonization of construction materials is well documented, and essential oils (EOs) have been widely studied for their antimicrobial properties against pathogenic bacteria and fungi, their potential against aerophytic algae remains largely unexplored. To date, no dedicated studies have investigated the effects of EOs, hydrolates, or plant extracts on terrestrial algal species colonizing building materials. In the present study, Salvia officinalis and Equisetum arvense, plants known for their antibacterial and antifungal activity [29,30], were selected as EO sources, although their efficacy against algae has not previously been assessed. Therefore, the aim of this study was to explore the anti-algal properties of EOs from Equisetum arvense and Salvia officinalis as natural algicidal biocides against terrestrial algae, considering their effectiveness and safety for materials.
The study examines essential oils, hydrolates, and extracts from two plant species, Salvia officinalis and Equisetum arvense, against a mixed culture of five terrestrial algae strains. Fluorescence measurements and digital inhibition zone analyses determined the minimum inhibitory (MIC) and algicidal concentrations (MAC). Based on these results, further tests were conducted using S. officinalis EO in liquid culture, measuring biomass optical density, fluorescence, colour change, chlorophyll a concentration, and morphological changes. A study was also conducted on the impact of S. officinalis EO on the colour change of building materials in the context of its application in cultural heritage objects.
2. Materials and Methods
2.1. Materials
2.1.1. Algae Strains
The five non-axenic algae strains Bracteacoccus minor PNK015, Stichococcus bacillaris PNK040, Klebsormidium nitens PNK013, Chloroidium saccharophilum PNK010, and Diplosphaera chodatii PNK021 were used to prepare a mixed culture for inoculation of laboratory medium (BG 11, liquid and solid with 1.5% agar) [48] and building materials. Selected strains were isolated from building materials in previous studies described in [9,25,49].
All algal strains used for the experiment are deposited in the Department of Algology and Mycology, University of Lodz. Biological material was cultivated on BG-11 agar plates under artificial light from fluorescent tubes (Osram FLUORA T8 L 36W/77) of 2800 Lux in a 16 h/8 h day/night period, with a temperature of 22 ± 0.2 °C and air humidity of 50 ± 5%.
Cultures of each algal strain in liquid medium were prepared, and biomass density was determined spectrophotometrically (UV VIS DR6000 spectrophotometer, Hach Lange, Wrocław, Poland) at the wavelength with the largest absorbance peak for each strain (in the range 678–681 nm) to achieve an absorbance of 1.0 AU for each strain.
2.1.2. Building Materials
For building materials representative of Central European architecture, red bricks and thin-layer plaster were selected. Brick samples with average dimensions of 5.0 × 4.0 × 1.5 cm were obtained by cutting commercially available full red bricks (Euroclass A1) to shape. Only the most homogenous specimens were selected for the purpose of this study. Plaster samples without biocides were prepared on demand (courtesy of ATLAS, Łodz, Poland) by applying thin-layer dispersion plaster on a cardboard and primer scaffold. Samples were standardized to the dimensions of 4.3 × 4.3 × 0.3 cm. Prior to the experiment, samples were surface-sterilized with UV light.
2.1.3. Plant Materials
Salvia officinalis and Equisetum arvense (purchased as dried, shredded herbs stalks and leaves from Natur-Vit, Pińczów, Poland) were used in the study. Plant materials were stored at 4 °C until use. For each species, essential oils and hydrolates were obtained by hydro-distillation, and extracts were prepared by ethanol-based solvent extraction.
2.1.4. Hydro-Distillation and Extraction of Plant Materials
Essential oils were obtained by hydro-distillation using a Deryng apparatus (LAB-SZKŁO s.c., Kraków, Poland). For each of three cycles, 60 g of Salvia officinalis and 85 g of Equisetum arvense were distilled, with 1.5 mL of diethyl ether added for E. arvense to improve EO yield. Collected EOs were air-dried to remove diethyl ether and diluted in dimethyl sulfoxide (DMSO, Merck Life Science Sp. z o.o., Poznań, Poland) to 1 mg/mL. Hydrolates were obtained by collecting the water-soluble fraction. Plant extracts were prepared by Soxhlet extraction (~3 g plant material) in ethanol (99.9%) for 3 h, followed by solvent removal via rotary evaporation (Buchi R-215 Rotary Evaporator System, BÜCHI Labortechnik AG, Flawil, Switzerland) and overnight air drying. Dried extracts were weighed, sonicated in DMSO, and diluted to 1 mg/mL. All extractions were performed in triplicate.
2.1.5. Algal Culture with Essential Oil
Algal cultures were grown in liquid M7 medium purchased from Coimbra Collection of Algae, Portugal (ACOI). Cultures (200 mL) were treated with Salvia officinalis EO at 0.1% and 0.5% (v/v) diluted with DMSO, with three replicates per treatment and controls without EO. After inoculation with 5% algal suspension, flasks were incubated on an Endeavor™ 5000 shaker (130 rpm, OHAUS Europe GmbH, Nänikon, Switzerland) under 3.2 Klux light. Salvia officinalis EO was added after 12 days of incubation to the cultures. During the cultivation of algae on a liquid medium with 0.1% and 0.5% concentration of S. officinalis EO, the morphology of algal cells was observed, and the optical density change, colour change, chlorophyll a concentration, and fluorescence were measured.
For the tests performed on the building materials, brick and plaster samples were inoculated with 1 mL of AU = 1.0 mixed algal cultures (each algal species was individually adjusted to AU = 1.0 before mixing in equal volumes (1:1 v/v ratio)) and cultivated for 1-month to enable biofilm formation. Afterwards, on each sample, a 6 mm cellulose disc containing 40 µL of 0.5% Salvia officinalis EO was diluted with DMSO. The volume of EO was increased to counteract rapid evaporation of active compounds and to enable the observation of any undesirable changes in material parameters. After 1, 3 and 7 days the samples were subjected to macroscopic observations, and colour change and fluorescence were measured. Assays were performed against control samples containing biofilm not subjected to EO and non-inoculated materials.
2.2. Materials Characteristics
2.2.1. Gas Chromatography–Mass Spectrometry (GC-MS)
Active chemical compounds in EOs, hydrolates, and extracts were identified by gas chromatography–mass spectrometry (GC-MS) using a Pegasus 4D (LECO, Tychy, Poland) coupled with an Agilent 7890 A GC and time-of-flight (TOF) analyzer. Separation was performed on a BPX5 column (30 m × 0.25 mm × 0.25 μm; Trajan Scientific and Medical, Ringwood, Australia) with helium (1.0 mL/min) as the carrier gas. The oven was held at 50 °C for 2 min, ramped at 8 °C/min to 300 °C, and held for 1 min. The injector was set at 200 °C. MS operated in EI mode at 70 eV, scanning 29–450 amu, with ion source and transfer line temperatures at 200 °C and 250 °C, respectively. A mixture of saturated n-alkanes (C7–C30, 97%) was used for the prediction of retention indices and the identification of compounds. For double-checking purposes, our own databases based on numerous standards were used [50].
2.2.2. Determination of Inhibitory and Biocidal Concentrations (MIC and MAC) of Plant Extracts Against Algal Biofilm
The susceptibility of mixed algal cultures to different concentrations of tested isolates was evaluated based on modified disc-diffusion Minimum Inhibitory Concentration (MIC) and Minimum Algicidal Concentration (MAC) tests [51,52]. For MIC, 100 µL of algal inoculum was applied to BG11 agar, followed by a 6 mm cellulose disc with 20 µL of essential oil, hydrolate, or extract diluted with DMSO. The MAC test used the same method but included a 14-day algal incubation before treatment. Six concentrations (1:19; 3:17; 5:15; 10:10; 15:5; 20:0 µL isolate/µL DMSO) were tested in triplicate including negative controls containing 20 µL pure DMSO. Inhibition zones were analyzed using MATLAB ver. R2024b image processing software, with areas calculated as percentages and diameters converted to mm [53].
2.2.3. Morphological Observations
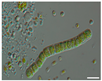
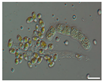
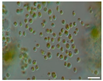
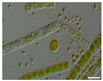
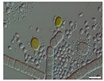
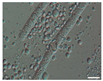
The morphological observations of algal cells in the liquid cultures were performed using a Nikon Eclipse 50i light microscope (Precoptic Co., Warsaw, Poland) equipped with an Opta-Tech digital camera. The observations were conducted at magnifications of 60× and 100×, employing the DIC (Differential Interference Contrast) Nomarski method [25]. The morphological observation was performed to examine the appearance of algal cells before and after the addition of Salvia officinalis EO at concentrations of 0.1% and 0.5%. The analyses were conducted on day 12 of the algal culture, as well as on days 15 and 19 corresponding to 3 and 7 days after the addition of the EO.
2.2.4. Cell Density Measurement
The optical density measurement was performed to determine the algal biomass growth during the incubation periods. A 3 mL sample of biomass from the liquid culture was transferred into a glass cuvette, and the absorbance was measured at 680 nm, corresponding to the highest peak recorded for the mixed algal culture prepared in M7 media [25].
2.2.5. Chlorophyll a Measurement
Chlorophyll a, the primary photosynthetic pigment, was used to assess the effect of essential oils on algal metabolism. Samples (1.5 mL each) were transferred into 2 mL Eppendorf tubes and centrifuged (MPW-351R, MPW, Warsaw, Poland) at 6000 rpm for 2 min. After discarding the supernatant, 100 µL of methanol was added to the biomass, and samples were homogenized for 1 min (Mini-homogenizer E0357-01, EURx, Gdańsk, Poland). Methanol was added to reach 1.5 mL; then, the samples were vortexed (VM300S, Alchem, Toruń, Poland) and incubated overnight at 4 °C. The next day, samples were centrifuged at 6000 rpm for 2 min, and absorbance was measured at 665 nm and 650 nm. Chlorophyll a concentration (mg/mL) was calculated using Formula (1):
where Chl − a: The chlorophyll a concentration (mg/mL); A665: Absorbance value measured at 665 nm; and A650: Absorbance value measured at 650 nm.
The final volume in which chlorophyll a was measured is 3 mL per sample, and the concentration of chlorophyll a is expressed in mg/mL [25].
2.2.6. Fluorescence Measurement
Fluorescence measurements were performed to assess biofilm growth and the photosynthetic efficiency of algal cells using a Handy FluorCam FC 1000 H Pulse-Amplitude Modulation (PAM) fluorimeter (Photon System Instruments, Drásov, Czech Republic) and FluorCam 7 software. Various light types, including measuring flashes, actinic light, saturating pulses, and far-red light, were applied. Light intensities corresponded to incubation conditions. For liquid media, 10 mL of culture was transferred into a 35 mm Petri dish for measurement. For algal cultures cultivated on building materials, fluorescence was measured directly in sterile conditions. All samples were dark-adapted for 30 min prior to measurement. Data analysis included calculation of mean and standard deviation for F0 (minimum fluorescence) and Fm (maximum fluorescence), following established protocols [54].
2.2.7. Colour Change Evaluation
The colour change measurements were conducted in the algal culture in liquid medium and on the surface of tested building materials using an CIE L*a*b* trichromatic colour model employing a portable spectrophotometer CM-700d (Konica Minolta, Warsaw, Poland) and CM-S100w SpectraMagicTM NX v.2.0 software. Illuminant D65 was applied, and measurements were performed in both SCI (Specular Component Included) and SCE (Specular Component Excluded) modes. For the liquid media, the algal culture without essential oil was used as a reference sample. The colour change was measured before inoculation, and subsequently, measurements were taken at additional time points. Each time, 10 measurements were performed for each sample, and the mean value was used for analysis. For liquid cultures, the spectrophotometer was inverted, and the volumetric flask containing the stirred culture was placed directly on the device. Measurements were performed directly on the surfaces of the building materials in sterile conditions. As a reference, identical specimens without algal biofilm were used. To avoid distortion from surface reflections, only data obtained in SCE mode were considered in the analysis. Three replicate samples were used for each material type. Colour change was calculated using Formula (2):
where ΔE—value of colour change between tested and reference sample; ΔL—difference in lightness (white—black) between tested and reference sample (0 represents black, and 100 represents the brightest light possible); Δa—difference in colour between tested and reference sample (green—red; +a = red, −a = green); and Δb—difference in colour (blue—yellow; +b = blue, −b = yellow) between tested and reference sample.
For evaluation of colour change (ΔE) the following scale was used: 0 < E < 1—the difference is not noticeable to the average person; 1 < E < 2—only a trained observer can see the difference; 2 < E < 3.5—an untrained person can notice a difference; 3.5 < E < 5—a clear colour difference is seen; and 5 < E—the viewer sees two distinct colours [55].
3. Results and Discussion
Salvia officinalis showed the highest ethanol extraction yield (23.12%) compared to Equisetum arvense (9.58%) and also outperformed it in hydro-distillation (1.62% for S. officinalis and 0.28% for E. arvense, respectively). The recorded values were consistent with those available in the scientific literature for isolation of different EOs [56,57,58]. Overall, Salvia officinalis is more suitable for both extraction methods due to its higher yield. The compound categories identified by the GC-MS method; the number and type of compounds; and the percentage composition (%) obtained for the hydrolates, extracts and EOs from the plants are presented in the Supplementary Materials (Tables S1–S4). The selected chemical compounds with the highest percentage shares (%) are shown in Table 1. Chromatograms for the analyses of EOs and exemplary mass spectra have also been added to the Supplementary Materials (Figures S1–S4).

Table 1.
Chemical compounds identified from Salvia officinalis and Equisetum arvense with the highest percentage shares [%] identified by GC-MS.
The predominant compounds in the EO, extract and hydrolate of Salvia officinalis are α-thujone (40.19–47.8%), eucalyptol (10.89–20.53%), camphor (11.54–17.62%) and β-thujone (8.69–14.26%) (Table 1), of which thujones is found in the highest concentration and might play a significant role in inhibiting algal growth. Thujone was also identified as the most abundant compound in S. officinalis essential oil in previous literature [59,60,61]. Furthermore, the inhibitory effect may be influenced by the bioactive properties of compounds such as camphor and α-pinene, but also eucalyptol (up to 20.53% identified in the EO and extract). In addition, other terpene compounds were also identified for the different Salvia officinalis variants: humulene, caryophyllene and aromadendrene (up to 6.93% in the extract), and camphene (2.58% in the EO). The composition of active compounds in the obtained EO was close to that recommended by the ISO 9909 [62] regulation for medicinal uses: cis-thujone (18.0–43.0%), camphor (4.5–24.5%), 1,8-cineole (5.5–13.0%), trans-thujone (3.0–8.5%), α-humulene (≤12.0%), α-pinene (1.0–6.5%), camphene (1.5–7.0%), limonene (0.5–3.0%), bornyl acetate (≤2.5%) and linalool + linalyl acetate (≤1.0%) [63,64].
Despite the presence of similar compounds, the Salvia officinalis extract and hydrolate did not show equally high antimicrobial efficacy (Table 2), compared to the EO, which may be due to the lower concentration of active ingredients or their different chemical compositions (Tables S2 and S3).

Table 2.
Evaluation of minimum inhibitory concentration (MIC) on the base of the inhibition zones [mm] of algal culture for Salvia officinalis and Equisetum arvense variants with various concentrations after 21st day of incubation.
In the case of Equisetum arvense, the predominant compounds are carvone (53.81–68.06%) and terpineol acetate (3.95–18.37%). Two aliphatic aldehydes also had a significant percentage share: nonanal and 2-hexenal (up to 8.37%). Relatively high contents were also noted for linalool, eugenol and caryophyllene (up to 4.17, 3.83 and 5.36%, respectively) (Table 1). However, these formulations did not show a significant inhibitory effect on algae (Table 2), which may be due to the low content of the active ingredients, their loss during distillation or their lower bioavailability. The chemical composition of Equisetum arvense essential oil also differs from literature data [65,66], which may be due to the volatilisation, the differing origins of the plant or the extraction methods. Radulović et al. (2006) [67] identified hexahydrofarnesyl acetone (18.3%), geranyl acetone (13.7%), thymol (12.1%) and phytol (10.1%) as the predominant active compounds in E. arvense EO; in our study only some of these compounds—like geranyl acetone, phytol, bornyl acetate, caryophyllene and others (Tables S1–S4)—were detected, which may affect antimicrobial activity. Most interestingly, although many identified compounds overlapped with those present in the existing literature, in our study, carvone—found for all E. arvense isolates—was scarcely reported. This finding signifies the importance of further research into the composition and bioactivity of horsetail extracts. Carvone, the main chemical component identified in EO, extracts, and hydrolates of Equisetum arvense, was ineffective for algal strains. The antimicrobial activity of carvone is known against numerous species of pathogenic bacteria, fungi, and protozoa [68], but its activity against algal species has not been described so far. In addition, studies to date have been conducted on this ingredient in its pure form, while EO from Equisetum arvense has not been tested. The low activity of this EO may be due to low extraction efficiency (low concentration of active ingredients), low sensitivity of algae to its components, or biodegradation of active ingredients in the culture medium by algae, which can result in both oxidation and reduction of active compounds.
Tests on the anti-algal activity of Salvia officinalis EO after 21 days of incubation with algae showed statistically significant algal growth inhibition effects (Table 2). It was determined that dilution of S. officinalis EO in DMSO (15:5 v/v EO/DMSO) inhibited algal growth and the observed zones of inhibition were very large (70 mm), Even at the lowest tested concentration (1:19 v/v EO/DMSO), an average inhibition zone of 26.98 mm was observed, demonstrating effectiveness at low concentrations. In contrast, S. officinalis extracts and hydrolates, as well as all Equisetum arvense variants, did not produce statistically significant inhibition of algal growth. However, some moderate anti-algal activity was noted for S. officinalis hydrolates and Equisetum arvense EO (Table 2).
Moreover, the EO of Salvia officinalis demonstrated a biocidal (lethal) effect against mature algal biofilm (Table 3). At a dilution of 10:10 v/v EO/DMSO, a statistically significant high inhibition zone (biofilm decolourisation) of 69.9 mm was observed, indicating strong biofilm-eradicating potential. Even at a lower dilution of 3:17 v/v EO/DMSO, the EO maintained significant activity, producing an inhibition zone of 24.4 mm. In contrast, none of the other tested variants showed the ability to remove mature algal biofilm.

Table 3.
Evaluation of minimum biocidal concentration (MAC) on the base of the inhibition zones [mm] of algal culture for Salvia officinalis and Equisetum arvense variants with various concentrations after 21st day of incubation.
The antialgal potential of plant extracts containing fatty acids, polyphenols, terpenoids tannins and flavonoids as potential allelochemicals, in terms of making significant contributions to algal inhibition, has been reported in the literature [40]. The current study has extended the knowledge to include the plant oil from Salvia officinalis, which may have applications for algal removal. The MIC and MAC results confirm that S. officinalis EO is effective not only in preventing algal growth but also in removing mature biofilms, supporting its potential use in anti-algal treatments.
Culturing the algae in a liquid medium supplemented with Salvia officinalis EO on the 13th day revealed noticeable morphological changes in the algal cells (Table 4). As early as three days after EO addition, several alterations were observed, including degradation of the photosynthetic apparatus, loss of chlorophyll, disruption of the cell membrane, accumulation of numerous lipid droplets, and deformations of the algal cells. These effects are likely due to the toxic action of the S. officinalis EO. Morphological changes were observed at both 0.1% and 0.5% EO concentrations, with more rapid and pronounced effects noted at the higher concentration (Table 4).

Table 4.
Morphological observations of algal cultures treated with 0.1% and 0.5% S. officinalis essential oil (EO) and in control sample (without EO). Scale bars 10 μm.
The addition of Salvia officinalis EO on the 12th day of algae cultivation also caused significant changes in cell density (Figure 1a) and chlorophyll a concentration (Figure 1b). Optical cell density decreased following the addition of the Salvia EO; 0.5% EO concentration exhibited a more extended stress response resulting in higher optical density, whereas the 0.1% EO concentration resulted in quick death of cells. The chlorophyll a concentration results confirmed that 0.5% caused more severe cellular damage of the photosynthetic apparatus, while 0.1% induced stress without fully disrupting photosynthesis.

Figure 1.
Cell density change (a) and chlorophyll a concentration (b) in algal cultures treated with 0.1% and 0.5% S. officinalis essential oil (EO) and in control sample (without EO). 12th day—addition of EO to the algal culture.
Moreover, the results show that chlorophyll a concentration is a more reliable indicator of live and dead cells than optical density as it degrades rapidly once released from cells.
Studies on the effect of Thymus satureioides Coss and Artemisia herba alba L. plant extracts on photosynthetic pigment concentrations in the cyanobacteria Microcystis aeruginosa showed a reduction in chlorophyll a concentration by 96% [69]; in our study the reduction in chlorophyll a concentration in green algal cultures was also high, and after the addition of S. officinalis EO, it decreased by 90–100% depending on the EO concentration.
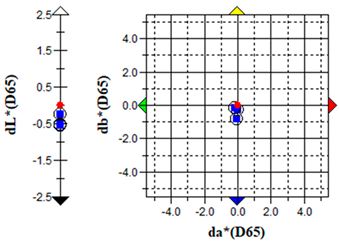
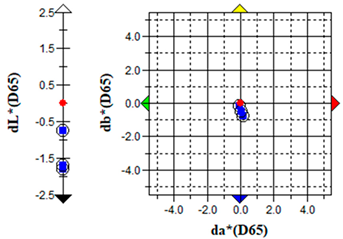
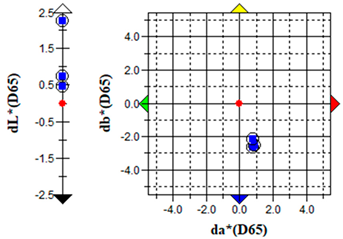
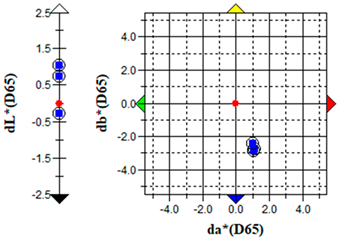
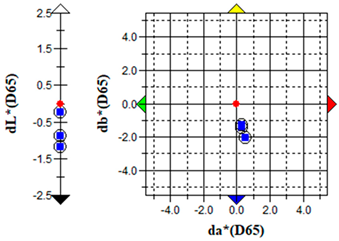
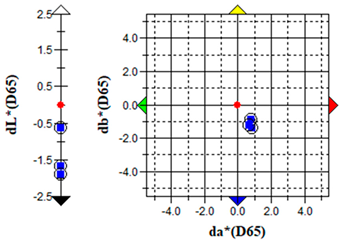
An important parameter in terms of the practical application of S. officinalis EO for the removal of algae from cultural heritage stone objects was to test the effect of the EO on colour changes in the algal culture treated with the EO. The results of the colour change measurements before and after the addition of the EO are presented graphically (Table 5) and in the form of the ΔE parameter (Table 6).

Table 5.
Colour change in algal culture samples treated with 0.1% and 0.5% concentration of S. officinalis essential oil (EO) before and after the addition of S. officinalis essential oil (EO).

Table 6.
Calculation of colour change measurements of ∆E for algal culture before and after the addition of S. officinalis essential oil (EO).
The brightness (L), red-green (a) and yellow-blue (b) values of the samples were relatively constant before the addition of S. officinalis EO. The natural pigmentation balance of the algal culture indicated a colour trend towards green (−a) and yellow (−b). Visible colour changes were observed within the first day after adding Salvia EO at both 0.1% and 0.5% concentrations. The Δb (yellow-blue) axis indicated a shift toward blue, with the 0.5% sample appearing more transparent (Table 5).
The values of the ∆E parameter obtained up to day 12 of the algal culture did not indicate significant visual colour differences—the ∆E parameter did not exceed 1; after the addition of S. officinalis EO, values of this parameter were recorded in the range ∆E = 1.03–1.89, indicating that only a trained observer could detect colour differences (Table 6).
Importantly, no dark colouration appeared, which is favourable for applications requiring colour stability. The addition of S. officinalis EO caused a final discolouration of the algal culture at both concentrations tested; a visual change from a green culture to a transparent one was observed, which may be important in the preservation and removal of coloured algal blooms on the surface of materials.
The ability of plant extracts to decolourise cyanobacterial cells has already been observed by other authors [69]; cyanobacterial cell lysis and chlorophyll degradation under the influence of plant extracts at concentrations of 0.5–1.0% was also observed by Meng et al., 2015 and Li et al., 2016 [70,71].
Fluorescence measurements in algal cultures were performed before and after the addition of S. officinalis EO to assess biofilm growth and the efficiency of algal cells’ activity (Table 7 and Table S3). Following the addition of essential oil, a significant reduction in fluorescence (F0-minimum and Fm-maximum fluorescence values) was observed at both 0.1% and 0.5% concentrations, indicating a potential inhibitory effect on biofilm photosynthetic activity (Table S3). Control samples maintained higher fluorescence, confirming the effect was EO-related. The 0.5% concentration caused a stronger decline, suggesting a dose-dependent response. Fluorescence imaging also showed reduced photosynthetic activity, particularly at the higher concentration (Table 7).

Table 7.
Fluorescence measurement images (F0 value) taken of algae culture before and after addition of S. officinalis essential oil (EO).
According to Ni et al. (2011) [72], allelopathic compounds derived from plants function similarly to natural algicides, exhibiting multiple modes of action and exerting diverse effects on target organisms. Certain allelochemicals act by inhibiting photosynthetic processes and decelerating algal cell growth. Additionally, they can target reactive oxygen species (ROS) at the level of cell membranes, leading to the degradation of unsaturated phospholipids and increased membrane permeability, ultimately disrupting cellular organization [70]. The antialgal activity of plant essential oils is similarly multifaceted [73]. Their mechanisms involve alterations in cell membrane permeability, which result in the leakage of cytoplasmic contents and nucleic acids. In some cases, EOs may also compromise the integrity of mitochondrial membranes, contributing to cellular dysfunction [74].
The mechanisms of Salvia EO’s antimicrobial activity have already been described for bacteria and fungi [75]; current research also confirms its high efficacy against terrestrial green algae and points to several mechanisms of action. The main mechanism of action of Salvia EO on algae is related to damage to the photosynthetic apparatus resulting in degradation and a reduction in chlorophyll concentration, with consequent inhibition of photosynthetic activity (reduced fluorescence). Microscopic images also showed disruption of the cell membrane, accumulation of numerous lipid droplets, and deformation of algal cells.
The efficacy of S. officinalis EO in treating colonized substrates was confirmed through investigations on building materials, with results showing strong agreement with previous findings. Application of 0.5% S. officinalis EO resulted in a significant reduction in substrate discolouration, biofilm photosynthetic activity, and coverage area within 7 days of treatment. It should be noted that the concentration used in this study corresponds to the amount of biocides typically added to building coatings (0.5–2.5% by weight) [76,77,78].
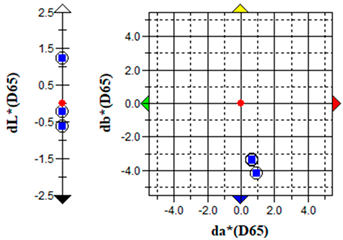
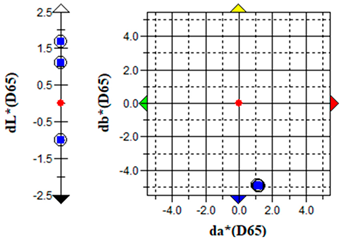
The ΔE parameter values (Table 8) confirmed biofilm formation, which caused notable discolouration of both substrate types, characterized predominantly by green (−a) and yellowish (+b) colour shifts. The extent of these colour changes was consistent with previous observations of photoautotrophic biofilms on building materials [9,25]. Treatment with 0.5% S. officinalis EO significantly reversed the algae-induced colour changes. Initial effects were observed after 24 h of exposure (Table 9), with clearance zones forming around cellulose discs containing EOs. After 7 days, overall discolouration decreased from ΔE = 17.46 ± 1.61 for brick substrates and ΔE = 43.93 ± 6.81 for plaster substrates to ΔE = 2.04 ± 1.44 and 15.49 ± 2.72, respectively. The treatment achieved nearly complete colour recovery for brick samples. Although a significant effect was observed for plaster specimens, the rate and extent of colour restoration were considerably lower, suggesting the need for treatment reapplication. For plaster samples, green colour reduction (Δa) was achieved; however, the yellow component (Δb) persisted, indicating the necessity for post-treatment washing. The remaining yellow colouration was most likely due to the production of carotenoids and release from dying algal cells rather than direct essential oil–substrate interactions. Importantly, no adverse effects were observed when S. officinalis EO was applied to non-inoculated samples. For brick substrates, the colour change parameter remained below ΔE < 2, indicating minimal alteration. It should be noted that lower-grade bricks with structural defects such as cracks, fissures, and low homogeneity may naturally exhibit higher ΔE values. Similarly, negligible colour changes were recorded for plaster samples (ΔE < 0.5).

Table 8.
Colour change measurements (∆E) for studies performed on building substrates.

Table 9.
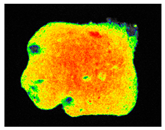
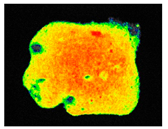
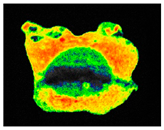
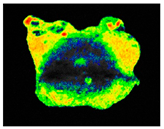
PAM fluorescence imaging (F0 value) of inoculated building materials treated with 0.5% S. officinalis essential oil.
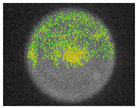
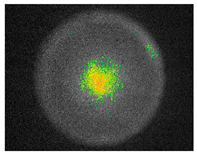
Pulsed amplitude modulation (PAM) chlorophyll a fluorescence imaging (Table 9) confirmed the algicidal effect of S. officinalis EO against biofilms formed on building materials. Within the first day of exposure, 39% and 37% reduction in F0 and Fm parameters was achieved for treated biofilm on brick samples. After 7 days of exposure, an 81% reduction was observed with mean F0 and Fm values of 46.48 ± 1.07 and 52.00 ± 3.46, respectively. In comparison, untreated samples showed an average minimum fluorescence of 215.84 ± 10.58 (Table S4).
Although still significant, lower efficiency of S. officinalis EO was observed for biofilms colonizing plaster substrates. During the first day of treatment, fluorescence increased slightly by 6%, reaching F0 = 529.86 ± 36.17 and Fm = 534.32 ± 36.25. From the third day of exposure, chlorophyll fluorescence decreased, reaching F0 = 220.08 ± 40.17 and Fm = 223.43 ± 40.20 after 7 days, representing a 56% reduction for both parameters. For untreated samples, overall fluorescence decreased slightly throughout the experiment but remained at levels of 354.33 ± 21.20 and 358.08 ± 21.31 for minimum and maximum fluorescence, respectively.
The results demonstrated that treatment with 0.5% S. officinalis EO effectively reduced both the photosynthetic activity and the overall size of the algal biofilm, as shown in Table 9. Importantly, no adverse effects were observed on uninoculated substrates, indicating that the EO does not negatively impact the underlying building materials when no biofilm is present. The data also revealed that biofilms growing on plaster samples exhibited greater resistance to the treatment compared to those on brick, reinforcing the need for repeated applications when treating plaster surfaces. Despite these differences, the findings confirm the potential of S. officinalis EO as a safe and effective agent for the treatment of colonized building materials, regardless of substrate type.
It is important to emphasize that, for S. officinalis EO to be effectively applied in the conservation of building materials and cultural heritage objects, further research is required. In particular, studies conducted under real environmental conditions are necessary, as such factors can significantly influence the activity of EOs. Moreover, exploring alternative solvents is recommended to improve the economic feasibility of this approach and to address the limitations associated with dimethyl sulfoxide, which is sensitive to temperature fluctuations. By optimizing both the application conditions and the formulation, the practical use of S. officinalis EO in heritage conservation can be further advanced. When considering the use of plant extracts as algicidal biocides for buildings and stone materials in the conservation of historic objects, future consideration should be given to the development of appropriate extraction procedures and the development of efficient stabilization processes (e.g., microencapsulation) to ensure their homogeneity and effectiveness over time [40].
This is especially crucial in view of the volatility of EOs, as well as their sensitivity to oxygen, light and/or heat [79]. Recent studies show that immobilization of EOs within hydrogels [80,81], encapsulates [82] and emulsions [83,84] can be effectively used for the treatment of various substrates of cultural importance. As described by [80], alginate hydrogel allowed researchers to achieve a one-month mitigation effect after the initial application, with [79] mentioning positive results even four months after treatment for some emulsions. Building on the results obtained in this study for Salvia officinalis EO, immobilization techniques could be employed to reduce or eliminate the need for repeated treatments, particularly on plaster-type substrates where reapplication was found to be necessary. Additionally, the effectiveness of the treatment could be further improved by using blends of EOs, as suggested by Spada et al. (2021) [85], especially since many potential combinations remain unexplored. A comparative analysis of the efficacy and cost between commonly used commercial algicidal biocides such as quaternary ammonium salts (QAS) and the essential oils (EOs) derived from Salvia officinalis and Equisetum arvense evaluated in this study provides valuable insights into the practical and ecological implications of their use. Market data from 2022 to 2024 indicate that the cost of QAS ranges from approximately USD 1.5 to USD 26 per kilogram [86], depending on the specific compound and purchase volume. High-purity laboratory-grade QAS (≥95%) can cost as much as USD 100–USD 870/kg [86,87,88,89]. In contrast, the cost of EOs is harder to estimate and varies considerably depending on factors such as plant origin, extraction method, regional availability, and packaging volume. For S. officinalis, prices per 10 mL do not usually exceed USD 12 [90,91,92]. Due to the more complex production process of E. arvense oil, market availability is significantly lower and the prices are higher (USD 29.99) [93]. On the other hand, based on local availability, the cost of 1 kg of pre-cut dried Salvia officinalis leaves ranges from USD 10 to USD 15 [94,95,96], allowing for the isolation of 16.2 mL of S. officinalis essential oil (based on the yield calculated for this study). Given the high effectiveness of the tested EO observed even for low concentrations, the acquired findings suggest potential high economic viability.
In terms of efficacy, quaternary ammonium salts (QAS) exhibit strong antimicrobial activity primarily through disruption of cell membranes and denaturation of proteins [97]. Essential oils (EOs), by contrast, act via diverse mechanisms including membrane destabilization, oxidative stress induction, and enzyme inhibition. Due to their volatile and chemically complex nature, EOs often display non-specific, algistatic effects that inhibit growth rather than causing rapid cell death [98]. Comparative studies on green algal genera such as Chlorella and Microcystis have demonstrated that QAS (e.g., benzalkonium chloride, didecyldimethylammonium chloride) are effective at low concentrations (1–10 ppm), whereas EO constituents like thymol and eugenol require higher concentrations (≥50–100 ppm) to achieve similar efficacy [98,99,100].
However, a critical distinction lies in their environmental impact. QAS are known for their high toxicity toward non-target organisms and ecosystems, and they exhibit poor biodegradability, raising concerns about their long-term ecological safety [101]. In contrast, EOs are generally considered to have low toxicity (though some may exhibit phytotoxic effects) and are readily biodegradable, attributes that enhance their profile as environmentally safe alternatives [102]. Furthermore, while microbial resistance to QAS is well-documented [103], the likelihood of developing resistance to EOs is significantly lower due to their complex and multi-component nature.
In summary, while QAS remain highly effective algicidal agents at low concentrations, essential oils despite requiring higher doses offer a more sustainable, biodegradable, and ecologically responsible alternative, with a reduced risk of developing resistance. These findings underscore the importance of continued research into plant-based biocides for use in environmental conservation and biofilm control applications.
4. Conclusions
Among all tested variants of EOs, hydrolates, and extracts from Salvia officinalis and Equisetum arvense, the EO derived from Salvia officinalis demonstrated the highest efficacy against a mixed algal culture. It significantly inhibited algal proliferation (growth inhibition zones: 23–84 mm) and effectively removed algal biofilms (biofilm inhibition zones: 15–94 mm). This was confirmed in all analyses, which showed a decrease in algal cell density, chlorophyll a content, and fluorescence related to photosynthetic activity and morphological changes in the algal cells after EO treatment. Optical cell density declined after EO application; however, the 0.5% EO concentration elicited a more prolonged stress response, resulting in relatively higher optical density values, whereas the 0.1% concentration led to rapid cell death. A pronounced reduction (90–100%) in chlorophyll a concentration further confirmed the EO’s damaging effects on the photosynthetic apparatus, especially at the higher 0.5% concentration. These findings indicate that chlorophyll a levels provide a more reliable measure of algal viability than optical density alone. Microscopic examination revealed additional mechanisms of action, including disruption of cell membranes, accumulation of lipid droplets, and structural deformations of algal cells. Colorimetric assays also showed distinct cell discoloration post-treatment, while the treated substrates remained visually unaffected (∆E < 2), indicating no adverse esthetic impact.
These findings highlight the potential of Salvia officinalis EO as a sustainable, natural alternative to synthetic algicidal biocides important in the conservation of stone heritage sites. Future studies should assess the effectiveness of the EO on stone substrates, including historical objects, as well as evaluate its impact on the physicochemical properties of these materials after repeated applications. Additional consideration should be placed on immobilization strategies. The search for natural algicidal biocides should also be expanded to include blends and other plant species and focus on developing formulations effective both in preventing algal growth and in eradicating mature biofilms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17156996/s1, Figure S1: Chromatogram of S. officinalis essential oil analysis; Figure S2: MS spectrum similarity for peak 4 to library hit; Figure S3: Chromatogram of E. arvense essential oil analysis; Figure S4: MS spectrum similarity for peak 2 to library hit; Table S1: Identified compounds for S. officinalis; Table S2: Identified compounds for E. arvense; Table S3: Florescence measurements in an algal culture treated with S. officinalis essential oil (EO); Table S4: Fluorescence measurements of inoculated building materials treated with 0.5% S. officinalis essential oil.
Author Contributions
Conceptualization, M.K. and B.G.; methodology, M.K. and K.S.; software, M.K. and K.S.; validation, M.K. and K.S.; investigation: M.K., N.D., P.N.-K. and K.S.; resources, B.G. and M.K.; data curation, N.D., M.K., K.S., P.N.-K. and B.G.; writing—original draft preparation, M.K. and B.G.; writing—review and editing, M.K. and B.G.; visualization, M.K., N.D., P.N.-K. and B.G.; supervision, B.G.; project administration, M.K. and B.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No wild or endangered flora species were used in this study. Commercially available, dried and pre-shredded stalks and leaves of Salvia officinalis herbs were used in this study. S. officinalis herbs were purchased from certified retailer (ISO 9001:2015, Reg. No. 0198 100 00820) courtesy of Natur-Vit Sp. z o. o. (Kopernia 9, 28-400 Pinczów, Poland). Commercially available, dried and pre-shredded stalks and leaves of Equisetum arvense herbs were used in this study. S. officinalis herbs were purchased from certified retailer (ISO 9001:2015, Reg No. 0198 100 00820) courtesy of Natur-Vit Sp. z o. o. (Kopernia 9, 28-400 Pinczów, Poland).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GC-MS | Gas Chromatography–Mass Spectrometry |
| EO | Essential Oil |
| MIC | Minimum Inhibitory Concentration |
| MAC | Minimum Algicidal Concentration |
| DMSO | Dimethyl Sulfoxide |
| TOF | Time-Of-Flight |
| DIC | Differential Interference Contrast |
| PAM | Pulse-Amplitude Modulation |
| F0 | Minimum Fluorescence |
| Fm | Maximum Fluorescence |
References
- Samad, L.; Adhikary, S. Diversity of Micro-Algae and Cyanobacteria on Building Facades and Monuments in India. ALGAE 2008, 23, 91–114. [Google Scholar] [CrossRef]
- Karsten, U.; Lembcke, S.; Schumann, R. The Effects of Ultraviolet Radiation on Photosynthetic Performance, Growth and Sunscreen Compounds in Aeroterrestrial Biofilm Algae Isolated from Building Facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Gray, D.W.; Lewis, L.A.; Cardon, Z.G. Photosynthetic Recovery Following Desiccation of Desert Green Algae (Chlorophyta) and Their Aquatic Relatives. Plant. Cell Environ. 2007, 30, 1240–1255. [Google Scholar] [CrossRef]
- Rindi, F. Terrestrial Green Algae: Systematics, Biogeography and Expected Responses to Climate Change. In Climate Change, Ecology and Systematics; Cambridge University Press: Cambridge, UK, 2011; pp. 201–227. ISBN 9780511974540. [Google Scholar]
- Tomaselli, L.; Lamenti, G.; Bosco, M.; Tiano, P. Biodiversity of Photosynthetic Micro-Organisms Dwelling on Stone Monuments. Int. Biodeterior. Biodegrad. 2000, 46, 251–258. [Google Scholar] [CrossRef]
- May, E.; Zamarreño, D.; Hotchkiss, S.; Mitchell, J.; Inkpen, R. Bioremediation of Algal Contamination on Stone. In Biocolonization of Stone: Control and Preventive Methods: Proceedings from the MCI Workshop Series; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of Cyanobacteria and Green Algae on Monuments in the Mediterranean Basin: An Overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Zelazna-Wieczorek, J.; Otlewska, A.; Koziróg, A.; Rajkowska, K.; Piotrowska, M.; Gutarowska, B.; Żydzik-Białek, A. Diversity of an Aerial Phototrophic Coating of Historic Buildings in the Former Auschwitz II-Birkenau Concentration Camp. Sci. Total Environ. 2014. [Google Scholar] [CrossRef]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Konca, P.; Gutarowska, B. Metabolomic Analysis of Photosynthetic Biofilms on Building Façades in Temperate Climate Zones. Int. Biodeterior. Biodegrad. 2022, 169, 105374. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Komar, M.; Gutarowska, B. Towards Understanding the Link between the Deterioration of Building Materials and the Nature of Aerophytic Green Algae. Sci. Total Environ. 2022, 802, 149856. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, B.S.C.; Callow, M.E. Formation, Composition and Physiology of Algal Biofilms. In BT—Biofilms—Science and Technology; Melo, L.F., Bott, T.R., Fletcher, M., Capdeville, B., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 149–162. ISBN 978-94-011-1824-8. [Google Scholar]
- Palla, F. Biotechnology and Cultural Heritage Conservation; Springer: Cham, Switzerland, 2020; ISBN 978-1-83881-924-8. [Google Scholar]
- Borderie, F.; Tête, N.; Cailhol, D.; Alaoui-Sehmer, L.; Bousta, F.; Rieffel, D.; Aleya, L.; Alaoui-Sossé, B. Factors Driving Epilithic Algal Colonization in Show Caves and New Insights into Combating Biofilm Development with UV-C Treatments. Sci. Total Environ. 2014, 484, 43–52. [Google Scholar] [CrossRef]
- Dakal, T.C.; Cameotra, S.S. Microbially Induced Deterioration of Architectural Heritages: Routes and Mechanisms Involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Chapter 5 Microbial Deterioration of Stone Monuments—An Updated Overview. In Advances in Applied Microbiology; Academic Press: New York, NY, USA, 2009; Volume 66, pp. 97–139. ISBN 0065-2164. [Google Scholar]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.D. Microbial Deterioration and Sustainable Conservation of Stone Monuments and Buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Cutler, N.A.; Viles, H.A.; Ahmad, S.; McCabe, S.; Smith, B.J. Algal ‘Greening’ and the Conservation of Stone Heritage Structures. Sci. Total Environ. 2013, 442, 152–164. [Google Scholar] [CrossRef]
- May, E. Stone Biodeterioration. In Cultural Heritage Microbiology: Fundamental Studies in Conservation Science; Ralph Mitchell, C.J.M., Ed.; ASM Press: Washington, DC, USA, 2010; pp. 221–230. [Google Scholar]
- Ferrari, C.; Santunione, G.; Libbra, A.; Muscio, A.; Sgarbi, E.; Siligardi, C.; Barozzi, G.S. Review on the Influence of Biological Deterioration on the Surface Properties of Building Materials: Organisms, Materials, and Methods. Int. J. Des. Nat. Ecodynamics 2015, 10, 21–39. [Google Scholar] [CrossRef]
- Borderie, F.; Alaoui-Sossé, B.; Aleya, L. Heritage Materials and Biofouling Mitigation through UV-C Irradiation in Show Caves: State-of-the-Art Practices and Future Challenges. Environ. Sci. Pollut. Res. 2015, 22, 4144–4172. [Google Scholar] [CrossRef]
- Javaherdashti, R.; Nikraz, H.; Borowitzka, M.; Moheimani, N.; Olivia, M. On the Impact of Algae on Accelerating the Biodeterioration/Biocorrosion of Reinforced Concrete: A Mechanistic Review. Eur. J. Sci. Res. 2009, 36, 394–406. [Google Scholar]
- Koziróg, A.; Otlewska, A.; Piotrowska, M.; Rajkowska, K.; Nowicka-Krawczyk, P.; Hachułka, M.; Wolski, G.J.; Gutarowska, B.; Kunicka-Styczyńska, A.; Libudzisz, Z.; et al. Colonising Organisms as a Biodegradation Factor Affecting Historical Wood Materials at the Former Concentration Camp of Auschwitz II—Birkenau. Int. Biodeterior. Biodegrad. 2014, 86, 171–178. [Google Scholar] [CrossRef]
- Mustoe, G.E. Biogenic Weathering: Solubilization of Iron from Minerals by Epilithic Freshwater Algae and Cyanobacteria. Microorganisms 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.O.; Onwukwe, C.; Peesor, S. Assessment of Microalgae-Influenced Biodeterioration of Concrete Structures. Niger. J. Biotechnol. 2018, 34, 19. [Google Scholar] [CrossRef]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Dudek, M.; Gutarowska, B. Biodeterioration Potential of Algae on Building Materials - Model Study. Int. Biodeterior. Biodegrad. 2023, 180, 105593. [Google Scholar] [CrossRef]
- Pfendler, S.; Borderie, F.; Bousta, F.; Alaoui-Sosse, L.; Alaoui-Sosse, B.; Aleya, L. Comparison of Biocides, Allelopathic Substances and UV-C as Treatments for Biofilm Proliferation on Heritage Monuments. J. Cult. Herit. 2018, 33, 117–124. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Gustavs, L.; Pandolfini, G.; Mauro, T. Heat Shock Treatments for the Control of Lithobionts: A Case Study with Epilithic Green Microalgae. Int. Biodeterior. Biodegrad. 2017, 123. [Google Scholar] [CrossRef]
- Barreiro, P.; González, P.; Pozo-Antonio, J.S. IR Irradiation to Remove a Sub-Aerial Biofilm from Granitic Stones Using Two Different Laser Systems: An Nd: YAG (1064 nm) and an Er:YAG (2940 nm). Sci. Total Environ. 2019, 688, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; Rodríguez, A.; Aguiar, U. Medium-Term Field Evaluation of Several Widely Used Cleaning-Restoration Techniques Applied to Algal Biofilm Formed on a Granite-Built Historical Monument. Int. Biodeterior. Biodegrad. 2019, 147. [Google Scholar] [CrossRef]
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm Colonization of Metamorphic Lithotypes of a Renaissance Cathedral Exposed to Urban Atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef]
- Becerra, J.; Ortiz, P.; Zaderenko, A.; Karapanagiotis, I. (Yiannis) Assessment of Nanoparticles/Nanocomposites to Inhibit Micro-Algal Fouling on Limestone Façades. Build. Res. Inf. 2019, 48, 180–190. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Krakova, L.; Pangallo, D.; Bruno, L. Effects of Biocide Treatments on the Biofilm Community in Domitilla’s Catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef]
- Genova, C.; Zoppis, E.; Grottoli, A.; Cencetti, C.; Matricardi, P.; Favero, G. An Integrated Approach to the Recovery of Travertine Biodegradation by Combining Phyto-Cleaning with Genomic Characterization. Microchem. J. 2020, 156, 104918. [Google Scholar] [CrossRef]
- Marin, E.; Vaccaro, C.; Leis, M. Biotechnology Applied to Historic Stoneworks Conservation: Testing the Potential Harmfulness of Two Biological Biocides. Int. J. Conserv. Sci. 2016, 7, 227–238. [Google Scholar]
- Urzì, C.; De Leo, F. Evaluation of the Efficiency of Water-Repellent and Biocide Compounds against Microbial Colonization of Mortars. Int. Biodeterior. Biodegrad. 2007, 60, 25–34. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Pavlou, A.; Manoudis, P.N.; Aifantis, K.E. Water Repellent ORMOSIL Films for the Protection of Stone and Other Materials. Mater. Lett. 2014, 131, 276–279. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Doni, M.; Chican, I.E.; Fierascu, R.C. A Short Overview of Recent Developments in the Application of Polymeric Materials for the Conservation of Stone Cultural Heritage Elements. Materials 2022, 15, 6294. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products; European Parliament and Council: Luxembourg, 2012. [Google Scholar]
- Sterflinger, K.; Piñar, G. Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural Biocides for the Conservation of Stone Cultural Heritage: A Review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Romani, M.; Warscheid, T.; Nicole, L.; Marcon, L.; Di Martino, P.; Suzuki, M.T.; Lebaron, P.; Lami, R. Current and Future Chemical Treatments to Fight Biodeterioration of Outdoor Building Materials and Associated Biofilms: Moving Away from Ecotoxic and towards Efficient, Sustainable Solutions. Sci. Total Environ. 2022, 802, 149846. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Sekhavati, M.H.; Moghaddam, M. Antimicrobial Activity of Some Plant Essential Oils and an Antimicrobial-Peptide against Some Clinically Isolated Pathogens. Chem. Biol. Technol. Agric. 2020, 7, 13. [Google Scholar] [CrossRef]
- Acimovic, M.; Tešević, V.; Smiljanic, K.; Cvetkovic, M.; Stankovic, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Zembrzuska, J. Advances in Biological Activities and Application of Plant Extracts. Appl. Sci. 2023, 13, 9324. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sorlini, C. New Environmentally Friendly Approaches against Biodeterioration of Outdoor Cultural Heritage. In Biocolonization of Stone: Control and Preventive Methods: Proceedings from the MCI Workshop Series; Smithsonian Institu-tion Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Barani, M.; Yousefzadi, M.; Moezi, M. Essential Oils, New Source of Algicidal Compounds. J. Appl. Phycol. 2015, 27, 267–273. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Ferraz, E.; Perna, V.; Sales, P.; Hipólito-Correia, V. Essential Oils and Extracts of Plants as Biocides against Microorganisms Isolated from the Ruins of the Roman City of Conímbriga (Portugal). Environ. Sci. Pollut. Res. 2024, 31, 40669–40677. [Google Scholar] [CrossRef]
- Andersen, R. Algal Culturing Techniques; Academic Press: Burlington, NJ, USA, 2005. [Google Scholar]
- Komar, M.; Szulc, J.; Kata, I.; Szafran, K.; Gutarowska, B. Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling. Appl. Sci. 2023, 13, 8009. [Google Scholar] [CrossRef]
- Bonikowski, R.; Paoli, M.; Szymczak, K.; Agnieszka, M.-K.; Wajs-Bonikowska, A.; Tomi, F.; Danuta, K. Chromatographic and Spectral Characteristic of Some Esters of a Common Monoterpene Alcohols. Flavour Fragr. J. 2016, 31. [Google Scholar] [CrossRef]
- Dahms, H.-U.; Hellio, C. 12—Laboratory Bioassays for Screening Marine Antifouling Compounds. In Woodhead Publishing Series in Metals and Surface Engineering; Hellio, C., Yebra, D., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 275–307. ISBN 978-1-84569-386-2. [Google Scholar]
- Desbois, A.; Smith, V. Disk Diffusion Assay to Assess the Antimicrobial Activity of Marine Algal Extracts. Methods Mol. Biol. 2015, 1308, 403–410. [Google Scholar] [CrossRef]
- Higham, D.J.; Highham, N.J. MATLAB Guide; Siam: Philadelphia, PA, USA, 2016; Volume 150. [Google Scholar]
- Eggert, A.; Häubner, N.; Klausch, S.; Karsten, U.; Schumann, R. Quantification of Algal Biofilms Colonising Building Materials: Chlorophyll α Measured by PAM-Fluorometry as a Biomass Parameter. Biofouling 2006, 22, 79–90. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color Difference Delta E - A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Huzar, E.; Dzięcioł, M.; Wodnicka, A.; Örün, H.; İçöz, A.; Çiçek, E. Influence of Hydrodistillation Conditions on Yield and Composition of Coriander (Coriandrum Sativum L.) Essential Oil. Polish J. Food Nutr. Sci. 2018, 68, 243–249. [Google Scholar] [CrossRef]
- Dao, T.P.; Tran, T.; Nhan, N.; Ngô, Q.; Tien, L.; Trieu, T.-A.; Quan, P.; Nguyen, N.; Anh, L.; Linh, H. Optimization of Essential Oil Yield from Vietnamese Green Pepper ( Piper Nigrum ) Using Hydro-Distillation Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 22039. [Google Scholar] [CrossRef]
- Marques, S.D.; Pinheiro, R.O.; Nascimento, R.A.; Andrade, E.H.; Faria, L.J. Effects of Harvest Time and Hydrodistillation Time on Yield, Composition, and Antioxidant Activity of Mint Essential Oil. Molecules 2023, 28, 7583. [Google Scholar] [CrossRef]
- Aćimović, M.; Pezo, L.; Čabarkapa, I.; Trudić, A.; Stanković Jeremić, J.; Varga, A.; Lončar, B.; Šovljanski, O.; Tešević, V. Variation of Salvia officinalis L. Essential Oil and Hydrolate Composition and Their Antimicrobial Activity. Processes 2022, 10, 1608. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the Essential Oil of Salvia officinalis L. from Various European Countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Jažo, Z.; Glumac, M.; Paštar, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Composition and Biological Activity of Salvia officinalis L. Essential Oil. Plants 2023, 12, 1794. [Google Scholar] [CrossRef]
- ISO 9909:1997; Oil of Dalmatian Sage (Salvia officinalis L.). International Organization for Standardization: Geneva, Switzerland, 1997.
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis Essential Oil: Chemical Analysis and Evaluation of Anti-Enzymatic and Antioxidant Bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Organ- and Season-Dependent Variation in the Essential Oil Composition of Salvia Officinalis L. Cultivated at Two Different Sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yi, T.; Lin, P.; Hu, J. Study on Essential Oil, Antioxidant Activity, Anti-Human Prostate Cancer Effects, and Induction of Apoptosis by Equisetum Arvense. Open Chem. 2022, 20, 1187–1195. [Google Scholar] [CrossRef]
- Bácsi, I.; Gonda, S.; Nemes-Kókai, Z.; B-Béres, V.; Vasas, G. Horseradish Essential Oil as a Promising Anti-Algal Product for Prevention of Phytoplankton Proliferation and Biofouling. Plants 2021, 10, 1550. [Google Scholar] [CrossRef]
- Radulović, N.; Stojanović, G.; Palić, R. Composition and Antimicrobial Activity of Equisetum Arvense L. Essential Oil. Phytother. Res. 2006, 20, 85–88. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and Its Pharmacological Activities: A Systematic Review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef]
- Tebaa, L.; Douma, M.; Tazart, Z.; Manaut, N.; Kh, M.; Loudiki, M. Assessment of the Potentially Algicidal Effects of Thymus Satureioides Coss. and Artemisia Herba Alba L. against Microcystis Aeruginosa. Appl. Ecol. Environ. Res. 2018, 16, 903–912. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Liu, Z.; Li, X.; Xu, H. Allelopathic Effects of Ailanthus Altissima Extracts on Microcystis Aeruginosa Growth, Physiological Changes and Microcystins Release. Chemosphere 2015, 141, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Zhang, P.; Zeng, G.; Cai, X.; Liu, S.; Yin, Y.; Hu, X.; Hu, X.; Tan, X. Growth Inhibition and Oxidative Damage of Microcystis Aeruginosa Induced by Crude Extract of Sagittaria Trifolia Tubers. J. Environ. Sci. 2016, 43, 40–47. [Google Scholar] [CrossRef]
- Ni, L.; Hao, X.; Li, S.; Chen, S.; Ren, G.; Zhu, L. Inhibitory Effects of the Extracts with Different Solvents from Three Compositae Plants on Cyanobacterium Microcystis Aeruginosas. Sci. China-Chem. 2011, 54, 1123–1129. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Fletcher, M.H.; Jennings, M.C.; Wuest, W.M. Draining the Moat: Disrupting Bacterial Biofilms with Natural Products. Tetrahedron 2014, 70, 6373–6383. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial Activity and Mechanisms of Salvia Sclarea Essential Oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Pedroso, P.F.; Silvestre, J.D.; Borsoi, G.; Flores-Colen, I. Life Cycle Assessment of Protection Products for External Thermal Insulation Composite Systems. Sustainability 2022, 14, 16969. [Google Scholar] [CrossRef]
- Kędzierski, M.; Wiejak, A.; Janiszewska, J.; Wiśniewska, A.; Grzywa-Niksińska, I.; Kurzepa, K. Efficiency of Selected Biocide Compounds in the Protection of Building Coatings against Colonization by Mold Fungi, Cyanobacteria and Algae. Polimery 2020, 65, 371–379. [Google Scholar] [CrossRef]
- Krueger, N.; Hofbauer, W.; Krus, M.; Fitz, C.; Mayer, F.; Melzer, A.; Breuer, K. Effectiveness and Durability of Biocides in Building Coatings: Biological Aspects BT. In Hygrothermal Behavior, Building Pathology and Durability; de Freitas, V.P., Delgado, J.M.P.Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 45–60. ISBN 978-3-642-31158-1. [Google Scholar]
- Reale, R.; Medeghini, L.; Botticelli, M. Stealing from Phytotherapy—Heritage Conservation with Essential Oils: A Review, from Remedy to Sustainable Restoration Product. Sustainability 2024, 16, 5110. [Google Scholar] [CrossRef]
- Gabriele, F.; Vetrano, A.; Bruno, L.; Casieri, C.; Germani, R.; Rugnini, L.; Spreti, N. New Oxidative Alginate-Biocide Hydrogels against Stone Biodeterioration. Int. Biodeterior. Biodegrad. 2021, 163, 105281. [Google Scholar] [CrossRef]
- Bruno, L.; Casieri, C.; Gabriele, F.; Ranaldi, R.; Rugnini, L.; Spreti, N. In Situ Application of Alginate Hydrogels Containing Oxidant or Natural Biocides on Fortunato Depero’s Mosaic (Rome, Italy). Int. Biodeterior. Biodegrad. 2023, 183, 105641. [Google Scholar] [CrossRef]
- Macedo-Arantes, S.; Piçarra, A.; Caldeira, A.T.; Candeias, A.E.; Martins, M.R. Essential Oils of Portuguese Flavouring Plants: Potential as Green Biocides in Cultural Heritage. Eur. Phys. J. Plus 2021, 136, 1106. [Google Scholar] [CrossRef]
- Di Vito, M.; Vergari, L.; Mariotti, M.; Proto, M.R.; Barbanti, L.; Garzoli, S.; Sanguinetti, M.; Sabatini, L.; Peduzzi, A.; Bellardi, M.G.; et al. Anti-Mold Effectiveness of a Green Emulsion Based on Citrus Aurantium Hydrolate and Cinnamomum Zeylanicum Essential Oil for the Modern Paintings Restoration. Microorganisms 2022, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Ioan, M.; Anghel, D.F.; Gifu, I.C.; Alexandrescu, E.; Petcu, C.; Diţu, L.M.; Sanda, G.A.; Bala, D.; Cinteza, L.O. Novel Microemulsions with Essential Oils for Environmentally Friendly Cleaning of Copper Cultural Heritage Artifacts. Nanomaterials 2023, 13, 2430. [Google Scholar] [CrossRef]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential Oils Mixtures as an Eco-Friendly Biocidal Solution for a Marble Statue Restoration. Int. Biodeterior. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- IndexBox. Price for Quaternary Ammonium Salts and Hydroxides; Choline and Its Salts in Finland. 2025. Available online: https://www.indexbox.io/search/price-for-quaternary-ammonium-salts-and-hydroxides-choline-and-its-salts-finland/ (accessed on 15 July 2025).
- Alkali. Scientific Benzalkonium Chloride, 1 X 100 g (12060-100G). Available online: https://alkalisci.com/benzalkonium-chloride-1-x-100-g-12060-100g/ (accessed on 15 July 2025).
- CP Lab. Safety Benzalkonium Chloride, 1 Kg. Available online: https://www.calpaclab.com/benzalkonium-chloride-1kg-each/spc-b3775-1kg (accessed on 15 July 2025).
- Spectrum Chemical Mfg. Corp. Benzalkonium Chloride. Available online: https://www.spectrumchemical.com/chemical/fine-chemicals/organic-laboratory-fine-chemicals/benzalkonium-chloride-b3775 (accessed on 15 July 2025).
- NHR Organic Oils Ltd. Organic Common Sage Essential Oil (Salvia officinalis). Available online: https://www.nhrorganicoils.com/products.php?id=2858 (accessed on 15 July 2025).
- IMMORTELLA. Fresh Cosmetics Co. Essential Oil Sage (Salvia officinalis), 10 ml. Available online: https://immortella.eu/en/p/essential-oil-sage-salvia-officinalis-10-ml/ (accessed on 15 July 2025).
- Nature In Bottle. Common Sage (Dalmatian Sage) Essential Oil. Available online: https://www.natureinbottle.com/product/sage_essential_oil (accessed on 15 July 2025).
- GoSupps.com 2014–2025. All Rights Reserved. Pure Horsetail Oil (Equisetum arvense) Cold Pressed 10 ml. Available online: https://www.gosupps.com/pure-horsetail-oil-equisetum-arvense-cold-pressed-10ml-0-33-oz-fragrance-free-0-33-fl-oz-pack-of-1.html (accessed on 23 July 2025).
- Natura Wita. Salvia Officinallis Ref. 5902194544870. Available online: https://sklepnaturawita.pl/en/home/407-szalwia-lekarska-500-g-5902194544870.html?SubmitCurrency=1&id_currency=3 (accessed on 15 July 2025).
- Aromatika. Salvia officinalis Leaf. Available online: https://www.aromatika.com.pl/pl/p/SZALWIA-LEKARSKA-LISC/410 (accessed on 15 July 2025).
- Polish Herbs Sage. Available online: https://polishherbs.com/en/produkty/sage/ (accessed on 15 July 2025).
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a Bacterial Membrane Permeabilization Assay for Quantitative Evaluation of Benzalkonium Chloride as a Membrane-Disrupting Agent. Medchemcomm 2017, 8, 1408–1413. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Huang, W.; He, L.; Li, J.; Zhou, J.; Zhou, J. Influence of Eugenol on Algal Growth, Cell Physiology of Cyanobacteria Microcystis Aeruginosa and Its Interaction with Signaling Molecules. Chemosphere 2020, 255, 126935. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, F.; Zhang, L. Composition and Anti-Cyanobacterial Activity of Essential Oils from Six Different Submerged Macrophytes. Polish J. Environ. Stud. 2015, 24, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tanasă, F.; Nechifor, M.; Teacă, C.-A. Essential Oils as Alternative Green Broad-Spectrum Biocides. Plants 2024, 13, 3442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in the Environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Oh, S.; Tandukar, M.; Pavlostathis, S.G.; Chain, P.S.G.; Konstantinidis, K.T. Microbial Community Adaptation to Quaternary Ammonium Biocides as Revealed by Metagenomics. Environ. Microbiol. 2013, 15, 2850–2864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).