Soil Organic Carbon Sequestration Mechanisms and the Chemical Nature of Soil Organic Matter—A Review
Abstract
1. Introduction
1.1. Soil Processes and Carbon Sequestration
1.2. On the Variable Effectiveness of Soil C Sequestration
1.3. Considerations on the Side-Effects of C Sequestration
1.4. Basic Research on the C Sequestration in Soil
1.5. Experimental Approaches to Monitor Soil C Sequestration Processes
1.6. The Soil Organic Matter Constituents: Biomacromolecules and Humic Substances
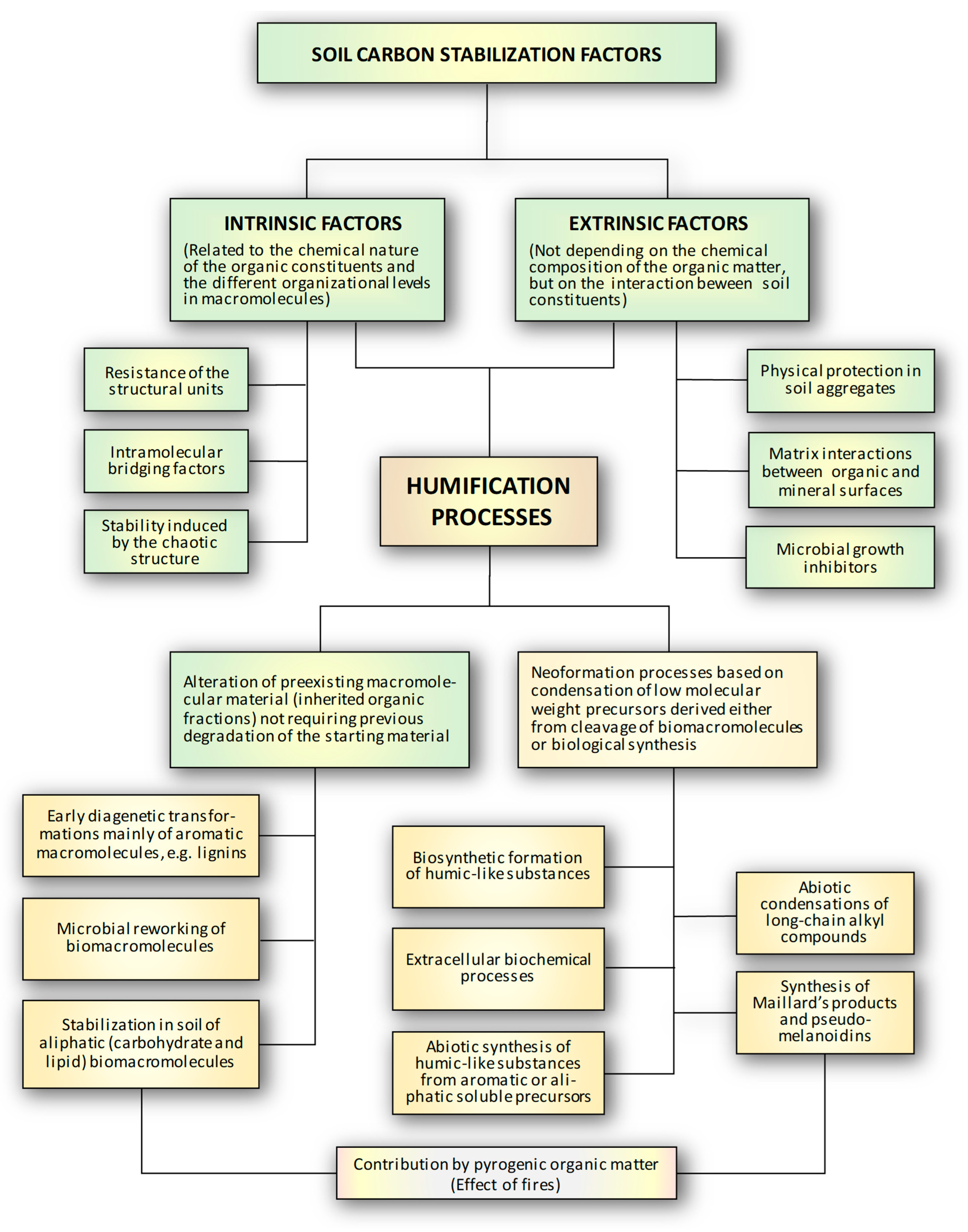
2. Soil Carbon Stabilization Factors
2.1. Intrinsic Factors
2.1.1. The Resistance of Structural Units
2.1.2. Intramolecular Bridging Factors
2.1.3. Stability Induced by the Chaotic Structure
2.2. Extrinsic Factors
2.2.1. Physical Protection
2.2.2. Matrix Interactions Between Organic and Mineral Surfaces
2.2.3. Microbial Growth Inhibitors
3. The Formation Processes of Humic Substances
3.1. Stabilization Mechanisms Involving Alteration of Pre-Existing Macromolecular Material (Inherited Organic Fractions) Not Requiring Complete Previous Degradation of the Starting Material
3.2. Neoformation Processes Based on the Condensation of Low Molecular Weight Precursors, or Structures Not Present in the Starting Material Derived Either from the Cleavage of Biomacromolecules or from Biological Synthesis
3.2.1. Biosynthetic Formation of Humic-like Substances
3.2.2. Extracellular Biochemical Processes
3.2.3. Abiotic Synthesis of Humic-like Substances from Aromatic or Aliphatic Precursors
3.2.4. Synthesis of Maillard’s Products
3.2.5. Fire-Mediated Production of Pyogenic Organic Matter and Its Evolution into Humic-like Substances
3.2.6. Abiotic Condensations of Long-Chain Alkyl Compounds
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lan, X.; Thoning, K.W.; Dlugokencky, E.J. Trends in Globally-Averaged CH4, N2O, and SF6 Determined from NOAA Global Monitoring Laboratory Measurements. Version 2025-06. Available online: https://gml.noaa.gov/ccgg/trends_doi.html (accessed on 27 June 2025).
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Rodrigues, C.I.D.; Brito, L.M.; Nunes, L.J.R. Soil carbon sequestration in the context of climate change mitigation: A review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Post, W.M.; Emmanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den Berg, E.; Reich, P. Organic carbon in soils of the world. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Gerke, J. The central role of soil organic matter in soil fertility and carbon storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Schnitzer, M. Some observations on the chemistry of humic substances. Agrochimica 1978, 22, 216–225. [Google Scholar]
- Buringh, P. Organic carbon in soils of the world. In The Role of Terrestrial Vegetation in the Global Carbon Cycle: Measurement by Remote Sensing; Woodwell, G.M., Ed.; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Eswaran, H.; Van den Berg, P.; Reich, P.; Kimble, J. Global soil carbon resources. In Soils and Global Change; Lal, R., Kimble, J.M., Levine, E., Stewart, B.A., Eds.; CRC/Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 25–43. [Google Scholar]
- Batjes, N.H.; Sombroek, W.G. Possibilities for carbon sequestration in tropical and subtropical soils. Global Change Biol. 1997, 3, 161–173. [Google Scholar] [CrossRef]
- Glenn, E.; Squires, V.; Olsen, M.; Frye, R. Potential for carbon sequestration in the drylands. Water Air Soil Pollut. 1993, 70, 341–355. [Google Scholar] [CrossRef]
- Hall, D.O. Carbon flows in the biosphere: Present and future. J. Geogr. Soc. 1989, 146, 175–181. [Google Scholar] [CrossRef]
- Batjes, N.H. Mitigation of atmospheric CO2 concentrations by increased carbon sequestration in the soil. Biol. Fertil. Soils 1998, 27, 230–235. [Google Scholar] [CrossRef]
- Schaeffer, A.; Nannipieri, P.; Kästner, M.; Schmidt, B.; Botterweck, J. From humic substances to soil organic matter–microbial contributions. In honour of Konrad Haider and James P. Martin for their outstanding research contribution to soil science. J. Soils Sediments 2015, 15, 1865–1881. [Google Scholar] [CrossRef]
- Post, W.M.; King, A.W.; Wullschleger, S.D. Soil organic matter models and global estimates of soil organic carbon. In Evaluation of Soil Organic Matter Models; NATO ASI Series (ASII); Springer: Berlin/Heidelberg, Germany, 1993; Volume 38, pp. 201–222. [Google Scholar] [CrossRef]
- Rodríguez Murillo, J.C. Organic carbon content under different types of land use and soil in Peninsular Spain. Biol. Fertil. Soils 2001, 33, 53–61. [Google Scholar] [CrossRef]
- Hontoria, C.; Rodríguez-Murillo, J.C. Relationships between soil organic carbon and site characteristics in Peninsular Spain. Soil Sci. Soc. Am. J. 1999, 63, 614–621. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; Virto, I.; Arias-González, A.; Enrique, A.; Fernández-Ugalde, O.; Barré, P. Mineral control of organic carbon storage in acid temperate forest soils in the Basque Country. Geoderma 2020, 358, 113998. [Google Scholar] [CrossRef]
- United Nations. Kyoto Protocol to the United Nations Framework Convention on Climate Change; United Nations: Kyoto, Japan, 1997; Available online: https://unfccc.int/process-and-meetings/the-kyoto-protocol/history-of-the-kyoto-protocol/text-of-the-kyoto-protocol (accessed on 27 June 2025).
- Duxbury, J.M. The significance of greenhouse emissions from soils of tropical agroecosystems. In Soil Management and Greenhouse Effect; Lal, R., Kimble, J., Levine, E., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 279–292. [Google Scholar]
- Fisher, M.J.; Rao, I.M.; Lascano, C.E.K.; Sanz, J.I.; Thomas, R.J.K.; Vera, R.R.; Ayarza, M.A. Pasture soils as carbon sink. Nature 1995, 376, 473. [Google Scholar] [CrossRef]
- Devevre, O.C.; Horwath, W.R. Carbon sequestration into soil organic matter under organic–based management. In Proceedings of the 10th International Meeting of the International Humic Substances Society (IHSS10), Tolouse, France, 24–28 July 2000; pp. 707–711. [Google Scholar]
- Khan, S.U.; Schnitzer, M. The retention of hydrophobic organic compounds by humic acid. Geochim. Cosmochim. Acta 1972, 36, 745–754. [Google Scholar] [CrossRef]
- Hargitai, L. The role of humus status of soils in binding toxic elements and compounds. Sci. Total Environ. 1989, 81–82, 643–651. [Google Scholar] [CrossRef]
- Almendros, G. Sorptive interactions of pesticides in soils treated with modified humic acids. Eur. J. Soil Sci. 1995, 46, 287–301. [Google Scholar] [CrossRef]
- Maffia, A.; Oliva, M.; Marra, F.; Mallamaci, C.; Nardi, S.; Muscolo, A. Humic substances: Bridging ecology and agriculture for a greener future. Agronomy 2025, 15, 410. [Google Scholar] [CrossRef]
- Kröger, N.; Brunner, E.; Estroff, L.; Marin, F. The role of organic matrices in biomineralization. Discov. Mater. 2021, 1, 21. [Google Scholar] [CrossRef]
- Mujuru, L.; Gotora, T.; Velthorst, E.J.; Nyamangara, J.; Hoosbeek, M.R. Soil carbon and nitrogen sequestration over an age sequence of Pinus patula plantations in Zimbabwean eastern highlands. Forest Ecol. Manag. 2014, 313, 254–265. [Google Scholar] [CrossRef]
- Almendros, G. Carbon Sequestration in Soil. In Kluwer Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 315–323. ISBN 978-1-4020-3994-2. [Google Scholar]
- Goudriaan, J. Global Carbon Cycle and Carbon Sequestration. In Carbon Sequestration in the Biosphere; Beran, M.A., Ed.; NATO ASI Series I: Global Environmental Change; Springer: Berlin/Heidelberg, Germany, 1995; Volume 33, pp. 3–18. [Google Scholar] [CrossRef]
- Fang, C.; Smith, P.; Moncrieff, J.B.; Smith, J.U. Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 1995, 433, 57–59. [Google Scholar] [CrossRef]
- Xiao, C. Soil Organic Carbon Storage (Sequestration) Principles and Management. In Potential Role for Recycled Organic Materials in Agricultural Soils of Washington State; Waste 2 Resources Program, Washington State Department of Ecology: Olympia, WA, USA, 2015; 90p. [Google Scholar]
- Olk, D.C.; Bloom, P.R.; De Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using humic fractions to understand natural organic matter processes in soil and water: Selected studies and applications. J. Environ. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef]
- Myneni, S.C. Chemistry of natural organic matter—The next step: Commentary on a humic substances debate. J. Environ. Qual. 2019, 48, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J. Biochemistry of the formation of humic substances. In Humus Chemistry; Stevenson, F.J., Ed.; Wiley: New York, NY, USA, 1892; pp. 195–220. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular Structure in Soil Humic Substances: The New View. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Almendros, G. Humic Substances. In Kluwer Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 97–99. ISBN 978-1-4020-3994-2. [Google Scholar]
- Almendros, G.; Tinoco, M.P.; De la Rosa, J.M.; Knicker, H.; González-Pérez, J.A.; González-Vila, F.J. Selective effects of forest fires on the structural domains of soil humic acids as shown by dipolar dephasing 13C NMR and graphical-statistical analysis of pyrolysis compounds. J. Soil Sediments 2018, 18, 1303–1313. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Rumpel, C. Advances in molecular approaches for understanding soil organic matter composition, origin, and turnover: A historical overview. Adv. Agron. 2018, 149, 1–48. [Google Scholar] [CrossRef]
- Tinoco, P.; Almendros, G.; González-Vila, F.J.; Sanz, J.; González-Pérez, J.A. Revisiting molecular characteristics responsive for the aromaticity of soil humic acids. J. Soils Sediments 2015, 15, 781–791. [Google Scholar] [CrossRef]
- Falloon, P.D.; Smith, P. Modelling refractory soil organic matter. Biol. Fertil. Soils 2000, 30, 388–398. [Google Scholar] [CrossRef]
- Almendros, G.; Sanz, J.; Velasco, F. Signature of lipid assemblages in soils under continental Mediterranean forests. Eur. J. Soil Sci. 1996, 47, 183–196. [Google Scholar] [CrossRef]
- Wilson, M.A. Application of nuclear magnetic resonance spectroscopy to the study of the structure of soil organic matter. J. Soil Sci. 1981, 32, 167–186. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Lüdemann, H.-D.; Martín, F. 13C-NMR structural features of soil humic acids and their methylated, hydrolyzed and extracted derivatives. Geoderma 1983, 31, 3–15. [Google Scholar] [CrossRef]
- Almendros, G.; Guadalix, M.E.; González-Vila, F.J.; Martin, F. Preservation of aliphatic macromolecules in soil humins. Org. Geochem. 1996, 24, 651–659. [Google Scholar] [CrossRef]
- Preston, C.M.; Ripmeester, J.A. Application of solution and solid-state 13C NMR to four organic soils, their humic acids, fulvic acids, humins and hydrolysis residues. Can. J. Spectrosc. 1982, 27, 99–105. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, G.; Logan, G.A. Molecular preservation. Phil. Trans. R. Soc. Lond. B 1991, 333, 315–328. [Google Scholar] [CrossRef]
- Hempfling, R.; Schulten, H.R. Selective preservation of biomolecules during humification of forest litter studied by pyrolysis-field ionization mass spectrometry. Sci. Total Environ. 1989, 81, 31–40. [Google Scholar] [CrossRef]
- Stevenson, F.J. Structural Basis of Humic Substances. In Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994; p. 337. [Google Scholar]
- Wershaw, R.L.; Pinckney, D.J.; Booker, S.E. Chemical structure of humic acids– Part 1. A generalized structural model. J. Res. US Geol. Surv. 1977, 5, 565–569. [Google Scholar]
- Orsi, M. Molecular dynamics simulation of humic substances. Chem. Biol. Technol. Agric. 2014, 1, 10. [Google Scholar] [CrossRef]
- De Nobili, M. Comment on “Humic Substances Extracted by Alkali Are Invalid Proxies for the Dynamics and Functions of Organic Matter in Terrestrial and Aquatic Ecosystems,” by Kleber and Lehmann (2019). J. Environ. Qual. 2019, 48, 787–789. [Google Scholar] [CrossRef]
- Wang, M.C.; Chang, S.H. Mean residence times and characteristics of humic substances extracted from a Taiwan soil. Can. J. Soil Sci. 2001, 81, 299–307. [Google Scholar] [CrossRef]
- Piccolo, A.; Drosos, M. The essential role of humified organic matter in preserving soil health. Chem. Biol. Technol. Agric. 2025, 12, 21. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of soil organic matter Individual fractions (fulvic acids, humic acids, and humins) by spectroscopic and electrochemical techniques in agricultural soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- Goh, K.M. Carbon sequestration and stabilization in soils: Implications for soil productivity and climate change. Soil Sci. Plant Nutr. 2004, 50, 467–476. [Google Scholar] [CrossRef]
- Haworth, R.D. The chemical nature of humic acid. Soil Sci. 1971, 111, 71–79. [Google Scholar] [CrossRef]
- Martín, F.; Saíz-Jiménez, C.; González-Vila, F.J. The persulfate oxidation of a soil humic acid. Soil Sci. 1981, 132, 200–203. [Google Scholar] [CrossRef]
- Almendros, G.; Sanz, J. Compounds released from humic acids upon BF3-MeOH transesterification. Sci. Total Environ. 1989, 81–82, 51–60. [Google Scholar] [CrossRef]
- Almendros, G.; Sanz, J. A structural study of alkyl polymers in soil after perborate degradation of humin. Geoderma 1992, 53, 79–95. [Google Scholar] [CrossRef]
- Almendros, G.; Hernández, Z.; Sanz, J.; Jiménez-González, M.A.; Rodríguez-Sánchez, S.; González-Pérez, J.A. Graphical statistical approach to soil organic matter resilience using analytical pyrolysis data. J. Chromatogr. A 2018, 1533, 164–173. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; Almendros, G.; Waggoner, D.C.; Álvarez, A.M.; Hatcher, P.G. Assessment of the molecular composition of humic acid as an indicator of soil carbon levels by ultra-high-resolution mass spectrometric analysis. Org. Geochem. 2020, 143, 104012. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; Álvarez, A.; Carral, P.; Almendros, G. Chemometric assessment of soil organic matter storage and quality from humic acid infrared spectra. Sci. Total Environ. 2019, 685, 1160–1168. [Google Scholar] [CrossRef]
- Hatakka, A.; Hammel, K.E. Fungal Biodegradation of Lignocelluloses. In The Mycota X: Industrial Applications, 2nd ed.; Hofrichter, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 319–340. Available online: https://link.springer.com/chapter/10.1007/978-3-642-11458-8_15 (accessed on 14 July 2025).
- Almendros, G.; González-Vila, F.J. Degradative studies on a soil humin fraction. Sequential degradation of inherited humin. Soil Biol. Biochem. 1987, 19, 513–520. [Google Scholar] [CrossRef]
- Almendros, G.; Sanz, J. A structural study on the soil humin fraction—Boron trifluoride-methanol transesterification of soil humin preparations. Soil Biol. Biochem. 1991, 23, 1147–1154. [Google Scholar] [CrossRef]
- Almendros, G.; Guadalix, M.E.; González-Vila, F.J.; Martín, F. Distribution of structural units in humic substances as revealed by multi–step selective degradations and 13C–NMR of successive residues. Soil Biol. Biochem. 1998, 30, 755–765. [Google Scholar] [CrossRef]
- Almendros, G.; Dorado, J. Molecular characteristics related to the biodegradability of humic acid preparations. Eur. J. Soil Sci. 1999, 50, 227–236. [Google Scholar] [CrossRef]
- Tombácz, E.; Rice, J.; Ren, Z. Fractal structure of polydisperse humic acid particles in solution studied by scattering methods. Models Chem. 1997, 134, 877–888. [Google Scholar]
- Saiz-Jimenez, C.; De Leeuw, J.W. Chemical characterization of soil organic matter fractions by analytical pyrolysis-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis 1986, 9, 99–119. [Google Scholar] [CrossRef]
- Rahmonov, O.; Kowalski, W.J.; Bednarek, R. Characterization of the soil organic matter and plant tissues in an initial stage of the plant succession and soil development by means of Curie-point pyrolysis coupled with GC-MS. Eurasian Soil Sci. 2010, 43, 1557–1568. [Google Scholar] [CrossRef]
- Pandao, M.R.; Rathod, S.R.; Sirsat, D.D.; Lingayat, N.R. The role of soil in carbon sequestration: Mechanisms and implications. Asian J. Environ. Ecol. 2024, 23, 66–75. [Google Scholar] [CrossRef]
- Duchaufour, P.; Jacquin, F. Comparaison des procesus d’humification dans les principaux types d’humus forestiers. Sci. Sol. 1975, 1, 29–36. [Google Scholar]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Basile-Doelsch, I.; Balesdent, J.; Pellerin, S. Reviews and syntheses: The mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Derrien, D.; Barré, P.; Basile-Doelsch, I.; Cécillon, L.; Chabbi, A.; Crème, A.; Fontaine, S.; Henneron, L.; Janot, N.; Lashermes, G.; et al. Current controversies on mechanisms controlling soil carbon storage: Implications for interactions with practitioners and policy-makers. A review. Agron. Sustain. Dev. 2023, 43, 21. [Google Scholar] [CrossRef] [PubMed]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Clarke, P.; Taylor, J.A.; Oades, J.M.; McClure, S.G. The chemistry and nature of protected carbon in soil. Aust. J. Soil Res. 1996, 34, 251–271. [Google Scholar] [CrossRef]
- Ladd, J.N.; Foster, R.C.; Skjemstad, J.O. Soil structure: Carbon and nitrogen metabolism. Geoderma 1993, 56, 401–434. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Soil structure and carbon cycling. Aust. J. Soil Res. 1994, 32, 1043–1068. [Google Scholar] [CrossRef]
- Oades, J.M.; Vassallo, A.M.; Waters, A.G.; Wilson, A. Characterization of organic matter in particle size and density fractions from a Red–brown Earth by solid–state 13C NMR. Aust. J. Soil Res. 1987, 25, 71–82. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Schnitzer, M. Aliphatics in soil organic matter in fine-clay fractions. Soil Sci. Soc. Am. J. 1990, 54, 98–105. [Google Scholar] [CrossRef]
- Song, X.; Yuan, Z.-Q.; Fang, C.; Hu, Z.-H.; Li, F.-M.; Sardans, J.; Penuelas, J. The formation of humic acid and micro-aggregates facilitated long-time soil organic carbon sequestration after Medicago sativa L. introduction on abandoned farmlands. Geoderma 2024, 445, 116889. [Google Scholar] [CrossRef]
- Bottner, P.; Couteaux, M.M.; Vallejo, V.R. Soil organic matter in Mediterranean-type ecosystems and global climatic changes: A case study—The soils of the Mediterranean basin. In Global Change and Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1995; pp. 306–325. [Google Scholar] [CrossRef]
- Spaccini, R.; Conte, P.; Piccolo, A.; Haberhauer, G.; Gerzabek, M.H. Increased soil organic carbon sequestration through hydrophobic protection by humic substances. In Proceedings of the 10th International Meeting of the International Humic Substances Society (IHSS10), Tolouse, France, 24–28 July 2000; p. 419. [Google Scholar]
- Spaccini, R.; Piccolo, A.; Conte, P.; Haberhauer, G.; Gerzabek, M.H. Increased soil organic carbon sequestration through hydrophobic protection by humic substances. Soil Biol. Biochem. 2002, 34, 1839–1851. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon management and climate change. Carbon Manag. 2013, 4, 439–462. [Google Scholar] [CrossRef]
- Greenland, D.J. Interactions between humic and fulvic acids and clays. Soil Sci. 1971, 111, 34–41. [Google Scholar] [CrossRef]
- Calvo de Anta, R.M.; Díaz-Fierros, F. Mineralización del carbono y del nitrógeno en suelos forestales de Galicia. An. Edafol. Agrobiol. 1982, 41, 1–10. [Google Scholar]
- Deuel, H. Interactions between inorganic and organic soil constituents. Trans. 7th Int. Congr. Soil Sci. 1960, 38–53. [Google Scholar]
- Aran, D.; Gury, M.; Jeanroy, E. Organo-metallic complexes in an Andosol: A comparative study with a Cambisol and Podzol. Geoderma 2001, 99, 65–79. [Google Scholar] [CrossRef]
- Saggar, S.; Tate, K.R.; Feltham, C.W.; Childs, C.W.; Parshotam, A. Carbon turnover in a range of allophanic soils amended with 14C-labelled glucose. Soil Biol. Biochem. 2006, 26, 1263–1271. [Google Scholar] [CrossRef]
- Derenne, S.; Quénéa, K. Analytical pyrolysis as a tool to probe soil organic matter. J. Anal. Appl. Pyrolysis 2015, 111, 108–120. [Google Scholar] [CrossRef]
- Hernández, Z.; Almendros, G. Biogeochemical factors related with organic matter degradation and C storage in agricultural volcanic ash soils. Soil Biol. Biochem. 2012, 44, 130–142. [Google Scholar] [CrossRef]
- Hernández, Z.; Almendros, G.; Álvarez, A.; Figueiredo, T.; Carral, P. Soil carbon stabilization pathways as reflected by the pyrolytic signature of humic acid in agricultural volcanic soils. J. Anal. Appl. Pyrolysis. 2019, 137, 14–28. [Google Scholar] [CrossRef]
- Merlet, D. Mise au Point Technique Concernant l’Extraction et la Caractérisation des Composés Organiques Dans les Sols; Centre de Pédologie Biologique: Nancy, France, 1971. [Google Scholar]
- Davies, R.I. Relation of polyphenols to decomposition of organic matter and to pedogenetic processes. Soil Sci. 1971, 111, 80–85. [Google Scholar] [CrossRef]
- Basaraba, J.; Starkey, R.L. Effect of plant tannins on decomposition of organic substances. Soil Sci. 1966, 101, 17–23. [Google Scholar] [CrossRef]
- Lynch, J.M. Products of soil microorganisms in relation to plant growth. Crit. Rev. Microbiol. 1976, 5, 67–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.C.; Yang, T.-K.; Chuang, T.-T. Soil phenolic acids as plant growth inhibitors. Soil Sci. 1967, 103, 239–246. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. Adv. Agron. 2020, 163, 1–37. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 3, 237–249. [Google Scholar] [CrossRef]
- De Nobili, M. La complessità della sostanza organica del suolo e le nuove prospettive di ricerca sull’umificazione. In Chimica del Suolo: Ieri, Oggi e Domani; Accademia dei Georgofili: Firenze, Italy, 2022; pp. 370–380. [Google Scholar]
- Dou, S.; Shan, J.; Song, X.; Cao, R.; Wu, M.; Li, C.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Goulden, J.D.S.; Jenkinson, D.S. Studies on the organic material extracted from soil and compost. II. The infra-red spectra of ligno-proteins isolated from compost. J. Soil Sci. 1959, 10, 264–270. [Google Scholar] [CrossRef]
- Almendros, G.; Polo, A.; Dorado, E. Estudio de los compuestos húmicos en diversos tipos de compost preparados a partir de paja de trigo.– II: Caracterización fisicoquímica de los compuestos húmicos. Agrochimica 1983, 27, 311–325. [Google Scholar]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Lignin contribution to aliphatic constituents of humic acids through fungal degradation. J. Environ. Qual. 2019, 48, 1565–1570. [Google Scholar] [CrossRef]
- Nip, M.; Tegelaar, E.W.; de Leeuw, J.W.; Schenck, P.A. A new non-saponifiable highly aliphatic and resistant biopolymer in plant cuticles. Naturwissenschaften 1986, 73, 579–585. [Google Scholar] [CrossRef]
- van Aarssen, B.G.K.; Leeuw, J.W.; Tegelaar, E.W. Recently discovered aliphatic biopolymers of vascular plants and algae and their impact on the environment. In Proceedings of the IV Workshop Chemistry and Analysis of Environmental Hydrocarbons, Strasbourg, France, 19–21 April 1990; p. 21. [Google Scholar]
- Holloway, P.J. The composition of suberin from the corks of Quercus suber L. and Betula pendula Roth. Chem. Phys. Lipids 1972, 9, 158–170. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Biosynthesis and degradation of lipid polymers. In Lipids and Lipid Polymers in Higher Plants; Tevini, M., Lichtenthaler, H.K., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1977; Chapter 15; pp. 271–292. [Google Scholar]
- Almendros, G.; Sanz, J.; González-Vila, F.J.; Martin, F. Evidence for a polyalkyl nature of soil humin. Naturwissenschaften 1991, 78, 359–362. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Maciel, G.E.; Dennis, L.W. Aliphatic structures of humic acids, a clue to their origin. Org. Geochem. 1981, 3, 43–48. [Google Scholar] [CrossRef]
- Waksman, S.A. Humus, Origin, Chemical Composition and Importance in Nature; Williams and Wilkins: Baltimore, MD, USA, 1936. [Google Scholar]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Almendros, G.; Dorado, J.; González-Vila, F.J.; Blanco, M.J.; Lankes, U. 13C NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol. Biochem. 2000, 32, 793–804. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Microbial activity in relation to soil humus formation. Soil Sci. 1971, 111, 54–63. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Ann. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P. Synthesis and transformation of phenolic compounds by Epicoccum nigrum in relation to humic acid formation. Soil Sci. Soc. Amer. Proc. 1967, 31, 766–772. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Saiz-Jimenez, C.; Lentz, H.; Lüdemann, H.-D. 13C nuclear magnetic resonance spectra of fungal melanins. Z. Naturforsch. C 1978, 33, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Jimenez, C.; Martin-Sanchez, P.M.; González-Pérez, J.A.; Hermosín, B. Analytical pyrolysis of the fungal melanins from Ochroconis spp. isolated from Lascaux cave, France. Appl. Sci. 2021, 11, 1198. [Google Scholar] [CrossRef]
- Paim, S.; Linhares, L.E.; Mangrich, A.S.; Martin, J.P. Characterization of fungal melanins and soil humic acids by chemical analysis and infrared spectroscopy. Biol. Fertil. Soils 1990, 10, 72–76. [Google Scholar] [CrossRef]
- Zavgorodnyaya, Y.A.; Demin, V.V.; Kurakov, A.V. Biochemical degradation of soil humic acids and fungal melanins. Org. Geochem. 2002, 33, 347–355. [Google Scholar] [CrossRef]
- Kumada, K.; Hurst, H.M. Green humic acid and its possible origin as a fungal metabolite. Nature 1967, 214, 631–633. [Google Scholar] [CrossRef]
- Almendros, G.; Martínez, A.T.; Dorado, E. Production of brown and green humic-like substances by Ulocladium atrum. Soil Biol. Biochem. 1985, 17, 257–259. [Google Scholar] [CrossRef]
- Valmaseda, M.; Martínez, A.T.; Almendros, G. Contribution by pigmented fungi to P–type humic acid formation in two forest soils. Soil Biol. Biochem. 1989, 21, 23–28. [Google Scholar] [CrossRef]
- Sato, O. A green pigment similar to the Pg fraction of P type humic acids and related compounds produced by litter-decomposing fungi. Soil Sci. Plant Nutr. 1976, 22, 269–275. [Google Scholar] [CrossRef]
- Kumada, K.; Sato, O. Studies on the chemical properties of P type humic acid. Trans. Int. Symp. Humus Planta 1967, 4, 131–133. [Google Scholar]
- Kobayashi, T.; Rasmussen, C.; Sumida, H. Characterization of the perylenequinone pigments in Japanese Andosols and Cambisol. Soil Sci. Plant Nutr. 2019, 65, 1–10. [Google Scholar] [CrossRef]
- Itoh, N.; Sakagami, N.; Torimura, M.; Watanabe, M. Perylene in Lake Biwa sediments originating from Cenococcum geophilum in its catchment area. Geochim. Cosmochim. Acta 2012, 95, 241–251. [Google Scholar] [CrossRef]
- Watanabe, A.; Fujimori, H.; Nagai, Y.; Miyajima, T.; Kuwatsuka, S. Analysis of the green fraction of humic acids. Eur. J. Soil Sci. 1996, 47, 197–204. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanaka, H.; Sakagami, K.; Aoki, K.; Sugiyama, S. Evaluation of Pg absorption strength of humic acids as a paleoenvironmental indicator in buried paleosols on tephra beds, Japan. Quat. Int. 1996, 34, 197–203. [Google Scholar] [CrossRef]
- Almendros, G.; Dorado, E. Estudio de ácidos húmicos de tipo P. Distribución de los pigmentos verdes en las diferentes fracciones húmicas del suelo. An. Edafol. Agrobiol. 1985, 43, 547–559. [Google Scholar]
- Kumada, K.; Sato, O. Characteristics of the green fraction of P-type humic acid. Soil Sci. Plant Nutr. 1980, 26, 181–190. [Google Scholar] [CrossRef]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Ertel, J.R.; Hedges, J.I. The lignin component of humic substances: Distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim. Cosmochim. Acta 1984, 48, 2065–2074. [Google Scholar] [CrossRef]
- Andreux, F. Contribution à L’étude des Processus de Melanification des Autolysats Végétaux (Juglans regia). Ph.D. Thesis, Université de Nancy, Nancy, France, 1969. [Google Scholar]
- Schnitzer, M.; Khan, S.U. Humic Substances in the Environment; Marcel Dekker Inc.: New York, NY, USA, 1972. [Google Scholar]
- Suflita, J.M.; Bollag, J.M. Polymerization of phenolic compounds by a soil-enzyme complex. Soil Sci. Soc. Am. J. 1981, 45, 297–302. [Google Scholar] [CrossRef]
- Andreux, F.; Golebiowska, D.; Chone, T.; Jacquin, F.; Metche, M. Caractérisation et transformation en milieu mull d’un modèle humique issu de l’autoxidation du système catechol-glycine et marqué séléctivement au carbone-14. In Soil Organic Matter Studies, Proceedings of the 5th Symposium IAEA, Braunschweig, Germany, 6–10 September 1976; International Atomic Energy Agency: Vienna, Austria, 1977; Volume II, p. 43. [Google Scholar]
- Shindo, H. Catalytic synthesis of humic acids from phenolic compounds by Mn(IV) oxide (Birnessite). Soil Sci. Plant Nutr. 1990, 36, 679–682. [Google Scholar] [CrossRef]
- Zou, J.; Huang, J.; Zhang, H.; Yue, D. Evolution of humic substances in polymerization of polyphenol and amino acid based on non-destructive characterization. Front. Environ. Sci. Eng. 2021, 15, 5. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Truong, H.B.; Hong, S.; Li, X.; Hur, J. Biotic and abiotic catalysts for enhanced humification in composting: A comprehensive review. J. Clean. Prod. 2023, 402, 136832. [Google Scholar] [CrossRef]
- Sun, K.; Niu, Z.; Xiao, S.; Qi, X.; Li, S.; Chen, M.; Dai, L.; Si, Y. Artificially regulated humification in creating humic-like biostimulators. NPJ Clean Water 2024, 7, 47. [Google Scholar] [CrossRef]
- Kiprop, A.K.; Marie-Camille, J.; Pourtier, E.; Kimutai, S.; Kirui, S. Synthesis of humic and fulvic acids and their characterization using optical spectroscopy (ATR-FTIR and UV-Visible). Int. J. Appl. Sci. Technol. 2013, 3, 28–35. [Google Scholar]
- Min, D.W.; Kim, K.; Lui, K.H.; Kim, B.; Kim, S.; Cho, J.; Choi, W. Abiotic formation of humic-like substances through freezing-accelerated reaction of phenolic compounds and nitrite. Environ. Sci. Technol. 2019, 53, 7410–7418. [Google Scholar] [CrossRef]
- Spiteller, M.; Schnitzer, M. A comparison of the structural characteristics of polymaleic acid and a soil fulvic acid. J. Soil Sci. 1983, 34, 525–537. [Google Scholar] [CrossRef]
- Martin, F.; González-Vila, F.J.; Lüdemann, H.-D. About the similarity between polymaleic acid and water soluble humic fractions. Z. Naturforschung 1984, 369, 244–248. [Google Scholar] [CrossRef]
- Almendros, G.; Martin, F.; González-Vila, F.J. Depolymerization and degradation of humic acids with sodium perborate. Geoderma 1987, 39, 235–247. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Drosos, M.; Vinci, G.; Cozzolino, V. The molecular composition of humus carbon: Recalcitrance and reactivity in soils. In The Future of Soil Carbon its Conservation and Formation; Garcia, C., Nannipieri, P., Hernandez, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 4. [Google Scholar] [CrossRef]
- Ellis, G.P. The Maillard reaction. Adv. Carbohydr. Chem. 1959, 14, 63–134. [Google Scholar]
- Rubinsztain, Y.; Ioselis, P.; Ikan, R.; Aizenshtat, Z. Investigations on the structural units of melanoidins. Org. Geochem. 1984, 6, 791–804. [Google Scholar] [CrossRef]
- Maillard, M.L.-C. Synthèse des matières humiques par action des acides aminés sur les sucres réducteurs. Ann. Chim. 1916, 5, 258–317. [Google Scholar]
- Nakaya, Y.; Okada, K.; Ikuno, Y.; Nakashima, S. Spectroscopic study of effects of goethite surfaces on the simulated Maillard reaction forming humic-like substances. Surf. Sci. Nanotechnol. 2018, 16, 411–418. [Google Scholar] [CrossRef]
- Benzing-Purdie, L.; Ripmeester, J.A. Melanoidins and soil organic matter: Evidence of strong similarities revealed by 13CP–MAS NMR. Soil Sci. Soc. Am. J. 1983, 47, 56–61. [Google Scholar] [CrossRef]
- Hardie, A.G.; Dynes, J.J.; Kozaka, L.M.; Huang, P.M. Abiotic catalysis of the Maillard and polyphenol-Maillard humification pathways by soil clays from temperate and tropical environments. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Xu, J., Huang, P.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, D.; Ma, H. Darkening mechanism and kinetics of humification process in catechol-Maillard system. Chemosphere 2015, 130, 40–45. [Google Scholar] [CrossRef]
- Jokic, A.; Wang, M.C.; Liu, C.; Frenkel, A.I.; Huang, P.M. Integration of the polyphenol and Maillard reactions into a unified abiotic pathway for humification in nature: The role of δ-MnO2. Org. Geochem. 2004, 35, 747–762. [Google Scholar] [CrossRef]
- Wang, N.; Cui, Y.; Zhou, Y.; Liu, P.; Wang, M.; Sun, H.; Huang, Y.; Wang, S. Changes in the glucose concentration affect the formation of humic-like substances in polyphenol–Maillard reactions involving gibbsite. Molecules 2024, 29, 2115. [Google Scholar] [CrossRef]
- Popoff, T.; Theander, O. Formation of aromatic compounds from carbohydrates. Part III. Reaction of D-glucose and D-fructose in slightly acidic, aqueous solution. Acta Chem. Scand. 1976, 30, 397–402. [Google Scholar] [CrossRef]
- Ikan, R.; Ioselis, P.; Rubinsztain, Y.; Aizenshtat, Z.; Pugmire, R.; Anderson, L.L.; Ishiwatari, R. Carbohydrate origin of humic substances. Naturwissenschaften 1986, 73, 150–151. [Google Scholar] [CrossRef]
- Almendros, G.; Sanz, J.; Sobrados, I. Characterization of synthetic carbohydrate-derived humic-like polymers. Sci. Total Environ. 1989, 81–82, 91–98. [Google Scholar] [CrossRef]
- Hodge, J.E. Chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Feather, M.S.; Harris, J.F. Dehydration reactions of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1973, 28, 161–224. [Google Scholar] [CrossRef]
- Almendros, G.; Dorado, J.; González-Vila, F.J.; Martin, F. Pyrolysis of carbohydrate–derived macromolecules: Its potential in monitoring the carbohydrate signature of geopolymers. J. Anal. Appl. Pyrolysis 1997, 40–41, 599–610. [Google Scholar] [CrossRef]
- Kumada, K. Carbonaceous materials as a possible source of soil humus. Soil Sci. Plant Nutr. 1983, 29, 383–386. [Google Scholar] [CrossRef]
- Shindo, H.; Matsui, Y.; Higashi, T. Humus composition of charred plant residues. Soil Sci. Plant Nutr. 1986, 32, 475–478. [Google Scholar] [CrossRef]
- Haumaier, L.; Zech, W. Black carbon–possible source of highly aromatic components of soil humic acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Poirier, N.; Derenne, S.; Balesdent, J.; Rouzaud, J.-N.; Mariotti, A.; Largeau, C. Abundance and composition of the refractory organic fraction of an ancient, tropical soil (Pointe Noire, Congo). Org. Geochem. 2002, 38, 383–391. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Taylor, J.A. Does the Walkley–Black method determine soil charcoal? Commun. Soil Sci. Plant Anal. 1999, 30, 2299–2310. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819. [Google Scholar] [CrossRef]
- Contreras, E.; García, R.; Martínez, F.; Hernández, M.; Linares, J. Efecto de la temperatura, tiempo de oxidación y relación sustrato: Oxidante en el rendimiento y composición de los ácidos húmicos derivados del carbón, monitoreado por RMN de 1H y FT-IR. Rev. Téc. Ing. Univ. Zulia 2004, 27, 132–140. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0254-07702004000200006 (accessed on 14 July 2025).
- Almendros, G.; González-Vila, F.J.; Martin, F. Fire-induced transformation of soil organic matter from an oak forest. An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- Almendros, G.; Knicker, H.; González-Vila, F.J. Rearrangement of carbon and nitrogen forms in peat after progressive thermal oxidation as determined by solid-state 13C- and 15N spectroscopy. Org. Geochem. 2003, 34, 1559–1568. [Google Scholar] [CrossRef]
- Almendros, G.; Fründ, R.; González-Vila, F.J.; Haider, K.M.; Knicker, H.; Lüdemann, H.-D. Analysis of 13C and 15N CPMAS NMR-spectra of soil organic matter and composts. FEBS Lett. 1991, 282, 119–121. [Google Scholar] [CrossRef] [PubMed]
- DeBano, L.F.; Mann, L.D.; Hamilton, D.A. Translocation of hydrophobic substances by burning organic litter. Soil Sci. Soc. Am. Proc. 1970, 34, 130–133. [Google Scholar] [CrossRef]
- Giovannini, G.; Luchessi, S.; Cerevelli, S. Water-repellent substances and aggregate stability in hydrophobic soil. Soil Sci. 1983, 135, 110–113. [Google Scholar] [CrossRef]
- Savage, S.M.; Osborn, J.; Letey, J.; Heaton, C. Substances contributing to fire–induced water repellency in soil. Soil Sci. Soc. Am. Proc. 1972, 36, 674–678. [Google Scholar] [CrossRef]
- de Deus, M.; Miller, A.Z.; Jiménez-Morillo, N.T. Molecular characterization of burned organic matter at different soil depths and its relationship with soil water repellency: A preliminary result. Agronomy 2021, 11, 2560. [Google Scholar] [CrossRef]
- Shindo, H.; Matsui, Y.; Higashi, T. A possible source of humic acids in volcanic ash soils in Japan- Charred residue of Miscanthus sinensis. Soil Sci. 1986, 141, 84–87. [Google Scholar] [CrossRef]
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Chebykina, E.; Abakumov, E. Characteristics of humic acids isolated from burned and unburned topsoils in sub-boreal Scotch pine forests by 13C-NMR spectroscopy. One Ecosyst. 2022, 7, e82720. [Google Scholar] [CrossRef]
- Harvey, G.R.; Boran, D.A.; Chesal, L.A.; Tokar, J.M. The structure of marine fulvic and humic acids. Mar. Chem. 1983, 12, 119–132. [Google Scholar] [CrossRef]
- Harvey, G.R.; Boran, D.A. Geochemistry of humic substances in seawater. In Humic Substances in Soil, Sediment and Water; Aiken, R.G., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; Wiley-Interscience: New York, NY, USA, 1985; Chapter 9; pp. 233–247. [Google Scholar]
- Huc, C.; Durand, B.; Jacquin, F. Caractérisation des acides humiques de sédiments marins récents et comparaison avec leurs homologues terrestres. Bull. ENSAIA 1974, 16, 59–75. [Google Scholar]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Humin: Its Composition and Importance in Soil Organic Matter. Adv. Agron. 2017, 143, 47–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almendros, G.; González-Pérez, J.A. Soil Organic Carbon Sequestration Mechanisms and the Chemical Nature of Soil Organic Matter—A Review. Sustainability 2025, 17, 6689. https://doi.org/10.3390/su17156689
Almendros G, González-Pérez JA. Soil Organic Carbon Sequestration Mechanisms and the Chemical Nature of Soil Organic Matter—A Review. Sustainability. 2025; 17(15):6689. https://doi.org/10.3390/su17156689
Chicago/Turabian StyleAlmendros, Gonzalo, and José A. González-Pérez. 2025. "Soil Organic Carbon Sequestration Mechanisms and the Chemical Nature of Soil Organic Matter—A Review" Sustainability 17, no. 15: 6689. https://doi.org/10.3390/su17156689
APA StyleAlmendros, G., & González-Pérez, J. A. (2025). Soil Organic Carbon Sequestration Mechanisms and the Chemical Nature of Soil Organic Matter—A Review. Sustainability, 17(15), 6689. https://doi.org/10.3390/su17156689






