From Waste to Value: Investigating Mushroom Stems from Pleurotus ostreatus Grown on Mealworm Frass as a Nutritional Source for Aquaculture Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Frass
2.2. Mushroom Cultivation
2.3. Nutritional Analysis
2.3.1. Protein Quantification
2.3.2. Lipid Content
2.3.3. Glucan Determination
2.3.4. Chitin Quantification
2.4. Fatty Acid Profiling and Quantification by GC-FID
2.5. Amino Acid Profiling and Quantification by UPLC-DAD-MS
2.6. Heavy Metal Analysis
2.7. Preparation of Crude Extracts for Antimicrobial Analysis
2.8. Antimicrobial ASSAY
2.9. Statistical Analysis
3. Results
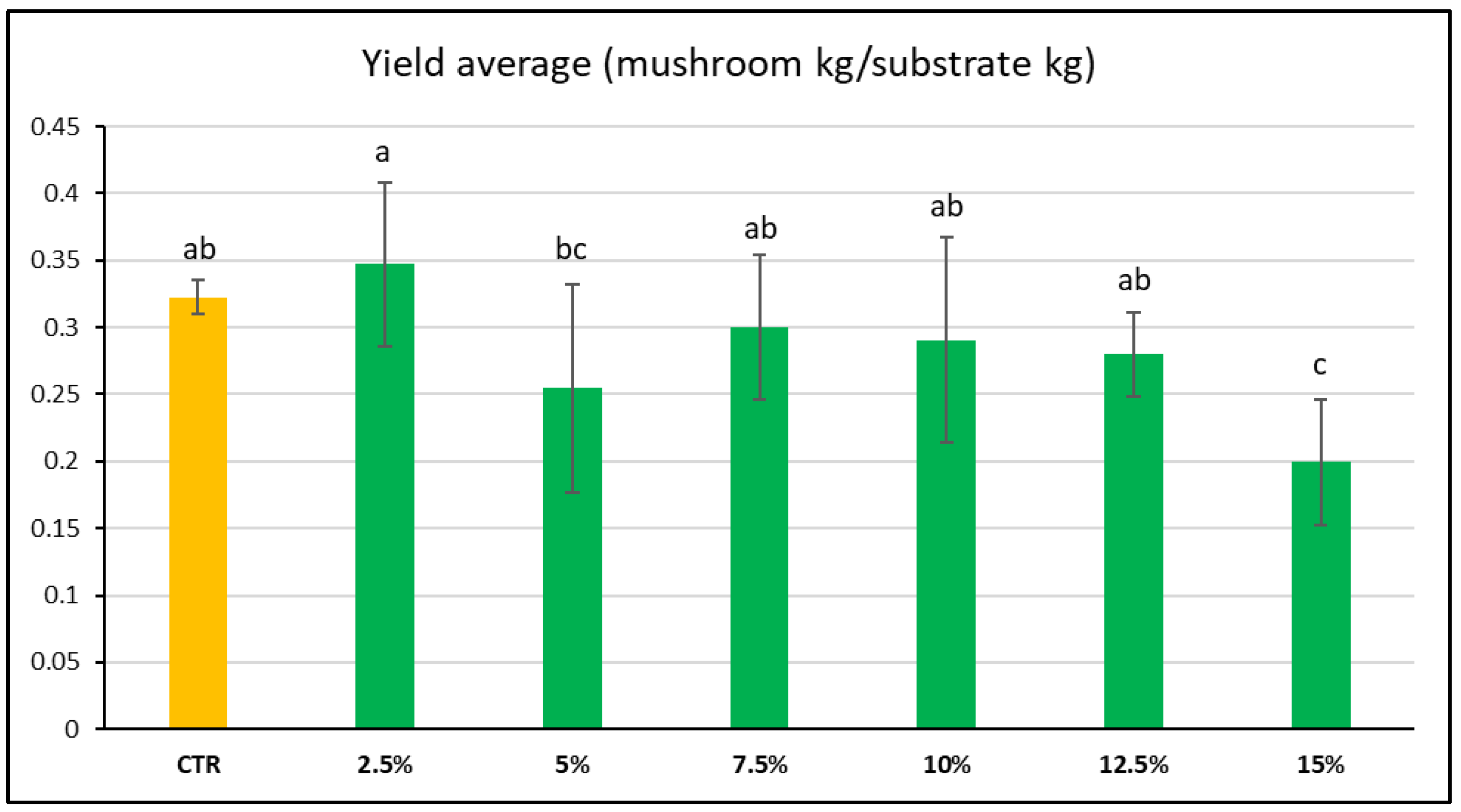
3.1. Mushroom Yield Due to Substrate Variation
3.2. Nutritional Composition of Mushroom Stems and Fruiting Body
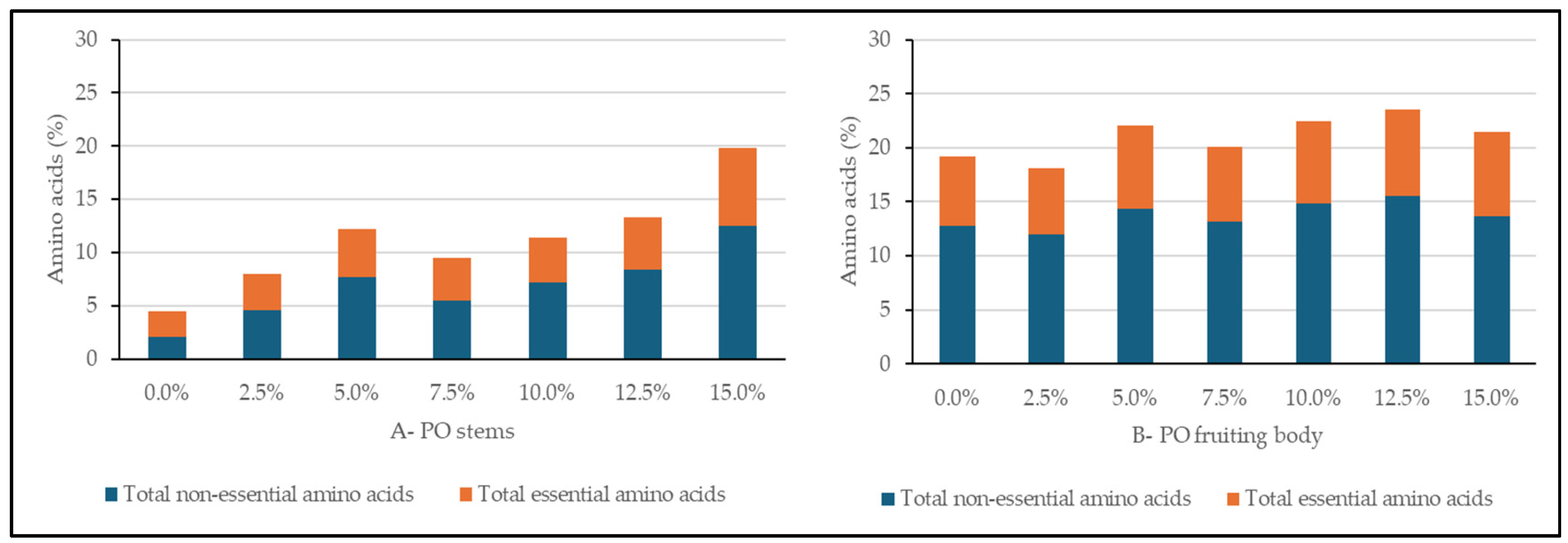
3.3. Amino Acid Composition
3.4. Fatty Acid Composition
3.5. Heavy Metal Content Analysis
3.6. Analysis of Antimicrobial Activity
4. Discussion
4.1. Yield, Nutritional and Microbiological Benefits, and Potential of Mushroom Stems
4.1.1. Yield Analysis
4.1.2. Nutritional Potential
4.1.3. Safety Analysis
4.1.4. Antimicrobial Potential
4.2. SWOT Analysis: Valorization of Mushroom Stems Cultivated Using Insect Frass for Aquaculture
4.2.1. Strengths
- Enhance nutritional values:
- Inhibitory activity and immunostimulant potential:
- Use of waste products (frass and mushroom stems):
- Cost-effectiveness:
- Food vs. feed competition:
4.2.2. Weaknesses
- Nutritional variability due to substrate differences
- Market hesitation about unconventional ingredients
4.2.3. Opportunities
- Growing demand for sustainable aquaculture solutions
- Consumers preference for sustainability in aquaculture and support from governments and eco-friendly policies
4.2.4. Threats
- Regulatory barriers and strict feed safety standards
- Strong competition from established feed ingredients
- Environmental risks and economic viability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strain | Culture Medium | Incubation Time (h) | Incubation Temperature (°C) |
|---|---|---|---|
| Edwardsiella tarda DSM 30052 | Tryptic soy | 24 | 28 |
| Aeromonas hydrophila DSM 3018 | Tryptic soy | 24 | 28 |
| Pseudomonas anguilliseptica DSM 12111 | Tryptic soy | 48–72 | 25 |
| Yersinia ruckeri ATCC 29473 | Tryptic soy | 24–48 | 28 |
| Listonella (Vibrio) anguillarum ATCC 19264 | Tryptic soy | 48 | 28 |
| Tenacibaculum maritimum ATCC 43397 | Marine agar | 24–48 | 25 |
| Lactococcus garvieae DSM 20684 | Tryptic soy yeast | 24–48 | 28 |
| Escherichia coli ATCC 25922 | Mueller–Hinton | 24 | 37 |
| Staphylococcus aureus ATCC 29213 | Mueller–Hinton | 24 | 37 |
| Salmonella enterica ATCC 25241 | Mueller–Hinton | 24 | 37 |
| Candida albicans ATCC 10231 | Sabouraud dextrose | 24 | 37 |
Appendix B. Amino Acid Content in P. ostreatus Stems and Fruiting Body
| (a) PO Stems | 0% | 2.5% | 5% | 7.5% | 10% | 12.5% | 15% |
|---|---|---|---|---|---|---|---|
| Alanine | 0.46 | 0.68 | 0.93 | 0.82 | 0.84 | 1.03 | 1.52 |
| Arginine | 0.34 | 0.54 | 0.79 | 0.72 | 0.73 | 0.88 | 1.57 |
| Aspartic acid | LQ | LQ | 1.37 | LQ | 1.33 | 1.51 | 2.22 |
| Cystine | LQ | 0.11 | 0.16 | 0.12 | 0.11 | 0.11 | 0.21 |
| Glutamic acid | LQ | 1.51 | 2.04 | 1.89 | 1.98 | 2.22 | 3.10 |
| Glycine | 0.41 | 0.56 | 0.72 | 0.63 | 0.65 | 0.80 | 1.15 |
| Histidine | 0.16 | 0.21 | 0.29 | 0.27 | 0.30 | 0.34 | 0.49 |
| Hydroxyproline | LQ | LQ | LQ | LQ | LQ | LQ | LQ |
| IsoLeucine | 0.29 | 0.43 | 0.55 | 0.47 | 0.50 | 0.61 | 0.92 |
| Leucine | 0.44 | 0.66 | 0.88 | 0.75 | 0.78 | 0.98 | 1.48 |
| Lysine | 0.37 | 0.51 | 0.68 | 0.63 | 0.67 | 0.82 | 1.20 |
| Methionine | 0.08 | 0.11 | 0.17 | 0.13 | 0.14 | 0.17 | 0.31 |
| Ornithine | 0 | 0 | 0.08 | LQ | 0.16 | 0.15 | 0.17 |
| Phenylalanine | 0.36 | 0.48 | 0.60 | 0.53 | 0.56 | 0.65 | 0.92 |
| Proline | 0.36 | 0.49 | 0.60 | 0.54 | 0.56 | 0.66 | 0.94 |
| Serine | 0.31 | 0.45 | 0.63 | 0.53 | 0.57 | 0.68 | 1.00 |
| Threonine | 0.36 | 0.50 | 0.62 | 0.55 | 0.58 | 0.66 | 0.97 |
| Tryptophan | LQ | LQ | LQ | LQ | LQ | LQ | LQ |
| Tyrosine | 0.19 | 0.26 | 0.36 | 0.29 | 0.30 | 0.32 | 0.60 |
| Valine | 0.39 | 0.54 | 0.68 | 0.61 | 0.63 | 0.76 | 1.08 |
| Total non-essential amino acids | 2.07 | 4.59 | 7.68 | 5.53 | 7.24 | 8.36 | 12.49 |
| Total essential amino acids | 2.44 | 3.44 | 4.47 | 3.94 | 4.14 | 4.98 | 7.37 |
| Total | 4.51 | 8.04 | 12.16 | 9.48 | 11.38 | 13.34 | 19.86 |
| (b) PO Fruiting Body | 0% | 2.5% | 5% | 7.5% | 10% | 12.5% | 15% |
|---|---|---|---|---|---|---|---|
| Alanine | 1.23 | 1.19 | 1.40 | 1.20 | 1.36 | 1.46 | 1.43 |
| Arginine | 1.20 | 1.49 | 1.81 | 1.75 | 1.94 | 2.14 | 1.71 |
| Aspartic acid | 1.93 | 1.85 | 2.19 | 1.84 | 2.17 | 2.39 | 2.22 |
| Cystine | 0.21 | 0.21 | 0.27 | 0.25 | 0.25 | 0.24 | 0.24 |
| Glutamic acid | 4.90 | 4.06 | 4.58 | 4.35 | 4.99 | 4.71 | 3.84 |
| Glycine | 1.01 | 0.97 | 1.08 | 0.97 | 1.07 | 1.20 | 1.13 |
| Histidine | 0.46 | 0.44 | 0.57 | 0.53 | 0.64 | 0.64 | 0.59 |
| Hydroxyproline | LQ | LQ | LQ | LQ | LQ | LQ | LQ |
| IsoLeucine | 0.77 | 0.74 | 0.95 | 0.80 | 0.87 | 0.91 | 0.90 |
| Leucine | 1.27 | 1.19 | 1.37 | 1.15 | 1.34 | 1.45 | 1.42 |
| Lysine | 1.03 | 1.03 | 1.32 | 1.21 | 1.32 | 1.45 | 1.34 |
| Methionine | 0.27 | 0.26 | 0.36 | 0.34 | 0.35 | 0.38 | 0.37 |
| Ornithine | 0.00 | 0.00 | 0.23 | 0.23 | 0.28 | 0.31 | 0.22 |
| Phenylalanine | 0.80 | 0.77 | 0.97 | 0.92 | 0.95 | 0.99 | 0.99 |
| Proline | 0.78 | 0.76 | 0.98 | 0.88 | 0.96 | 1.03 | 1.01 |
| Serine | 0.94 | 0.89 | 1.16 | 1.04 | 1.14 | 1.20 | 1.11 |
| Threonine | 0.88 | 0.82 | 1.03 | 0.96 | 1.04 | 1.09 | 1.02 |
| Tryptophan | LQ | LQ | LQ | LQ | LQ | LQ | LQ |
| Tyrosine | 0.55 | 0.52 | 0.66 | 0.65 | 0.70 | 0.81 | 0.77 |
| Valine | 0.91 | 0.88 | 1.11 | 1.03 | 1.10 | 1.17 | 1.16 |
| Total non-essential amino acids | 12.74 | 11.95 | 14.36 | 13.16 | 14.87 | 15.50 | 13.69 |
| Total essential amino acids | 6.41 | 6.13 | 7.67 | 6.93 | 7.63 | 8.08 | 7.78 |
| Total | 19.14 | 18.08 | 22.02 | 20.10 | 22.50 | 23.58 | 21.47 |
Appendix C. Fatty Acid Profile in PO Stems and Fruiting Body
| (a) PO Stems | 0.0% | 2.5% | 5.0% | 7.5% | 10.0% | 12.5% | 15.0% |
|---|---|---|---|---|---|---|---|
| Myristic acid | 0.00 | 0.00 | 0.00 | 0.12 | 0.11 | 0.11 | 0.14 |
| Caproic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pentadecanoic acid | 1.47 | 1.53 | 1.45 | 1.93 | 1.55 | 1.44 | 1.47 |
| Palmitic acid | 7.16 | 7.72 | 7.82 | 0.93 | 8.19 | 8.83 | 10.16 |
| Palmitoleic acid | 0.00 | 0.00 | 0.00 | 0.11 | 0.07 | 0.09 | 0.25 |
| Heptadecanoic acid | 0.00 | 0.11 | 0.10 | 0.15 | 0.13 | 0.13 | 0.12 |
| 10-heptadecenoic acid | 0.35 | 0.30 | 0.19 | 0.28 | 0.25 | 0.20 | 0.13 |
| Stearic acid | 0.94 | 0.72 | 0.70 | 0.84 | 0.69 | 0.71 | 0.83 |
| Elaidic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oleic acid | 4.29 | 3.76 | 3.96 | 4.66 | 3.89 | 4.30 | 6.76 |
| Linolelaidic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 |
| Linoleic acid | 37.81 | 42.45 | 45.10 | 53.22 | 46.17 | 49.32 | 54.06 |
| Arachidic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| g-Linolenic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 |
| Alpha-linolenic acid (ALA) | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | 0.00 | 0.07 |
| 11-Eicosenic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 |
| Eicosadienoic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.06 |
| Behenic acid | 0.00 | 0.00 | 0.08 | 0.09 | 0.09 | 0.08 | 0.09 |
| Gamma-Eicosatrienoic acid (DGLA) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Erucic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tricosanoic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lignoceric acid | 0.12 | 0.13 | 0.14 | 0.21 | 0.16 | 0.16 | 0.20 |
| Nervonic acid | 0.12 | 0.16 | 0.20 | 0.23 | 0.18 | 0.20 | 0.38 |
| Docosahexaenoic acid (DHA) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| (b) PO Fruiting Body | 0.0% | 2.5% | 5.0% | 7.5% | 10.0% | 12.5% | 15.0% |
|---|---|---|---|---|---|---|---|
| Myristic acid | 0.08 | 0.09 | 0.10 | 0.10 | 0.09 | 0.10 | 0.10 |
| Caproic acid | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pentadecanoic acid | 1.93 | 1.73 | 1.63 | 1.81 | 1.83 | 1.58 | 1.26 |
| Palmitic acid | 8.57 | 8.80 | 8.71 | 9.14 | 8.65 | 8.82 | 8.96 |
| Palmitoleic acid | 0.11 | 0.15 | 0.17 | 0.17 | 0.12 | 0.15 | 0.16 |
| Heptadecanoic acid | 0.10 | 0.09 | 0.08 | 0.10 | 0.11 | 0.10 | 0.09 |
| 10-heptadecenoic acid | 0.16 | 0.15 | 0.11 | 0.10 | 0.10 | 0.10 | 0.09 |
| Stearic acid | 1.16 | 1.06 | 0.95 | 0.95 | 0.84 | 0.78 | 0.92 |
| Elaidic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oleic acid | 9.38 | 9.16 | 8.33 | 7.94 | 6.59 | 6.95 | 11.63 |
| Linolelaidic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Linoleic acid | 47.78 | 48.49 | 50.86 | 52.34 | 52.59 | 53.09 | 52.12 |
| Arachidic acid | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 |
| g-Linolenic acid | 0.09 | 0.10 | 0.09 | 0.10 | 0.10 | 0.10 | 0.09 |
| Alpha-linolenic acid (ALA) | 0.26 | 0.04 | 0.23 | 0.48 | 0.06 | 0.10 | 0.55 |
| 11-Eicosenic acid | 0.00 | 0.00 | 0.05 | 0.07 | 0.54 | 0.00 | 0.07 |
| Eicosadienoic acid | 0.00 | 0.04 | 0.19 | 0.00 | 0.06 | 0.12 | 0.08 |
| Behenic acid | 0.23 | 0.18 | 0.10 | 0.17 | 0.11 | 0.09 | 0.17 |
| Gamma-Eicosatrienoic acid (DGLA) | 0.17 | 0.14 | 0.09 | 0.23 | 0.33 | 0.16 | 0.00 |
| Erucic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| Tricosanoic acid | 0.06 | 0.00 | 0.00 | 0.04 | 0.03 | 0.03 | 0.00 |
| Lignoceric acid | 0.58 | 0.51 | 0.32 | 0.39 | 0.38 | 0.32 | 0.25 |
| Nervonic acid | 0.33 | 0.29 | 0.27 | 0.26 | 0.23 | 0.24 | 0.24 |
| Docosahexaenoic acid (DHA) | 0.00 | 1.94 | 0.00 | 0.00 | 1.80 | 0.00 | 0.00 |
Appendix D. Trace Elements Content in PO Stems and Fruiting Body
| Trace Metals (mg/kg DM%) | Stems 0% | Stems 2.5% | Stems 5% | Stems 7.5% | Stems 10% | Stems 12.5% | Stems 15% |
|---|---|---|---|---|---|---|---|
| Al ** | 21.3 ± 0 | <20 | <20 | <20 | <20 | <20 | <20 |
| As ** | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Ca ** | 159.5 ± 6.5 | 122 ± 9 | 128.5 ± 8.5 | 107.9 ± 25.1 | 81.65 ± 8.1 | 99.4 ± 16.6 | 107.8 ± 9.2 |
| Cd ** | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Co ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Cr ** | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cu ** | <10 | <10 | <10 | <10 | <10 | 12.2 ± 1.9 | 13.6 ± 0.15 |
| Fe ** | 72.85 ± 4.15 | 67.5 ± 4.1 | 74.6 ± 0.8 | 66.65 ± 0.15 | 64.7 ± 00 | 62.85 ± 3.95 | 61.5 ± 1 |
| Hg ** | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| K * | 31,250 ± 550 | 32,150 ± 450 | 33,050 ± 250 | 30,700 ± 200 | 30,850 ± 350 | 29,750 ± 50 | 27,600 ± 400 |
| Mg * | 1525 ± 5 | 1670 ± 20 | 1650 ± 10 | 1515 ± 15 | 1615 ± 5 | 1445 ± 25 | 1340 ± 10 |
| Mn * | 9.7 ± 0.1 | 11 ± 0.2 | 11.8 ± 0.1 | 11.1 ± 00 | 12.75 ± 0.15 | 12.8 ± 1.2 | 11.55 ± 0.05 |
| Mo ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Ni ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| P * | 6580 ± 30 | 9845 ± 155 | 11,300 ± 200 | 10,650 ± 50 | 12,000 ± 100 | 11,300 ± 100 | 10,600 ± 200 |
| Pb ** | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| S * | 2550 ± 20 | 2535 ± 75 | 2685 ± 15 | 2620 ± 20 | 2910 ± 10 | 2850 ± 10 | 3025 ± 55 |
| Se ** | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Si * | 63.15 ± 0.75 | 36.95 ± 14.15 | 25.6 ± 7.3 | 21.65 ± 2.25 | 38.35 ± 0.9 | 27.8 ± 6.3 | 44 ± 6.3 |
| Sn ** | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| V ** | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Zn ** | 84.2 ± 2.4 | 82.15 ± 2.35 | 78.7 ± 0.4 | 73.7 ± 1 | 82.65 ± 0.15 | 95.45 ± 11.55 | 74.3 ± 1.9 |
| Trace Metals (mg/kg DM%) | Stems 0% | Stems 2.5% | Stems 5% | Stems 7.5% | Stems 10% | Stems 12.5% | Stems 15% |
|---|---|---|---|---|---|---|---|
| Al * | 40.45 ± 7.05 | 43 ± 9.8 | 60.9 ± 4 | 45.75 ± 3.55 | 32.7 ± 1 | 26.8 ± 3.9 | <20 |
| As ** | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Ca * | 410.5 ± 21.5 | 616 ± 60 | 777.5 ± 55.5 | 536.5 ± 2.5 | 325 ± 9 | 253 ± 6 | 248.5 ± 48.5 |
| Cd ** | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Co ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Cr ** | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cu ** | <10 | <10 | 14.9 ± 0.4 | 15.25 ± 0.65 | 16.1 ± 0.5 | 17.65 ± 1.75 | 15.75 ± 0.05 |
| Fe ** | <50 | <50 | 70.8 ± 2.4 | 49.85 ± 2.45 | <50 | <50 | 71.5 |
| Hg ** | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| K * | 20,800 ± 600 | 24,300 ± 0 | 25,550 ± 50 | 21,300 ± 300 | 20,800 ± 200 | 20,400 ± 100 | 23,400 ± 400 |
| Mg * | 1240 ± 60 | 1305 ± 15 | 1160 ± 10 | 1020 ± 10 | 965.5 ± 10.5 | 871.5 ± 9.5 | 1055 ± 25 |
| Mn * | 7.25 ± 0.32 | 9.83 ± 0.46 | 9.89 ± 0.41 | 7.59 ± 0.09 | 7.8 ± 0 | 6.92 ± 0.17 | 9.41 ± 0.35 |
| Mo ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Ni ** | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| P * | 1715 ± 5 | 4215 ± 5 | 5860 ± 40 | 5610 ± 10 | 5715 ± 125 | 5475 ± 115 | 7815 ± 35 |
| Pb ** | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| S * | 1510 ± 30 | 1655 ± 30 | 2040 ± 70 | 1530 ± 10 | 1480 ± 20 | 1430 ± 50 | 2200 ± 30 |
| Se ** | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Si * | 145 ± 19 | 157.5 ± 4.5 | 190 ± 9 | 155.5 ± 2.5 | 126 ± 5 | 123 ± 2 | 88.75 ± 2.95 |
| Sn ** | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| V ** | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Zn | <50 | <50 | 51 ± 0 | <50 | <50 | <50 | 62.8 |
References
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as Dietary Protein Source for Rainbow Trout: Body and Fillet Quality Traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Shurson, G.C.; Dierenfeld, E.S.; Dou, Z. Rules Are Meant to Be Broken—Rethinking the Regulations on the Use of Food Waste as Animal Feed. Resour. Conserv. Recycl. 2023, 199, 107273. [Google Scholar] [CrossRef]
- Zermeño-Cervantes, L.A.; González-Acosta, B.; Martínez-Díaz, S.F.; Cardona-Félix, C.S. Antibacterial Proteins and Peptides as Potential Treatment in Aquaculture: Current Status and Perspectives on Delivery. Rev. Aquac. 2020, 12, 1135–1156. [Google Scholar] [CrossRef]
- Saman, P.; Chaiongkarn, A.; Moonmangmee, S.; Sukcharoen, J.; Kuancha, C.; Fungsin, B. Evaluation of Prebiotic Property in Edible Mushrooms. Biol. Chem. Res. 2016, 3, 75–85. [Google Scholar]
- Radzki, W.; Ziaja-Sołtys, M.; Nowak, J.; Rzymowska, J.; Topolska, J.; Sławińska, A.; Michalak-Majewska, M.; Zalewska-Korona, M.; Kuczumow, A. Effect of Processing on the Content and Biological Activity of Polysaccharides from Pleurotus Ostreatus Mushroom. LWT-Food Sci. Technol. 2016, 66, 27–33. [Google Scholar] [CrossRef]
- Cohen, L.; Persky, Y.; Hadar, R. Biotechnological Applications and Potential of Wood-Degrading Mushrooms of the Genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Bilen, S.; Ünal, S.; Güvensoy, H. Effects of Oyster Mushroom (Pleurotus ostreatus) and Nettle (Urtica dioica) Methanolic Extracts on Immune Responses and Resistance to Aeromonas Hydrophila in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2016, 454, 90–94. [Google Scholar] [CrossRef]
- Katya, K.; Yun, Y.; Yun, H.; Lee, J.-Y.; Bai, S.C. Effects of Dietary Fermented By-Product of Mushroom, Pleurotus ostreatus, as an Additive on Growth, Serological Characteristics and Nonspecific Immune Responses in Juvenile Amur Catfish, Silurus asotus. Aquac. Res. 2016, 47, 1622–1630. [Google Scholar] [CrossRef]
- Baba, E.; Uluköy, G.; Öntaş, C. Effects of Feed Supplemented with Lentinula edodes Mushroom Extract on the Immune Response of Rainbow Trout, Oncorhynchus mykiss, and Disease Resistance against Lactococcus Garvieae. Aquaculture 2015, 448, 476–482. [Google Scholar] [CrossRef]
- Van Doan, H.; Doolgindachbaporn, S.; Suksri, A. Effects of Eryngii Mushroom (Pleurotus eryngii) and Lactobacillus plantarum on Growth Performance, Immunity and Disease Resistance of Pangasius Catfish (Pangasius bocourti, Sauvage 1880). Fish Physiol. Biochem. 2016, 42, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, K.; Zhong, W.; Liu, N.; Wu, X.; Li, W.; Zheng, L.; Yu, Z.; Zhang, J. Bioconversion-Composting of Golden Needle Mushroom (Flammulina velutipes) Root Waste by Black Soldier Fly (Hermetia illucens, Diptera: Stratiomyidae) Larvae, to Obtain Added-Value Biomass and Fertilizer. Waste Biomass Valorization 2019, 10, 265–273. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Rumbos, C.I. Frass and Furious: Unfolding the Potential of Insect Frass as Soil Fertilizer. Agrochemicals 2025, 4, 1. [Google Scholar] [CrossRef]
- Putri, M.J.; Arsal, A.F.; Pagarra, H.; Rachmawaty, R.; Asiz, A.A.; Ali, A.; Muis, A.; Junda, M.; Djawad, Y.A.; Jumadi, O. The Effect of The Addition of Mealworm Frass (Tenebrio molitor) and Molasses on the Increasing of the Proximate Value of White Oyster Mushroom (Pleurotus ostreatus). Bionature 2023, 24, 180. [Google Scholar] [CrossRef]
- Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Rubio-Moraga, Á. Food and Human Health Applications of Edible Mushroom By-Products. New Biotechnol. 2024, 81, 43–56. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- AOAC International. Maryland, USA Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Girão, M.; Ribeiro, I.; Ribeiro, T.; Azevedo, I.C.; Pereira, F.; Urbatzka, R.; Leão, P.N.; Carvalho, M.F. Actinobacteria Isolated From Laminaria Ochroleuca: A Source of New Bioactive Compounds. Front. Microbiol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendaño-Herrera, R.; Rodkhum, C. Tenacibaculosis Caused by Tenacibaculum maritimum: Updated Knowledge of This Marine Bacterial Fish Pathogen. Front. Cell. Infect. Microbiol. 2023, 12, 1068000. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Amorim, H.C.S.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect Frass Composition and Potential Use as an Organic Fertilizer in Circular Economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Muñoz-Seijas, N.; Fernandes, H.; Outeiriño, D.; Morán-Aguilar, M.G.; Domínguez, J.M.; Salgado, J.M. Potential Use of Frass from Edible Insect Tenebrio molitor for Proteases Production by Solid-State Fermentation. Food Bioprod. Process. 2024, 144, 146–155. [Google Scholar] [CrossRef]
- Habte-Tsion, H.-M. A Review on Fish Immuno-Nutritional Response to Indispensable Amino Acids in Relation to TOR, NF-κB and Nrf2 Signaling Pathways: Trends and Prospects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 241, 110389. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary Requirements of “Nutritionally Non-Essential Amino Acids” by Animals and Humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ferraz, P.L.; Bozza, T.; Nicastro, H.; Lancha, A.H. Distinct Effects of Leucine or a Mixture of the Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) Supplementation on Resistance to Fatigue, and Muscle and Liver-Glycogen Degradation, in Trained Rats. Nutrition 2013, 29, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Mata-Sotres, J.A.; Marques, V.H.; Barba, D.; Braga, A.; Araújo, B.; Viana, M.T.; Rombenso, A.N. Increasing Dietary SFA:MUFA Ratio with Low Levels of LC-PUFA Affected Lipid Metabolism, Tissue Fatty Acid Profile and Growth of Juvenile California Yellowtail (Seriola Dorsalis). Aquaculture 2021, 543, 737011. [Google Scholar] [CrossRef]
- Pogue, R.; Murphy, E.J.; Fehrenbach, G.W.; Rezoagli, E.; Rowan, N.J. Exploiting Immunomodulatory Properties of β-Glucans Derived from Natural Products for Improving Health and Sustainability in Aquaculture-Farmed Organisms: Concise Review of Existing Knowledge, Innovation and Future Opportunities. Curr. Opin. Environ. Sci. Health 2021, 21, 100248. [Google Scholar] [CrossRef]
- Huang, L.; Qin, D.; Tang, S.; Wang, P.; Gao, L. Trace Element Content and Health Risk Assessment of Main Aquaculture Products in Northeast China. Qual. Assur. Saf. Crops Foods 2025, 17, 86–105. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace Minerals in Fish Nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Öztürk, M.; Tel-Çayan, G.; Muhammad, A.; Terzioğlu, P.; Duru, M.E. Mushrooms. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 363–456. ISBN 978-0-444-63473-3. [Google Scholar]
- Onomu, A.J.; Okuthe, G.E. The Application of Fungi and Their Secondary Metabolites in Aquaculture. J. Fungi 2024, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, L.E.; Rio-Zaragoza, O.B.D.; Tintos-Gómez, A.; Hernández-Rodríguez, M.; Guzmán, L.; Zapata, D.B. El uso de hongos macroscópicos como inmunoestimulantes en peces teleósteos: Estado del arte al 2018 The use of macroscopic fungi as immunostimulants in fish: State of the art in 2018. Hidrobiológica 2018, 28, 209–217. [Google Scholar]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom Immunomodulators: Unique Molecules with Unlimited Applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Uluköy, G.; Baba, E.; Öntaş, C. Effect of Oyster Mushroom, Pleurotus ostreatus, Extract on Hemato-Immunological Parameters of Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2016, 47, 676–684. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; De Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.J. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Kiron, V.; Kulkarni, A.; Dahle, D.; Vasanth, G.; Lokesh, J.; Elvebo, O. Recognition of Purified Beta 1,3/1,6 Glucan and Molecular Signalling in the Intestine of Atlantic Salmon. Dev. Comp. Immunol. 2016, 56, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of Dietary β-Glucan on the Growth and Innate Immune Response of Juvenile Persian Sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Sahoo, P.K. Dietary β -1,3 Glucan Potentiates Innate Immunity and Disease Resistance of Asian Catfish, Clarias Batrachus (L.). J. Fish Dis. 2006, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, J.H.; Buchmann, K. Effects of Short- and Long-Term Glucan Feeding of Rainbow Trout (Salmonidae) on the Susceptibility to Ichthyophthirius Multifiliis Infections. Acta Ichthyol. Piscat. 2010, 40, 61–66. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Yin, F.; Rossi, W.; Hume, M.; Gatlin, D.M. β-1,3 Glucan Derived from Euglena Gracilis and AlgamuneTM Enhances Innate Immune Responses of Red Drum (Sciaenops ocellatus L.). Fish Shellfish Immunol. 2018, 77, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Diet Enriched with Mushroom Phellinus linteus Extract Enhances the Growth, Innate Immune Response, and Disease Resistance of Kelp Grouper, Epinephelus bruneus against Vibriosis. Fish Shellfish Immunol. 2011, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Effect of Inonotus Obliquus Enriched Diet on Hematology, Immune Response, and Disease Protection in Kelp Grouper, Epinephelus bruneus against Vibrio harveyi. Aquaculture 2012, 344–349, 48–53. [Google Scholar] [CrossRef]
- Sakai, M. Current Research Status of Fish Immunostimulants. Aquaculture 1999, 172, 63–92. [Google Scholar] [CrossRef]
- Mancini, L.; Valente, A.; Barbero Vignola, G.; Sanyé Mengual, E.; Sala, S. Social Footprint of European Food Production and Consumption. Sustain. Prod. Consum. 2023, 35, 287–299. [Google Scholar] [CrossRef]
- Pedersen, C.S. The UN Sustainable Development Goals (SDGs) Are a Great Gift to Business! Procedia CIRP 2018, 69, 21–24. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Fang, Z. Valorization of Mushroom By-products: A Review. J. Sci. Food Agric. 2022, 102, 5593–5605. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-Starch Polysaccharides and Their Role in Fish Nutrition—A Review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Vos, R.; Glauber, J.; Hebebrand, C.; Rice, B. Global Shocks to Fertilizer Markets: Impacts on Prices, Demand and Farm Profitability. Food Policy 2025, 133, 102790. [Google Scholar] [CrossRef]
- Fernández Sánchez, J.L.; Basurco, B.; Aguilera, C. Economic Assessment of Investment in Automatic Feeding Systems for Sea Bass Grow-out Farms of Different Sizes. J. World Aquac. Soc. 2023, 54, 625–634. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review: Feed Demand Landscape and Implications of Food-Not Feed Strategy for Food Security and Climate Change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Rulli, M.C.; Santini, M. Human Food vs. Animal Feed Debate. A Thorough Analysis of Environmental Footprints. Land Use Policy 2017, 67, 652–659. [Google Scholar] [CrossRef]
- Harvey, M. The Food-Energy-Climate Change Trilemma: Toward a Socio-Economic Analysis. Theory Cult. Soc. 2014, 31, 155–182. [Google Scholar] [CrossRef]
- Søndergaard, N.; Adrien Fernandes, J.F.; Potent, J.; Karl, K.; Furtado, M.; Baethgen, W. A Governance Framework to Manage the Food-Environment-Livelihood Trilemma of Alternative Proteins. One Earth 2023, 6, 843–853. [Google Scholar] [CrossRef]
- Breeman, G.; Dijkman, J.; Termeer, C. Enhancing Food Security through a Multi-Stakeholder Process: The Global Agenda for Sustainable Livestock. Food Secur. 2015, 7, 425–435. [Google Scholar] [CrossRef]
- Frankic, A.; Hershner, C. Sustainable Aquaculture: Developing the Promise of Aquaculture. Aquac. Int. 2003, 11, 517–530. [Google Scholar] [CrossRef]
- Fantatto, R.R.; Mota, J.; Ligeiro, C.; Vieira, I.; Guilgur, L.G.; Santos, M.; Murta, D. Exploring Sustainable Alternatives in Aquaculture Feeding: The Role of Insects. Aquac. Rep. 2024, 37, 102228. [Google Scholar] [CrossRef]




| Sampe | Humidity (%) | Ashes (%s.m.s.) | Nitrogen (%) | pH | Conductivity (mS/cm) | Organic Matter (%) | Lignin (%) | Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | 72.5 ± 2.1 | 17.0 ± 4.0 | 0.9 ± 1.1 | 8.4 ± 0.5 | 1.8 ± 0.6 | 83.0 ± 4.0 | 9.9 ± 2.4 | 42.2 ± 3.6 | 24.5 ± 4.4 |
| Insect frass | 6.9 ± 2.5 | 7.1 ± 0.1 | 3.4 ± 0.2 | 5.8 ± 0.2 | 6.2 ± 0.9 | 92.9 ± 0.1 | 7.4 ± 2.6 | 16.0 ± 3.1 | 41.6 ± 4.6 |
| Inclusion Percentage | Protein | β-Glucan (%) | Chitin (%) | |||
|---|---|---|---|---|---|---|
| PO. St * | PO. FB | PO. St * | PO. FB * | PO. St | PO. FB | |
| 0% | 7.8 ± 0.6 d | 24.7 ± 0.5 a | 37.9 ± 0.8 a | 26.4 ± 0.4 a | 5.5 ± 1.2 a | 6.1 ± 0.5 a |
| 2.5% | 10.5 ± 0.8 cd | 26.5 ± 0.7 a | 36.6 ± 0.8 ab | 19.2 ± 0.3 b | 6.2 ± 0.8 a | 6.5 ± 0.7 a |
| 5% | 15.0 ± 0.7 bc | 27.8 ± 1.7 a | 26.7 ± 0.1 cd | 14.9 ± 0.6 c | 6.6 ± 0.6 a | 6.9 ± 0.5 a |
| 7.5% | 14.5 ± 0.1 bc | 30.5 ± 1.0 a | 25.3 ± 3.1 cd | 16.1 ± 0.4 c | 6.6 ± 0.7 a | 6.8 ± 0.3 a |
| 10% | 15.2 ± 1.2 b | 30.6 ± 2.2 a | 29.8 ± 0.8 bc | 15.1 ± 0.4 c | 7.3 ± 0.6 a | 7.3 ± 0.6 a |
| 12.5% | 14.8 ± 1.0 bc | 29.1 ± 3.8 a | 24.4 ± 0.4 cd | 14.2 ± 0.2 c | 6.7 ± 0.5 a | 7.1 ± 0.5 a |
| 15% | 22.3 ± 0.7 a | 31.0 ± 2.1 a | 20.3 ± 0.9 d | 14.3 ± 0.8 c | 6.4 ± 0.6 a | 6.4 ± 0.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilali, S.; Stierlin, E.; Tello Martín, M.L.; Amaral, D.; Pérez-Clavijo, M.; Girão, M.; Carvalho, M.d.F.; Pérez Bonilla, A.M.; de Diego, S.; Ramírez, P.; et al. From Waste to Value: Investigating Mushroom Stems from Pleurotus ostreatus Grown on Mealworm Frass as a Nutritional Source for Aquaculture Feed. Sustainability 2025, 17, 6496. https://doi.org/10.3390/su17146496
Hilali S, Stierlin E, Tello Martín ML, Amaral D, Pérez-Clavijo M, Girão M, Carvalho MdF, Pérez Bonilla AM, de Diego S, Ramírez P, et al. From Waste to Value: Investigating Mushroom Stems from Pleurotus ostreatus Grown on Mealworm Frass as a Nutritional Source for Aquaculture Feed. Sustainability. 2025; 17(14):6496. https://doi.org/10.3390/su17146496
Chicago/Turabian StyleHilali, Soukaina, Emilie Stierlin, María Luisa Tello Martín, Diogo Amaral, Margarita Pérez-Clavijo, Mariana Girão, Maria de Fátima Carvalho, Andrea María Pérez Bonilla, Sabas de Diego, Pablo Ramírez, and et al. 2025. "From Waste to Value: Investigating Mushroom Stems from Pleurotus ostreatus Grown on Mealworm Frass as a Nutritional Source for Aquaculture Feed" Sustainability 17, no. 14: 6496. https://doi.org/10.3390/su17146496
APA StyleHilali, S., Stierlin, E., Tello Martín, M. L., Amaral, D., Pérez-Clavijo, M., Girão, M., Carvalho, M. d. F., Pérez Bonilla, A. M., de Diego, S., Ramírez, P., & Ozorio, R. (2025). From Waste to Value: Investigating Mushroom Stems from Pleurotus ostreatus Grown on Mealworm Frass as a Nutritional Source for Aquaculture Feed. Sustainability, 17(14), 6496. https://doi.org/10.3390/su17146496







