Abstract
With increasing global challenges related to water scarcity and phosphorus depletion, the recovery and reuse of wastewater-derived nutrients offer a sustainable path forward. This study evaluates the dual role of lanthanides (Ce3+ and La3+) in recovering phosphorus from municipal wastewater and supporting corn (Zea mays) cultivation through lanthanide phosphate (Ln-P) and lanthanide-reclaimed wastewater (LRWW, wastewater spiked with lanthanide). High-purity precipitates of CePO4 (98%) and LaPO4 (92%) were successfully obtained without pH adjustment, as confirmed by X-ray photoelectron spectroscopy (XPS) and energy-dispersive spectroscopy (EDS). Germination assays revealed that lanthanides, even at concentrations up to 2000 mg/L, did not significantly alter germination rates compared to traditional coagulants, though root and shoot development declined above this threshold—likely due to reduced hydrogen peroxide (H2O2) production and elevated total dissolved solids (TDSs), which induced physiological drought. Greenhouse experiments using desert-like soil amended with Ln-P and irrigated with LRWW showed no statistically significant differences in corn growth parameters—including plant height, stem diameter, leaf number, leaf area, and biomass—when compared to control treatments. Photosynthetic performance, including stomatal conductance, quantum efficiency, and chlorophyll content, remained unaffected by lanthanide application. Metal uptake analysis indicated that lanthanides did not inhibit phosphorus absorption and even enhanced the uptake of calcium and magnesium. Minimal lanthanide accumulation was detected in plant tissues, with most retained in the root zone, highlighting their limited mobility. These findings suggest that lanthanides can be safely and effectively used for phosphorus recovery and agricultural reuse, contributing to sustainable nutrient cycling and aligning with the United Nations’ Sustainable Development Goals of zero hunger and sustainable cities.
1. Introduction
Water scarcity and food security remain among the most pressing global challenges of the 21st century. With climate change intensifying drought frequency and population growth increasing demand, the strain on freshwater resources is projected to worsen. According to the Food and Agriculture Organization (FAO), approximately 1.2 billion people already reside in water-stressed regions, and hunger continues to claim the lives of 17 people every minute [1,2]. Agriculture, which accounts for about 70% of global freshwater withdrawals, is particularly vulnerable to water shortages. At the same time, food production systems are under additional pressure due to the dwindling availability of essential nutrients, especially phosphorus.
Phosphorus (P) is a non-substitutable nutrient that is vital for plant development, yet it is primarily obtained from finite phosphate rock reserves. The extraction of phosphorus is energy-intensive and costly, ranging from $13 to $350 per metric ton depending on market and processing conditions [3,4,5]. Current estimates suggest that global phosphorus reserves may be exhausted within the next 80 years, especially under existing technological constraints and growing agricultural demand. Consequently, there is an urgent need to explore alternative and sustainable sources of phosphorus that can reduce dependence on mined fertilizers and mitigate environmental degradation associated with conventional fertilizer use.
One such promising strategy involves the recovery of phosphorus from municipal wastewater. Municipal wastewater is continuously generated and relatively stable in composition, making it a reliable resource for nutrient recovery. Unlike mineral sources, it is not subject to geopolitical supply risks. Enhanced biological phosphorus removal (EBPR) systems are commonly employed in wastewater treatment plants to accumulate phosphorus biologically. During the aerobic phase, phosphorus-accumulating organisms (PAOs) uptake phosphate, which is later released during sludge dewatering. This process produces a centrate stream enriched in phosphate, with concentrations reaching up to 600 mg PO43−/L [6].
To recover phosphorus from these waste streams, coagulants such as ferric and aluminum salts are often used. However, these traditional coagulants require high dosages and may introduce toxicity concerns or generate large volumes of sludge. Recent studies have identified rare earth elements (REEs), particularly lanthanum (La3+) and cerium (Ce3+), as effective and less toxic alternatives for phosphate precipitation [6,7,8,9,10]. These lanthanides form highly stable lanthanide phosphate (Ln-P) compounds under ambient conditions, making them suitable for nutrient recovery.
In this study, we aim to recover phosphate from municipal wastewater using lanthanum and cerium and to evaluate the agricultural potential of the resulting byproducts. Specifically, we investigate two outputs: (1) the lanthanide phosphate precipitate (Ln-P) and (2) lanthanide-reclaimed wastewater (LRWW), which is the treated effluent containing residual lanthanides. We assess their use as phosphorus sources and irrigation water, respectively, for growing corn (Zea mays). Although lanthanides are not classified as essential plant nutrients, prior research suggests that they can enhance plant growth, stimulate physiological processes, and improve nutrient uptake [11,12,13,14,15,16,17,18]. Additionally, they may offer benefits such as improved root structure and enhanced photosynthetic activity [19,20,21,22,23].
By demonstrating the dual value of wastewater-derived lanthanide products for both nutrient recovery and crop production, this research supports a circular economy model. It also contributes to the advancement of sustainable agricultural practices and aligns with the United Nations’ Sustainable Development Goals, particularly Goal 2 (Zero Hunger) and Goal 11 (Sustainable Cities and Communities).
2. Materials and Methods
2.1. Lanthanide Phosphate Recovery and Treated Effluent Preparation
The centrate wastewater utilized in this study originated from the dewatering process of activated sludge at the Clark County Water Reclamation Facility, located in Las Vegas, NV, USA. Upon collection, samples were either processed immediately or kept refrigerated at 4 °C until further use.
Phosphorus recovery was achieved using a standard jar test protocol adapted from Kajjumba et al. [6]. The precipitation process involved adding cerium chloride (CeCl3) and lanthanum chloride (LaCl3) directly to the centrate without altering its native pH (~6.7), as lanthanides are known to facilitate phosphate precipitation effectively across a broad pH range (4.0–9.0). A molar ratio of 1:1.2 (PO43−:M3+) was maintained for optimal performance. Details on the chemical reagents used, including their purity and source, are presented in Table 1.

Table 1.
Summary of chemicals used in the study.
After the addition of coagulants, each mixture underwent rapid stirring at 120 rpm for 1 min, followed by gentle mixing at 40 rpm for 20 min. The mixture was then allowed to settle for 30 min. The resulting lanthanide phosphate (Ln-P) solids were separated by filtration and subsequently dried in an oven at 105 °C for three days. These solids were later used as soil amendments. Their composition and bonding structure were verified via energy-dispersive X-ray spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS).
In parallel, treated secondary effluent from the same facility was repurposed for irrigation trials. This effluent was fortified with one of the selected metal salts—cerium (III) chloride (CeCl3), lanthanum (III) chloride (LaCl3), ferric chloride (FeCl3), and aluminum sulfate (Al2(SO4)3)—to achieve a final metal ion concentration of 3.0 mg/L (M3+), thereby simulating metal-amended reclaimed water. The full analytical profile of this irrigation water is presented in Table 2, including key metrics such as pH, nutrient levels, and salinity indicators.

Table 2.
Composition of effluent water that was used to irrigate the corn plants [24].
2.2. Corn Germination Assay
To assess the impact of lanthanide ions on corn germination, corn seeds were exposed to distilled water containing a range of lanthanide concentrations. Five seeds (Burpee®, Warminster, PA, USA) were placed on moistened paper towels inside 90 mm Petri dishes filled with solutions containing 0, 50, 100, 500, 1000, 2000, or 5000 mg/L of trivalent metal ions (M3+). The dishes were covered and incubated at ambient temperature (~20 °C) in complete darkness to simulate optimal germination conditions. Seed germination was observed daily over a five-day period. A seed was classified as germinated when its radicle extended at least 2 mm. On the fifth day, the germination rate, along with root and shoot lengths, was measured and recorded. To enable comparison, additional test groups were treated with conventional coagulants commonly used in wastewater treatment—ferric (Fe3+) and aluminum (Al3+) salts—alongside the lanthanide treatments (Ce3+ and La3+). This allowed evaluation of whether lanthanides present greater, lesser, or similar effects on early plant development relative to traditional coagulants.
2.3. Oxidative Stress Assessment
The concentration of hydrogen peroxide (H2O2) was used as a biomarker to evaluate oxidative stress in corn seedlings exposed to metal-based coagulants. Approximately 0.30–0.40 g of germinated seed tissue was weighed and homogenized in 5.0 mL of chilled distilled water maintained at 4 °C. The homogenized mixture was then centrifuged at 9000 rpm to remove coarse debris and large particulates. From the supernatant, 4.0 mL was collected and treated with 100 µL of a titanium chloride solution (99.9%) [25], which reacts with H2O2 to form a detectable complex. After a second centrifugation step at 9000 rpm for 5 min, residual solids, including titanium oxide, were removed. The clarified solution was then passed through a 0.45 µm nylon filter (Ks-Tek, China) to eliminate any remaining fine particles. The absorbance of the resulting filtrate was measured at 415 nm using a UV-Vis spectrophotometer [24]. The H2O2 concentration in each sample was calculated using a calibration curve generated from known standards processed under identical conditions.
2.4. Soil Preparation and Irrigation
Soil formulation for this study followed the approach outlined by Rader and colleagues [26], which was designed to mimic arid soil environments. A custom blend was created using washed sand (Quikrete®, Atlanta, GA, USA) and tree bark (Miracle-Gro®, Marysville, OH, USA) mixed in a 4:1 volumetric ratio. To minimize microbial interference, the soil was sterilized and allowed to cool in a sterile environment for 48 h before planting. Particle size analysis using dry sieving revealed a composition dominated by coarse-to-medium sand, with low gravel content (approximately 8.1%). The bulk density of the blend was about 1.2 g/cm3, and among particles smaller than 2 mm, 99.6% were sand, of which 87.8% were coarse to medium-sized.
Based on standard agricultural nutrient guidelines, a typical one-acre plot requires about 82 kg of nitrogen, 41 kg of P2O5, and 73 kg of K2O [27]. Given that an acre-furrow slice contains approximately 0.9 million kg of topsoil, this equates to 46 mg of P2O5 (or 20 mg of elemental phosphorus) and 91 mg of nitrogen per kg of soil. In this study, phosphorus was supplemented by blending phosphate-rich precipitates (derived from different metal coagulants) into 2 kg of prepared soil to provide 15 mg P/kg, as outlined in Table 3 [24]. The remaining 5 mg P/kg was supplied through irrigation using lanthanide-reclaimed wastewater (LRWW), which also served as the moisture source. Since LRWW already contained 15.58 ± 0.64 mg/L of nitrogen and 58.80 ± 4.65 µg/L of phosphate (see Table 2), no extra nitrogen amendments were necessary.

Table 3.
The amount of phosphate precipitate added to 2 kg of soil [24].
Corn seeds were soaked overnight in distilled water prior to planting and sown 2–3 cm deep in 17 cm diameter pots. Each pot was initially planted with five seeds and irrigated every 2–4 days using LRWW dosed with 3.0 mg/L of metal ions (Ce3+, La3+, Fe3+, or Al3+). To reduce leaching and mimic drip irrigation conditions, 200 mL or 250 mL of the solution was added per irrigation event. Separate sets of pots were used for each metal treatment and the control group. The cultivation experiment was conducted in a controlled greenhouse setting at the University of Nevada, Las Vegas, from 7 October 2021 to 9 January 2022, under environmental conditions of approximately 23.4 ± 2.2 °C and 32.1 ± 8.4% relative humidity. On day 25, each pot was thinned to retain three corn plants for the remainder of the study.
2.5. Physical and Photosynthetic Parameters
This study evaluated several morphological and physiological characteristics of corn plants, including plant height, stem thickness, leaf count, and dry biomass. Biomass was assessed by drying a known portion of fresh stem tissue at 105 °C for 48 h, after which the dry mass was recorded using a precision analytical balance. Plant height and stem diameter were recorded using a measuring tape and digital caliper, respectively. Leaf chlorophyll levels were measured using a Chlorophyll Content Meter−300 (Opti-Sciences CCM−300, Hudson, NH, USA). For photosynthetic function, an LI−600 Porometer/Fluorometer (LI-COR, Lincoln, NE, USA) was used to assess the following variables: stomatal conductance, gsw (mol/m2/s); transpiration rate, E (mmol/m2/s); quantum efficiency of photosynthetic electron transport through photosystem II, PhiPS2; electron transport rate, ETR (µmol/m2/s), vapor pressure deficit (kPa); humidity; and temperature. All physiological measurements were performed during daylight hours to capture active photosynthetic responses.
2.6. Metal Uptake and Elemental Mapping
To assess the absorption and spatial distribution of lanthanides, phosphorus, and related nutrients in plant tissues, electron probe microanalysis (EPMA) was conducted using a JEOL JXA−8900 analyzer (Peabody, MA, USA). In living plant tissues, minerals may redistribute due to internal water movement (capillary action), which can compromise localization accuracy. To prevent this, samples were frozen at −20 °C for a minimum of 24 h prior to sectioning, as described by [24,28]. Following freezing, thin tissue sections were prepared from the roots, stems, and leaves, then thawed and mounted onto microscope slides. Graphite adhesive (Electron Microscopy Sciences, Hatfield, PA, USA) was used to secure the sections to the slides for EPMA scanning. Root tissue was sampled at the junction of seminal and nodal roots. Stem tissue was collected from a point approximately 20% up the plant’s height, and leaf sections were taken along the central midrib. Acquired EPMA images were further processed using the ImageJ software, Version 6 (University of Wisconsin, Madison, WI, USA) to visualize and quantify elemental distribution across the different plant tissues.
3. Results and Discussion
3.1. Lanthanide Phosphate Precipitates
High-resolution X-ray photoelectron spectroscopy (XPS) was used to analyze the interactions between lanthanides (Ln) and phosphorus (P), focusing on the shifts in binding energies that indicate chemical bonding. In the La matrix, peaks corresponding to the La 3d5/2 and La 3d3/2 orbitals—sublevels within the 3d electron shell—were observed. These orbitals represent core-level electrons, and their binding energies are sensitive indicators of the chemical environment. The La 3d5/2 and La 3d3/2 peaks appeared at 836.0 eV and 853.0 eV, respectively, consistent with inner-sphere complex formation [29]. Similarly, in the Ce system, binding energies for the Ce 3d3/2 (902.4 eV) and Ce 3d5/2 (883.8 eV) orbitals—also core electron states—were identified, which play key roles in Ce-P interactions. For phosphorus, the most reactive electrons reside in the 2p1/2 and 2p3/2 subshells, with binding energies at 136 eV and 135 eV, respectively [29].
The formation of inner-sphere bonds between phosphorus and lanthanides altered these valence electron energies. Figure 1A,B present the XPS spectra of Ln-P, showing clear peaks for Ce, La, P, and O. In the Ce-PO4 complex, the Ce 3d3/2 binding energy shifted to 904.0 eV—an increase of 1.6 eV. Likewise, in the La-PO4 system, the La 3d5/2 peak shifted by 1.4 eV. These shifts confirm the successful formation of La-O-P and Ce-O-P bonding structures. Figure 1C displays EDS results, showing elemental ratios of La, Ce, O, and P that closely match the theoretical stoichiometry for pure Ce-/La-PO4. Impurity levels were minimal, with CePO4 containing up to 1.9% impurities and LaPO4 up to 7.9%. Given their purity, these precipitates are suitable for use as phosphorus sources in plant growth applications.
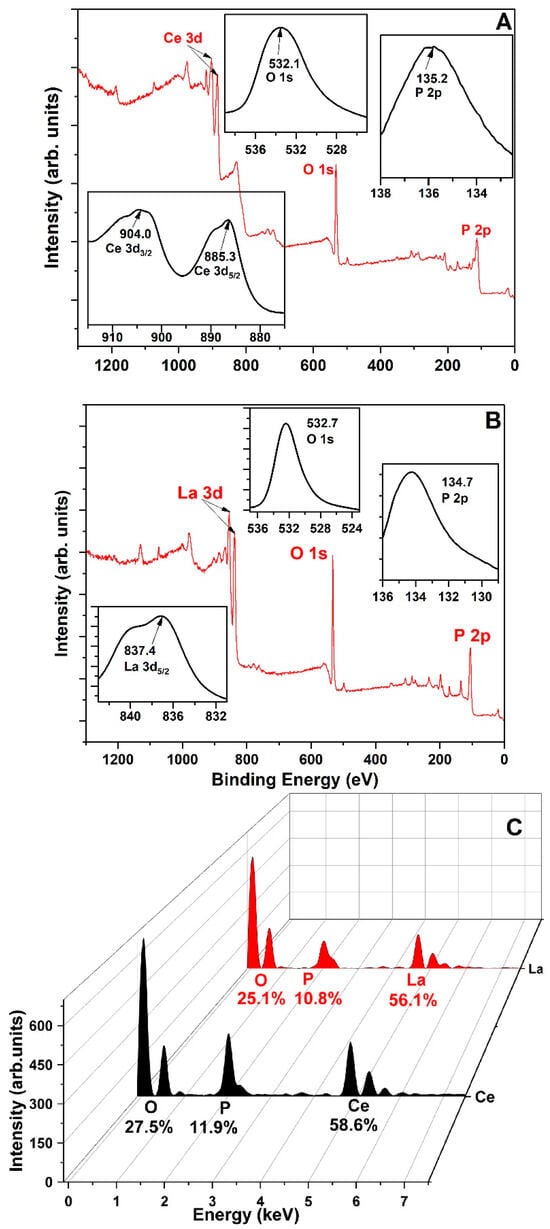
Figure 1.
XPS analysis of (A) CePO4 and (B) LaPO4 and (C) EDS pattern of CePO4 and LaPO4 precipitated from wastewater. Impurities detected include carbon, calcium, iron, and sulfur [24].
Table 4 summarizes the characteristics of the centrate wastewater used for lanthanide treatment, including the initial concentrations and the percentage removal of phosphorus (PO43−-P) and other key parameters following treatment with CeCl3 and LaCl3. The results highlight the high efficiency of both lanthanides in phosphorus removal (>98%) with moderate effects on other water quality indicators.

Table 4.
Characteristics of centrate that was collected from wastewater treatment and the performance of lanthanide. Reported as the average value (n ≥ 2) with standard deviation [24].
3.2. Germination Rate and Hydrogen Peroxide
Seed germination begins with water uptake, which activates metabolic processes essential for transforming a dormant seed into a growing plant. These metabolic activities not only determine the likelihood of germination but also influence the future health of the plant. To assess the effect of metal salts on germination, corn kernels (n = 5 per treatment) were grown in Petri dishes with metal solutions at concentrations of 0, 50, 100, 500, 1000, 2000, and 5000 mg M3+/L. After five days of incubation in the dark, the seeds were assessed for germination rate, root and shoot lengths, and hydrogen peroxide (H2O2) production. A seed was considered germinated if it developed a visible embryonic axis, regardless of root or shoot length. As shown in Table A1 and Table A2, no clear correlation was observed between metal concentration and germination rate. This inconsistency could be attributed to seed dormancy at the time of planting, which may have hindered germination even under otherwise optimal conditions (i.e., proper moisture, light, oxygen, pH, and temperature). However, root and shoot lengths showed a more consistent trend. At concentrations below 1000 mg M3+/L, the root and shoot lengths were comparable to those observed in the control group treated with deionized water. At concentrations above 1000 mg M3+/L, both root and shoot lengths decreased significantly (Table A1 and Table A3).
To further investigate these changes, H2O2 levels were measured in the germinated kernels, as H2O2 is a key reactive oxygen species (ROS) involved in seed metabolism. Water uptake during germination triggers a cascade of biochemical reactions, including the production of ROS such as H2O2 [30]. It is estimated that 1−5% of the oxygen consumed during germination is converted into H2O2 [31]. Most H2O2 in seeds is generated from the dismutation of superoxide anions (O2•⁻), which are produced via electron leakage from mitochondrial complexes I, II, and III [32,33].
Another significant source of H2O2 is NADPH oxidase, located on the plasma membrane, which is especially active during biotic or abiotic stress. This process—known as the “oxidative burst” or respiratory burst oxidase homologs (RBOHs)—is triggered by melatonin and modulated by S-nitrosylation and phosphorylation, which enhance O2•⁻ and, subsequently, H2O2 production [31,34,35]. Additional contributors to ROS production during germination include polyamine oxidase [35,36] and peroxisomal metabolism [37,38]. Unlike other ROS, H2O2 is relatively stable and can migrate throughout the seed and even into the surrounding environment [39]. While moderate levels of H2O2 are beneficial and function as signaling molecules, excessive accumulation can damage DNA, proteins, and lipids, thereby inhibiting germination [40].
Figure 2 illustrates the ratio of H2O2 production relative to metal concentration, compared to the control. Despite increasing metal concentrations, H2O2 production remained relatively unchanged up to 1000 mg/L. This suggests that low-to-moderate metal levels did not significantly impact oxidative stress. This observation aligns with reports that exogenous H2O2 can promote germination by modulating the proteome, transcriptome, and hormone signaling pathways [41]. Specifically, H2O2 activates mitogen-activated protein kinases (MAPKs) that suppress abscisic acid while also promoting protein carbonylation and activating the pentose phosphate pathway (PPP). This activation leads to increased NADPH production, which has antioxidant properties that support seed germination and early plant development [30,42]. However, at 2000 mg/L and above, a decline in H2O2 levels was observed. This reduction coincided with the suppression of root and shoot development, suggesting that excess metal concentrations may have impaired the physiological processes critical to germination and early growth.
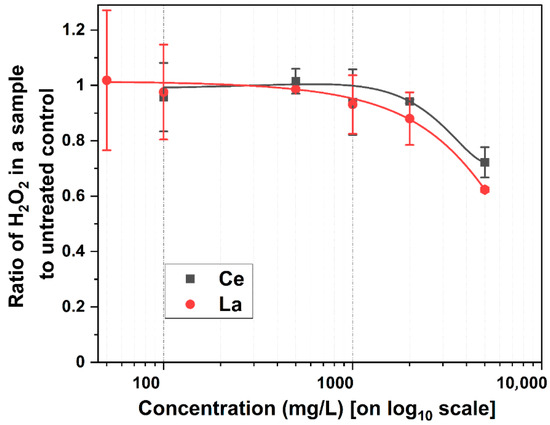
Figure 2.
Generation of H2O2 at different metal concentrations during kernel germination after five days of incubation in darkness. Measured H2O2 in the control (untreated) = 0.0013% ± 0.0003% per 0.39 g of wet weight. Error bars represent one standard deviation, with n = 2 [24].
The reduction in root and shoot lengths at higher metal concentrations may be attributed to pH changes, particularly in environments treated with Al3+ and Fe3+. As the metal concentration increased, the pH of the Al3+ and Fe3+ solutions decreased significantly, as shown in Table A4. These coagulants are typically formulated using strong acids such as sulfuric acid and hydrochloric acid [6]. While a slight reduction in pH can help weaken the seed coat—facilitating water absorption into the endosperm—excessive acidification can lead to “water flooding.” This condition disrupts oxygen availability within the seed, thereby inhibiting the germination signaling pathways. In contrast, the pH in Ce3+ and La3+ treatments remained relatively stable, with Ce3+ at 5.3 ± 0.1 and La3+ at 5.2 ± 0.2 (Table A5). In these cases, the decline in root and shoot development is more likely due to elevated total dissolved solids (TDSs), which increase electrical conductivity. Excessive TDSs create osmotic stress, limiting water uptake into the seed—a phenomenon often referred to as physiological drought [43]. This water deficit prevents the initiation of germination or restricts early root development. Figure 3 illustrates corn kernel germination in a Ce3+ environment. At concentrations between 0 and 2000 mg/L, kernels were frequently covered by a fungal mat. The presence of this fungal growth is believed to aid in nutrient mobilization from more distant parts of the root system [44], thereby improving germination and seedling development. However, at 5000 mg/L, no fungal development was observed. Without fungal assistance, the kernels likely depended solely on localized nutrients near the root surface, which may have limited their growth potential.
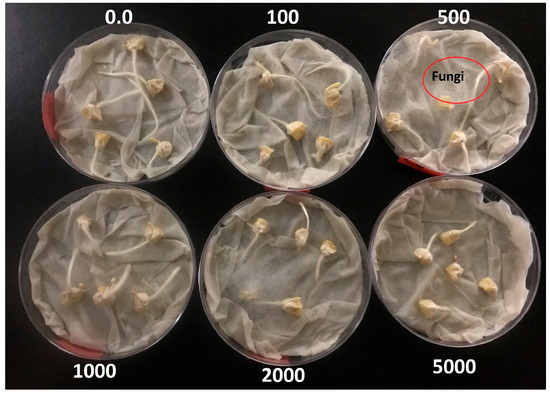
Figure 3.
Germination of kernels under a Ce3+ environment and the development of fungi at different metal ion concentrations (0–5000 mg/L) [24].
3.3. Corn Physical Characteristics
Throughout the corn growth cycle, key physical characteristics were monitored, including plant height, stem diameter, the number of leaves, leaf area, and dry biomass. These parameters serve as indicators of overall plant health and development. By day 75, each pot had received a total of 4.59 L of LRWW. The average plant height was 42.0 ± 1.7 cm, with corn grown under lanthanum treatment exhibiting the shortest average height at 39.9 ± 11.5 cm (Figure 4A). The average stem diameter across all treatments was 7.44 ± 0.65 mm, with no statistically significant differences observed between treatments (Figure 4B, ρ > 0.05). Leaf number increased steadily until day 75, at which point tassels (male flowers) had emerged on all plants. At maturity, plants produced between 11 and 12 leaves (Figure 4C). Leaves play a crucial role in plant physiology, particularly in photosynthesis, where they facilitate CO2 uptake and water vapor release. Consequently, leaf development influences sugar production and has been correlated with crop yield in several studies [45,46]. At maturity (day 75), the average leaf area per plant was 70.6 cm2. Plants treated with aluminum showed the largest average leaf area, 72.2 cm2 (Figure 4D). However, no statistically significant differences (ρ > 0.05) were observed among treatments, including lanthanides, the control, and other coagulants. These results suggest that lanthanide treatments do not adversely affect corn leaf development.
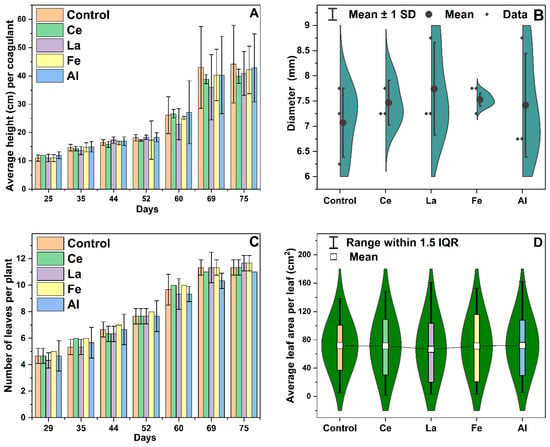
Figure 4.
(A) Height of corn, (B) diameter of corn plant stem on day 75, (C) number of leaves per plant, and (D) leaf area per plant on day 75. SD: standard deviation; IQR: interquartile range. Error bars represent one standard deviation, with n = 3. There was no statistical difference between the control and plants treated with cerium (Ce), lanthanum (La), iron (Fe), or aluminum (Al) when measured on the same day at a 95% confidence level [24].
3.4. Photosynthetic Parameters
The primary function of stomata is to regulate the exchange of gases—specifically, minimizing water loss while maximizing carbon dioxide (CO2) uptake. While CO2 uptake is essential for photosynthesis, excessive water loss poses a threat to plant survival. Plants mitigate this trade-off by optimizing photosynthesis to offset the cost of water loss. Stomatal conductance (gsw) reflects the rate at which CO2 enters and water vapor exits the leaf. Throughout the corn growth cycle, gsw remained relatively consistent across all treatments involving different coagulants (Figure 5A). As a result, transpiration rates also remained stable, showing no significant variation among treatments (Figure 5B). To assess photosynthetic induction, the electron transport rate (ETR) and quantum efficiency (PhiPS2) were measured. ETR values were not statistically different from the control under any coagulant treatment throughout the experiment (Figure 5C). Similarly, PhiPS2 values remained above 0.5 regardless of metal addition, indicating that the photosynthetic machinery of the plants was not adversely affected (Figure 5D). Quantum efficiency values greater than 0.5 suggest that lanthanides did not negatively impact corn photosynthesis. This consistency is likely due to stable chlorophyll production throughout the experiment (Figure 6A) [47]. A slight decline in chlorophyll levels observed after day 35 can be attributed to reduced daylight hours during the winter season when the study was conducted.
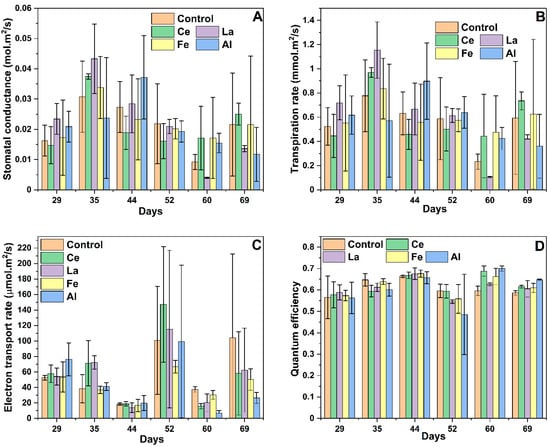
Figure 5.
Stomatal conductance (A), transpiration rate (B), electron transport rate (C), and quantum efficiency (D) of corn grown with different coagulants. Error bars represent one standard deviation, with n = 3. There was no statistical difference between the control and plants treated with Ce, La, Fe, or Al when measured on the same day at a 95% confidence level.
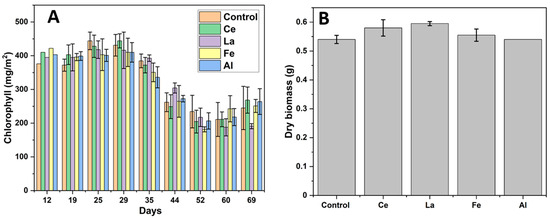
Figure 6.
(A) The amount of chlorophyll per m2 under different coagulant environments. (B) Dry biomass of corn stems after 94 days. Wet mass of each stem = 1.50 g. Stems were dried for 48 h at 105 °C. Error bars represent standard error, with n ≥ 2 [24].
3.5. Metals Uptake
Due to the strong bond formed between lanthanides and phosphate (Ln–P), it was initially anticipated that Ln3+ might reduce phosphorus availability to corn by forming insoluble Ln–P precipitates. Metal uptake analysis was performed on 94-day-old corn plants. Each pot treated with cerium or lanthanum received 165.9 mg of Ce3+ and 164.9 mg of La3+, respectively. Figure 7A,B show the uptake of Ce, La, P, Ca, and Mg in the roots of plants grown in Ce and La environments. Compared to the control (Figure 7C), Ln3+ treatments did not inhibit or enhance phosphorus uptake. If any interaction between Ln and phosphorus occurred, it is likely that soil fungi and/or mycorrhizae may have played a role in breaking down the Ln-O-P bond to release phosphorus [48], although this mechanism remains unclear and warrants further research. Trace amounts of cerium and lanthanum were detected in the roots, but none were found in the stems or leaves. This absence suggests limited translocation of lanthanides within the plant, likely due to their moderate to high binding affinity with silica-based substrates like sand [49,50], which may immobilize them in the soil matrix.
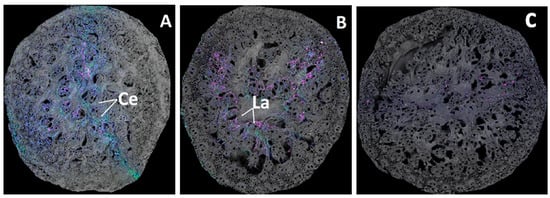
Figure 7.
Metal mapping in roots for plants grown with cerium (A), lanthanum (B), and (C) the control. The red spots represent Ce/La, and magenta represents P. Blue is Ca, and green is Mg. Plants were 94 days old, and each respective pot had received 165.9 mg of Ce3+ and 164.9 mg of La3+ [24].
The presence of other metals (P, Ca, and Mg) in the stems and leaves likely resulted from capillary transport through the plant’s vascular system, as the irrigation method used (drip irrigation) avoided direct application of LRWW to aerial parts. Based on fluorescence intensity results (Figure 8), the presence of rare earth elements (REEs) in the root zone appeared to enhance the uptake of P, Ca, and Mg compared to the control. Similar findings have been reported in soybeans, where exposure to lanthanum improved the uptake of metals such as Ca, K, and P [51,52]. Although the precise mechanism by which lanthanides enhance nutrient uptake is not fully understood, one hypothesis is that their interaction with soil silica may liberate micro- and macronutrients, increasing their bioavailability to plant roots and associated fungi. Additionally, as lanthanides enter root tissues, they may create micro-pores or “gates” that facilitate the entry of smaller ions such as Fe and P [53]. This enhanced metal uptake may explain the slightly higher biomass observed in corn plants irrigated with LRWW (Figure 6B). These findings support the potential benefits of using Ln-P and LRWW as phosphorus sources in crop production.
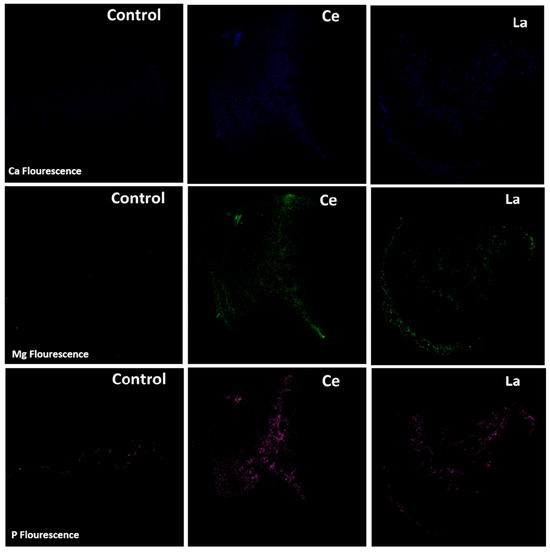
Figure 8.
Metal fluorescence in corn roots. Plants were 94 days old, and each respective pot had received 165.9 mg of Ce3+ and 164.9 mg of La3+ [24].
4. Conclusions
Achieving the United Nations’ Sustainable Development Goals—particularly Goal 2 (Zero Hunger) and Goal 11 (Sustainable Cities and Communities)—requires sustainable and integrated strategies that promote nutrient recovery and water reuse. This study demonstrates that lanthanides can effectively recover phosphorus from municipal wastewater, forming high-purity lanthanide phosphate (Ln-P) precipitates (≥98% purity), as confirmed by XPS/EDS analysis.
To assess the potential phytotoxicity of lanthanides, corn seeds were exposed to varying concentrations (0–5000 mg/L) of Ln3+. Compared to traditional wastewater coagulants such as aluminum and ferric salts, lanthanides did not significantly affect germination rates. However, at concentrations above 2000 mg/L, all tested metal treatments—including lanthanides—led to a reduction in root and shoot development. This inhibition was attributed to decreased H2O2 production, a critical signaling molecule during germination, and to increased TDS, which imposed osmotic stress and induced physiological drought.
When corn was grown in desert-like soil amended with Ln-P and irrigated using lanthanide-reclaimed wastewater (LRWW), plant development metrics—including height, stem diameter, the number of leaves, and leaf area—showed no statistically significant differences compared to the control group. Likewise, photosynthetic parameters such as stomatal conductance, chlorophyll content, and quantum efficiency remained stable, indicating that lanthanides did not disrupt key physiological processes.
Metal uptake analysis revealed that lanthanide treatments did not inhibit phosphorus absorption. In fact, plants exposed to lanthanides exhibited increased uptake of Ca and Mg, suggesting a potential synergistic effect. Notably, minimal concentrations of lanthanides were detected in plant tissues after 94 days, indicating limited mobility and translocation, with most lanthanides retained in the soil. This immobility not only supports their environmental safety but also raises questions about their role as a long-term phosphorus source.
Overall, the application of Ln-P and LRWW appears to be a promising strategy for sustainable agriculture, especially in arid regions. However, further research is needed to better understand the mechanisms of phosphorus release from Ln-P compounds and their long-term performance as slow-release fertilizers. These insights are essential for advancing circular economy practices in wastewater management and agriculture.
Author Contributions
Conceptualization, G.W.K. and E.J.M.; methodology, G.W.K. and E.J.M.; validation, E.J.M.; formal analysis, G.W.K. and S.V.; investigation, G.W.K. and S.V.; resources, E.J.M.; writing—original draft preparation, G.W.K., S.V. and E.J.M.; writing—review and editing, G.W.K., S.V. and E.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors acknowledge the contribution and advice from James Gaskin, Jeffery Q. Shen, Minghua Ren, Lindsay Chiquoine, Joshua Greenwood, Serdar Aydin, and Anne J. Villacastin.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Germination rate of kernels [24].
Table A1.
Germination rate of kernels [24].
| mg M3+/L | La3+ | Ce3+ | Fe3+ | Al3+ |
|---|---|---|---|---|
| 0 | 70.0% | 100.0% | 80.0% | 40.0% |
| 50 | 80.0% | 100.0% | 100.0% | 80.0% |
| 100 | 90.0% | 100.0% | 40.0% | 80.0% |
| 500 | 90.0% | 80.0% | 100.0% | 80.0% |
| 1000 | 100.0% | 100.0% | 80.0% | 100.0% |
| 2000 | 90.0% | 100.0% | 100.0% | 100.0% |
| 5000 | 100.0% | 100.0% | 60.0% | 100.0% |

Table A2.
Root length (cm) after 5 days of kernel planting [24].
Table A2.
Root length (cm) after 5 days of kernel planting [24].
| mg M3+/L | La3+ | Ce3+ | Fe3+ | Al3+ |
|---|---|---|---|---|
| 0 | 4.4 | 2.8 | 2.8 | 1.8 |
| 50 | 4.6 | 3.2 | 3.1 | 3.5 |
| 100 | 4.6 | 3.6 | 2.6 | 4.3 |
| 500 | 1.9 | 3.3 | 3.4 | 3.9 |
| 1000 | 1.6 | 2.2 | 4.1 | 1.8 |
| 2000 | 0.9 | 0.6 | 0.8 | 0.7 |
| 5000 | 0.4 | 1.0 | 0.5 | 0.7 |

Table A3.
Shoot length (cm) after 5 days of kernel planting [24].
Table A3.
Shoot length (cm) after 5 days of kernel planting [24].
| mg M3+/L | La3+ | Ce3+ | Fe3+ | Al3+ |
|---|---|---|---|---|
| 0 | 1.2 | 1.2 | 1.2 | 2.3 |
| 50 | 1.5 | 1.3 | 1.5 | 2.2 |
| 100 | 1.4 | 1.1 | 1.1 | 2.8 |
| 500 | 1.0 | 1.6 | 1.0 | 1.6 |
| 1000 | 1.0 | 1.4 | 1.2 | 1.5 |
| 2000 | 0.6 | 0.6 | 0.9 | 0.8 |
| 5000 | 0.5 | 0.2 | 0.3 | 0.4 |

Table A4.
pH after 5 days of kernel planting [24].
Table A4.
pH after 5 days of kernel planting [24].
| mg M3+/L | La3+ | Ce3+ | Fe3+ | Al3+ |
|---|---|---|---|---|
| 0 | 5.5 | 5.5 | 5.5 | 5.5 |
| 50 | 5.1 | 5.3 | 3.1 | 4.0 |
| 100 | 5.1 | 5.3 | 2.8 | 3.9 |
| 500 | 5.2 | 5.3 | 2.3 | 3.7 |
| 1000 | 5.2 | 5.3 | 2.1 | 3.5 |
| 2000 | 5.1 | 5.3 | <0.0 | 3.4 |
| 5000 | 5.0 | 5.2 | <0.0 | 3.3 |

Table A5.
Conductivity (µS/cm) after 5 days of kernel planting [24].
Table A5.
Conductivity (µS/cm) after 5 days of kernel planting [24].
| mg M3+/L | La3+ | Ce3+ | Fe3+ | Al3+ |
|---|---|---|---|---|
| 0 | 1.5 | 1.5 | 1.5 | 1.5 |
| 50 | 148.3 | 147.1 | 528.0 | 378.0 |
| 100 | 285.0 | 285.0 | 1014.0 | 628.0 |
| 500 | 1204.0 | 1233.0 | 3760.0 | 2108.0 |
| 1000 | 2340.0 | 2390.0 | 6680.0 | 3550.0 |
| 2000 | 4340.0 | 4500.0 | 11,780.0 | 6060.0 |
| 5000 | 9760.0 | 10,230.0 | 23,500.0 | 12,070.0 |
The number of kernels per Petri dish = 5. Each value represents an average of at least two kernels. M3+ = La3+, Ce3+, Fe3+, or Al3+.
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: Rome, Italy; WFP: Rome, Italy; WHO: Rome, Italy, 2020. [Google Scholar]
- Leghari, S.J.; Han, W.; Hu, K.; Laghari, Y.; Wei, Y.; Cui, L. What Should We Do for Water Security? A Technical Review on More Yield per Water Drop. J. Environ. Manag. 2024, 370, 122832. [Google Scholar] [CrossRef] [PubMed]
- Endfield, G.H.; Tejedo, I.F. Decades of Drought, Years of Hunger: Archival Investigations of Multiple Year Droughts in Late Colonial Chihuahua. Clim. Change 2006, 75, 391–419. [Google Scholar] [CrossRef]
- Mew, M.C. Phosphate Rock Costs, Prices and Resources Interaction. Sci. Total Environ. 2016, 542, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, W.J.; Sutton, M.A.; Cordell, D.; Reay, D.S.; Heal, K.V.; Withers, P.J.A.; Vanderbeck, I.; Spears, B.M. Phosphorus Price Spikes: A Wake-up Call for Phosphorus Resilience. Front. Sustain. Food Syst. 2023, 7, 1088776. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Fischer, D.; Risso, L.; Koury, D.; Marti, E.J. Application of Cerium and Lanthanum Coagulants in Wastewater Treatment—A Comparative Assessment to Magnesium, Aluminum, and Iron Coagulants. Chem. Eng. J. 2021, 426, 131268. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Lu, Y.; Tariq, M.; Yuan, L.; Yang, J.; Li, Z.; Lyu, S. Development of Lanthanum Hydroxide Loaded on Molecular Sieve Adsorbent and Mechanistic Study for Phosphate Removal. J. Alloys Compd. 2018, 768, 953–961. [Google Scholar] [CrossRef]
- Su, Y.; Yang, W.; Sun, W.; Li, Q.; Shang, J.K. Synthesis of Mesoporous Cerium-Zirconium Binary Oxide Nanoadsorbents by a Solvothermal Process and Their Effective Adsorption of Phosphate from Water. Chem. Eng. J. 2015, 268, 270–279. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Attene-Ramos, M.; Marti, E.J. Toxicity of Lanthanide Coagulants Assessed Using Four in Vitro Bioassays. Sci. Total Environ. 2021, 800, 149556. [Google Scholar] [CrossRef] [PubMed]
- Kajjumba, G.W.; Marti, E.J. A Review of the Application of Cerium and Lanthanum in Phosphorus Removal during Wastewater Treatment: Characteristics, Mechanism, and Recovery. Chemosphere 2022, 309, 136462. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, K.; Wang, F.; Zhao, X.; Bai, R.; Liu, B. Effects of Rare Earth Elements on Growth and Determination of Secondary Metabolites under in Vitro Conditions in Salvia Miltiorrhiza. Am. Soc. Hortic. Sci. 2020, 55, 310–316. [Google Scholar] [CrossRef]
- Chen, W.J.; Tao, Y.; Gu, Y.H.; Zhao, G.W. Effect of Lanthanide Chloride on Photosynthesis and Dry Matter Accumulation in Tobacco Seedlings. Biol. Trace Elem. Res. 2001, 79, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Gong, X.; Liu, C.; Hong, M.; Wang, L.; Hong, F. Effects of Manganese Deficiency and Added Cerium on Photochemical Efficiency of Maize Chloroplasts. Biol. Trace Elem. Res. 2012, 146, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Fashui, H.; Ling, W.; Chao, L. Study of Lanthanum on Seed Germination and Growth of Rice. Biol. Trace Elem. Res. 2003, 94, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Kaineder, K.; Mezricky, D.; Řezanka, M.; Bišová, K.; Zachleder, V.; Vítová, M. The Effect of Lanthanides on Photosynthesis, Growth, and Chlorophyll Profile of the Green Alga Desmodesmus Quadricauda. Photosynth. Res. 2016, 130, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, F.S.; Wu, K.; Ma, H.B.; Zhang, X.G.; Hong, C.J.; Wu, C.; Gao, F.Q.; Yang, F.; Zheng, L.; et al. Effect of Nd3+ Ion on Carboxylation Activity of Ribulose−1,5-Bisphosphate Carboxylase/Oxygenase of Spinach. Biochem. Biophys. Res. Commun. 2006, 342, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Li, D.; Peng, A. Application of Rare-Earth Elements in the Agriculture of China and Its Environmental Behavior in Soil. Environ. Sci. Pollut. Res. 2002, 9, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, L.; Zhou, Q. Lanthanum (III) Regulates the Nitrogen Assimilation in Soybean Seedlings under Ultraviolet-B Radiation. Biol. Trace Elem. Res. 2013, 151, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Q.; Xu, X.J.; Wang, S.; Shan, C.J. Lanthanum Improves Salt Tolerance of Maize Seedlings. Photosynthetica 2016, 54, 148–151. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed Priming to Alleviate Salinity Stress in Germinating Seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.P.; Fasciano, C.; d’Aquino, L.; Tommasi, F. Responses of Antioxidant Systems to Lanthanum Nitrate Treatments in Tomato Plants during Drought Stress. Plant Biosyst. 2011, 145, 248–252. [Google Scholar] [CrossRef]
- Wang, J.; Bian, C. Strengthening Effects of LaCl 3 Treatment on Resistance of Capsicum Seeds to Acid Rain Stress. Zhongguo Xitu Xuebao/J. Chin. Rare Earth Soc. 2012, 30, 373–379. [Google Scholar]
- Wahid, P.A.; Valiathan, M.S.; Kamalam, N.V.; Eapen, J.T.; Vijayalakshmi, S.; Prabhu, R.K.; Mahalingam, T.R. Effect of Rare Earth Elements on Growth and Nutrition of Coconut Palm and Root Competition for These Elements between the Palm and Calotropis Gigantea. J. Plant Nutr. 2000, 23, 329–338. [Google Scholar] [CrossRef]
- Kajjumba, G.W. Application of Lanthanides in Wastewater Treatment and Water Reuse. Ph.D. Thesis, University of Nevada, Las Vegas, NV, USA, 2022. [Google Scholar]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen Peroxide Signaling Integrates with Phytohormones during the Germination of Magnetoprimed Tomato Seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef] [PubMed]
- Rader, A.J.; Chiquoine, L.P.; Weigand, J.F.; Perkins, J.L.; Munson, S.M.; Abella, S.R. Biotic and Abiotic Treatments as a Bet-Hedging Approach to Restoring Plant Communities and Soil Functions. Restor. Ecol. 2022, 30, e13527. [Google Scholar] [CrossRef]
- Dekalb Benefits of Phosphorus for Corn Production. Available online: https://www.dekalbasgrowdeltapine.com/en-us/agronomy/benefits-phosphorus-corn-production.html#:~:text=Corndoesnotrequireas,canlimitcornyieldpotential (accessed on 5 December 2021).
- Zhao, Y.; Peralta-Videa, J.R.; Lopez-Moreno, M.L.; Ren, M.; Saupe, G.; Gardea-Torresdey, J.L. Kinetin Increases Chromium Absorption, Modulates Its Distribution, and Changes the Activity of Catalase and Ascorbate Peroxidase in Mexican Palo Verde. Environ. Sci. Technol. 2011, 45, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- CRC. Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498754293. [Google Scholar]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action during Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Małecka, A.; Ciereszko, I.; Staszak, A.M. Mitochondria Are Important Determinants of the Aging of Seeds. Int. J. Mol. Sci. 2019, 20, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yan, Y.; Wen, D.; Shi, Q. Hydrogen Peroxide Produced by NADPH Oxidase: A Novel Downstream Signaling Pathway in Melatonin-Induced Stress Tolerance in Solanum Lycopersicum. Physiol. Plant. 2017, 160, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Yang, K.; Wang, Y.; Yang, L.; Hu, L.; Liu, R.; Shi, Z. Melatonin Facilitates Lateral Root Development by Coordinating PAO-Derived Hydrogen Peroxide and Rboh-Derived Superoxide Radical. Free Radic. Biol. Med. 2019, 143, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The Signalling Role of ROS in the Regulation of Seed Germination and Dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.X.; Li, W.Y.; Gao, Y.T.; Chen, Z.J.; Zhang, W.N.; Liu, Q.J.; Chen, Z.; Liu, J. Involvement of Polyamine Oxidase-Produced Hydrogen Peroxide during Coleorhiza-Limited Germination of Rice Seeds. Front. Plant Sci. 2016, 7, 1219. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal Hydrogen Peroxide Is Coupled to Biotic Defense Responses by ISOCHORISMATE SYNTHASE1 in a Daylength-Related Manner. Plant Physiol. 2010, 153, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed Birth to Death: Dual Functions of Reactive Oxygen Species in Seed Physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Colville, L. Metals and Seeds: Biochemical and Molecular Implications and Their Significance for Seed Germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in Pea Seed Germination. Plant Signal. Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of Protein Oxidation in Arabidopsis Seeds and during Germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Fan, R.; Zheng, R.; Li, C.M.; Yu, D.Y. Proteomic Analysis of Seed Germination under Salt Stress in Soybeans. J. Zhejiang Univ. Sci. 2011, 12, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Schlüter, S.; Vogel, H.J.; Vetterlein, D. Roots Compact the Surrounding Soil Depending on the Structures They Encounter. Sci. Rep. 2019, 9, 16236. [Google Scholar] [CrossRef] [PubMed]
- Mark Kliewer, W.; Dokoozlian, N.K. Leaf Area/Crop Weight Ratios of Grapevines: Influence on Fruit Composition and Wine Quality. In Proceedings of the ASEV 50th Anniversary Annual Meeting, Seattle, WA, USA, 19–23 June 2000. [Google Scholar]
- Sun, J.; Gao, J.; Wang, Z.; Hu, S.; Zhang, F.; Bao, H.; Fan, Y. Maize Canopy Photosynthetic Efficiency, Plant Growth, and Yield Responses to Tillage Depth. Agronomy 2019, 9, 3. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-Translational Coordination of Chlorophyll Biosynthesis and Breakdown by BCMs Maintains Chlorophyll Homeostasis during Leaf Development. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jalali, J.; Lebeau, T. The Role of Microorganisms in Mobilization and Phytoextraction of Rare Earth Elements: A Review. Front. Environ. Sci. 2021, 9, 213. [Google Scholar] [CrossRef]
- Ballinger, B.; Motuzas, J.; Miller, C.R.; Smart, S.; Diniz, D.; Costa, J.C. Nanoscale Assembly of Lanthanum Silica with Dense and Porous Interfacial Structures. Sci. Rep. 2015, 5, 8210. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Wang, X.; Yin, X.; Luo, N.; Khayambashi, A.; Wei, Y. The Synthesis and Characterization of Hydrous Cerium Oxide Nanoparticles Loaded on Porous Silica Micro-Sphere as Novel and Efficient Adsorbents to Remove Phosphate Radicals from Water. Microporous Mesoporous Mater. 2019, 279, 73–81. [Google Scholar] [CrossRef]
- de Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; de Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; e Silva, P.H.P.; Schnug, E.; et al. Bioaccumulation and Effects of Lanthanum on Growth and Mitotic Index in Soybean Plants. Ecotoxicol. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Zhu, J.G.; Chu, H.Y.; Zhang, Y.L.; Zeng, Q.; Ma, H.L.; Cao, Z.H. Effect of Lanthanum on Rice Production, Nutrient Uptake, and Distribution. J. Plant Nutr. 2006, 25, 2315–2331. [Google Scholar] [CrossRef]
- Ahmad, H.R.; ZiaurRehman, M.; Sohail, M.I.; Anwar ul Haq, M.; Khalid, H.; Ayub, M.A.; Ishaq, G. Effects of Rare Earth Oxide Nanoparticles on Plants. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).