Productivity, Biodiversity and Forage Value of Meadow Sward Depending on Management Intensity and Silicon Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Weather Conditions
2.3. Evaluation of Botanical Composition and Plant Productivity
2.4. Nutritive Value Analyses
2.5. Statistical Analysis
3. Results
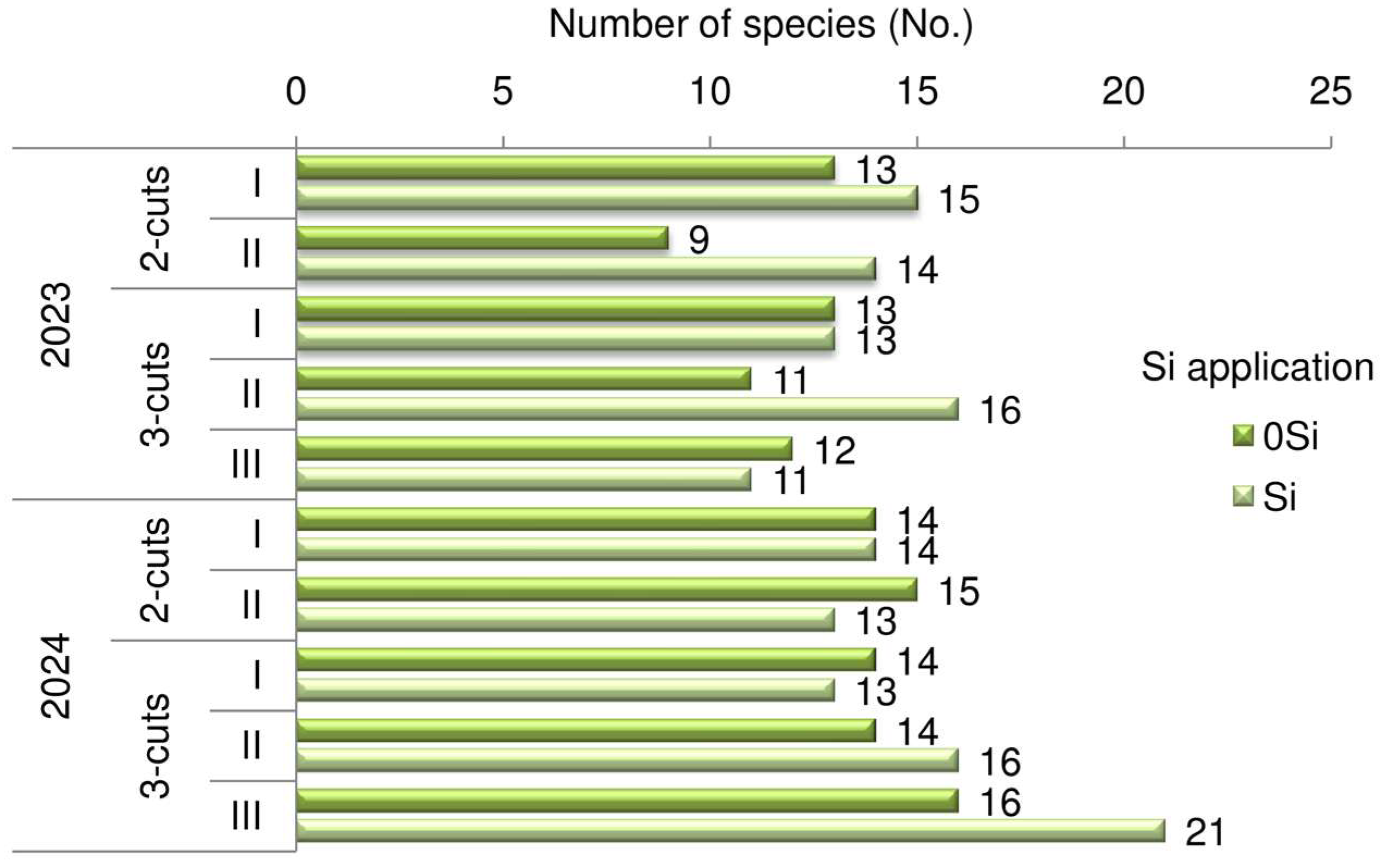
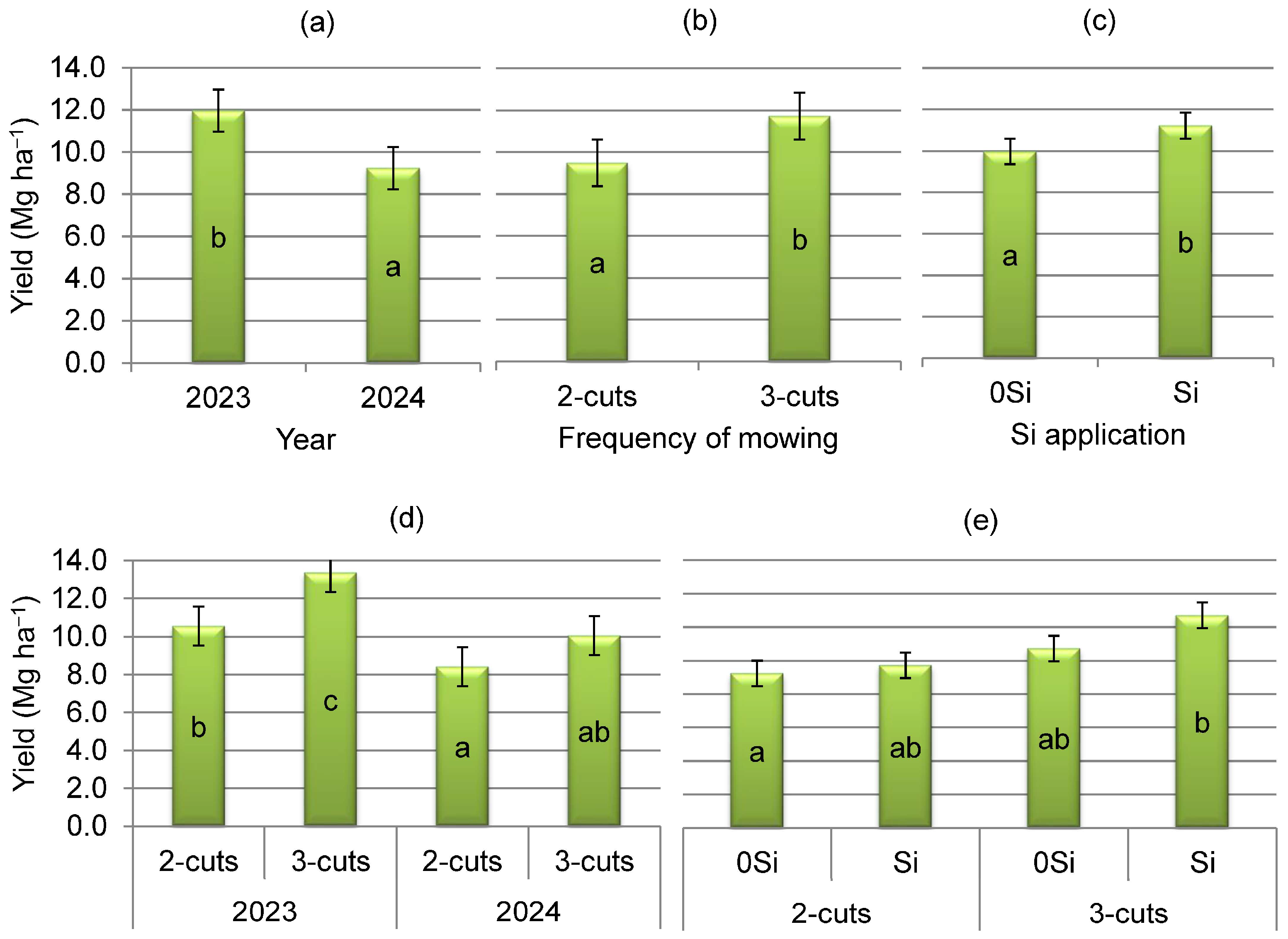
3.1. Botanical Composition and Plant Productivity
3.2. Nutritive Value
4. Discussion
4.1. Botanical Composition and Plant Productivity
4.2. Nutritive Value
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, A.; Wilkins, R.J. Temperate grassland: Key developments in the last century and future perspectives. J. Agric. Sci. 2006, 144, 503–523. [Google Scholar] [CrossRef]
- Mălinas, A.; Rotar, I.; Vidican, R.; Iuga, V.; Păcurar, F.; Mălinas, C.; Moldovan, C. Designing a sustainable temporary grassland system by monitoring nitrogen use efficiency. Agronomy 2020, 10, 149. [Google Scholar] [CrossRef]
- Søegaard, K.; Gierus, M.; Hopkins, A.; Halling, M. Temporary grassland-challenges in the future. Permanent and temporary grassland: Plant, environment and economy. In Proceedings of the 14th Symposium of the European Grassland Federation, Ghent, Belgium, 3–5 September 2007; Belgian Society for Grassland and Forage Crops: Ghent, Belgium, 2007; pp. 27–38. [Google Scholar]
- Hajduk, E.; Właśniewski, S.; Szpunar-Krok, E. Influence of legume crops on content of organic carbon in sandy soil. Soil Sci. Ann. 2015, 66, 52–56. [Google Scholar] [CrossRef]
- Grzelak, M.; Gaweł, E.; Barszczewski, J. Impact of the occurrence of herbs and weeds on variations in meadow sward economic value. Prog. Plant Prot. 2013, 53, 182–185. [Google Scholar] [CrossRef]
- Vogel, A.; Scherer-Lorenzen, M.; Weigelt, A. Grassland resistance and resilience after drought depends on management intensity and species richness. PLoS ONE 2012, 7, e36992. [Google Scholar] [CrossRef] [PubMed]
- Thumm, U.; Tonn, B. E ect of precipitation on dry matter production of a meadow with varied cutting frequency. Grassl. Sci. Eur. 2010, 15, 90–92. [Google Scholar]
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land Dev. 2021, 51, 243–249. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 72, 1212–1226. [Google Scholar] [CrossRef]
- Ciepiela, G.A.; Godlewska, A. Changes in protein compounds and monosaccharides in select grass species following application of a seaweed extract. Pol. J. Environ. Stud. 2014, 23, 29–35. [Google Scholar]
- Wolski, K.; Biernacik, M.; Świerszcz, S.; Talar-Krasa, M.; Leshchenko, O. Effect of the application of a biostimulant and mineral fertilizers on the concentration of mineral elements in the sward of forage mixtures cultivated on light soil. J. Elem. 2019, 24, 385–397. [Google Scholar]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73–86. [Google Scholar] [CrossRef]
- Sacała, E. Role of silicon in plant resistance to water stress. J. Elementol. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Hameed, M. Improving drought tolerance potential in wheat (Triticum aestivum L.) through exogenous silicon supply. Pak. J. Bot. 2015, 47, 1641–1648. [Google Scholar]
- Kowalska, J.; Tyburski, J.; Jakubowska, M.; Krzymińska, J. Effect of different forms of silicon on growth of spring wheat cultivated in organic farming system. Silicon 2021, 13, 211–217. [Google Scholar] [CrossRef]
- Sierra, M.J.; Schmid, T.; Guirado, M.; Escolano, O.; Millan, R. How management practices affect silicon uptake by Hordeum vulgare grown in a highly calcareous soil. Soil Use Manag. 2021, 37, 802–814. [Google Scholar] [CrossRef]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Janicka, M.; Wróbel, B. Effect of silicon-containing fertilizers on the nutritional value of grass-legume mixtures on temporary grasslands. Agriculture 2022, 12, 145. [Google Scholar] [CrossRef]
- Radkowski, A.; Sosin-Bzducha, E.; Radkowska, I. Effects of silicon foliar fertilization of meadow plants on nutritional value of silage fed to dairy cows. J. Elem. 2017, 22, 1311–1322. [Google Scholar]
- Zhang, P.; Ouyang, N.; Wei, X.; Zhang, Y.; Hu, B.; Lu, Z.; Peng, H.; Zhang, J.; Li, X.; Xie, M. Factors affecting the vertical distribution of silicon in paddy soils in Mid-Subtropical China. Silicon 2023, 15, 7477–7487. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Sujkowska-Rybkowska, M.; Bederska-Błaszczyk, M.; Borucki, W.; Dąbrowski, P. Silicon mitigates the adverse effects of drought on Lolium perenne physiological, morphometric and anatomical characters. PeerJ 2025, 13, e18944. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. Soil Sci. Plant Nutr. 2006, 67, 10–17. [Google Scholar] [CrossRef]
- Falkowski, M.; Kukułka, I.; Kozłowski, S. Chemical Properties of Meadow Plants; Wyd. Akademii Rolniczej w Poznaniu: Poznań, Poland, 2000; p. 132. [Google Scholar]
- Peoples, M.B. Legumes root nitrogen in cropping system nitrogen cycling. Graine Legume 2001, 33, 8–9. [Google Scholar]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Søegaard, K.; Høgh-Jensen, H.; Eriksen, J.; Sanderson, M.A.; Rasmussen, J.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage: Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef]
- Gaweł, E. Nutritive value of legume-grass mixtures cultivated in organic farms. J. Res. Appl. Agric. Eng. 2012, 57, 91–97. [Google Scholar]
- Lüscher, A.; Mueller-Harve, I.; Soussana, I.F.; Rees, R.M.; Peyraud, J.L. Potential of legume-based grassland-livestock systems in Europe: A review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef]
- Regulation (EU) 2021/2115 of the European Parliament and of the Council of 2 December 2021 Establishing Rules on Support for Strategic Plans to be Drawn up by Member States Under the Common Agricultural Policy (CAP Strategic Plans) and Financed by the European Agricultural Guarantee Fund (EAGF) and by the European Agricultural Fund for Rural Development (EAFRD) and Repealing Regulations (EU) No 1305/2013 and (EU) No 1307/2013. Available online: http://data.europa.eu/eli/reg/2021/2115/2024-05-25 (accessed on 16 July 2025).
- Brink, G.E.; Pederson, G.A.; Sistani, K.R.; Fairbrother, E. Uptake of selected nutrients by temperate grasses and legumes. Agron. J. 2001, 93, 887–890. [Google Scholar] [CrossRef]
- Marijanušić, K.; Manojlović, M.; Bogdanović, D.; Čabilovski, R.; Lombnaes, P. Mineral composition of forage crops in respect to dairy cow nutrition. Bulg. J. Agric. Sci. 2017, 23, 204–212. [Google Scholar]
- Grzyś, E. The role and importance of micronutrients in plants nutrition. Zesz. Probl. Post. Nauk Rol. 2004, 502, 89–99. [Google Scholar]
- Jones, G.B.; Tracy, B.F. Evaluating seasonal variation in mineral concentration of cool-season pasture herbage. Grass Forage Sci. 2013, 70, 94–101. [Google Scholar] [CrossRef]
- Darch, T.; McGrath, S.P.; Lee, M.R.F.; Beaumont, D.A.; Blackwell, M.S.A.; Horrocks, C.A.; Evans, J.; Storkey, J. The mineral composition of wild-type and cultivated varieties of pasture species. Agronomy 2020, 10, 1463. [Google Scholar] [CrossRef]
- Høgh-Jensen, H.; Søegaard, K. Robustness in the mineral supply from temporary grasslands. Acta Agric. Scand. 2012, 62, 79–90. [Google Scholar] [CrossRef]
- Olszewska, M.; Mackiewicz-Walec, E. The effect of different proportions of Medicago media Pers. in two-component alfalfa-grass mixtures on the macronutrient content of aboveground biomass. J. Elem. 2023, 28, 1055–1072. [Google Scholar] [CrossRef]
- Lindström, B.E.M.; Frankow-Lindberg, B.E.; Dahlin, A.S.; Wivstad, M.; Watson, C.A. Micronutrient concentrations in common and novel forage species and varieties grown on two contrasting soils. Grass Forage Sci. 2012, 68, 427–436. [Google Scholar] [CrossRef]
- Zemenchik, R.A.; Albrecht, K.A.; Shaver, R.D. Improved nutritive value of kura clover and birdsfoot trefoil grass mixtures compared with grass monocultures. Agron. J. 2002, 94, 1131–1138. [Google Scholar] [CrossRef]
- Allana, E.; Weissera, W.; Weigelta, A.; Roschere, C.; Fischerb, M.; Hillebrandf, H. More diverse plant communities have higher functioning over time due to turnover in complementary dominant species. Proc. Natl. Acad. Sci. USA 2011, 108, 17034–17039. [Google Scholar] [CrossRef] [PubMed]
- Kocięcka, J.; Liberacki, D.; Kupiec, J.M.; Stróżecki, M.; Dłużewski, P. Effects of silicon application and groundwater level in a subirrigation system on yield of a three-cut meadow. Water 2023, 15, 2103. [Google Scholar] [CrossRef]
- Schaub, S.; Finger, R.; Leiber, F.; Probst, S.; Kreuzer, M.; Weigelt, A.; Buchmann, N.; Scherer-Lorenzen, M. Plant diversity effects on forage quality, yield and revenues of semi-natural grasslands. Nat Commun. 2020, 7, 768. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.; Grzegorczyk, S.; Bałuch-Małecka, A. The effect of different proportions of Medicago media Pers. in mixtures with Festulolium braunii (K. Richt.) A. Camus on the yield and feed value of green fodder. Agric. Food Sci. 2019, 28, 18–26. [Google Scholar] [CrossRef]
- Gaweł, E. Skład gatunkowy i mineralny mieszanek motylkowato-trawiastych w ekologicznej uprawie i kośno-pastwiskowym użytkowaniu. Pol. J. Agron. 2011, 6, 17–26. [Google Scholar]
- Staniak, M.; Harasim, E. Changes in nutritive value of alfalfa (Medicago x varia T. Martyn) and Festulolium (Festulolium braunii (K. Richt.) A. Camus) under drought stress. J. Agron. Crop Sci. 2018, 204, 456–466. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Dąbrowski, P.; Szara, E.; Perzanowska, A.; Wróbel, B. Can the application the silicon improve the productivity and nutritional value of grass-clover sward in conditions of rainfall shortage in organic management? Agronomy 2020, 10, 1007. [Google Scholar] [CrossRef]
- Terlikowski, J. The effect of permanent grassland sward enrichment with special varieties of grasses and legumes on the quality of produced bulk fodder. J. Res. Appl. Agric. Eng. 2014, 59, 107–110. [Google Scholar]
- Boller, B.; Nüesch, B. Wann kann man sehr frühreife Englische Raigras Arion im Fruhjahr zum ersten Mal nutzen? Landwirtsch. Schweiz 1991, 4, 462–467. [Google Scholar]
- Adams, M.A.; Buchmann, N.; Sprent, J.; Buckley, T.N.; Turnbull, T.L. Crops, nitrogen, water: Are legumes friend, foe, or misunderstood ally? Trends Plant Sci. 2018, 23, 539–550. [Google Scholar] [CrossRef]
- White, P.J. Long-distance transport in the xylemand phloem. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Waltham, MA, USA, 2012; pp. 49–70. [Google Scholar]
- Tiley, G.E.D.; Frame, J. Evaluation of forage herbs in grass/herbs mixtures. In Proceedings of the 14th General Meeting of the European Grassland Federation, Lahti, Finland, 8–11 June 1992; pp. 542–544. [Google Scholar]
- Grzegorczyk, S.; Grabowski, K.; Olszewska, M. Content of potassium, calcium, magnesium, phosphorus and sodium in meadow sward irrigated with wastewater. J. Elem. 2020, 25, 249–257. [Google Scholar] [CrossRef]
- Kuusela, E. Annual and seasonal changes in mineral contents (Ca, Mg, P, K and Na) of grazed clovergrass mixtures in organic farming. Agric Food Sci. 2006, 15, 23–34. [Google Scholar] [CrossRef]
- Mastalerczuk, G. Nutrient content in organs of meadow plants in different conditions of management intensity. Grassl. Sci. Pol. 2007, 9, 131–140. [Google Scholar]
- Miller, E.R.; Lei, X.; Ullrey, D.E. Trace Elements in Animal Nutrition. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Schuman, L.M., Welch, R.M., Eds.; SSSA Soil Science Society of America: Madison, WI, USA, 1991; pp. 593–662. [Google Scholar]
- Romheld, V.; Marschner, H. Function of Micronutrients in Plants. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; SSSA Soil Science Society of America: Madison, WI, USA, 1991; pp. 297–328. [Google Scholar]
- Żarczyńska, K.; Żarczyński, P.; Sobiech, P.; Snarska, A.; Stopyra, A.; Wieteska, M.; Płaczek, A. The effect of micronutrient deficiencies on the health status of transition cows. J. Elem. 2017, 22, 1223–1234. [Google Scholar] [CrossRef]
- Roche, J.R.; Turner, L.R.; Lee, J.M.; Edmeades, D.C.; Donaghy, D.J.; MacDonald, K.A.; Penno, J.W.; Berry, D.P. Weather, herbage quality and milk production in pastoral systems, 3: Interrelationships and associations between weather variables and herbage growth rate, quality and mineral concentration. Anim. Prod. Sci. 2009, 49, 211–221. [Google Scholar] [CrossRef]
- Markovic, J.; Štrbanovi, R.; Cvetkovi, M.; Andjelkovic, B.; Živkovi, B. Effects of growth stage on the mineral concentrations in alfalfa (Medicago sativa L.) leaf, stem and the whole plant. Biotechnol. Anim. Husb. 2009, 25, 1225–1231. [Google Scholar]
- NRC. National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Vigh, A.; Criste, A.D.; Corcionivoschi, N.; Gerard, C. Rumen solubility of copper, manganese and zinc and the potential link between the source and rumen function: A systematic review. Agriculture 2023, 13, 2198. [Google Scholar] [CrossRef]
- López-Alonso, M.; Miranda, M. Copper supplementation, a challenge in cattle. Animals 2020, 10, 1890. [Google Scholar] [CrossRef]
- Olszewska, M. Micronutrient content of aboveground biomass as influenced by different proportions of Medicago media Pers. in two-component alfalfa-grass mixtures. Agriculture 2024, 14, 2205. [Google Scholar] [CrossRef]
- Sloup, V.; Jankowská, I.; Nechybová, S.; Pesinková, P.; Langrová, I. Zinc in the animal organism: A review. Sci. Agric. Bohem. 2017, 48, 13–21. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 548. [Google Scholar]
- Grzegorczyk, S.; Olszewska, M.; Alberski, J. Accumulation of copper, zinc, manganese and iron by selected species of grassland legumes and herbs. J. Elem. 2014, 19, 109–118. [Google Scholar] [CrossRef]
- Abaturov, B.D. Variability of grassland forage resources and their division by a complex of herbivorous mammals in the case of joint grazing. Arid. Ecosyst. 2024, 14, 202–208. [Google Scholar] [CrossRef]

| Year | Month | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | Mean IV-IX | Mean I-XII | |
| Temperature (°C) | ||||||||||||||
| 2023 | 3.4 | 1.3 | 4.4 | 8.5 | 13.1 | 18.4 | 20.6 | 20.5 | 17.4 | 10.7 | 4.0 | 2.0 | 16.4 | 10.4 |
| 2024 | −0.1 | 6.1 | 6.6 | 11.1 | 16.5 | 19.4 | 21.7 | 20.4 | 16.8 | 9.5 | 3.4 | 2.5 | 17.7 | 11.2 |
| Precipitation (mm) | ||||||||||||||
| 2023 | 42.5 | 38.1 | 47.4 | 47.9 | 36.8 | 43.2 | 44.0 | 106.4 | 8.6 | 68.7 | 62.6 | 62.1 | 286.9 | 608.3 |
| 2024 | 56.5 | 47.3 | 31.0 | 31.4 | 42.7 | 56.9 | 37.0 | 67.8 | 37.6 | 26.1 | 27.9 | 15.0 | 273.4 | 477.2 |
| Yield | Crude Protein | P | K | Na | Ca | Mg | |
|---|---|---|---|---|---|---|---|
| Main effects | |||||||
| Year (A) | 0.000 | 0.941 | 0.505 | 0.017 | 0.013 | 0.003 | 0.176 |
| Frequency of mowing (B) | 0.025 | 0.000 | 0.038 | 0.414 | 0.018 | 0.000 | 0.031 |
| Si application (C) | 0.001 | 0.345 | 0.509 | 0.408 | 0.746 | 0.991 | 0.207 |
| Regrowh (D) | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 |
| Interactions | |||||||
| A × B | 0.024 | 0.011 | 0.039 | 0.012 | 0.008 | 0.001 | 0.041 |
| A × C | 0.517 | 0.346 | 0.826 | 0.027 | 0.007 | 0.030 | 0.420 |
| A × D | 0.730 | 0.002 | 0.044 | 0.333 | 0.118 | 0.075 | 0.864 |
| B × C | 0.048 | 0.021 | 0.043 | 0.268 | 0.009 | 0.024 | 0.009 |
| C × D | 0.216 | 0.049 | 0.045 | 0.025 | 0.009 | 0.022 | 0.009 |
| S | Fe | Mn | Cu | Zn | B | Si | |
| Main effects | |||||||
| Year (A) | 0.000 | 0.430 | 0.506 | 0.007 | 0.245 | 0.002 | 0.000 |
| Frequency of mowing (B) | 0.000 | 0.028 | 0.000 | 0.002 | 0.030 | 0.000 | 0.377 |
| Si application (C) | 0.579 | 0.834 | 0.019 | 0.509 | 0.157 | 0.949 | 0.408 |
| Regrowh (D) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Interactions | |||||||
| A × B | 0.045 | 0.030 | 0.020 | 0.000 | 0.018 | 0.001 | 0.028 |
| A × C | 0.009 | 0.521 | 0.038 | 0.008 | 0.044 | 0.023 | 0.046 |
| A × D | 0.001 | 0.000 | 0.132 | 0.001 | 0.127 | 0.039 | 0.386 |
| B × C | 0.006 | 0.028 | 0.006 | 0.028 | 0.024 | 0.010 | 0.878 |
| C × D | 0.007 | 0.006 | 0.030 | 0.007 | 0.045 | 0.005 | 0.007 |
| Effect | Crude Protein | P | K | Na | Ca | Mg |
|---|---|---|---|---|---|---|
| Year (A) | ||||||
| 2023 | 134.16 a | 2.93 a | 27.05 b | 0.25 a | 10.56 a | 2.32 a |
| 2024 | 140.82 a | 2.91 a | 22.55 a | 0.59 b | 12.26 b | 2.53 a |
| Frequency of mowing (B) | ||||||
| 2-cuts | 113.10 a | 2.70 a | 23.34 a | 0.24 a | 9.50 a | 2.14 a |
| 3-cuts | 153.75 b | 3.07 b | 25.78 a | 0.53 b | 12.69 b | 2.61 b |
| Si application (C) | ||||||
| 0Si | 138.40 a | 2.96 a | 25.41 a | 0.41 a | 11.21 a | 2.47 a |
| Si | 136.58 a | 2.88 a | 24.19 a | 0.42 a | 11.62 a | 2.37 a |
| Regrowh (D) | ||||||
| 1st | 86.00 a | 2.27 a | 19.79 a | 0.19 a | 6.78 a | 1.55 a |
| 2nd | 171.38 b | 3.25 b | 25.47 b | 0.54 b | 14.43 b | 2.86 b |
| 3rd | 172.68 b | 3.56 b | 33.49 c | 0.63 b | 14.65 b | 3.29 c |
| Crude Protein | P | K | Na | Ca | Mg | |
|---|---|---|---|---|---|---|
| Interaction A × B | ||||||
| 2023 × 2-cuts | 115.09 a | 2.75 ab | 23.69 a | 0.15 a | 10.07 ab | 2.09 a |
| 2023 × 3-cuts | 146.87 b | 3.05 ab | 29.29 b | 0.31 a | 10.90 b | 2.47 ab |
| 2024 × 2-cuts | 111.10 a | 2.64 a | 22.99 a | 0.33 a | 8.93 a | 2.19 a |
| 2024 × 3-cuts | 160.64 b | 3.08 b | 22.27 a | 0.76 b | 14.48 c | 2.75 b |
| Interaction A × C | ||||||
| 2023 × 0Si | 133.97 a | 2.96 a | 26.82 b | 0.25 a | 10.16 a | 2.32 a |
| 2023 × Si | 134.35 a | 2.91 a | 27.27 b | 0.25 a | 10.97 ab | 2.32 a |
| 2024 × 0Si | 142.84 a | 2.96 a | 24.00 ab | 0.57 ab | 12.26 b | 2.62 a |
| 2024 × Si | 138.81 a | 2.85 a | 21.11 a | 0.60 b | 12.27 b | 2.43 a |
| Interaction B × C | ||||||
| 2-cuts × 0Si | 116.70 a | 2.76 ab | 25.23 a | 0.24 a | 9.47 a | 2.31 ab |
| 2-cuts × Si | 109.49 a | 2.63 a | 21.44 a | 0.24 a | 9.53 a | 1.97 a |
| 3-cuts × 0Si | 152.88 b | 3.10 b | 25.53 a | 0.52 b | 12.37 b | 2.58 b |
| 3-cuts × Si | 154.63 b | 3.04 ab | 26.02 a | 0.55 b | 13.01 b | 2.64 b |
| Interaction C × D | ||||||
| 0Si × 1st | 84.91 a | 2.26 a | 20.07 a | 0.20 a | 6.23 a | 1.59 a |
| 0Si × 2nd | 172.83 b | 3.34 b | 27.04 bc | 0.54 b | 14.27 b | 2.93 b |
| 0Si × 3rd | 176.54 b | 3.60 b | 32.83 c | 0.57 b | 15.04 b | 3.31 b |
| Si × 1st | 87.10 a | 2.27 a | 19.51 a | 0.18 a | 7.33 a | 1.51 a |
| Si × 2nd | 169.93 b | 3.16 b | 23.89 ab | 0.54 b | 14.60 b | 2.79 b |
| Si × 3rd | 168.81 b | 3.52 b | 34.15 c | 0.68 b | 14.25 b | 3.27 b |
| Interaction B × D | ||||||
| 2-cuts × 1st | 72.17 a | 1.97 a | 17.31 a | 0.12 a | 6.33 a | 1.48 a |
| 2-cuts × 2nd | 154.02 c | 3.42 c | 29.36 b | 0.36 abc | 12.68 b | 2.80 b |
| 3-cuts × 1st | 99.84 b | 2.57 b | 22.27 a | 0.26 ab | 7.23 a | 1.62 a |
| 3-cuts × 2nd | 188.74 d | 3.08 c | 21.57 a | 0.72 c | 16.19 c | 2.92 b |
| 3-cuts × 3rd | 172.68 cd | 3.56 c | 33.49 b | 0.63 bc | 14.65 bc | 3.29 b |
| S | Fe | Mn | Cu | Zn | B | Si | |
|---|---|---|---|---|---|---|---|
| Year (A) | |||||||
| 2023 | 1.16 a | 167.7 a | 103.5 a | 7.19 a | 23.67 a | 13.43 a | 4924 a |
| 2024 | 1.77 b | 169.3 a | 101.4 a | 8.68 b | 25.91 a | 19.55 b | 7052 b |
| Frequency of mowing (B) | |||||||
| 2-cuts | 1.23 a | 148.3 a | 118.0 b | 6.59 a | 22.45 a | 12.34 a | 5883 a |
| 3-cuts | 1.62 b | 181.9 b | 92.1 a | 8.83 b | 26.35 b | 19.26 b | 6058 a |
| Si application (C) | |||||||
| 0Si | 1.48 a | 173.0 a | 109.3 b | 8.00 a | 25.93 a | 16.30 a | 6147 a |
| Si | 1.44 a | 163.9 a | 95.6 a | 7.86 a | 23.65 a | 16.68 a | 5829 a |
| Regrowth (D) | |||||||
| 1st | 0.96 a | 113.2 a | 113.7 b | 4.89 a | 18.83 a | 9.97 a | 5140 a |
| 2nd | 1.76 b | 185.4 b | 91.1 a | 9.76 b | 27.95 b | 20.73 b | 5805 a |
| 3rd | 1.87 b | 245.1 c | 102.7 ab | 10.37 b | 30.42 b | 21.04 b | 8049 b |
| S | Fe | Mn | Cu | Zn | B | Si | |
|---|---|---|---|---|---|---|---|
| Interaction A × B | |||||||
| 2023 × 2-cuts | 1.00 a | 168.1 b | 112.2 bc | 6.67 a | 22.14 a | 12.21 a | 5178 a |
| 2023 × 3-cuts | 1.27 b | 167.4 b | 97.7 ab | 7.54 a | 24.70 ab | 14.25 a | 4755 a |
| 2024 × 2-cuts | 1.46 b | 128.5 a | 123.8 c | 6.51 a | 22.76 a | 12.47 a | 6587 b |
| 2024 × 3-cuts | 1.97 c | 196.5 b | 86.5 a | 10.12 b | 28.01 b | 24.26 b | 7362 b |
| Interaction A × C | |||||||
| 2023 × 0Si | 1.18 a | 173.7 a | 108.8 ab | 7.31 a | 24.57 ab | 12.37 a | 5012 a |
| 2023 × Si | 1.13 a | 161.6 a | 98.2 ab | 7.07 a | 22.77 a | 14.50 ab | 4836 a |
| 2024 × 0 Si | 1.79 b | 172.3 a | 109.9 b | 8.70 b | 27.29 c | 20.23 c | 7281 b |
| 2024 × Si | 1.75 b | 166.3 a | 93.0 a | 8.66 b | 24.54 ab | 18.87 bc | 6823 b |
| Interaction B × C | |||||||
| 2-cuts × 0Si | 1.28 a | 151.2 a | 126.8 c | 6.90 a | 24.28 ab | 11.91 a | 6084 a |
| 2-cuts × Si | 1.17 a | 145.4 a | 109.2 bc | 6.27 a | 20.62 a | 12.77 a | 5681 a |
| 3-cuts × 0Si | 1.62 b | 187.5 b | 97.6 ab | 8.74 b | 27.03 b | 19.22 b | 6189 a |
| 3-cuts × Si | 1.62 b | 176.3 ab | 86.6 a | 8.92 b | 25.68 b | 19.29 b | 5928 a |
| Interaction C × D | |||||||
| 0Si × 1st | 0.98 a | 113.4 a | 120.1 c | 4.81 a | 19.22 a | 8.88 a | 5156 a |
| 0Si × 2nd | 1.81 b | 180.5 b | 99.5 ab | 9.99 b | 29.66 b | 20.99 b | 6134 ab |
| 0Si × 3rd | 1.84 b | 277.3 c | 107.2 bc | 10.42 b | 31.90 b | 21.75 b | 8154 c |
| Si × 1st | 0.94 a | 113.1 a | 107.3 bc | 4.96 a | 18.45 a | 11.06 a | 5125 a |
| Si × 2nd | 1.70 b | 190.3 b | 82.7 a | 9.54 b | 26.23 b | 20.48 b | 5476 a |
| Si × 3rd | 1.91 b | 213.0 b | 98.1 ab | 10.32 b | 28.93 b | 20.33 b | 7944 bc |
| Interaction B × D | |||||||
| 2-cuts × 1st | 0.89 a | 110.7 a | 120.2 b | 4.30 a | 17.24 a | 7.75 a | 5644 ab |
| 2-cuts × 2nd | 1.56 b | 185.9 b | 115.8 b | 8.87 b | 27.67 b | 16.93 bc | 6121 b |
| 3-cuts × 1st | 1.03 a | 115.7 a | 107.3 b | 5.47 a | 20.43 a | 12.19 ab | 4636 a |
| 3-cuts × 2nd | 1.95 c | 184.9 b | 66.4 a | 10.65 c | 28.22 b | 24.54 c | 5489 ab |
| 3-cuts × 3rd | 1.87 c | 245.1 c | 102.7 b | 10.37 c | 30.42 b | 21.04 cd | 8049 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borawska-Jarmułowicz, B.; Mastalerczuk, G. Productivity, Biodiversity and Forage Value of Meadow Sward Depending on Management Intensity and Silicon Application. Sustainability 2025, 17, 6717. https://doi.org/10.3390/su17156717
Borawska-Jarmułowicz B, Mastalerczuk G. Productivity, Biodiversity and Forage Value of Meadow Sward Depending on Management Intensity and Silicon Application. Sustainability. 2025; 17(15):6717. https://doi.org/10.3390/su17156717
Chicago/Turabian StyleBorawska-Jarmułowicz, Barbara, and Grażyna Mastalerczuk. 2025. "Productivity, Biodiversity and Forage Value of Meadow Sward Depending on Management Intensity and Silicon Application" Sustainability 17, no. 15: 6717. https://doi.org/10.3390/su17156717
APA StyleBorawska-Jarmułowicz, B., & Mastalerczuk, G. (2025). Productivity, Biodiversity and Forage Value of Meadow Sward Depending on Management Intensity and Silicon Application. Sustainability, 17(15), 6717. https://doi.org/10.3390/su17156717





