Dynamic Temperature–Vacuum Swing Adsorption for Sustainable Direct Air Capture: Parametric Optimisation for High-Purity CO2 Removal
Abstract
1. Introduction
2. Materials and Methods
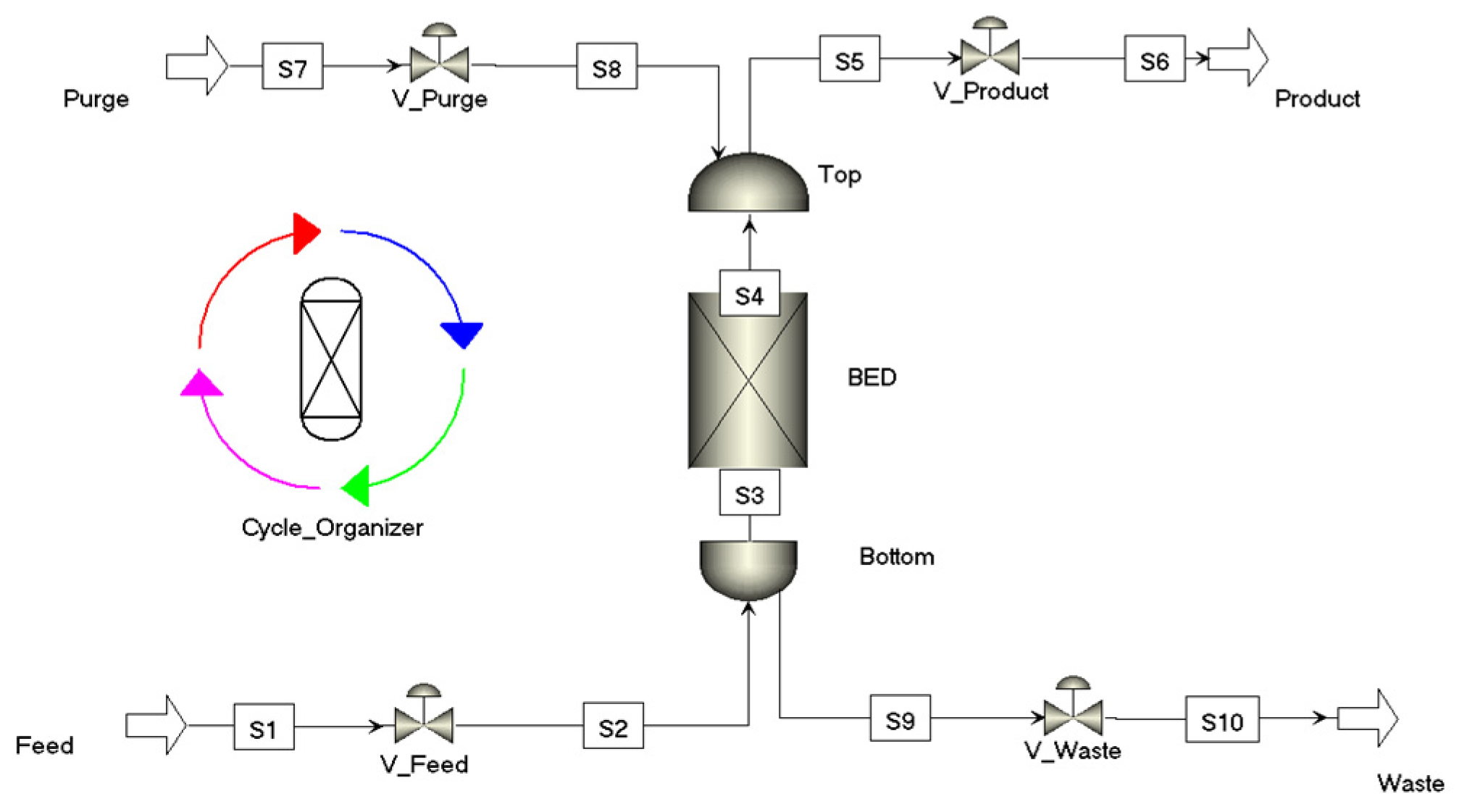
2.1. Simulation Framework (Numerical Modelling and Assumption)
- The sorbent has a spherical shape with uniform, continuous properties, as the selected particles used in the experimental studies were derived from MOF powders composed of fine crystallites that were agglomerated and sieved to obtain particles with controlled and consistent size [56].
- The gas phase follows ideal gas behaviour, which is a reasonable approximation under the low operating pressures and dilute CO2 concentrations typical of DAC systems.
- Constant adsorbed phase heat capacity is assumed because the amount of adsorbed CO2 is small in DAC.
- The column thickness effect is ignored, assuming the adiabatic conditions.
- No parasitic reactions between the gas and the adsorbent.
- A one-dimensional model incorporating convection.
- The air flow mixture consists of CO2 and N2, with CO2 as the sole adsorbed component.
- CO2 adsorption kinetics differ below and above Pₛₜₑₚ, and the model captures this through dual-regime rate constants derived from experimental fitting [56].
2.1.1. Packed-Bed Specification
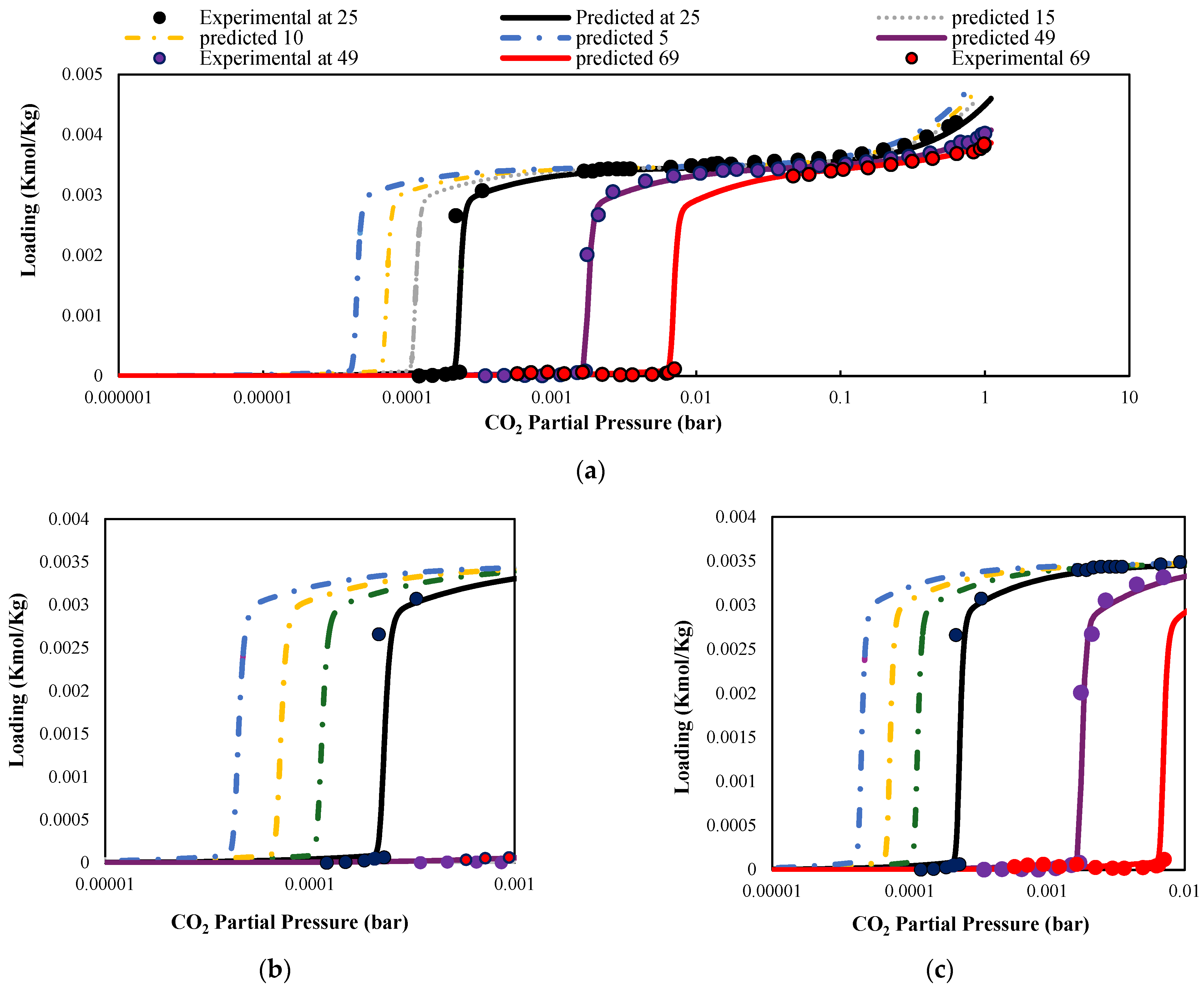
2.1.2. Adsorption Equilibrium and Kinetics
| Parameters | Unit | Values | Reference |
|---|---|---|---|
| CO2 heat of adsorption | KJ/mol | −71 | [68] |
| N2 heat of adsorption | KJ/mol | −18 | [57] |
| Heat capacity of the crystal | KJ/Kg·K | 1.6 | [51] |
| CO2 heat capacity | KJ/Kmol·K | 37.4673 | (Aspen Plus) |
| N2 heat capacity | KJ/Kmol·K | 29.1806 | (Aspen Plus) |
| Thermal conductivity | W/m·K | 0.3 | [69] |
2.1.3. Energy Balance
2.1.4. Pressure Drop
2.2. Process Design: Basis and Criteria
- Adsorption: Ambient air flows through the packed bed, where CO2 is selectivity adsorbed onto the sorbent while CO2-depleted air is released.
- Evacuation: The system pressure is reduced to remove N2.
- Heating + Evacuation: The bed is heated using a heat exchanger to reach the regeneration temperature while maintaining vacuum conditions to promote efficient CO2 desorption.
- Cooling: The heat exchanger cools the bed to maintain thermal stability, prevent amine degradation, and facilitate the next adsorption step.
- Pressurisation: The system is returned to atmospheric pressure by gradually reintroducing air, initiating the next adsorption cycle.
2.3. Performance Metrics
3. Results and Discussion
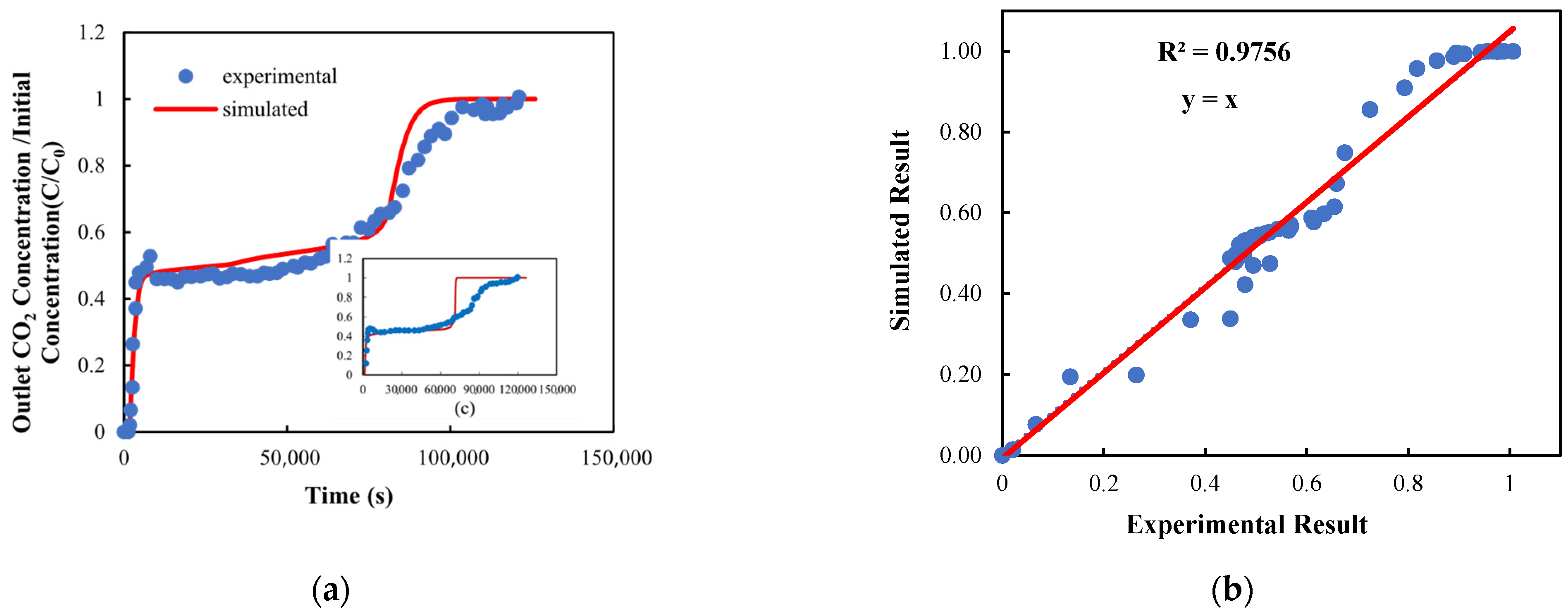
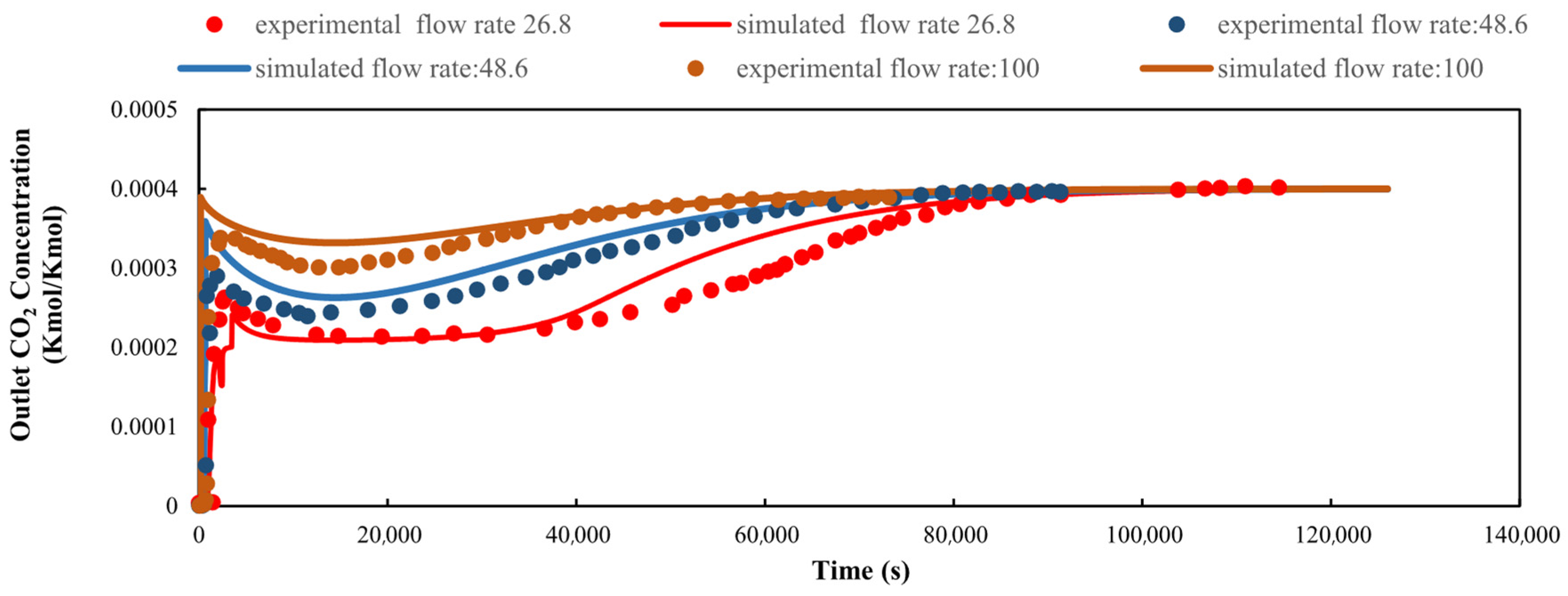
3.1. Mass Transfer Coefficient (MTC) Validation: Breakthrough Curve Comparison
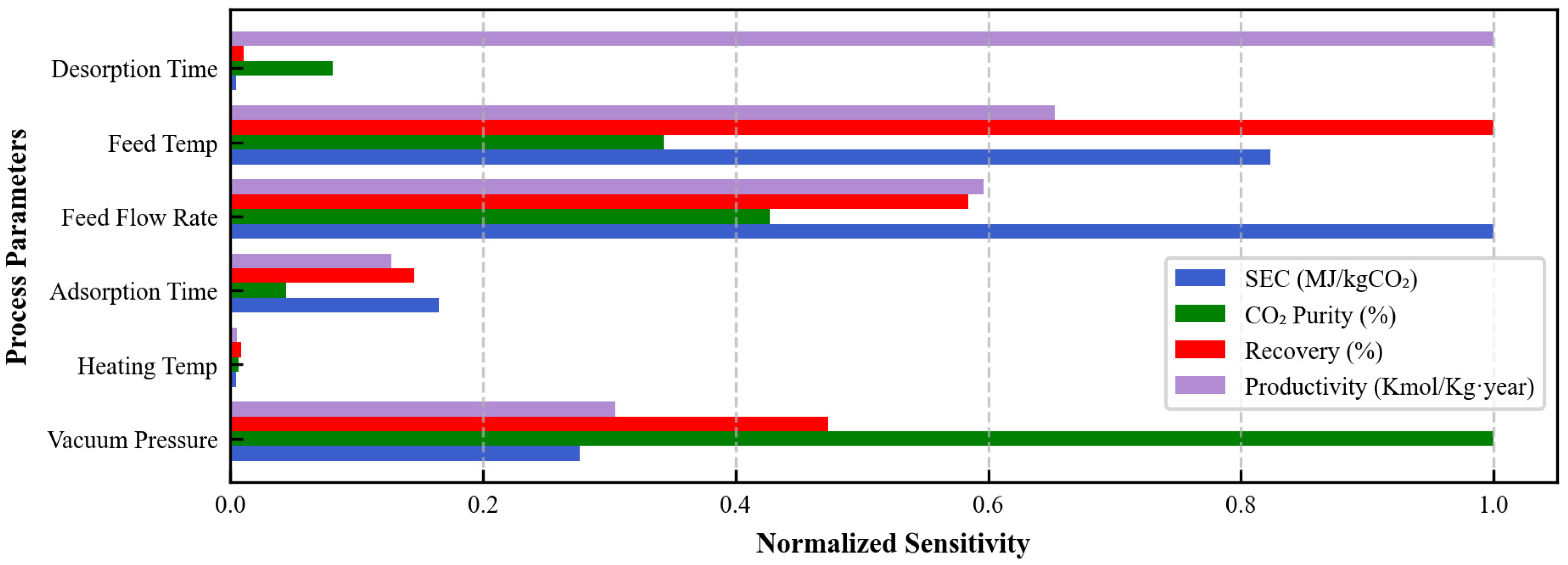
3.2. Parametric Evaluation
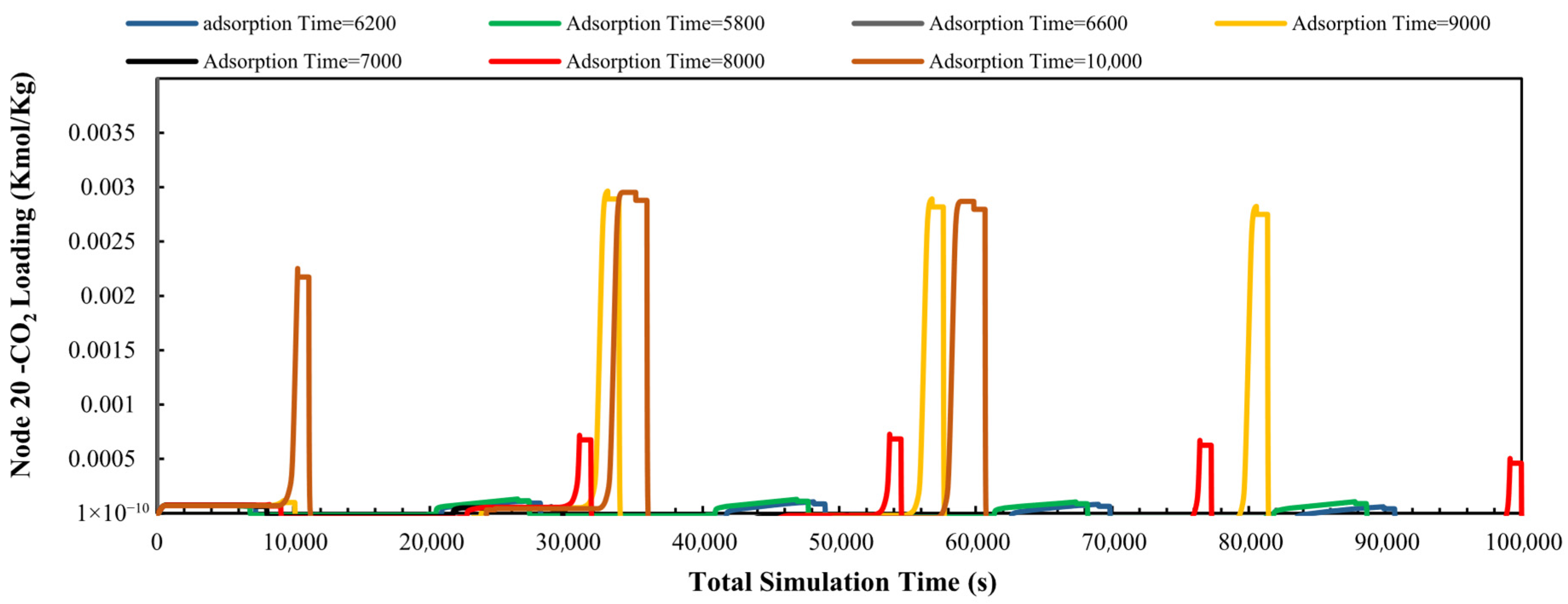
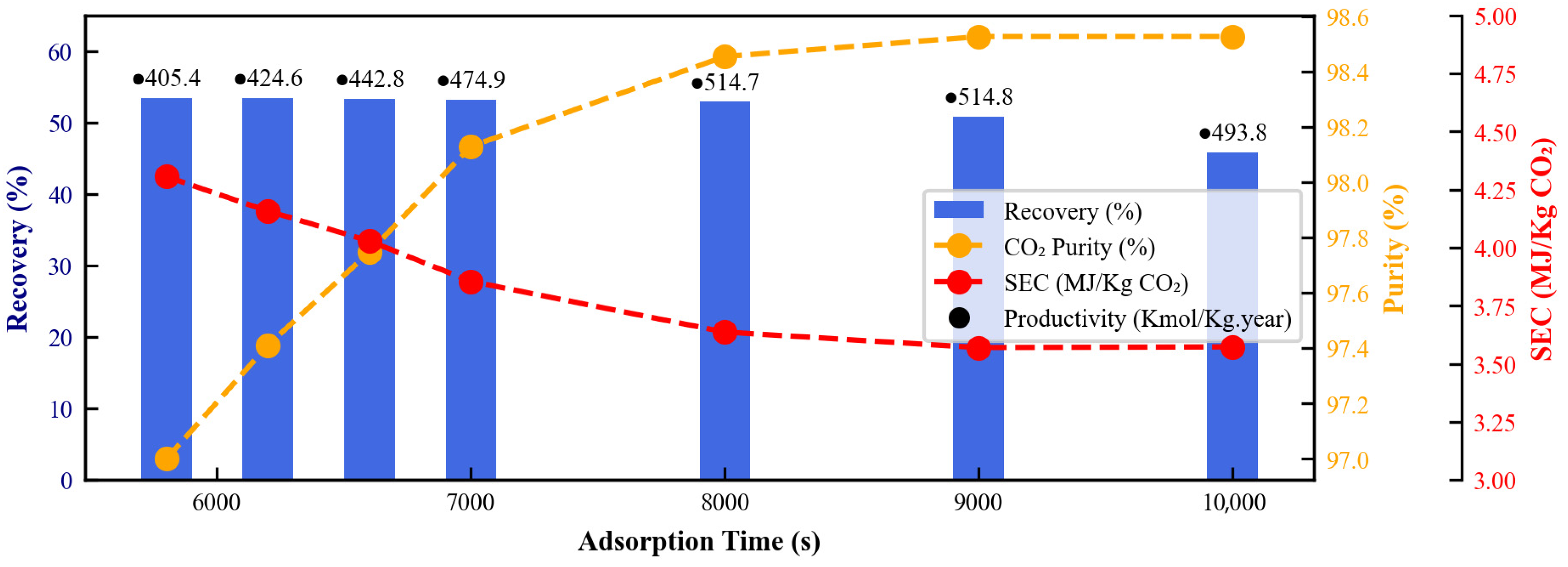
3.2.1. Adsorption Time
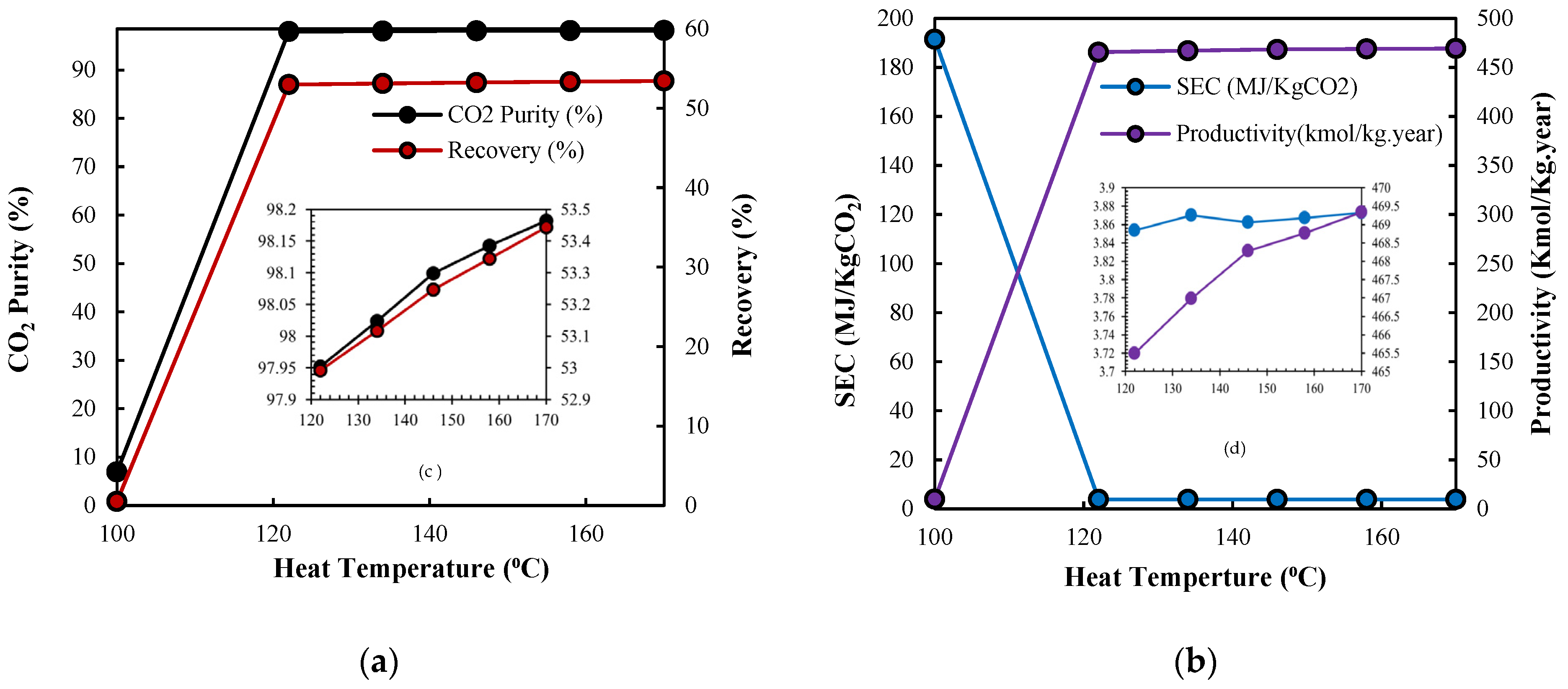
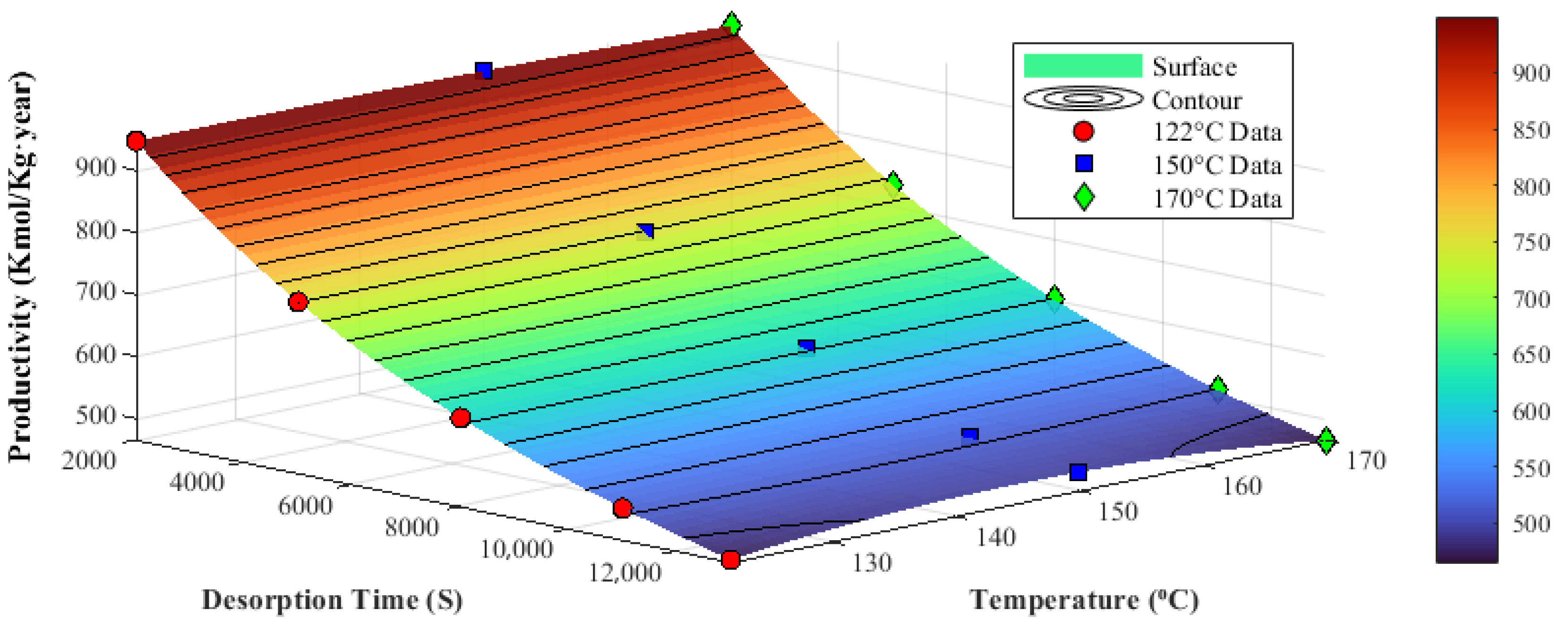
3.2.2. Desorption Temperature
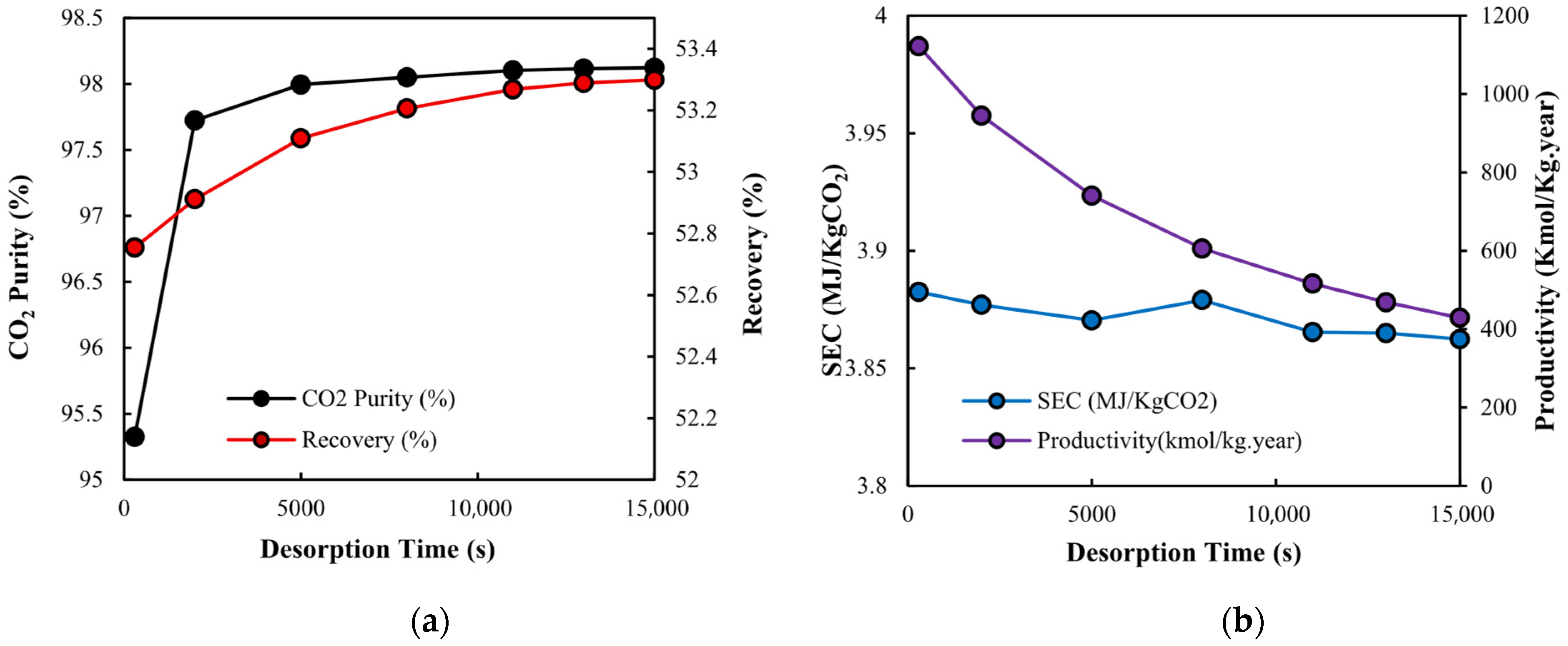
3.2.3. Desorption Time
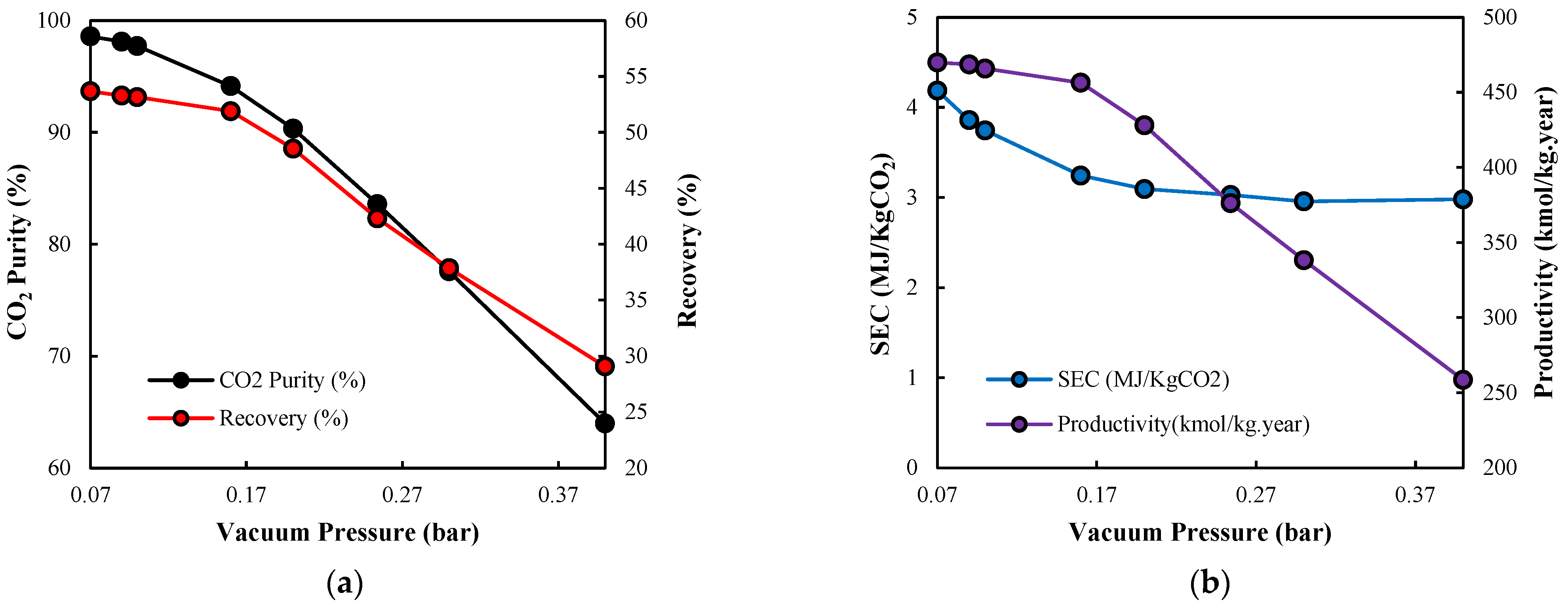
3.2.4. Evacuation Pressure
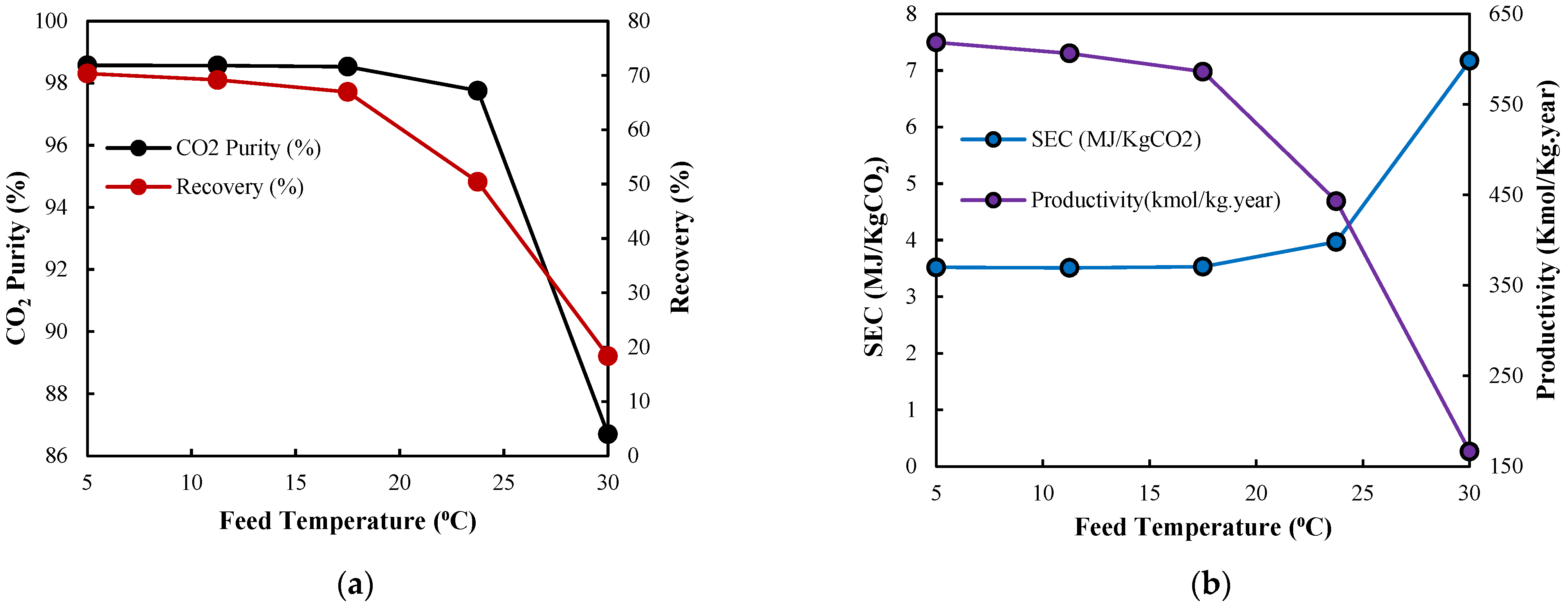
3.2.5. Inlet Feed Temperature
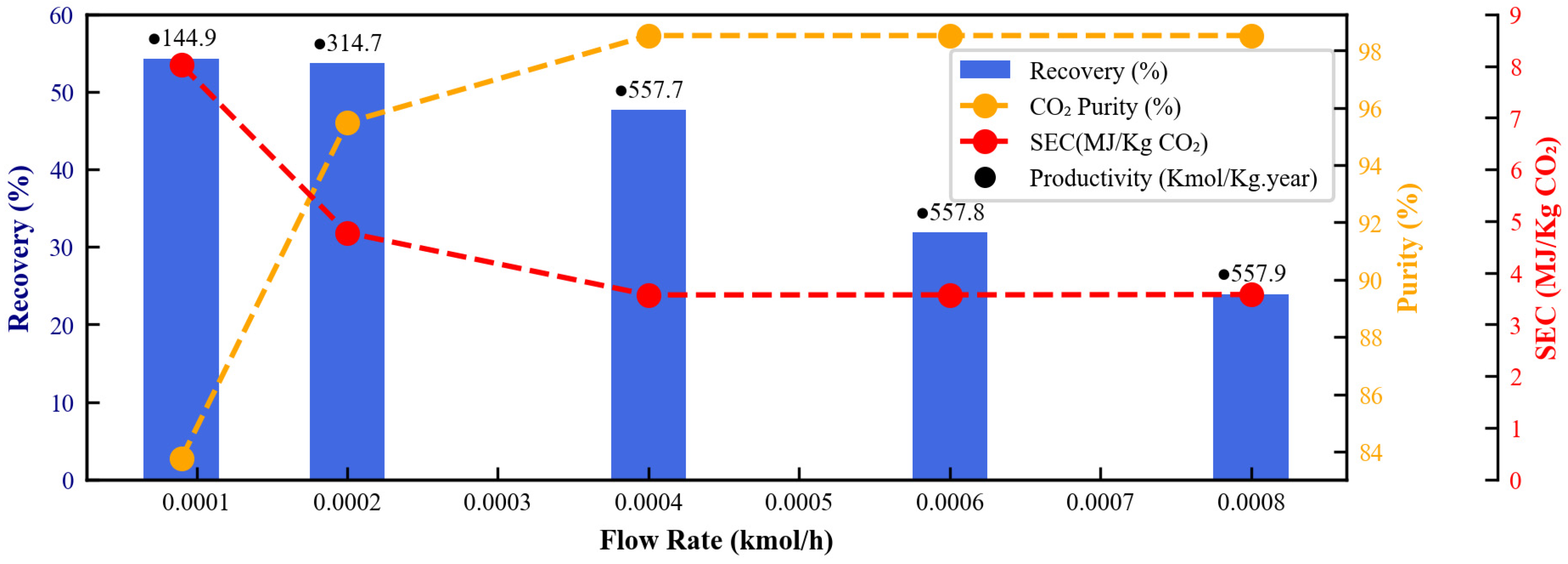
3.2.6. Inlet Feed Flow Rate
3.3. Optimal Design Discussion
- The current model assumes negligible pressure drop, thereby excluding blower energy consumption.
- Real-world vacuum equipment typically operates at lower efficiencies than assumed in ideal models.
- The Climeworks contactor design includes additional flow resistance due to the parallel flow path through structured adsorbent sheets, whereas the current model assumes axial flow through a thin sorbent layer, reducing resistance and energy loss.
- The current study does not consider the presence of water in the feed and associated energy required for water–CO2 separation. These factors would likely increase the energy demand in real-word systems.
| Performance Indicators | Process | Purity | Recovery | Specific Energy Consumption | Productivity | Reference |
|---|---|---|---|---|---|---|
| Unit | % | % | MJ/Kg | Kmol/Kg·year | ||
| Base case | Packed bed, TVSA | 98.13 | 53.26 | 3.85 | 474.85 | This work |
| Designed case | Packed bed, TVSA | 98.05 | 75 | 3.64 | 1373 | This work |
| Climeworks amine-based sorbent | TVSA | 99.9 | 85.4 | 6.12–8.18 | 1344 | [82,83] |
| Lewatit VP OC 1065 | Packed bed, TVSA | 99 | 77 | 5.42 | 1090 | [81] |
| Mmen-Mg2(dobpdc) | Coated monolith Steam assistance, TVSA | 95 | 60 | 3.52 | - | [53] |
| MIL-101(cr)-PEI-800 | Coated monolith Steam assistance, TVSA | 95 | 50 | 5.34 | - | [53] |
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature | Kfk | Film resistance coefficient (m/s) | |
| Rp | Particle radius (m) | Kpk | Macropore diffusion coefficient (m2/s) |
| Rb | Bed radius (m) | Kk | Overall mass transfer coefficient (1/s) |
| aP | External surface area per unit volume of the particle (1/m) | Dkk | Knudsen diffusion coefficient (m2/s) |
| q | Feed flow rate (Kmol/h) | Vg | Superficial gas velocity (m/s) |
| ΔHCO2 | CO2 heat of adsorption (KJ/mol) | Pfeed | Feed pressure (bar) |
| ΔHN2 | N2 heat of adsorption (KJ/mol) | Fproduct | Product flow rate (Kmol/h) |
| Cps | Crystal heat capacity (KJ/Kmol·K) | Ffeed | Feed flow rate (Kmol/h) |
| K | Thermal conductivity(W/m K) | kA | Avrami rate constant (1/s) |
| HTC | Heat transfer coefficient (W/m2·K) | nA | Avrami fractional constant |
| aHx | Heat exchanger surface area per unit volume (1/m) | T cycle | Full cycle time (s) |
| QHx | Heat supplied or removed by the heat exchanger (W/m3) | Wadsorbent | Adsorbent mass (Kg) |
| ΔH | Isosteric heat of adsorption (KJ/mol) | Pvac | Vacuum pressure (bar) |
| Cp | Heat capacity at constant pressure (KJ/mol·K) | y | Gas mole fraction |
| Cv | Heat capacity at constant volume (KJ/mol·K) | AARD | Average absolute relative deviation |
| Cps | Specific solid phase heat capacity (MJ/Kmol·K) | RMSE | Root mean squared error |
| Cvg | Specific gas phase heat capacity (MJ/kmol·K), | Greek letters | |
| P step | Isotherm step pressure (bar) | Gas density (Kg/m3) | |
| CO2 uptake before Pstep (mol/Kg) | Solid (crystal) density (Kg/m3) | ||
| CO2 uptake after Pstep (mol/Kg) | γ | Isotherm parameters (sharpness of the transition) | |
| qL | Low-affinity adsorption capacity (mol/Kg) | λ | Isotherm parameter controlling the effect of temperature (1/K) |
| qH | High-affinity adsorption capacity (mol/Kg) | µ | Fluid viscosity (N.s/m2) |
| qU | Ultimate affinity adsorption capacity(mol/Kg) | εp | Intraparticle void fraction (m3 void/m3particle) |
| n | Surface homogeneity factor | εb | Bed porosity (m3void/m3bed) |
| R | Gas constant (J/mol. K) | εt | Total bed porosity (m3void + m3pore)/m3bed |
| b | Langmuir constant (isotherm parameters) (1/bar) | γ | Specific heat ratio |
| W | Solid loading (Kmol/Kg) | η | Pump efficiency |
| W* | Equilibrium solid loading (Kmol/Kg) | ω | Isotherm parameter (smooth transition function) |
| Shk | Sherwood number | Abbreviations | |
| Re | Reynolds number | DAC | Direct air capture |
| Sck | Schmidt number | MOF | Metal-organic framework |
| MTC | Mass transfer coefficient (1/s) | CCUS | Carbon capture, utilisation, and storage |
| LDF | Linear driving force | TVSA | Temperature–vacuum swing adsorption |
References
- Nasriani, H.R.; Jamiolahmady, M. Maximizing fracture productivity in unconventional fields; analysis of post hydraulic fracturing flowback cleanup. J. Nat. Gas Sci. Eng. 2018, 52, 529–548. [Google Scholar] [CrossRef]
- Nasriani, H.R.; Kalantariasl, A. Choke Performance in High-rate Gas Condensate Wells Under Subcritical Flow Condition. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 37, 192–199. [Google Scholar] [CrossRef]
- Nasriani, H.R.; Jamiolahmady, M.; Saif, T.; Sánchez, J. A systematic investigation into the flowback cleanup of hydraulic-fractured wells in unconventional gas plays. Int. J. Coal Geol. 2018, 193, 46–60. [Google Scholar] [CrossRef]
- Nasriani, H.R.; Jamiolahmady, M. A Comparison of Clean-Up Efficiency of Multiple Fractured Horizontal Wells and Hydraulically Fractured Vertical Wells in Tight Gas Reservoirs. In Proceedings of the SPE Europec Featured at 80th EAGE Conference and Exhibition, Society of Petroleum Engineers, Copenhagen, Denmark, 11 June 2018. [Google Scholar] [CrossRef]
- Nasriani, H.R.; Jamiolahmady, M. Optimising Flowback Strategies in Unconventional Reservoirs: The Critical Role of Capillary Forces and Fluid Dynamics. Energies 2024, 17, 5822. [Google Scholar] [CrossRef]
- Medaiyese, F.J.; Nasriani, H.R.; Khan, K.; Khajenoori, L. Sustainable Hydrogen Production from Plastic Waste: Optimizing Pyrolysis for a Circular Economy. Hydrogen 2025, 6, 15. [Google Scholar] [CrossRef]
- Medaiyese, F.J.; Nasriani, H.R.; Khajenoori, L.; Khan, K.; Badiei, A. From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes. Sustainability 2024, 16, 4973. [Google Scholar] [CrossRef]
- Joonaki, E.; Rostaminikoo, E.; Ghanaatian, S.; Nasriani, H.R. Thermodynamic properties of hydrogen containing systems and calculation of gas critical flow factor. Meas. Sens. 2025, 38, 101587. [Google Scholar] [CrossRef]
- Gholami, S.; Rostaminikoo, E.; Khajenoori, L.; Nasriani, H.R. Density determination of CO2-Rich fluids within CCUS processes. Meas. Sens. 2025, 38, 101739. [Google Scholar] [CrossRef]
- Rostaminikoo, E.; Ghanaatian, S.; Joonaki, E.; Nasriani, H.R.; Whitton, J. Advanced thermodynamics of hydrogen and natural gas blends for gas transmission and distribution networks. Meas. Sens. 2025, 38, 101765. [Google Scholar] [CrossRef]
- Joonaki, E.; Rostaminikoo, E.; Ghanaatian, S.; Nasriani, H. Thermodynamics of Hydrogen; Analysing and Refining of Critical Flow Factor Through Comprehensive Uncertainty Assessment and Experimental Data Integration. In Proceedings of the ADIPEC, Abu Dhabi, United Arab Emirates, 4–7 November 2024. [Google Scholar] [CrossRef]
- IEA. Clean Energy Market MonitorParis, Mar. 2024. Available online: https://www.iea.org/reports/clean-energy-market-monitor-march-2024 (accessed on 1 March 2024).
- Stein, T. No Sign of Greenhouse Gases Increases Slowing in 2023. Available online: https://research.noaa.gov/no-sign-of-greenhouse-gases-increases-slowing-in-2023/ (accessed on 5 April 2024).
- Ozkan, M.; Custelcean, R. The status and prospects of materials for carbon capture technologies. MRS Bull. 2022, 47, 390–394. [Google Scholar] [CrossRef]
- UNFCCC. Key aspects of the Paris Agreement. In Proceedings of the COP21/CMP11, UNFCCC, Paris, Franch, 12 December 2015. Available online: https://unfccc.int/ (accessed on 1 December 2015).
- Lackner, K.; Ziock, A.H.-J.; Grimes, P.; Associates, G. Carbon Dioxide Extraction From Air: Is It An Option? In Proceedings of the Los A: 24th Annual Technical Conference on Coal Utilization and Fuel Systems. Clearwater, FL, USA, 1 February 1999; Available online: https://www.osti.gov/biblio/770509 (accessed on 1 February 1999).
- Custelcean, R. Direct Air Capture of CO2 Using Solvents. Annu. Rev. Chem. Biomol. Eng. 2025, 13, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Zeman, F. Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Farooqui, A.; McCoy, S.T. The impact of climate on solvent-based direct air capture systems. Appl. Energy 2022, 325, 119895. [Google Scholar] [CrossRef]
- Pidko, E.A.; Couck, S.; Remy, T.; Hensen, E.J.M.; Weckhuysen, B.M.; Denayer, J.; Gascon, J.; Kapteijn, F. Complexity behind CO2 Capture on NH2-MIL-53(Al). Langmuir 2011, 27, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Gandomkar, A.; Torabi, F.; Nasriani, H.R.; Enick, R.M. Decreasing Asphaltene Precipitation and Deposition during Immiscible Gas Injection Via the Introduction of a CO2-Soluble Asphaltene Inhibitor. SPE J. 2023, 28, 2316–2328. [Google Scholar] [CrossRef]
- Gandomkar, A.; Nasriani, H.R.; Enick, R.M.; Torabi, F. The effect of CO2-philic thickeners on gravity drainage mechanism in gas invaded zone. Fuel 2023, 331, 125760. [Google Scholar] [CrossRef]
- Nasriani, H.R.; Borazjani, A.A.; Iraji, B.; MoradiDowlatAbad, M. Investigation into the effect of capillary number on productivity of a lean gas condensate reservoir. J. Pet. Sci. Eng. 2015, 135, 384–390. [Google Scholar] [CrossRef]
- Mehra, P.; Paul, A. Decoding Carbon-Based Materials’ Properties for High CO2 Capture and Selectivity. ACS Omega 2022, 7, 34538–34546. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on Activated Carbons Prepared by Chemical Activation with Cupric Nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef] [PubMed]
- Casas, N.; Schell, J.; Pini, R.; Mazzotti, M. Fixed bed adsorption of CO2/H2 mixtures on activated carbon: Experiments and modeling. Adsorption 2012, 18, 143–161. [Google Scholar] [CrossRef]
- Serafin, Ł.; Baca, M.; Biegun, M.; Mijowska, E.; Kaleńczuk, R.J.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Li, P.; Wang, J.; Yu, J. CO2 capture from flue gas by two successive VPSA units using 13XAPG. Adsorption 2012, 18, 445–459. [Google Scholar] [CrossRef]
- Datta, S.J.; Khumnoon, C.; Lee, Z.H.; Moon, W.K.; Docao, S.; Nguyen, T.H.; Hwang, I.C.; Moon, D.; Oleynikov, P.; Terasaki, O.; et al. CO2 capture from humid flue gases and humid atmosphere using a microporous copper silicate. Science (1979) 2015, 350, 302–306. [Google Scholar]
- Amaraweera, S.M.; Gunathilake, C.A.; Gunawardene, O.H.; Dassanayake, R.S.; Cho, E.B.; Du, Y. Carbon Capture Using Porous Silica Materials. Nanomaterials 2023, 13, 2050. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Stilck, J.F.; Lee, J.H.; Neaton, J.B.; Prendergast, D.; Whitelam, S. Cooperative Gas Adsorption without a Phase Transition in Metal-Organic Frameworks. Phys. Rev. Lett. 2018, 121, 015701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, S.; He, S.; Wu, C. Direct air capture of CO2 by KOH-activated bamboo biochar. J. Energy Inst. 2022, 105, 399–405. [Google Scholar] [CrossRef]
- Ledesma, B.; Román, S.; Álvarez-Murillo, A.; Sabio, E.; González, J.F. Cyclic adsorption/thermal regeneration of activated carbons. J. Anal. Appl. Pyrolysis 2014, 106, 112–117. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: Application for gas purification. Ind. Eng. Chem. Res. 2010, 49, 359–365. [Google Scholar] [CrossRef]
- Guo, J.; He, S.; Liu, X.; Xu, M.; Liang, J.; Chu, Y. Hydrophobic modification of walnut shell biomass-derived porous carbon for the adsorption of VOCs at high humidity. Chem. Eng. J. 2024, 488, 150792. [Google Scholar] [CrossRef]
- Rodríguez-Mosqueda, R.; Bramer, E.A.; Roestenberg, T.; Brem, G. Parametrical Study on CO2 Capture from Ambient Air Using Hydrated K2CO3 Supported on an Activated Carbon Honeycomb. Ind. Eng. Chem. Res. 2018, 57, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 23, 2634–2656. [Google Scholar] [CrossRef]
- Wilson, S.M.W.; Tezel, F.H. Direct Dry Air Capture of CO2 Using VTSA with Faujasite Zeolites. Ind. Eng. Chem. Res. 2020, 59, 8783–8794. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal-Organic Frameworks for Past, Present, and Future. Am. Chem. Soc. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Lin, J.B.; Nguyen, T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, W.-H.; Jiang, W.; Yang, J.; Zhu, J.; Wang, L.; Ou, H.; Zhuang, Z.; Chen, M.; Sun, X.; et al. MOF Encapsulating N-Heterocyclic Carbene-Ligated Copper Single-Atom Site Catalyst towards Efficient Methane Electrosynthesis. Angew. Chem. 2021, 61, e202114450. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, X.; Zhu, P.; Li, Y.; Zhuang, Z.; Wu, H.; Zhu, J.; Xiao, C.; Chen, M.; Wang, P.; et al. Copper Atom Pairs Stabilize * OCCO Dipole Toward Highly Selective CO2 Electroreduction to C2H4. Angew. Chem. Int. Ed. 2024, 63, e202411591. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Chen, O.I.-F.; Hanikel, N.; Hossain, M.I.; Flaig, R.W.; Pei, X.; Amin, A.; Doherty, M.D.; Impastato, R.K.; Glover, T.G.; et al. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal-Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 2022, 144, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Hack, J.; Maeda, N.; Meier, D.M. Review on CO2 Capture Using Amine-Functionalized Materials. Am. Chem. Soc. 2022, 7, 39520–39530. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, S.; Lin, G.; Chen, S. Development and characterization of amine-functionalized hyper-cross-linked resin for CO2 capture. New J. Chem. 2018, 42, 420–428. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg 2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Darunte, L.A.; Terada, Y.; Murdock, C.R.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Monolith-Supported Amine-Functionalized Mg2(dobpdc) Adsorbents for CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 17042–17050. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sengupta, D.; Malliakas, C.D.; Idrees, K.B.; Xie, H.; Wang, X.; Barsoum, M.L.; Barker, N.M.; Dravid, V.P.; Islamoglu, T.; et al. Suitability of a diamine functionalized metal-organic framework for direct air capture. Chem. Sci. 2023, 14, 9380–9388. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]
- Joss, L.; Hefti, M.; Bjelobrk, Z.; Mazzotti, M. On the Potential of Phase-change Adsorbents for CO2 Capture by Temperature Swing Adsorption. Energy Procedia 2017, 114, 2271–2278. [Google Scholar] [CrossRef]
- Darunte, L.A. Application of Metal Organic Frameworks (mofs) to Capturing CO2 Directly from Air. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2018. Available online: https://smartech.gatech.edu/handle/1853/60614 (accessed on 25 March 2018).
- Darunte, L.A.; Sen, T.; Bhawanani, C.; Walton, K.S.; Sholl, D.S.; Realff, M.J.; Jones, C.W. Moving beyond Adsorption Capacity in Design of Adsorbents for CO2 Capture from Ultradilute Feeds: Kinetics of CO2 Adsorption in Materials with Stepped Isotherms. Ind. Eng. Chem. Res. 2019, 58, 366–377. [Google Scholar] [CrossRef]
- Shi, W.K.; Zhang, X.J.; Liu, X.; Wei, S.; Shi, X.; Wu, C.; Jiang, L. Temperature-vacuum swing adsorption for direct air capture by using low-grade heat. J. Clean. Prod. 2023, 414, 137731. [Google Scholar] [CrossRef]
- Gholami, M.; Van Assche, T.R.; Denayer, J.F. Temperature vacuum swing, a combined adsorption cycle for carbon capture. Curr. Opin. Chem. Eng. 2023, 39, 100891. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Y.C.; Ji, Y.; Yong, J.Y.; Zhang, X.J.; Jiang, L. Thermodynamic study on two adsorption working cycles for direct air capture. Appl. Therm. Eng. 2022, 214, 118920. [Google Scholar] [CrossRef]
- Elfving, J.; Bajamundi, C.; Kauppinen, J.; Sainio, T. Modelling of equilibrium working capacity of PSA, TSA and TVSA processes for CO2 adsorption under direct air capture conditions. J. CO2 Util. 2017, 22, 270–277. [Google Scholar] [CrossRef]
- Hughes, R.; Kotamreddy, G.; Ostace, A.; Bhattacharyya, D.; Siegelman, R.L.; Parker, S.T.; Didas, S.A.; Long, J.R.; Omell, B.; Matuszewski, M. Isotherm, Kinetic, Process Modeling, and Techno-Economic Analysis of a Diamine-Appended Metal-Organic Framework for CO2Capture Using Fixed Bed Contactors. Energy Fuels 2021, 35, 6040–6055. [Google Scholar] [CrossRef]
- Lee, J.H.; Siegelman, R.L.; Maserati, L.; Rangel, T.; Helms, B.A.; Long, J.R.; Neaton, J.B. Enhancement of CO2 binding and mechanical properties upon diamine functionalization of M2(dobpdc) metal-organic frameworks. Chem. Sci. 2018, 9, 5197–5206. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Realff, M.J. A parametric study of the techno-economics of direct CO2 air capture systems using solid adsorbents. AIChE J. 2019, 65, e16607. [Google Scholar] [CrossRef]
- Wilson, S.M.W. High purity CO2 from direct air capture using a single TVSA cycle with Na-X zeolites. Sep. Purif. Technol. 2022, 294, 121186. [Google Scholar] [CrossRef]
- Bagheri, M.; Fakhroleslam, M.; Fatemi, S. Ultra-Dilute CO2 capture in an ethane treatment plant via temperature swing adsorption: Simulation-based analysis and multi-objective optimal design. Sep. Purif. Technol. 2025, 356, 129968. [Google Scholar] [CrossRef]
- Mason, J.A.; McDonald, T.M.; Bae, T.H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, X.; Chen, Y.; Wang, R.; Ge, T. The analysis and evaluation of direct air capture adsorbents on the material characterization level. Chem. Eng. J. 2022, 450, 137958. [Google Scholar] [CrossRef]
- Ozkan, M.; Akhavi, A.A.; Coley, W.C.; Shang, R.; Ma, Y. Progress in carbon dioxide capture materials for deep decarbonization. Elsevier Inc. 2022, 8, 141–173. [Google Scholar] [CrossRef]
- Liu, D.; Purewal, J.J.; Yang, J.; Sudik, A.; Maurer, S.; Mueller, U.; Ni, J.; Siegel, D.J. MOF-5 composites exhibiting improved thermal conductivity. Int. J. Hydrogen Energy 2012, 37, 6109–6117. [Google Scholar] [CrossRef]
- Siegelman, R.L.; McDonald, T.M.; Gonzalez, M.I.; Martell, J.D.; Milner, P.J.; Mason, J.A.; Berger, A.H.; Bhown, A.S.; Long, J.R. Controlling Cooperative CO2 Adsorption in Diamine-Appended Mg2(dobpdc) Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 10526–10538. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ruthven, D.M.; Basmadjian, D. An Analysis of Adiabatic Sorption of Single Solutes in Fixed Beds: Pure Thermal Wave Formation and its Practical Implications; Academic Press: New York, NY, USA, 1990; Available online: https://pubs.acs.org/sharingguidelines (accessed on 6 July 2025).
- Luukkonen, A.; Elfving, J.; Inkeri, E. Improving adsorption-based direct air capture performance through operating parameter optimization. Chem. Eng. J. 2023, 471, 144525. [Google Scholar] [CrossRef]
- Stampi-Bombelli, V.; van der Spek, M.; Mazzotti, M. Analysis of direct capture of CO 2 from ambient air via steam-assisted temperature–vacuum swing adsorption. Adsorption 2020, 26, 1183–1197. [Google Scholar] [CrossRef]
- Wurzbacher, J.A. Development of a Temperature-Vacuum Swing Process for Co 2 Capture from Ambient Air. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2015. Available online: https://www.research-collection.ethz.ch/ (accessed on 26 May 2015).
- Martell, J.D.; Milner, P.J.; Siegelman, R.L.; Long, J.R. Kinetics of cooperative CO2adsorption in diamine-appended variants of the metal-organic framework Mg2(dobpdc). Chem. Sci. 2020, 11, 6457–6471. [Google Scholar] [CrossRef] [PubMed]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Balasubramaniam, B.M.; Thierry, P.T.; Lethier, S.; Pugnet, V.; Llewellyn, P.; Rajendran, A. Process-performance of solid sorbents for Direct Air Capture (DAC) of CO2 in optimized temperature-vacuum swing adsorption (TVSA) cycles. Chem. Eng. J. 2024, 485, 149568. [Google Scholar] [CrossRef]
- Wurzbacher, J.A.; Gebald, C.; Brunner, S.; Steinfeld, A. Heat and mass transfer of temperature–vacuum swing desorption for CO2 capture from air. Chem. Eng. J. 2016, 283, 1329–1338. [Google Scholar] [CrossRef]
- Mcdonald, T.M. Synthesis and Characterization of Alkylamine-Functionalized Metal-Organic Frameworks as Adsorbents for Carbon Dioxide. Ph.D. Thesis, University of Califirnia, Berkerly, CA, USA, 2015. Available online: https://escholarship.org/uc/item/8ph267b9 (accessed on 6 July 2025).
- Russbueldt, P.; Weitenberg, J.; Vernaleken, A.; Sartorius, T.; Schulte, J. Method and Arrangement For Spectral Broadening of Laser Pulses for Non-Linear Pulse Compression. U.S. Patent US 9,847,615, 19 December 2017. [Google Scholar]
- Deschamps, T.; Kanniche, M.; Grandjean, L.; Authier, O. Modeling of Vacuum Temperature Swing Adsorption for Direct Air Capture Using Aspen Adsorption. Clean. Technol. 2022, 4, 258–275. [Google Scholar] [CrossRef]
- Olga, F.; Christian, E.; Mahdi, B. Techno-economic assessment of CO2 direct air capture plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Bardow, S.; Deutz, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar]
| Parameters | Unit | Values |
|---|---|---|
| Bed Length | m | 0.055 |
| Bed Internal Radius | m | 0.004 |
| Desorption Temperature | °C | 115 |
| Adsorption Temperature | °C | 23 |
| Particle Radius | m | 2.25 × 10−4 |
| Crystal Density | Kg/m3 | 860 |
| Bed Porosity | fraction | 0.32 |
| Adsorbent Weight | g | 60 |
| Particle Porosity | fraction | 0.85 |
| Feed Flow rate | N mL/min | 17.2 |
| Parameters | Unit | Value | Parameters | Unit | Values |
|---|---|---|---|---|---|
| mol/Kg | 28.25 | KJ/mol | −62.49 | ||
| 1/bar | 2.51 × 10−15 | KJ/mol | 70.74 | ||
| - | 0.518 | KJ/mol | 1.35 | ||
| mol/Kg | 3.46 | KJ/mol | 67.72 | ||
| 1/bar | 2.42 × 10−11 | KJ/mol | 18.67 | ||
| mol/Kg.bar | 5.27 × 10−4 | - | 1.74 × 10−2 | ||
| ϒ | - | 4 | 1/0K | 6.53 | |
| Temperature-dependent variables’ formulas | |||||
| Cycles of Process | Unit | Duration |
|---|---|---|
| Adsorption | s | 7200 |
| Evacuation | s | 6 |
| Heating + Evacuation | s | 10,000 |
| Cooling | s | Temperature matched the feed temperature |
| Pressurising | s | Pressure matched the atmospheric pressure |
| Performance Indicators | Unit | Formula |
|---|---|---|
| CO2 Purity | % | |
| Recovery | % | |
| Productivity | Kmol/Kg·year | |
| SEC (vacuum) | MJ/Kg CO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiri, M.N.; Nasriani, H.R.; Khajenoori, L.; Mohammadkhani, S.; Williams, K.S. Dynamic Temperature–Vacuum Swing Adsorption for Sustainable Direct Air Capture: Parametric Optimisation for High-Purity CO2 Removal. Sustainability 2025, 17, 6796. https://doi.org/10.3390/su17156796
Ghiri MN, Nasriani HR, Khajenoori L, Mohammadkhani S, Williams KS. Dynamic Temperature–Vacuum Swing Adsorption for Sustainable Direct Air Capture: Parametric Optimisation for High-Purity CO2 Removal. Sustainability. 2025; 17(15):6796. https://doi.org/10.3390/su17156796
Chicago/Turabian StyleGhiri, Maryam Nasiri, Hamid Reza Nasriani, Leila Khajenoori, Samira Mohammadkhani, and Karl S. Williams. 2025. "Dynamic Temperature–Vacuum Swing Adsorption for Sustainable Direct Air Capture: Parametric Optimisation for High-Purity CO2 Removal" Sustainability 17, no. 15: 6796. https://doi.org/10.3390/su17156796
APA StyleGhiri, M. N., Nasriani, H. R., Khajenoori, L., Mohammadkhani, S., & Williams, K. S. (2025). Dynamic Temperature–Vacuum Swing Adsorption for Sustainable Direct Air Capture: Parametric Optimisation for High-Purity CO2 Removal. Sustainability, 17(15), 6796. https://doi.org/10.3390/su17156796








