Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Extract Preparation
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Applications of the Plant Extract in Pre- and Post-Germination Assays
2.3.1. Pre-Germination Tests
2.3.2. Post-Emergence Tests
2.4. Experimental Design and Statistical Analysis
3. Results
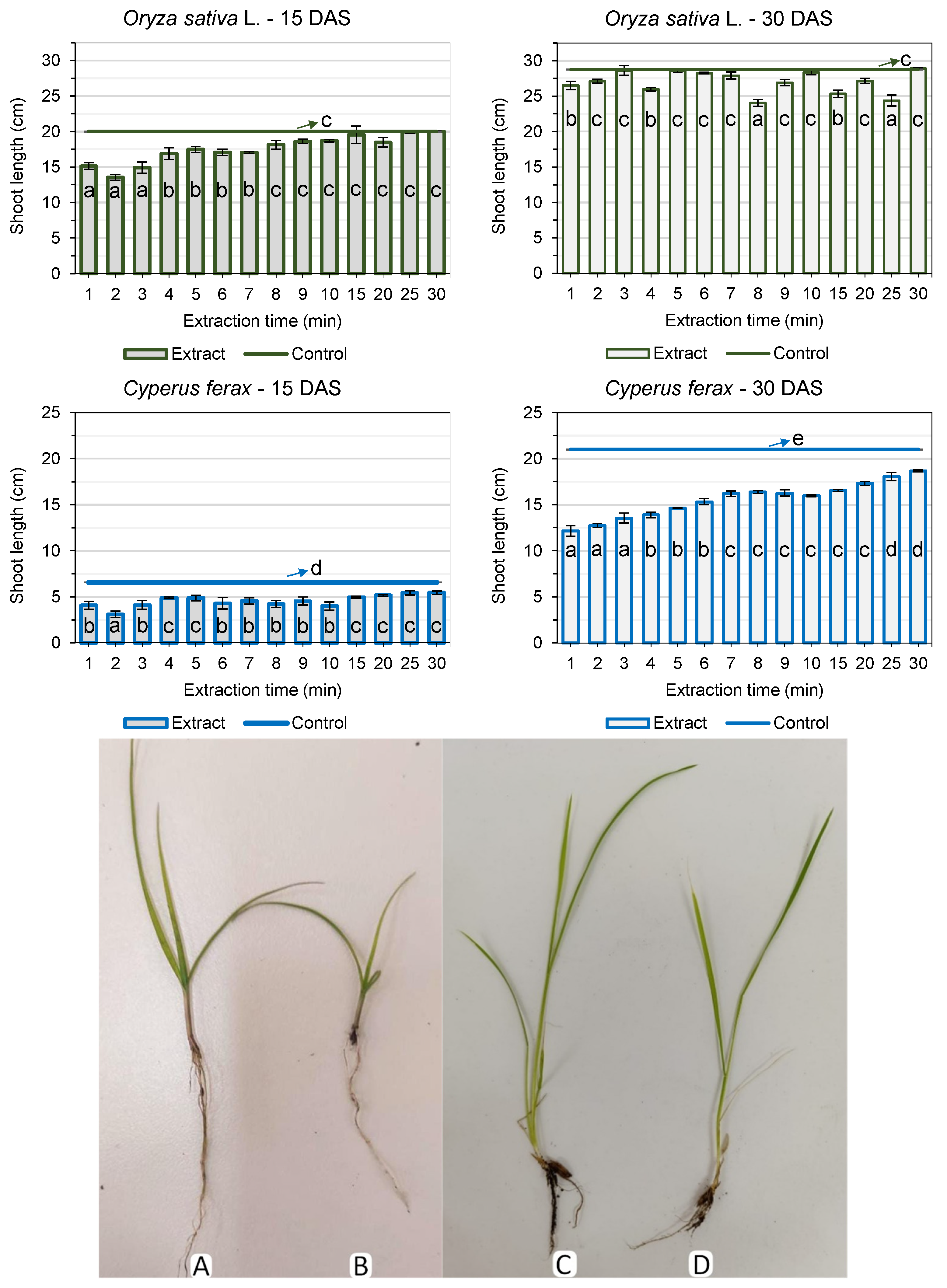
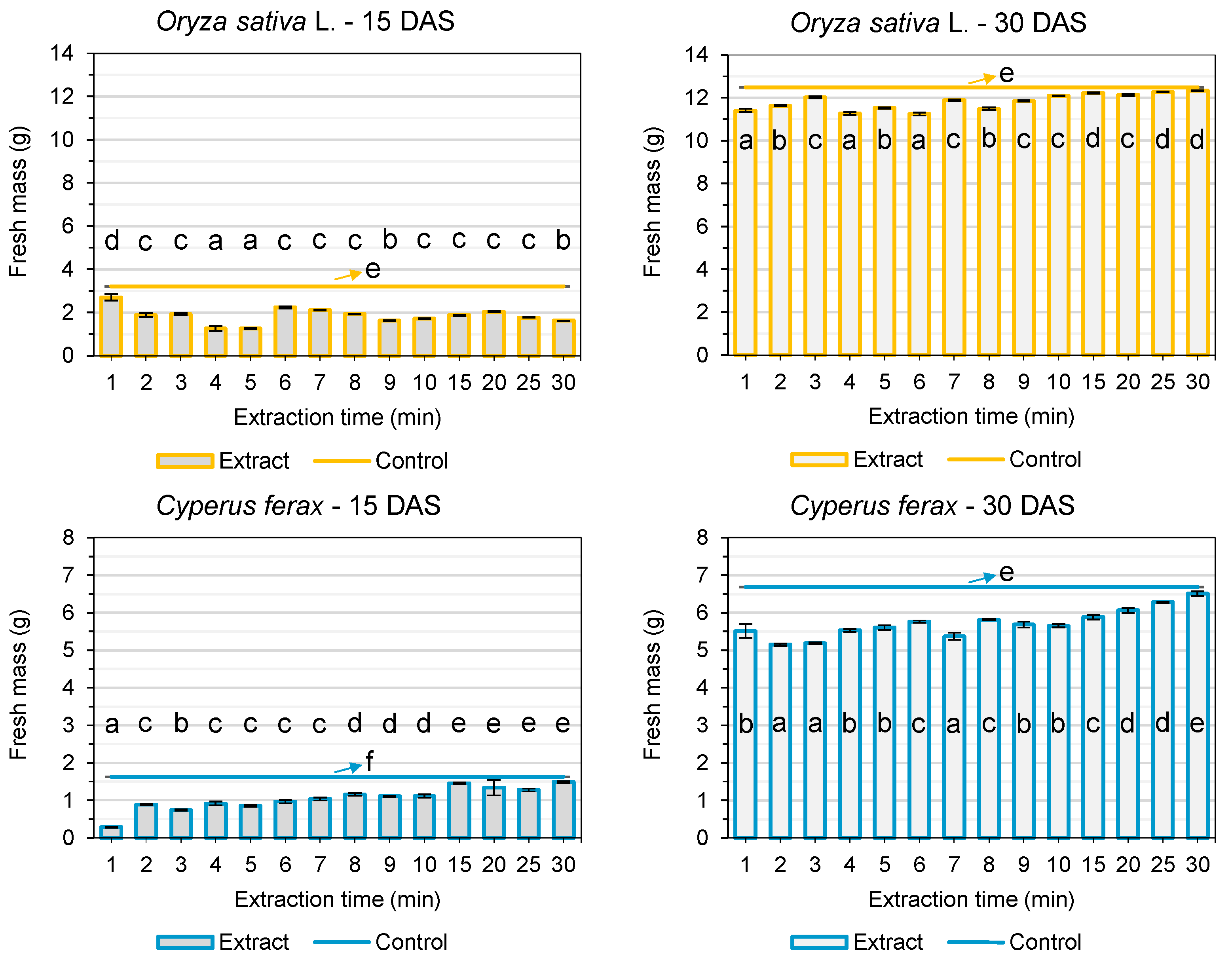
3.1. Pre-Germination Tests
3.2. Post-Emergence Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negi, B.; Khatri, K.; Bargali, S.S.; Bargali, K. Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield. Sustainability 2023, 15, 10748. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Duke, S.O. Implications of weakening of the United States Geological Survey Pesticide National Synthesis Project for Weed Scientists. Weed Sci. 2023, 71, 517–519. [Google Scholar] [CrossRef]
- Shrestha, S.; Stallworth, S.; Tseng, T.-M. Weedy rice: Competitive ability, evolution, and diversity. In Integrated View of Population Genetics; Maia, R.T., Campos, M.d.A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Panozzo, L.E.; Agostinetto, D.; Vignolo, G.; Galon, L.; Ferreira, E.A.; Silva, L.J.; Moraes, P.V.D. Diversity and sensitivity of Cyperus ferax to the herbicide Pe-noxsulam. Planta Daninha 2011, 29, 421–427. [Google Scholar] [CrossRef]
- Babalola, O.O.; Truter, J.C.; Van Wyk, J.H. Lethal and teratogenic impacts of imazapyr, diquat dibromide, and glufosinate ammonium herbicide formulations using frog embryo teratogenesis assay-xenopus (FETAX). Arch. Environ. Contam. Toxicol. 2021, 80, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Florentine, S.; Fernando, W.G.D.; Tennakoon, K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Confortin, T.C.; Todero, I.; Luft, L.; Schmaltz, S.; Ferreira, D.F.; Barin, J.S.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extraction of bioactive compounds from Senecio brasiliensis using emergent technologies. 3 Biotech 2021, 11, 101986. [Google Scholar] [CrossRef]
- Dolianitis, B.M.; Pfeifenberg, R.; Frescura, V.D.; Tres, M.V.; Zabot, G.L. Phytochemicals from Eucalyptus camaldulensis and Coleus barbatus Control eragrostis plana in Horticulture. Horticulturae 2025, 11, 291–306. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-Noguchi, H. Allelopathic and Herbicidal Effects of Crude Extract from Chromolaena odorata (L.) R.M.King and H.Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Uyun, Q.; Respatie, D.W.; Indradewa, D. Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management. Sustainability 2024, 16, 479. [Google Scholar] [CrossRef]
- Ody, L.P.; Santos, M.S.N.; Lopes, A.M.; Mazutti, M.A.; Tres, M.V.; Zabot, G.L. Review on biological and biochemical pesticides as a sustainable alternative in organic agriculture. Biocatal. Agric. Biotechnol. 2025, 67, 103655. [Google Scholar] [CrossRef]
- Nour, I.H.; Alhadead, K.; Ellmouni, F.Y.; Badr, R.; Saad, T.I.; EL-Banhawy, A.; Rahman, S.M.A. Morphological, anatomical and chemical characterization of Ricinus communis L. (Euphorbiaceae). Agronomy 2023, 13, 985. [Google Scholar] [CrossRef]
- Yang, S.; Mao, L.; Zheng, Z.; Chen, B.; Li, J. Pollen Atlas for Selected Subfamilies of Euphorbiaceae from Southern China: A Complementary Contribution to Quaternary Pollen Analysis. Palynology 2020, 44, 659–673. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profile of potent allelopathic plant fractions against major wheat weeds—A way forward to reduce the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.I.; Kim, M.J.; Hahm, J.-H.; Seo, H.D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Castor oil plant (Ricinus communis L.) leaves improve dexamethasone-induced muscle atrophy via Nrf2 activation. Front. Pharmacol. 2022, 5, 891762. [Google Scholar] [CrossRef]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind. Crops Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Setayeshnasab, M.; Sabzalian, M.R.; Rahimmalek, M. The relation between apomictic seed production and morpho-physiological characteristics in a world collection of castor bean (Ricinus communis L.). Sci. Rep. 2024, 14, 5013. [Google Scholar] [CrossRef]
- Zadra, M.; Menezes, B.B.d.; Frescura, L.M.; Essi, L.; Carvalho, C.A.d.; Rosa, M.B.d. Ruellia angustiflora (Nees) Lindau ex Rambo: Extraction and characterization of phenolic compounds and evaluation of antiradical, photoprotective and antimicrobial activities. Nat. Prod. Res. 2024, 38, 2082–2090. [Google Scholar] [CrossRef]
- Chung, I.M.; Ahn, J.K.; Yun, S.J. Assessment of allelopathic potential of barnyard grass (Echinochloa crus-galli) on rice (Oryza sativa L.) cultivars. Crop Prot. 2001, 20, 921–928. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of extraction methods for characterization of phenolic compounds in apricot fruit (Prunus armeniaca). Food Funct. 2019, 16, 6492–6502. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Meireles, M.A.A. Influence of the bed geometry on the kinetics of rosemary compounds extraction with supercritical CO2. J. Supercrit. Fluids 2014, 94, 234–244. [Google Scholar] [CrossRef]
- Schmaltz, S.; Silva, M.A.; Ninaus, R.G.; Guedes, J.V.C.; Zabot, G.L.; Tres, M.V.; Mazutti, M. Biomolecules in modern and sustainable agriculture. 3 Biotech 2023, 13, 70. [Google Scholar] [CrossRef]
- Borges, C.d.S.; Cuchiara, C.C.; Silva, S.D.d.A.e.; Bobrowsk, V.L. Cytotoxic and allelopathic effects of aqueous extracts of Ricinus communis using different bioindicators. Agric. Sci. Technol. 2011, 5, 15–20. [Google Scholar]
- Saadaoui, E.; Martín, J.J.; Ghazel, N.; Romdhane, C.B.; Massoudi, N.; Cervantes, E. Allelopathic effects of aqueous extracts of Ricinus communis L. on the germination of six cultivated species. Int. J. Plant Soil. Sci. 2015, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.W.N.; Morais, E.M.; Lacerda, J.J.d.J.; Araújo, F.D.d.S. Bioherbicidal potential of plant species with allelopathic effects on the weed Bidens bipinnata L. Sci. Rep. 2022, 12, 13476. [Google Scholar]
- Parreno, J.; Cempron, B.; Bucog, N.; Pepito, M.; Zamora, C. Allelopathic Potential of Castor plant (Ricinus communis L.) Aqueous Extracts on Germination on Selected Crop Species. Int. Res. J. Sci. Technol. 2020, 1, 120–125. [Google Scholar] [CrossRef]
- Othman, S.H.; Lazim, Z.S.; Al-Wakaa, A.H.A. Allelopathic Potential of Aqueous Plant Extracts Against Seed Germination and Seedling Growth of Weeds. IOP Conf. Ser. Earth Environ. Sci. 2023, 1262, 032041. [Google Scholar] [CrossRef]
- El-Gawad, A.M.A.; Mashaly, I.A.; Ziada, M.E.A.; Deweeb, M.R. Phytotoxicity of three Plantago species on germination and seedling growth of hairy beggarticks (Bidens pilosa L.). Egypt. J. Basic. Appl. Sci. 2015, 2, 303–309. [Google Scholar] [CrossRef]
- Castro, L.A.; Santos, P.G.A.; Carvalho, Y.B.; Motie, B. Allelopathic effect of crude plant extract on Ipomoea purpurea L. J. Res. Weed Sci. 2021, 4, 1988–1999. [Google Scholar]
- Rys, M.; Saja-Garbarz, D.; Skoczowski, A. Phytotoxic effects of selected herbal extracts on the germination, growth and metabolism of mustard and oilseed rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Duarte, D.; Galhano, C.; Dias, M.C.; Castro, P.; Lorenzo, P. Invasive plants and agri-food waste extracts as sustainable alternatives for the pre-emergence urban weed control in Portugal Central Region. Int. J. Sustain. Dev. World Ecol. 2023, 30, 605–619. [Google Scholar] [CrossRef]
- Abomughaid, M.M.; Teibo, J.O.; Akinfe, O.A.; Adewolu, A.M.; Teibo, T.K.A.; Afifi, M.; Al-Farga, A.M.H.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; et al. A phytochemical and pharmacological review of Ricinus communis L. Discover Appl. Sci. 2024, 6, 315. [Google Scholar]
- Hussain, W.S. Allelopathy: Allelochemicals a brief review. Plant Arch. 2020, 20, 5556–5560. [Google Scholar]
- Barros, V.M.d.S.; Pedrosa, J.L.F.; Gonçalves, D.R.; Medeiros, F.C.L.d.; Carvalho, G.R.; Gonçalves, A.H.; Teixeira, P.V.V.Q. Herbicides of biological origin: A review. J. Hortic. Sci. Biotechnol. 2021, 96, 288–296. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Env. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Imran, S.; Khatoon, Z.; Khan, M.Y.; Mechri, M.; Asghar, W.; Rehmani, M.I.A.; Villalobos, S.d.l.S.; Mumtaz, T.; et al. Bioherbicides: Revolutionizing weed management for sustainable agriculture in the era of One-health. Curr. Res. Microb. Sci. 2025, 8, 100394. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Karim, S.M.R.; Kheya, S.A.; Yeasmin, S. Unlocking the potential of bioherbicides for sustainable and environment friendly weed management. Heliyon 2024, 10, e36088. [Google Scholar] [CrossRef]
- Mittal, A.; Lunkand, A.S.; Singh, M.; Rathod, G.S.; Monsi, M.; Yadav, R.; Dubey, A.; Kunal. Computational Screening and Biochemical Analysis of Ricinus communis Linn Root. Afr. J. Biol. Sci. 2024, 6, 5864. [Google Scholar] [CrossRef]
- Nogueira, A.J.L.; Mendes, R.J.A.; Nino, C.R.C.F.; Oliveira, Y.D.S.; Rocha, C.Q.D.; Ambrósio, H.T.M.J.; Everton, G.O.; Rosa, I.G.; Pereira Filho, A.A. Evaluation of molluscicidal activity on Biomphalaria glabrata (Say, 1818) and phytochemical characterization of hydroalcoholic extract of leaves of Ricinus communis L. (EUPHORBIACEAE). Exp. Parasitol. 2023, 247, 108481. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Chen, G. Simultaneous determination of flavones and phenolic acids in the leaves of Ricinus communis Linn. by capillary electrophoresis with amperometric detection. J. Chromatogr. B. 2008, 863, 101–106. [Google Scholar] [CrossRef]
- Lima, B.M.F.V.; Oliveira, R.A.d.; Santos, E.A.d.; Bittencourt, M.A.L.; Santos, O.O.d. Phytochemical characterization and bioactivity of ethanolic extracts on eggs of Citrus blackfly. Cienc. Rural. 2017, 47, e20170092. [Google Scholar] [CrossRef]
- Lengai, G.; Muthomi, J. Biopesticides and Their Role in Sustainable Agricultural Production. J. Biosci. Med. 2018, 6, 7–41. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence. In Advances in Botanical Research; Kuete, V., Jacquot, J.-P., Eds.; Academic Press: New York, NY, USA, 2017; Volume 82, pp. 19–54. [Google Scholar]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Zhu, Y.; Guo, L.; Ji, R.; Miao, Y.; Guo, L.; Du, H.; Liu, D. Caffeic Acid, an Allelochemical in Artemisia argyi, Inhibits Weed Growth via Suppression of Mitogen-Activated Protein Kinase Signaling Pathway and the Biosynthesis of Gibberellin and Phytoalexin. Front. Plant Sci. 2022, 12, 809128. [Google Scholar] [CrossRef] [PubMed]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides. Agriculture 2022, 12, 1338. [Google Scholar] [CrossRef]
- Li, Z.R.; Amist, N.; Bai, L.Y. Allelopathy in sustainable weeds management. Allelopathy J. 2019, 48, 109–138. [Google Scholar]
- Franco, D.M.; Saldanha, L.L.; Neto, J.d.S.L.; Santos, L.C.d.; Dokkedal, A.L.; Almeida, L.F.R.d. Seasonal variation in allelopathic potential of the leaves of Copaifera langsdorffii Desf. Acta Bot. Bras. 2016, 30, 157–165. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S.; Evidente, A. Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: Quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants 2021, 10, 543. [Google Scholar] [CrossRef]


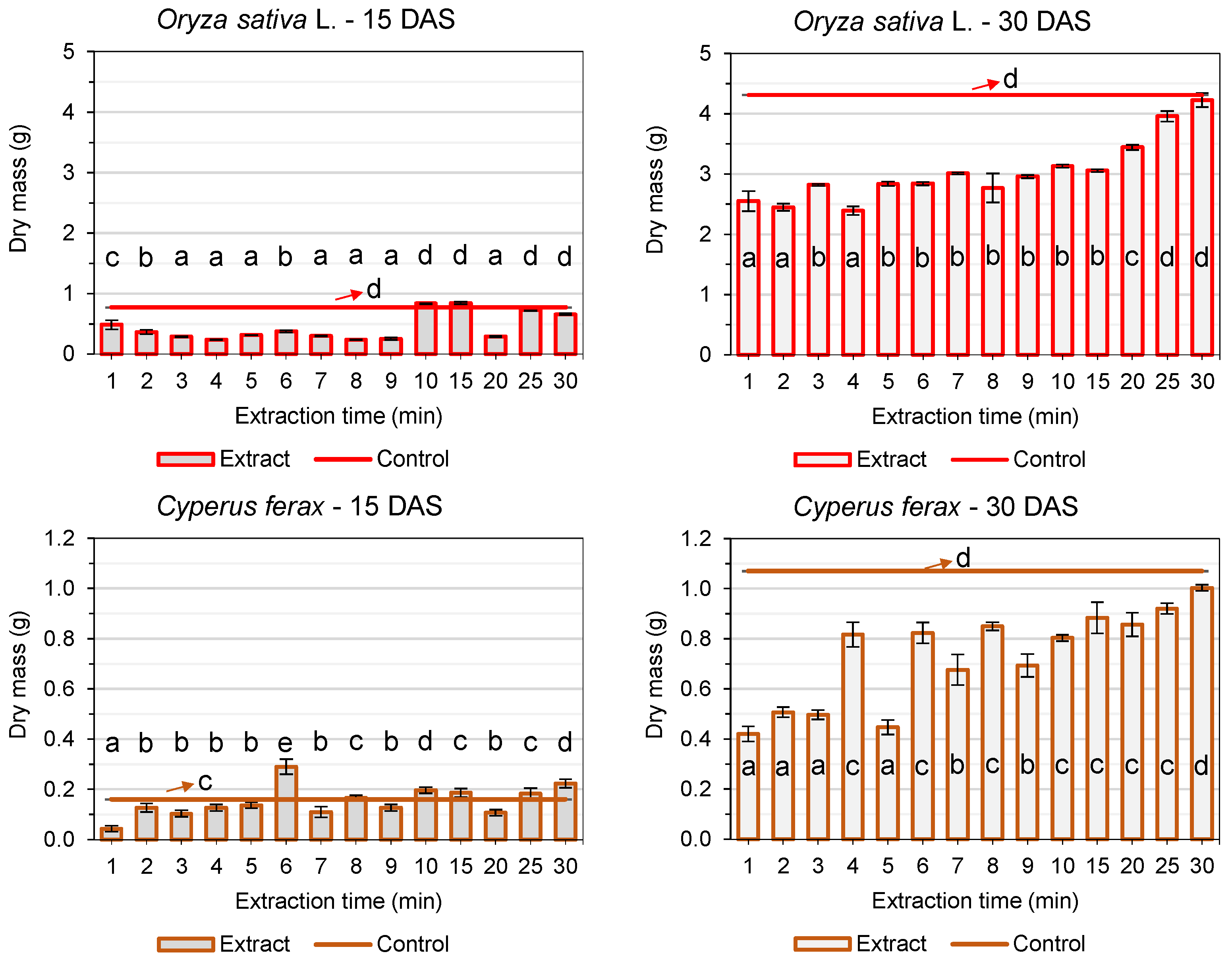
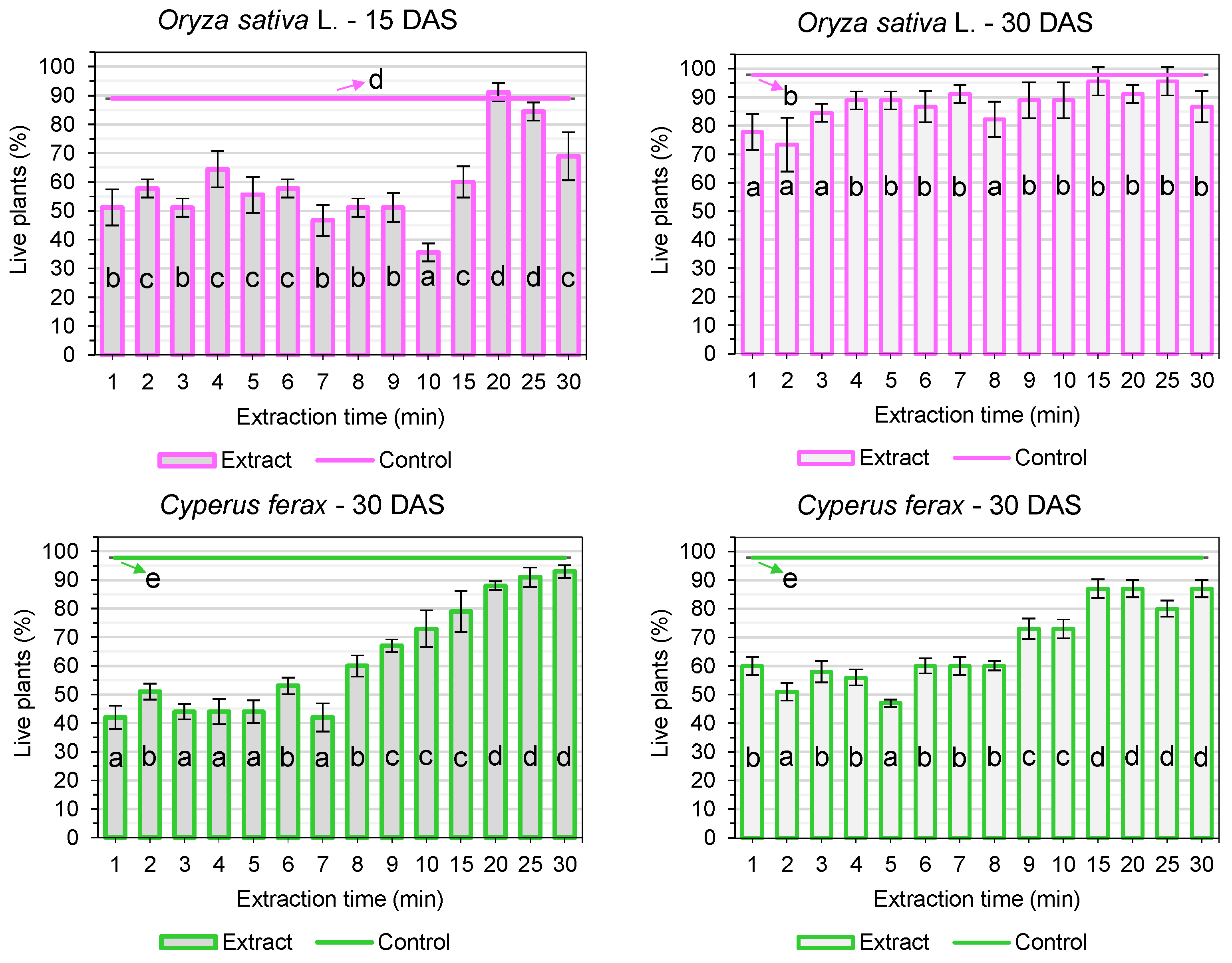
| Extraction Time (Minutes) | Germination of O. sativa L. (%) * | Germination of C. ferax (%) * |
|---|---|---|
| 1 | 18.7 ± 1.9 a | 9.0 ± 0.0 a |
| 2 | 21.0 ± 1.6 a | 7.3 ± 1.7 a |
| 3 | 31.3 ± 0.5 b | 8.0 ± 0.0 a |
| 4 | 35.7 ± 4.3 c | 9.3 ± 2.1 a |
| 5 | 40.0 ± 4.1 c | 8.3 ± 0.9 a |
| 6 | 49.7 ± 3.8 d | 10.3 ± 2.5 a |
| 7 | 50.3 ± 1.7 d | 9.7 ± 1.9 a |
| 8 | 46.7 ± 4.5 d | 9.7 ± 1.7 a |
| 9 | 48.3 ± 5.0 d | 9.7 ± 0.9 a |
| 10 | 46.3 ± 4.0 d | 9.3 ± 2.1 a |
| 15 | 54.7 ± 3.1 e | 19.0 ± 2.2 b |
| 20 | 52.0 ± 3.6 e | 23.3 ± 1.7 c |
| 25 | 54.0 ± 3.6 e | 23.0 ± 2.2 c |
| 30 | 58.0 ± 2.2 e | 26.3 ± 2.5 c |
| Control | 79.7 ± 0.9 f | 62.0 ± 1.4 d |
| Coefficient of variation (%) | 7.0 | 13.2 |
| Compound | Composition in the Extract (%) | ||
|---|---|---|---|
| Extract from 1 min | Extract from 15 min | Extract from 30 min | |
| Gallic acid | 9.8 | 6.5 | 3.3 |
| Caffeic acid | 24.2 | 23.9 | 14.1 |
| Rutin | 15.7 | 13.2 | 5.6 |
| Quercitrin | 13.4 | 12.6 | 4.2 |
| Kaempferol | 2.2 | 2.1 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.M.; Ribeiro, L.K.; Cogo, M.R.d.M.; Frescura, L.M.; da Rosa, M.B.; Schulz, A.; Mayer, F.D.; Abaide, E.R.; Tres, M.V.; Zabot, G.L. Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management. Sustainability 2025, 17, 6942. https://doi.org/10.3390/su17156942
Lopes AM, Ribeiro LK, Cogo MRdM, Frescura LM, da Rosa MB, Schulz A, Mayer FD, Abaide ER, Tres MV, Zabot GL. Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management. Sustainability. 2025; 17(15):6942. https://doi.org/10.3390/su17156942
Chicago/Turabian StyleLopes, Aline Mazoy, Lucas Kila Ribeiro, Maurício Ricardo de Melo Cogo, Lucas Mironuk Frescura, Marcelo Barcellos da Rosa, Alex Schulz, Flávio Dias Mayer, Ederson Rossi Abaide, Marcus Vinícius Tres, and Giovani Leone Zabot. 2025. "Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management" Sustainability 17, no. 15: 6942. https://doi.org/10.3390/su17156942
APA StyleLopes, A. M., Ribeiro, L. K., Cogo, M. R. d. M., Frescura, L. M., da Rosa, M. B., Schulz, A., Mayer, F. D., Abaide, E. R., Tres, M. V., & Zabot, G. L. (2025). Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management. Sustainability, 17(15), 6942. https://doi.org/10.3390/su17156942







