Evolutionary Characteristics of Sulphate Ions in Condensable Particulate Matter Following Ultra-Low Emissions from Coal-Fired Power Plants During Low Winter Temperatures
Abstract
1. Introduction
2. Methods
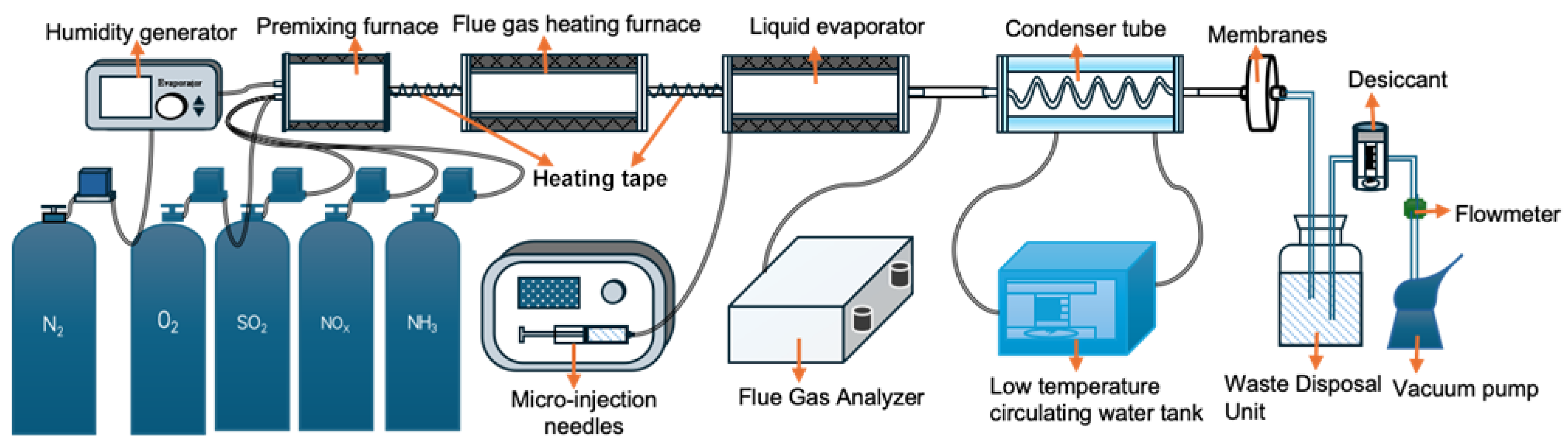
2.1. Experimental System
2.2. The Conditions Applied in the Experimen
2.3. Process of Analysis
3. Results and Analysis
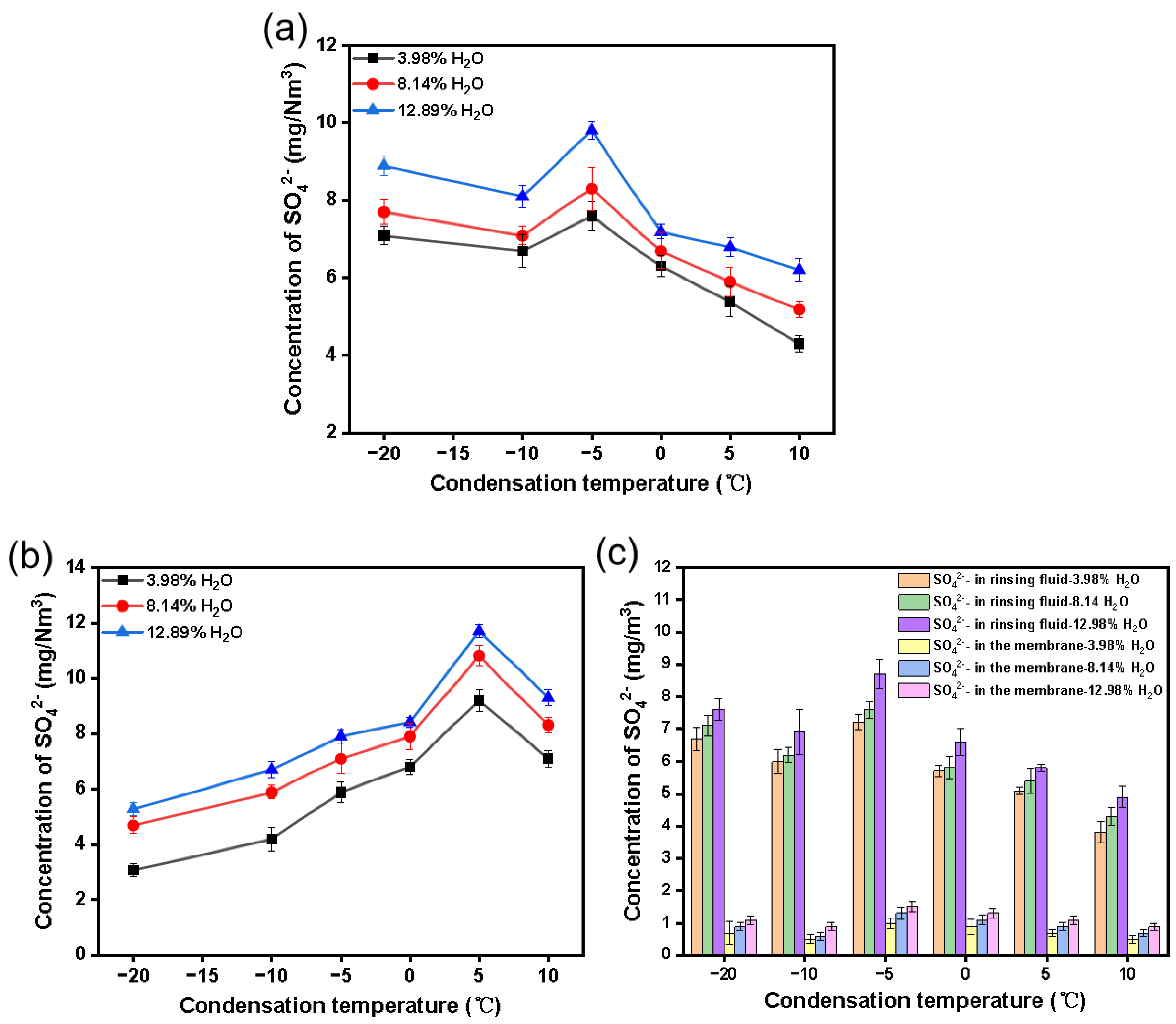
3.1. How Condensation Conditions Make an Impact
How Condensation Temperature Affects the Situation
3.2. Influence of H2O
3.3. The Impact of NH3 and NOX
3.3.1. Features and Traits of SO2
3.3.2. Features and Traits of SO3
3.3.3. Features and Traits of SO2 and SO3
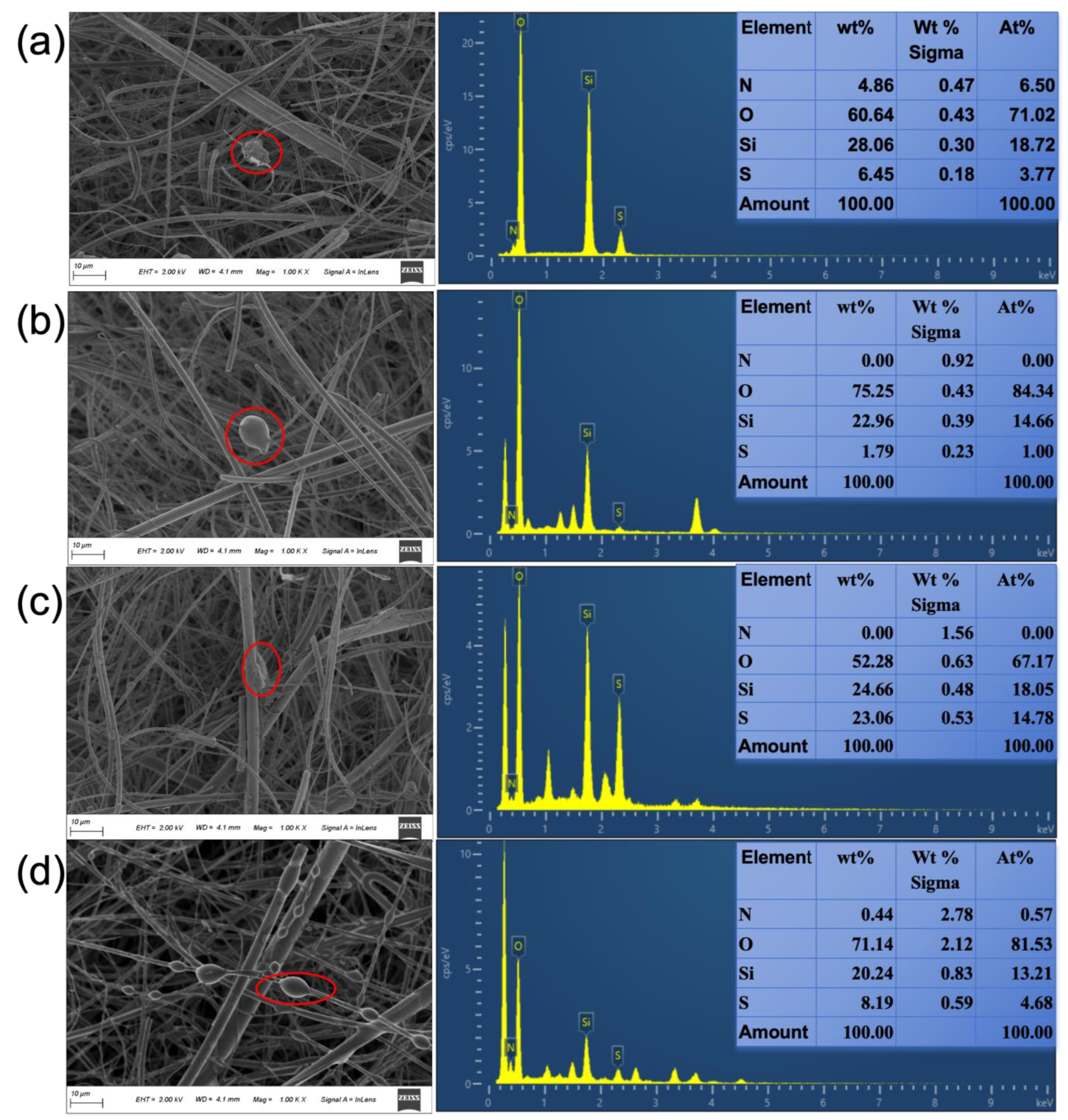
3.4. Morphological Characterization
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Zhao, M.; Hou, Y.; Li, S.; Lv, H.; Yang, L. Adsorption characteristics of organic pollutants in flue gas of coal-fired power plane by activated carbont. Electr. Power Technol. Environ. Prot. 2022, 38, 18–26. [Google Scholar]
- Fu, J.; Zhong, Z.; Xu, Y.; Xue, J.; Zhu, F.; Huang, W.; Xu, Y.; Lin, Z. Effect of chlorine addition on mercury speciation transformation and mercury—chlorine reaction mechanism in flue gast. Electr. Power Technol. Environ. Prot. 2022, 38, 27–35. [Google Scholar]
- Liu, Z.; Xie, Y.; Hu, B.; Wen, T.; Xin, J.; Li, X.; Wang, Y. Size-resolved aerosol water-soluble ions during the summer and winter seasons in Beijing: Formation mechanisms of secondary inorganic aerosols. Chemosphere 2017, 183, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lai, L.; Zhang, J. Research on the situation and path of green and low-carbon development in Jiangsu Provincet. Electr. Power Technol. Environ. Prot. 2023, 39, 603–610. [Google Scholar]
- Zhu, F.; Xu, J.; Wang, S. Processes and prospects of air pollutant control in coal-fired power plants in China. Electr. Power Technol. Environ. Prot. 2023, 39, 1–384. [Google Scholar]
- Morino, Y.; Chatani, S.; Tanabe, K.; Fujitani, Y.; Morikawa, T.; Takahashi, K.; Sato, K.; Sugata, S. Contributions of Condensable Particulate Matter to Atmospheric Organic Aerosol over Japan. Environ. Sci. Technol. 2018, 52, 8456–8466. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, Z.; Norris, P.; Romero, C.E.; Xu, H.; Pan, W.-P. Effect of Coordinated Air Pollution Control Devices in Coal-Fired Power Plants on Arsenic Emissions. Energy Fuels 2017, 31, 7309–7316. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Cui, L. Critical review of condensable particulate matter. Fuel 2018, 224, 801–813. [Google Scholar] [CrossRef]
- Corio, L.A.; Sherwell, J. In-Stack Condensible Particulate Matter Measurements and Issues. J. Air Waste Manag. Assoc. 2000, 50, 207–218. [Google Scholar] [CrossRef]
- Yang, H.H.; Lee, K.T.; Hsieh, Y.S.; Luo, S.W.; Li, M.S. Filterable and Condensable Fine Particulate Emissions from Stationary Sources. Aerosol Air Qual. Res. 2014, 14, 2010–2016. [Google Scholar] [CrossRef]
- Zheng, C.; Hong, Y.; Liu, S.; Yang, Z.; Chang, Q.; Zhang, Y.; Gao, X. Removal and Emission Characteristics of Condensable Particulate Matter in an Ultralow Emission Power Plant. Energy Fuels 2018, 32, 10586–10594. [Google Scholar] [CrossRef]
- Yang, H.H.; Lee, K.T.; Hsieh, Y.S.; Luo, S.W.; Huang, R.J. Emission Characteristics and Chemical Compositions of both Filterable and Condensable Fine Particulate from Steel Plants. Aerosol Air Qual. Res. 2015, 15, 1672–1680. [Google Scholar] [CrossRef]
- Wang, M.; Kong, W.; Marten, R.; He, X.-C.; Chen, D.; Pfeifer, J.; Heitto, A.; Kontkanen, J.; Dada, L.; Kürten, A.; et al. Rapid growth of new atmospheric particles by nitric acid and ammonia condensation. Nature 2020, 581, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, J.; Zhang, Y.; Li, Y.; Ma, Z.; Hao, J.; Jiang, J. Evaluating Airborne Condensable Particulate Matter Measurement Methods in Typical Stationary Sources in China. Environ. Sci. Technol. 2020, 54, 1363–1371. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, H.; Shen, J.; Gao, W.; Yang, Z.; Zhao, Z.; Wang, Y.; Zhang, H.; Gao, X. Evolution of Condensable Fine Particle Size Distribution in Simulated Flue Gas by External Regulation for Growth Enhancement. Environ. Sci. Technol. 2020, 54, 3840–3848. [Google Scholar] [CrossRef] [PubMed]
- Contini, D.; Cesari, D.; Conte, M.; Donateo, A. Application of PMF and CMB receptor models for the evaluation of the contribution of a large coal-fired power plant to PM10 concentrations. Sci. Total Environ. 2016, 560–561, 131–140. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Z.; Zhang, H.; Yang, Z. Influenced factors study and evaluation for SO2/SO3 conversion rate in SCR process. Fuel 2019, 245, 528–533. [Google Scholar] [CrossRef]
- Bin, H.; Lin, Z.; Yang, Y.; Fei, L.; Cai, L.; Linjun, Y. PM 2.5 and SO3 collaborative removal in electrostatic precipitator. Powder Technol. 2017, 318, 484–490. [Google Scholar] [CrossRef]
- Wu, B.; Bai, X.; Liu, W.; Lin, S.; Liu, S.; Luo, L.; Guo, Z.; Zhao, S.; Lv, Y.; Zhu, C.; et al. Non-Negligible Stack Emissions of Noncriteria Air Pollutants from Coal-Fired Power Plants in China: Condensable Particulate Matter and Sulfur Trioxide. Environ. Sci. Technol. 2020, 54, 6540–6550. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, H.; Jiang, W.; Chen, C.-W.; Pan, W.-P. Studies of the Fate of Sulfur Trioxide in Coal-Fired Utility Boilers Based on Modified Selected Condensation Methods. Environ. Sci. Technol. 2010, 44, 3429–3434. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, P.; Li, Q.; Jiang, Y.; Zheng, C.; Wang, Y.; Gao, X.; Lin, R. Comprehensive understanding of SO3 effects on synergies among air pollution control devices in ultra-low emission power plants burning high-sulfur coal. J. Clean. Prod. 2019, 239, 118096. [Google Scholar] [CrossRef]
- Fleig, D.; Alzueta, M.U.; Normann, F.; Abián, M.; Andersson, K.; Johnsson, F. Measurement and modeling of sulfur trioxide formation in a flow reactor under post-flame conditions. Combust. Flame 2013, 160, 1142–1151. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.; Sheng, Z.; Wu, T.; Hu, T. Study on the characteristics of sulfate ion in condensable particulate matter from ultra-low emission coal-fired power plants. J. Clean. Prod. 2023, 383, 135392. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Ge, S.; Liu, S.; Wu, C.; Wang, Y.; Chen, Y.; Lv, S.; Wang, F.; Meng, J.; et al. Rapid sulfate formation from synergetic oxidation of SO2 by O3 and NO2 under ammonia-rich conditions: Implications for the explosive growth of atmospheric PM2.5 during haze events in China. Sci. Total. Environ. 2021, 772, 144897. [Google Scholar] [CrossRef]
- Benner, W.; Ogorevc, B.; Novakov, T. Oxidation of SO2 in thin water films containing NH3. Atmos. Environ. Part A. Gen. Top. 1992, 26, 1713–1723. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef]
- Song, H.; Lu, K.; Ye, C.; Dong, H.; Li, S.; Chen, S.; Wu, Z.; Zheng, M.; Zeng, L.; Hu, M.; et al. A comprehensive observation-based multiphase chemical model analysis of sulfur dioxide oxidations in both summer and winter. Atmos. Chem. Phys. 2021, 21, 13713–13727. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, M.; Bertozzi, B.; Marie, G.; Rörup, B.; Schulze, B.; Bardakov, R.; He, X.C.; Shen, J.; Scholz, W.; et al. Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation. Nature 2022, 605, 483–489. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.J.; Yan, J.P.; Hong, G.X.; Yang, B. Improving the removal of fine particles by heterogeneous condensation during WFGD processes. Fuel Process. Technol. 2016, 145, 116–122. [Google Scholar] [CrossRef]
- Brachert, L.; Mertens, J.; Khakharia, P.; Schaber, K. The challenge of measuring sulfuric acid aerosols: Number concentration and size evaluation using a condensation particle counter (CPC) and an electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 67, 21–27. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Zhang, R. Formation Mechanism of Atmospheric Ammonium Bisulfate: Hydrogen-Bond-Promoted Nearly Barrierless Reactions of SO3 with NH3 and H2O. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2018, 19, 967–972. [Google Scholar] [CrossRef]
- Benson, D.R.; Yu, J.H.; Markovich, A.; Lee, S.H. Ternary homogeneous nucleation of H2SO4, NH3, and H2O under conditions relevant to the lower troposphere. Atmos. Chem. Phys. 2011, 11, 4755–4766. [Google Scholar] [CrossRef]
- Pitter, R.L.; Finnegan, W.G. Mechanism of single ice crystal growth in mixed clouds. Atmos. Res. 2010, 97, 438–445. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Liu, Y.; Yang, Z.; Qu, R.; Ye, D.; Liang, C.; Liu, S.; Gao, X. Formation, transformation, measurement, and control of SO3 in coal-fired power plants. Fuel 2019, 241, 327–346. [Google Scholar] [CrossRef]
- Liu, T.; Qin, H.; Yang, D.; Zhang, G. First Principles Study of Gas Molecules Adsorption on Monolayered β-SnSe. Coatings 2019, 9, 390. [Google Scholar] [CrossRef]
- Srivastava, R.; Miller, C.; Erickson, C.; Jambhekar, R. Emissions of Sulfur Trioxide from Coal-Fired Power Plants. J. Air Waste Manag. Assoc. 2004, 54, 750–762. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Clegg, S.; Davies, T. Observations of the preferential loss of major ions from melting snow and laboratory ice. Water Res. 1987, 21, 1279–1286. [Google Scholar] [CrossRef]
- Li, J.; Qi, Z.; Li, M.; Wu, D.; Zhou, C.; Lu, S.; Yan, J.; Li, X. Physical and Chemical Characteristics of Condensable Particulate Matter from an Ultralow-Emission Coal-Fired Power Plant. Energy Fuels 2017, 31, 1778–1785. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Li, J.; Sheng, Z.; He, Q.; Wu, K. Characteristics of condensable particulate matter before and after wet flue gas desulfurization and wet electrostatic precipitator from ultra-low emission coal-fired power plants in China. Fuel 2020, 278, 118206. [Google Scholar] [CrossRef]
- Liu, W.; Wu, B.; Bai, X.; Liu, S.; Liu, X.; Hao, Y.; Liang, W.; Lin, S.; Liu, H.; Luo, L.; et al. Migration and Emission Characteristics of Ammonia/Ammonium through Flue Gas Cleaning Devices in Coal-Fired Power Plants of China. Environ. Sci. Technol. 2020, 54, 390–399. [Google Scholar] [CrossRef]
- Xiang, B.; Tang, B.; Wu, Y.; Yang, H.; Zhang, M.; Lu, J. Predicting acid dew point with a semi-empirical model. Appl. Therm. Eng. 2016, 106, 992–1001. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Cui, L.; Yan, L.; Zhao, C.; Dong, Y. Cold condensing scrubbing method for fine particle reduction from saturated flue gas. Energy 2019, 171, 1193–1205. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Chen, Q.; Feng, Y.; Shang, Y.; Yang, X.; Gao, H.; Tian, C.; Li, J.; Zhang, G.; et al. Real-World Emission Factors of Gaseous and Particulate Pollutants from Marine Fishing Boats and Their Total Emissions in China. Environ. Sci. Technol. 2018, 52, 4910–4919. [Google Scholar] [CrossRef]
- Xue, J.; Yuan, Z.; Lau, A.K.; Yu, J.Z. Insights into factors affecting nitrate in PM2.5 in a polluted high NOx environment through hourly observations and size distribution measurements. J. Geophys. Res. 2014, 119, 4888–4902. [Google Scholar] [CrossRef]
- Kärcher, B.; Voigt, C. Formation of nitric acid/water ice particles in cirrus clouds. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Wang, A.; Song, Q.; Yao, Q. Thermophoretic capture of submicron particles by a droplet. Atmos. Environ. 2016, 147, 157–165. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Ma, Q.; Ma, J.; Liu, Y.; Liu, P.; Mu, Y. Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust. Phys. Chem. Chem. Phys. 2015, 18, 956–964. [Google Scholar] [CrossRef]
- Sparks, L.E.; Pilat, M.J. Effect of diffusiophoresis on particle collection by wet scrubbers. Atmos. Environ. 1970, 4, 651–660. [Google Scholar] [CrossRef]
- Kang, J.L.; Zhang, Y.; Fulk, S.; Rochelle, G.T. Modeling Amine Aerosol Growth in the Absorber and Water Wash. Energy Procedia 2017, 114, 959–976. [Google Scholar] [CrossRef]
- Chu, B.; Zhang, X.; Liu, Y.; He, H.; Sun, Y.; Jiang, J.; Li, J.; Hao, J. Synergetic formation of secondary inorganic and organic aerosol: Effect of SO2 and NH3 on particle formation and growth. Atmos. Chem. Phys. 2016, 16, 14219–14230. [Google Scholar] [CrossRef]
- Lehtipalo, K.; Rondo, L.; Kontkanen, J.; Schobesberger, S.; Jokinen, T.; Sarnela, N.; Kürten, A.; Ehrhart, S.; Franchin, A.; Nieminen, T.; et al. The effect of acid–base clustering and ions on the growth of atmospheric nano-particles. Nat. Commun. 2016, 7, 11594. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Praplan, A.P.; Sarnela, N.; Dommen, J.; Kurten, A.; Ortega, I.K.; Schobesberger, S.; Junninen, H.; Simon, M.; Tröst, J.; et al. Insight into Acid–Base Nucleation Experiments by Comparison of the Chemical Composition of Positive, Negative, and Neutral Clusters. Environ. Sci. Technol. 2014, 48, 13675–13684. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y.; Zhang, Q.; Wang, W.; Wang, Q. Mechanistic understanding of rapid H2SO4-HNO3-NH3 nucleation in the upper troposphere. Sci. Total. Environ. 2023, 883, 163477. [Google Scholar] [CrossRef] [PubMed]
- Sinanis, S.; Wix, A.; Ana, L.; Schaber, K. Characterization of sulphuric acid and ammonium sulphate aerosols in wet flue gas cleaning processes. Chem. Eng. Process. Process. Intensif. 2008, 47, 22–30. [Google Scholar] [CrossRef]


| Parameters | SO3 (mg/Nm3) | SO2 (mg/Nm3) | NH3 (mg/Nm3) | NOX (mg/Nm3) | Total Flow (L/Min) | Ratio of O2 (%) |
|---|---|---|---|---|---|---|
| Flow value | 12 | 100 | 12 | 110 | 1.95–2.05 | 6–9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Lu, H.; Zhou, K.; Zhuang, K.; Zhang, Y.; Zhang, C.; Yang, L.; Sheng, Z. Evolutionary Characteristics of Sulphate Ions in Condensable Particulate Matter Following Ultra-Low Emissions from Coal-Fired Power Plants During Low Winter Temperatures. Sustainability 2025, 17, 6342. https://doi.org/10.3390/su17146342
Xu Y, Lu H, Zhou K, Zhuang K, Zhang Y, Zhang C, Yang L, Sheng Z. Evolutionary Characteristics of Sulphate Ions in Condensable Particulate Matter Following Ultra-Low Emissions from Coal-Fired Power Plants During Low Winter Temperatures. Sustainability. 2025; 17(14):6342. https://doi.org/10.3390/su17146342
Chicago/Turabian StyleXu, Yun, Haixiang Lu, Kai Zhou, Ke Zhuang, Yaoyu Zhang, Chunlei Zhang, Liu Yang, and Zhongyi Sheng. 2025. "Evolutionary Characteristics of Sulphate Ions in Condensable Particulate Matter Following Ultra-Low Emissions from Coal-Fired Power Plants During Low Winter Temperatures" Sustainability 17, no. 14: 6342. https://doi.org/10.3390/su17146342
APA StyleXu, Y., Lu, H., Zhou, K., Zhuang, K., Zhang, Y., Zhang, C., Yang, L., & Sheng, Z. (2025). Evolutionary Characteristics of Sulphate Ions in Condensable Particulate Matter Following Ultra-Low Emissions from Coal-Fired Power Plants During Low Winter Temperatures. Sustainability, 17(14), 6342. https://doi.org/10.3390/su17146342






