Ichu Valorization by Pleurotus spp. Cultivation and Potential of the Residual Substrate as a Biofertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Selection of Biological Material
2.2. Proximal Analysis of the Ichu-Based Substrate
2.3. Cellulose and Lignin Analysis in the Ichu-Based Substrate
2.4. Pleurotus spp. Cultivation
2.5. Productivity Indicators of Pleurotus Cultivation
2.6. Proximal Analysis of Pleurotus
2.7. Potential of Pleurotus SMS Spent Substrate as Biofertilizer
2.8. Economic Analysis or Valorization of Pleurotus spp. Cultivation on Ichu
2.9. Data Analysis
3. Results and Discussion
3.1. Proximal Composition of Ichu
3.2. Cellulose and Lignin Content of Ichu
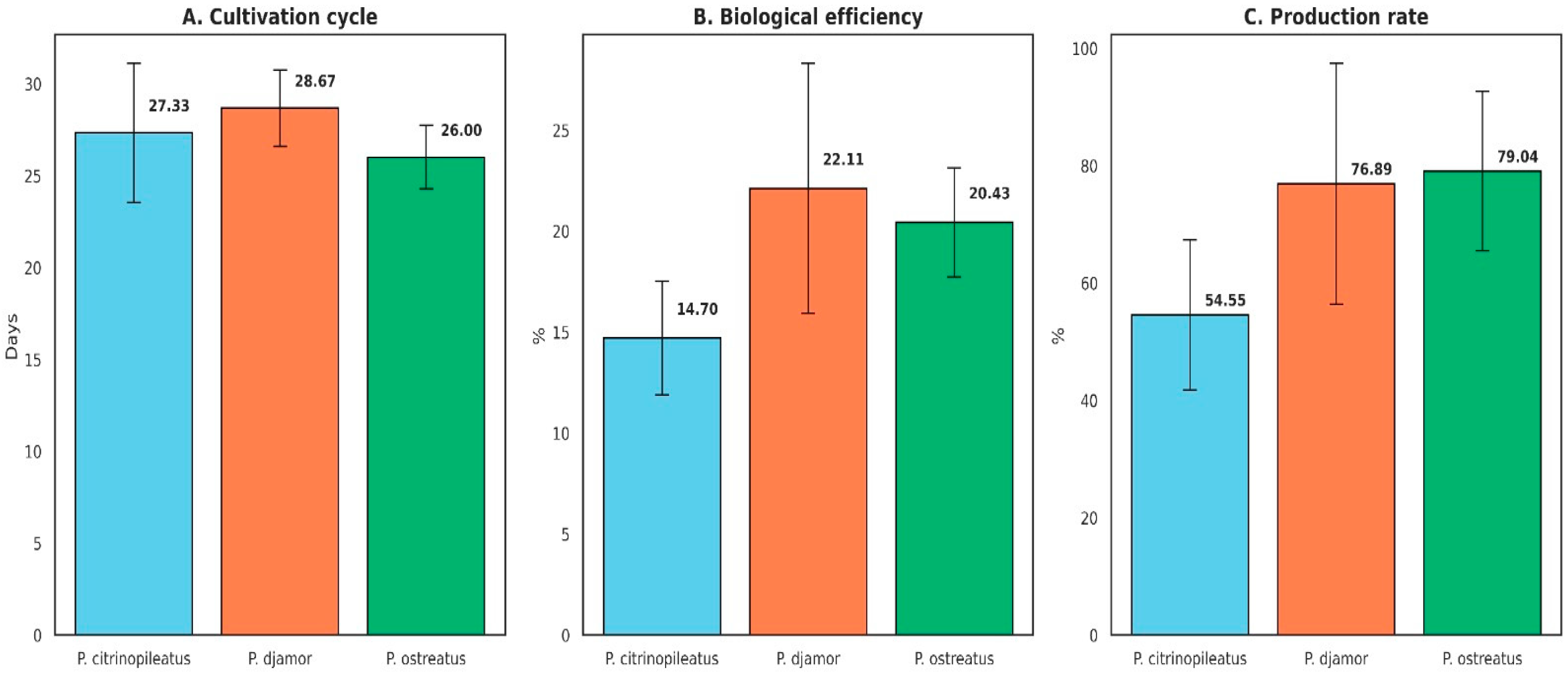
3.3. Performance of Pleurotus Cultivation Based on Productivity Indicators
3.4. Proximal Composition of Pleurotus spp.
3.5. Potential of Spent Mushroom Substrate as a Biofertilizer
3.6. Economic Analysis of Pleurotus spp. Production
3.7. Research Limitations and Perspectives for Future Exploration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valverde, H.; Fuentealba, B.; Blas, L.; Oropeza, T. La Importancia de los Pastizales Altoandinos Peruanos. Dir. Invest. Ecosist. Montaña—Inst. Nac. Invest. Glaciares Ecosist. Montaña (DIEM-INAIGEM). 2022. Available online: https://repositorio.inaigem.gob.pe/server/api/core/bitstreams/8f8bf505-e241-4af0-91ae-4c9e9033ee15/content (accessed on 7 January 2025).
- Zapana, J. Evaluación de pastizales naturales y determinación de la carga animal actual en la Comunidad Chila, Puno, Perú. Rev. Investig. Esc. Posgr. Univ. Nac. Altiplano—Puno 2019, 8, 1286–1296. Available online: http://revistas.unap.edu.pe/epg/index.php/investigaciones/article/view/1280/275 (accessed on 7 January 2025).
- Álvarez Cortez, S.; Peña Murillo, R.; Román Robalino, D. Ecosystem services linked to water and plant diversity in the Igualata Paramo of Hualcanga Region. ESPOCH Congr. Ecuad. J. S.T.E.A.M. 2021, 1, 1221–1235. [Google Scholar] [CrossRef]
- Hofstede, R.; Segarra, P.; Mena, P. Los Páramos del Mundo. In Proyecto Atlas Mundial de los Páramos; Global Peatland Initiative/NC-IUC/EcoCIENCIA: Quito, Ecuador, 2003; Available online: https://portals.iucn.org/library/sites/library/files/documents/2003-081.pdf (accessed on 7 January 2025).
- Foster, M.E.; Chen, D.; Kieser, M.S. Restauración y Conservación de Pastizales Altoandinos: Cuantificación de Mejoras Potenciales en el Caudal Base; Forest Trends. 2020. Available online: https://www.forest-trends.org/wp-content/uploads/2020/04/CUBHIC-Restauraci%C3%B3n-y-Conservaci%C3%B3n-de-Pastizales-Altoandinos-.pdf (accessed on 7 January 2025).
- Limay, E.; Vásquez, M. Resistencia a Compresión del Ladrillo de Arcilla con Adición de Ichu (Stipa Ichu). Tesis de Licenciatura, Universidad Privada del Norte, La Libertad, Perú, 2019. Available online: https://repositorio.upn.edu.pe/handle/11537/21089 (accessed on 7 January 2025).
- Tácuna, R.E.; Aguirre, L.; Flores, E.R. Influencia de la revegetación con especies nativas y la incorporación de materia orgánica en la recuperación de pastizales degradados. Ecol. Appl. 2015, 14, 181–191. [Google Scholar] [CrossRef]
- Álvarez, S.; Zubieta, R.; Martínez, A.; Ccanchi, Y. Uso del fuego y el rol de la Población Durante Quemas e Incendios Forestales en Cusco. Bol. Cient. Niño Inst. Geofis. Perú 2023, 10, 4–10. Available online: https://repositorio.igp.gob.pe/handle/20.500.12816/5509 (accessed on 8 January 2025).
- De La Cruz-Arango, J.; Cóndor Alarcón, R. Dinámica de regeneración natural post-incendio de ecosistemas altoandinos en el distrito de Chiara, Ayacucho-Perú. J. Selva Andin. Biosph. 2023, 11, 1. [Google Scholar] [CrossRef]
- Armenteras, D.; González, T.M.; Vargas, J.O.; Meza Elizalde, M.C.; Oliveras, I. Incendios en ecosistemas del norte de Suramérica: Avances en la ecología del fuego tropical en Colombia, Ecuador y Perú. Caldasia 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Charca, S.; Noel, J.; Andia, D.; Flores, J.; Guzman, A.; Renteros, C.; Tumialan, J. Assessment of Ichu fibers as a non-expensive thermal insulation system for the Andean regions. Energy Build. 2015, 108, 55–60. [Google Scholar] [CrossRef]
- Sánchez-Vega, I.; Dillon, M.O. Jalcas. In Botánica Económica de los Andes Centrales; Moraes, M., Øllgaard, B., Kvist, L.P., Borchsenius, F., Balslev, H., Eds.; Universidad Mayor de San Andrés: La Paz, Bolivia, 2006; pp. 77–90. [Google Scholar]
- Mori, S.; Tenazoa, C.; Candiotti, S.; Flores, E.; Charca, S. Assessment of Ichu Fibers Extraction and Their Use as Reinforcement in Composite Materials. J. Nat. Fibers 2020, 17, 700–715. [Google Scholar] [CrossRef]
- Mori, S.; Charca, S.; Flores, E.; Savastano, H., Jr. Physical and thermal properties of novel native Andean natural fibers. J. Nat. Fibers 2019, 18, 475–491. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Arzon, M.R. Pharmacological and nutritional importance of mushrooms. Int. J. Microbiol. 2022, 7, 1–10. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health-promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Sekan, A.; Myronycheva, O.; Karsson, O.; Gryganskyi, A.; Blume, Y. Green potential of Pleurotus spp. in biotechnology. Bio-Chem. Biophys. Mol. Biol. 2019, 7, e6664. Available online: https://peerj.com/articles/6664/ (accessed on 8 January 2025).
- Duddigan, S.; Alexander, P.D.; Shaw, L.J.; Collins, C.D. Effects of repeated application of organic soil amendments on horticultural soil physicochemical properties, nitrogen budget and yield. Horticulturae 2021, 7, 371. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Andrades, M.S.; Villalba Eguren, G.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Organic amendment for the recovery of vineyard soils: Effects of a single application on soil properties over two years. Processes 2022, 10, 317. [Google Scholar] [CrossRef]
- Yagüe, M.; Lobo, M. Reutilización del sustrato post-cultivo de hongos en semillero de hortícolas. ITEA-Inf. Téc. Econ. Agrar. 2021, 117, 347–359. Available online: https://www.aida-itea.org/aida-itea/files/itea/revistas/2021/117-4/ITEA%20117-4%20(347-359).pdf (accessed on 9 January 2025).
- Nakatsuka, H.; Oda, M.; Hayashi, Y.; Tamura, K. Effects of fresh spent mushroom substrate of Pleurotus ostreatus on soil micromorphology in Brazil. Geoderma 2016, 269, 54–60. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Zhou, X.; Baig, S.A.; Hu, B.; Xu, X. Composition variability of spent mushroom substrates during continuous cultivation, composting process and their effects on mineral nitrogen transformation in soil. Geoderma 2017, 307, 30–37. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y.; Li, H.; Li, R.; Ge, S.; Wang, H.; Wang, S.; Liu, H. Optimization of Manure-Based Substrate Preparation to Reduce Nutrients Losses and Improve Quality for Growth of Agaricus bisporus. Agriculture 2024, 14, 1833. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ling, N.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef]
- Feijóo-Vivas, K.; Bermúdez-Puga, S.A.; Rebolledo, H.; Figueroa, J.M.; Zamora, P.; Naranjo-Briceño, L. Bioproductos desarrollados a partir de micelio de hongos: Una nueva cultura material y su impacto en la transición hacia una economía sostenible. Bionatura 2021, 6, 1637–1652. [Google Scholar] [CrossRef]
- Kousar, A.; Khan, H.A.; Farid, S.; Zhao, Q.; Zeb, I. Recent advances on environmentally sustainable valorization of spent mushroom substrate: A review. Biofuels Bioprod. Biorefin. 2024, 18, 639–651. [Google Scholar] [CrossRef]
- Iglesias, H.; Ortiz, A.P.; Soriano Disla, J.M.; Lara-Guillén, A.J. Environmental and Economic Life Cycle Impacts of Using Spent Mushroom Substrate as a Soil Improver. Environments 2025, 12, 31. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef]
- Díaz Muñoz, K.; Casanova Guajardo, M.; León Torres, C.A.; Gil Ramírez, L.A.; Bardales Vásquez, C.B.; Cabos Sánchez, J. Producción de Pleurotus ostreatus (Pleurotaceae) ICFC 153/99 cultivado sobre diferentes residuos lignocelulósicos. Arnaldoa 2019, 26, 1177–1184. Available online: https://journal.upao.edu.pe/index.php/Arnaldoa/article/view/1413 (accessed on 9 January 2025).
- Nieto Juárez, J.I.; Cuzcano Ruiz, A.D.; Reyes López, W. Evaluación del cultivo del hongo Pleurotus ostreatus y de su composición nutricional en borra de café. Tecnia 2021, 31, 27–32. [Google Scholar] [CrossRef]
- Kumar, K. Nutraceutical potential and processing aspects of oyster mushrooms (Pleurotus species). Curr. Nutr. Food Sci. 2020, 16, 3–14. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 22nd ed.; AOAC: Rockville, MD, USA, 2023. [Google Scholar]
- Collazos, C. La Composición de Alimentos de Mayor Consumo en el Perú, 6th ed.; Ministerio de Salud, Instituto Nacional de Nutrición: Lima, Peru, 1993. [Google Scholar]
- ANKOM Technology. Methods for determining neutral detergent fiber. In Automated Fiber Analysis. Macedon; ANKOM Technology: Macedon, NY, USA, 2005. [Google Scholar]
- Akcay, C.; Ceylan, F.; Arslan, R. Production of oyster mushroom (Pleurotus ostreatus) from some waste lignocellulosic materials and FTIR characterization of structural changes. Sci. Rep. 2023, 13, 12897. [Google Scholar] [CrossRef]
- España-Rodríguez, M.; Hernández-Domínguez, E.M.; Velázquez-De Lucio, B.S.; Villa-García, M.; Álvarez-Cervantes, J. Productividad y análisis químico proximal de Pleurotus spp. crecidos sobre bagazo de Agave salmiana como sustrato alternativo. Agrociencia 2021, 55, 569–581. Available online: https://agrociencia-colpos.org/index.php/agrociencia/article/view/2604/2086 (accessed on 9 January 2025). [CrossRef]
- Kinge, T.R.; Adi, E.B.M.; Mih, A.M.; Ache, N.A.; Nji, T.M. Effect of substrate on the growth, nutritional and bioactive components of Pleurotus ostreatus and Pleurotus florida. Afr. J. Biotechnol. 2016, 15, 1476–1486. [Google Scholar] [CrossRef]
- Salmones, D.; Gaitán-Hernández, R.; Pérez, R.; Guzmán, G. Estudios sobre el género Pleurotus. VIII. Interacción entre crecimiento micelial y productividad. Rev. Iberoam. Micol. 1997, 14, 173–176. [Google Scholar] [PubMed]
- Reyes, G.R.; Abella, A.E.; Eguchi, F.; Lijima, T.; Higaki, M.; Quimio, T.H. Growing paddy straw mushroom. In Mushroom Grower’s Handbook 1: Oyster Mushroom Cultivation; Mushroom World: Seoul, Republic of Korea, 2004; pp. 262–269. [Google Scholar]
- NTP-ISO 1442:2006; (revisada en 2020). Carne y Productos Cárnicos. Determinación del Contenido de Humedad (Método de Referencia). Instituto Nacional de Calidad: Lima, Peru, 2020.
- NTP 201.022:2002; (revisada en 2015). Carne y Productos Cárnicos. Determinación del Contenido de Cenizas. Instituto Nacional de Calidad: Lima, Peru, 2015.
- NTP 201.016:2002; Carne y Productos Cárnicos. Determinación del Contenido de Grasa Total. Instituto Nacional de Calidad: Lima, Peru, 2002.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society: Crude Fiber in Oilseed By-Products, Ba 6-84, 4th ed.; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- AOAC 930.04; Loss on Drying (Moisture) in Plants. Official Methods of Analysis of AOAC INTERNATIONAL. AOAC INTERNATIONAL: Rockville, MD, USA, 2023.
- AOAC 981.12; pH of Acidified Foods. Official Methods of Analysis of AOAC INTERNATIONAL. AOAC INTERNATIONAL: Rockville, MD, USA, 2023.
- AOAC 970.39; Phosphorus in Fruits and Fruit Products—Gravimetric Quinoline Molybdate Method. Official Methods of Analysis of AOAC INTERNATIONAL. AOAC INTERNATIONAL: Rockville, MD, USA, 2023.
- AOAC 975.03; Potassium in Fertilizers—Gravimetric Tetraphenylborate Method. Official Methods of Analysis of AOAC INTERNATIONAL. AOAC INTERNATIONAL: Rockville, MD, USA, 2023.
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Linares, L.W.; Cayo-Rojas, F. Evaluación de la producción, composición botánica y contenido nutricional de pastos nativos en dos épocas del año en altiplano. J. Selva Andin. Anim. Sci. 2021, 8, 59–72. [Google Scholar] [CrossRef]
- Albarracín, K.; Jaramillo, L.; Albuja, M. Obtención de bioetanol anhidro a partir de paja (ichu). Rev. Politec. 2015, 36, 2. Available online: https://www.redalyc.org/articulo.oa?id=688773648015 (accessed on 9 January 2025).
- Rizki, M.; Tamai, Y. Effects of different nitrogen-rich substrates and their combination on the yield performance of oyster mushroom (Pleurotus ostreatus). World J. Microbiol. Biotechnol. 2011, 27, 1695–1702. [Google Scholar] [CrossRef]
- Baysal, E.; Peker, H.; Yalinkiliç, M.K.; Temiz, A. Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour. Technol. 2003, 89, 95–97. [Google Scholar] [CrossRef]
- Ruilova, M.; Hernández, A. Evaluación de residuos agrícolas para la producción del hongo Pleurotus ostreatus. ICIDCA Sobre Deriv. Caña Azúcar 2014, 48, 54–59. Available online: http://www.redalyc.org/articulo.oa?id=223131337008 (accessed on 9 January 2025).
- Saritha, M.; Arora, A.; Lata. Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Gómez, H.; Pabón, M.L.; Bolívar, D.M. Biomasa aérea y composición química de pastos Urochloa en diferentes edades de rebrote. Rev. Colomb. Cienc. Pecu. 2023, 36, 30–41. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=9968331 (accessed on 9 January 2025).
- Puliga, F.; Leonardi, P.; Minutella, F.; Zambonelli, A.; Francioso, O. Valorization of hazelnut shells as growing substrate for edible and medicinal mushrooms. Horticulturae 2022, 8, 214. [Google Scholar] [CrossRef]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; Taylor, M.; Saddler, J.; Mabee, E. From 1st- to 2nd-Generation Biofuel Technologies: An Overview of Current Industry and RD&D Activities; OECD/IEA: París, Francia, 2008. [Google Scholar]
- Badu, M.; Twumasi, M.; Boadi, N. Effects of lignocellulosic in wood used as substrate on the quality and yield of mushrooms. Food Nutr. Sci. 2011, 2, 780–784. [Google Scholar] [CrossRef]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express 2016, 6, 87. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid-state fermentation: A review. Bioresour. Bioprocess 2018, 5, 1. [Google Scholar] [CrossRef]
- Ríos, M.P.; Hoyos, J.L.; Mosquera, S.A. Evaluación de los parámetros productivos de la semilla de Pleurotus ostreatus propagada en diferentes medios de cultivo. Biotecnol. Sect. Agropec. Agroind. 2010, 8, 86–94. [Google Scholar]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The Effect of Alkaline Pretreatment Methods on Cellulose Structure and Accessibility. ChemSusChem 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Mohammad Rashed Iqbal, F.; Sharma, R.; Islam, F.; Mitra, S.; Emran, T.B. Nutritional value, medicinal importance, and health-promoting effects of dietary mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Caitano, C.E.C.; Ferrari, S.; Vieira Júnior, W.G.; Heinrichs, R.; de Almeida Moreira, B.R.; Pardo-Giménez, A.; Zied, D.C. Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing. Agronomy 2022, 12, 2884. [Google Scholar] [CrossRef]
- Elkanah, F.A.; Oke, M.A.; Adebayo, E.A. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Gardeli, C.; Mela, N.; Dedousi, M.; Kandyliari, A.; Kaparakou, E.; Diamantopoulou, P.; Pappas, C.; Mallouchos, A. The influence of substrate and strain on protein quality of Pleurotus ostreatus. Appl. Sci. 2024, 14, 4040. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef]
- Sales-Campos, C.; Araujo, L.M.; Minhoni, M.T.A.; de Andrade, M.C.N. Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon. Food Sci. Technol. 2011, 31, 456–461. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Kim, M.K.; Im, C.H.; Shin, P.-G. Development of cultivation media for extending the shelf-life and improving yield of king oyster mushrooms (Pleurotus eryngii). Sci. Hortic. 2015, 193, 121–126. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Bustamante, M.A.; Moral, R. Effects of spent mushroom substrates and inorganic fertilizer on the characteristics of a calcareous clayey-loam soil and lettuce production. Soil. Use Manag. 2016, 32, 487–491. [Google Scholar] [CrossRef]
- Peter, O.E.; Peter, G.R.; Obele, I.I.; Owuna, G.; Danladi, M.M.; Obiekieze, S.; Akwashiki, O. Utilization of some agro-wastes for cultivation of Pleurotus ostreatus (Oyster mushroom) in Keffi, Nigeria. Front. Environ. Microbiol. 2019, 5, 60–69. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Silva, R.M.; Carmo, C.O.; Oliveira, T.A.S.; Figueirêdo, V.R.; Duarte, E.A.A.; Soares, A.C.F. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated in agroindustrial wastes of palm oil fruits and cocoa almonds. Arq. Inst. Biol. 2020, 87, e0852018. [Google Scholar] [CrossRef]
- Irshad, A.; Tahir, A.; Sharif, S.; Khalid, A. Determination of nutritional and biochemical composition of selected Pleurotus spps. BioMed Res. Int. 2023, 2023, 8150909. [Google Scholar] [CrossRef]
- Kalač, P. Proximate composition and nutrients. In Edible Mushrooms; Elsevier: Amsterdam, The Netherlands, 2016; pp. 7–69. [Google Scholar] [CrossRef]
- Choi, J.W.; Yoon, Y.J.; Lee, J.H.; Kim, C.K.; Hong, Y.P.; Shin, I.S. Recent research trends of post-harvest technology for king oyster mushroom (Pleurotus eryngii). J. Mushroom 2018, 16, 131–139. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in Postharvest Storage and Preservation Strategies for Pleurotus eryngii. Foods 2023, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef]
- İnci, Ş.; Akyüz, M.; Kırbağ, S. Antimicrobial, antioxidant, cytotoxicity, and DNA protective properties of the pink oyster mushroom, Pleurotus djamor (Agaricomycetes). Int. J. Med. Mushrooms 2023, 25, 55–66. [Google Scholar] [CrossRef]
- Orngu, O.A.; Mbaeyi-Nwaoha, I.E.; Unagwu, B.O.; Etim, V.E. Oyster mushroom (Pleurotus ostreatus) cultivation using sawdust and different organic manures. Asian Food Sci. J. 2021, 20, 67–74. [Google Scholar] [CrossRef]
- Holgado-Rojas, M.E.; Aranzabal-Carrasco, R.L.; Lazarte-Lovatón, R.; Quispe-Peláez, A.; Pérez-Leguía, K.A.; Aguilar-Mainicta, F.B.; Aguilar-Pumahuillca, F. Cultivo de Pleurotus sp. y Lentinula edodes bajo condiciones artesanales en comunidades campesinas de la región Cusco, Perú. Rev. Investig. Altoandinas 2016, 18, 133–144. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- Silva, E.G.; Dias, E.S.; Siqueira, F.G.; Schwan, R.F. Análise química de corpos de frutificação de Pleurotus sajor-caju cultivado em diferentes concentrações de nitrogênio. Food Sci. Technol. 2007, 27, 72–75. [Google Scholar] [CrossRef][Green Version]
- Ragunathan, R.; Swaminathan, K. Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chem. 2003, 80, 371–375. [Google Scholar] [CrossRef]
- Cheung, P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ravlikovsky, A.; Symochko, L. Potential use of spent mushroom substrate of Lentinula edodes as a biofertilizer. Int. J. Ecosyst. Ecol. Sci. 2020, 10, 527–534. [Google Scholar] [CrossRef]
- Afsar, M.; Zia, A.; Salam, M.B.U.; Ahmad, M.N.; Khan, A.A.; Haq, T.U.; Aziz, T.; Alasmari, A.F. A multifaceted analysis of spent mushroom substrate of selected oyster mushrooms for enzymatic activity, proximate composition, and antimicrobial activity. Ital. J. Food Sci. 2024, 36, 165–174. [Google Scholar] [CrossRef]
- Lipiec, J.; Usowicz, B.; Kłopotek, J.; Turski, M.; Frąc, M. Effects of application of recycled chicken manure and spent mushroom substrate on organic matter, acidity, and hydraulic properties of sandy soils. Materials 2021, 14, 4036. [Google Scholar] [CrossRef]
- Ginterová, A.; Maxiaitová, A. The balance of nitrogen and composition of proteins in Pleurotus ostreatus grown on natural substrates. Folia Microbiol. 1975, 20, 246–250. [Google Scholar] [CrossRef]
- Uzun, I. Use of spent mushroom compost in sustainable fruit production. J. Fruit. Ornam. Plant Res. 2004, 12, 157–165. [Google Scholar]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Aamlid, T.S.; Landschoot, P.J. Effect of Spent Mushroom Substrate on Seed Germination of Cool-Season Turfgrasses. HortScience 2007, 42, 161–167. [Google Scholar] [CrossRef]
- Collela, C.F.; Costa, L.M.A.S.; Moraes, T.S.J.; Zied, D.C.; Rinker, D.L.; Dias, E.S. Potential utilization of spent Agaricus bisporus mushroom substrate for seedling production and organic fertilizer in tomato cultivation. Ciência Agrotecnol. 2019, 43, e017119. [Google Scholar] [CrossRef]
- Wei, H.; Di, Q.; Liang, T.; Liu, J.; Zhang, J. Effects of spent mushroom substrate biochar on growth of oyster mushroom (Pleurotus ostreatus). Environ. Technol. Innov. 2022, 28, 102729. [Google Scholar] [CrossRef]
- Luna Fontalvo, J.A.; Córdoba López, L.S.; Gil Pertuz, K.I.; Romero Borja, I.M. Effect of agroforestry residues partially biodegraded by Pleurotus ostreatus (Pleurotaceae) on tomato seedlings development. Acta Biol. Colomb. 2013, 18, 365. Available online: https://www.redalyc.org/articulo.oa?id=319028011012 (accessed on 9 January 2025).
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.; Luo, L.; Li, Y.; Chen, Z.; Gu, Y.; Chen, Q.; Deng, O.; Xu, X.; Lan, T.; et al. Short-term responses of soil nutrients, heavy metals and microbial community to partial substitution of chemical fertilizer with spent mushroom substrates (SMS). Sci. Total Environ. 2022, 844, 157064. [Google Scholar] [CrossRef]
- Peregrina, F.; Larrieta, C.; Colina, M.; Mariscal-Sancho, I.; Martín, I.; Martínez-Vidaurre, J.M.; García-Escudero, E. Spent mushroom substrates influence soil quality and nitrogen availability in a semiarid vineyard soil. Soil Sci. Soc. Am. J. 2012, 76, 1655–1666. [Google Scholar] [CrossRef]
- Zou, D.; Wang, J.; Wang, W.; Ye, X.; Yang, C.; He, F.; Zhang, M. Co-occurrences of soil nitrogen cycling and human-disease genes following spent mushroom substrate and nitrification inhibitor applications: A strategy for decreasing health risk. Land. Degrad. Dev. 2022, 33, 2769–2782. [Google Scholar] [CrossRef]

| Parameter | Unit | Result |
|---|---|---|
| Humidity | g∙100 g−1 | 8.8 |
| Ashes | g∙100 g−1 | 5.75 |
| Fat | g∙100 g−1 | 0.68 |
| Proteins | g∙100 g−1N × 6.25 | 5.53 |
| Crude fiber | g∙100 g−1 | 38.90 |
| Carbohydrates | g∙100 g−1 | 40.26 |
| Total energy | kcal∙100 g−1 | 189 |
| Carbohydrates | kcal∙100 g−1 | 161 |
| Proteins | kcal∙100 g−1 | 22 |
| Parameter | Species | Wet Basis (%) | Dry Basis (%) | SD |
|---|---|---|---|---|
| Humidity (g∙100 g−1) | P. citrinopileatus | 82.69 | - | 1.03 |
| P. djamor | 84.03 | - | 3.83 | |
| P. ostreatus | 82.29 | - | 6.41 | |
| Ash (g∙100 g−1) | P. citrinopileatus | 1.24 | 7.16 | 0.18 |
| P. djamor | 0.99 | 6.2 | 0.22 | |
| P. ostreatus | 0.98 | 5.53 | 0.3 | |
| Fat (g∙100 g−1) | P. citrinopileatus | 0.32 | 1.85 | 0.08 |
| P. djamor | 0.24 | 1.5 | 0.24 | |
| P. ostreatus | 0.69 | 3.9 | 0.3 | |
| Protein (g∙100 g−1N × 4.38) | P. citrinopileatus | 5.26 | 30.41 | 0.6 |
| P. djamor | 4.19 | 26.18 | 0.96 | |
| P. ostreatus | 4.27 | 24.1 | 0.92 | |
| Crude fiber (g∙100 g−1) | P. citrinopileatus | 1.83 | 10.57 | 0.33 |
| P. djamor | 2.17 | 13.59 | 0.93 | |
| P. ostreatus | 1.9 | 10.73 | 1.09 | |
| Carbohydrates (kcal∙100 g−1) | P. citrinopileatus | 8.65 | 49.97 | 0.04 |
| P. djamor | 8.08 | 50.59 | 1.83 | |
| P. ostreatus | 9.95 | 56.18 | 4.59 | |
| Total energy (kcal∙100 g−1) | P. citrinopileatus | 67.67 | - | 4.04 |
| P. djamor | 59 | - | 14.53 | |
| P. ostreatus | 70 | - | 25.94 | |
| Carbohydrates (kcal∙100 g−1) | P. citrinopileatus | 34.67 | - | 0.58 |
| P. djamor | 32.33 | - | 7.51 | |
| P. ostreatus | 40 | - | 18.33 | |
| Protein (kcal∙100 g−1) | P. citrinopileatus | 30 | - | 3.46 |
| P. djamor | 24 | - | 5.29 | |
| P. ostreatus | 24 | - | 5.2 | |
| Fat (kcal∙100 g−1) | P. citrinopileatus | 3 | - | 1 |
| P. djamor | 2.67 | - | 2.08 | |
| P. ostreatus | 6 | - | 2.65 |
| Cultivated Fungus | Humidity (g∙100 g−1) | Nitrogen (g∙100 g−1) | pH | Phosphorus (ppm) | Potassium (ppm) |
|---|---|---|---|---|---|
| P. ostreatus | 46.89 | 0.59 | 5.2 | 795.9 | 253.1 |
| P. citrinopileatus | 45.80 | 0.47 | 5.6 | 989.4 | 291.3 |
| P. djamor | 51.04 | 0.54 | 5.6 | 1296.9 | 262.6 |
| J. ichu (sin cultivo) | 5.40 | 4.02 | 5.75 | 0.11 | 7.77 |
| Coding | Treatment | Dose (mL) | Germination Percentage (%) |
|---|---|---|---|
| T1 | Ichu with P. citrinopileatus mycelium | 50 ml | 90 |
| T2 | Ichu with P. djamor mycelium | 50 mL | 100 |
| T3 | Ichu with P. ostreatus mycelium | 50 mL | 80 |
| T4 *-control | Chemical fertilizer | 50 mL | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano, R.; Dionisio, L.; Burga, L.; Javier-Astete, R.; Quispe-Apaza, C.; Oscco, P.; Johnson, L. Ichu Valorization by Pleurotus spp. Cultivation and Potential of the Residual Substrate as a Biofertilizer. Sustainability 2025, 17, 6695. https://doi.org/10.3390/su17156695
Solórzano R, Dionisio L, Burga L, Javier-Astete R, Quispe-Apaza C, Oscco P, Johnson L. Ichu Valorization by Pleurotus spp. Cultivation and Potential of the Residual Substrate as a Biofertilizer. Sustainability. 2025; 17(15):6695. https://doi.org/10.3390/su17156695
Chicago/Turabian StyleSolórzano, Richard, Luis Dionisio, Lyana Burga, Rosario Javier-Astete, Cinthia Quispe-Apaza, Persing Oscco, and Luis Johnson. 2025. "Ichu Valorization by Pleurotus spp. Cultivation and Potential of the Residual Substrate as a Biofertilizer" Sustainability 17, no. 15: 6695. https://doi.org/10.3390/su17156695
APA StyleSolórzano, R., Dionisio, L., Burga, L., Javier-Astete, R., Quispe-Apaza, C., Oscco, P., & Johnson, L. (2025). Ichu Valorization by Pleurotus spp. Cultivation and Potential of the Residual Substrate as a Biofertilizer. Sustainability, 17(15), 6695. https://doi.org/10.3390/su17156695







