1. Introduction
With the rapid pace of global industrialization, the environmental challenges associated with industrial waste have become increasingly pressing. Developing effective and sustainable waste management solutions is, therefore, a critical priority in environmental protection. Titanium dioxide (TiO
2), the third most produced inorganic salt globally, is widely used in coatings, plastics, inks, and other industries. As the world’s largest producer, China’s TiO
2 production capacity is projected to exceed 5 million tons by 2024, accounting for 53% of global output [
1]. The sulfuric acid process for producing TiO
2 generates large volumes of acidic wastewater, which, after neutralization, results in a byproduct known as titanium gypsum. Approximately 6–12 tons of titanium gypsum are generated per ton of TiO
2 produced [
2,
3]. Although primarily composed of calcium sulfate dihydrate (CaSO
4·2H
2O), it also contains potentially harmful elements such as arsenic and manganese. Improper disposal can lead to soil and groundwater contamination, posing risks to ecosystems and human health [
3,
4]. In recent years, the open-air stockpiling of millions of tons of titanium gypsum in the Yangtze River Basin has drawn widespread concern. Finding safe and sustainable ways to utilize titanium gypsum is therefore an urgent environmental challenge that is essential for promoting green and high-quality development in the TiO
2 industry.
The resourceful conversion of industrial byproduct gypsum—such as desulfurized gypsum, phosphogypsum, and titanogypsum—has become an important research focus in recent years. Yun Yi et al. [
5] found that titanium gypsum, despite its high salt content and mild pollution, shows potential for use in road green belts after appropriate modification, making it a viable resource for such applications. Li et al. [
6] demonstrated that properly treated industrial gypsum can fulfill compressive strength and performance requirements for road materials. Wang et al. [
7] investigated the hazardous components in gypsum and identified contaminants such as arsenic, manganese, barium, and zinc. Additionally, Wang et al. [
8] assessed the environmental risks of gypsum-based stabilizing materials and found that leached heavy metal concentrations met the Class III water quality standards specified in the Environmental Quality Standards for Surface Water. Wu et al. [
9,
10] enhanced the performance of phosphogypsum-based materials through optimized ball milling, meeting national standards and improving their applicability. Volodymyr Gunka et al. [
11] demonstrated that waste paper sludge ash and acid tar could replace traditional limestone powder in SMA after activation, enhancing water resistance and freeze–thaw performance.
Current risk assessments for solid waste resource utilization often rely on steady-state models based on traditional leaching experiments, which compare pollutant concentrations to groundwater quality standards but fail to capture the long-term unsteady-state release and complex migration processes of pollutants in applications such as road bases. This oversight can lead to underestimation of peak risk events, such as those exacerbated by heavy rainfall, and neglects regional meteorological variations, particularly precipitation differences, leading to incomplete risk evaluations. For instance, Luo et al. [
12] compared leaching concentrations of Zn, Pb, Cu, and Cr with regulatory limits without considering their long-term environmental behavior or accumulation effects; Li et al. [
13] assessed red mud-modified phosphogypsum self-leveling mortar’s feasibility without fully addressing the migration and transformation of contaminants like Cr and Cd; and Bai et al. [
14], while demonstrating that production process adjustments could reduce heavy metal leaching to meet regulatory standards in permeable concrete bricks made from industrial byproducts, emphasized the necessity of dynamic models to simulate long-term pollutant release and transformation for more accurate risk assessments.
To address this, this study used the Dutch standard NEN7371 method to characterize the source strength release characteristics of titanium gypsum utilized in road construction and compared the method to traditional leaching assessment methods. Then, an exponential decay model for source strength was integrated with Monte Carlo simulations and an instantaneous migration and diffusion model for pollutants to reveal the probability and temporal evolution characteristics of shallow groundwater contamination when titanium gypsum was used for road construction in the Yangtze River Basin, China. Regulatory thresholds for pollutants were derived and compared to existing limits in national standards to evaluate the rationality of current national standard values. This study provides a reference for risk assessment and the regionally differentiated management of titanium gypsum road applications.
2. Materials and Methods
2.1. Sample Collection and Analytical Detection
To assess its environmental impact, 100 titanogypsum samples were collected from a stockpile in the Yangtze River Basin using a 40 m × 40 m grid sampling method, in accordance with the requirements of the “Technical guidelines for risk assessment of soil contamination of land for construction” (HJ 25.3-2019) [
15] and “Technical specifications on identification for hazardous waste” (HJ 298-2019) [
16].
The Dutch standard NEN 7371 leaching method [
17,
18] is used to determine the maximum effective release of inorganic components from wastes and materials under extreme/adverse environmental conditions. This experimental method has been widely adopted by EU countries to assess the environmental safety of building materials. Based on the NEN 7371 protocol, a two-stage leaching experiment was conducted as follows: First, the pH of the leaching solution was stabilized between 6.5 and 7.5 for 3 h, and then the pH was maintained between 3.5 and 4.5 for another 3 h. The liquid-to-solid ratio was set at 50:1 (L/kg) for both stages. Equal volumes of the leachates obtained from each stage were thoroughly mixed before their concentrations were analyzed.
For comparative purposes, this study also used the “Solid Waste-Extraction Procedure for Leaching Toxicity Sulphuric Acid & Nitric Acid Method” (HJ/T 299-2007) [
19] to determine the heavy metal concentrations leached from the titanium gypsum samples.
The Pb, Ni, As, Cd, Zn, and Mn concentrations in the leachates were determined using the methods specified in “Water Quality-Determination of 65 Elements—Inductively Coupled Plasma-Mass Spectrometry” (HJ 700-2014) [
20] and “Water Quality—Determination of Mercury, arsenic, selenium, Bismuth and Antimonyatomic Fluorescence Spectrometry” (HJ 694-2014) [
21].
2.2. Characterization of Pollutant Release
Contaminants leach into the underlying soil and groundwater environment through rainfall infiltration when titanium gypsum is utilized in road construction. Pollutants within the solid waste progressively leach and are released as precipitation percolates through the ground. An exponential decay source model was used in this study to characterize the variations in contaminant release concentrations based on the source strength, as represented by Equations (1) and (2) [
22,
23]:
where
represents the release concentration of the contaminants, based on the source strength, at time
t (mg/L);
denotes the initial concentration of the contaminants in the leachate (mg/L);
α is the groundwater infiltration rate (mm/a);
d is the thickness of the road base layer (m);
f is the volume fraction of the titanium gypsum in the road base layer (dimensionless);
ρ is the density of the road base layer (kg/L);
is the leachate concentration of the contaminants obtained using the NEN 7371 method (mg/L); and
is the maximum effective release quantity for the contaminants (mg/kg).
2.3. Characterization of Pollutant Migration and Diffusion
This study characterized the most adverse exposure scenario where titanium gypsum sub-base material directly contacts the soil without any rain-shielding on top. Under the influence of rainfall, contaminants in the titanium gypsum sub-base material are leached out and released into the environment. They then migrate via diffusion and percolation into the saturated zone and spread through groundwater flow into monitoring wells, thereby impacting the environment and human health. The contaminant concentrations are diluted and reduced by diffusion and migration throughout the sub-base material release process. This natural attenuation process can be divided into two steps [
17,
23]: the first is the vertical leaching of heavy metals from the sub-base material into groundwater due to rainfall (the soil leaching factor, LF), and the second is the horizontal dilution and dispersion of contaminants in groundwater as it flows towards the monitoring wells (dilution attenuation coefficient for groundwater, DAF). Depending on the exposure pathways for characteristic pollutants in the sub-base material, the contaminant concentrations in the sub-base material, after dilution, attenuation, and becoming pollutants in groundwater, can be represented by Equation (3):
In the equation, represents the concentration of pollutants in groundwater at the observation well, in mg/L; represents the content of pollutants released from the pollution source, in mg/kg; LF is the soil leaching and dilution factor, in kg/L; and DAF is the groundwater dilution and attenuation coefficient, which is dimensionless.
2.3.1. Soil Leaching and Dilution Factor (LF)
To calculate the leaching and dilution factor (LF) for pollutants in road base materials, a typical heavy metal attenuation and diffusion model should be established that can simulate the actual use scenario of road base materials. Pollutants undergo adsorption–desorption, chemical reactions, as well as hydrodynamic migration and molecular diffusion in the unsaturated soil layer [
24]. However, in the long term, both adsorption and chemical reactions have certain capacities. For scenarios with high-concentration sources, such as the utilization of solid waste in road construction, from a conservative assessment perspective, adsorption and reactions can be neglected. Based on the leaching factor calculation model for pollutants migrating into groundwater specified in HJ 25.3, and considering various influencing factors such as soil density, permeability, pollutant partition coefficient, thickness of the mixing zone, and moisture content of the unsaturated zone, the dilution factor LF during the leaching process of metal pollutants from road base materials migrating and diffusing into groundwater is calculated using Equations (4)–(6) [
25]:
In the equations, LF represents the leaching factor for pollutants migrating from road base materials into groundwater, in kg/L; is the leaching factor for pollutants migrating from soil pore water into groundwater, which is dimensionless; is the partition coefficient of pollutants between soil and water, in cm3/g; is the Darcy velocity of groundwater, in cm/a; is the thickness of the groundwater mixing zone, in cm; I is the infiltration rate of water in the soil, in cm/a; W is the width of the road, in cm; is the partition coefficient of pollutants between the soil solid phase and water, in cm3/g; is the volumetric ratio of pore water in unsaturated soil, which is dimensionless; is the volumetric ratio of pore air in unsaturated soil, which is dimensionless; is the bulk density of soil, in kg/L; and H is the Henry’s constant for the pollutant, which is dimensionless.
2.3.2. Groundwater Dilution and Attenuation Factor (DAF)
The dilution and attenuation of pollutants in groundwater are controlled by processes such as the dilution effect of groundwater flow (i.e., convection) and the attenuation effect of the subsurface medium (i.e., degradation, dispersion, and retardation) [
26]. In homogeneous and isotropic soil–water systems, the transport process as well as the dilution and attenuation effects can be modeled using the one-dimensional advective–dispersive equation, as shown in Equation (7) [
27]:
In the equation, C represents the concentration of pollutants at a distance x and time t, measured in mg/L; x denotes the distance along the direction of groundwater flow, measured in meters (m); u is the groundwater velocity, measured in meters per second (m/s); n represents the effective porosity, which is a dimensionless quantity; R is the retardation factor; γ is the first-order decay rate, measured in seconds (s); and is the hydrodynamic longitudinal dispersion coefficient, measured in meters per second (m/s).
The analytical solution for a continuously injecting point source is given by Equation (8).
In the equation, DAF represents the groundwater dilution and attenuation factor, which is a dimensionless quantity; x denotes the distance along the direction of groundwater flow, measured in meters (m); u is the groundwater velocity, measured in meters per day (m/d); is the longitudinal dispersion coefficient, measured in square meters per day (m2/d); and erfc() is the complementary error function.
2.4. Risk Management Limit Values
The contaminants present in titanium gypsum need to be regulated if the risks associated with utilizing titanium gypsum in road construction are to be managed. If the Class III water quality limits stipulated in GB/T 14848-2017 [
28] are used as the acceptable exposure concentration, then the permissible regulatory limits can be inversely derived using the aforementioned equations. Equations (9) and (10) [
29] were applied to derive the regulatory limits for contaminants:
where
is the regulatory limit for the amount of contaminant
i released (mg/kg) and
is the limit value corresponding to contaminant
i as specified in the Class III water quality criteria in GB/T14848-2017 (mg/L).
The methods used to measure pollutant release are complex and both the “Identification Standards for Hazardous Wastes—Leaching Toxicity Identification” (GB5085.3-2007) [
30] and the “Standard for Pollution Control on the Storage and Landfill Site for General Industrial Solid Wastes” (GB18599-2020) [
31] use leaching concentration as their regulatory criterion. Therefore, Equation (10) was used to convert the regulatory limits for pollutant release into leaching concentration limits for control purposes:
where
is the leaching concentration regulatory limit for contaminant
i (mg/L);
is the regulatory limit for the amount of contaminant
i released (mg/kg);
is the leaching concentration (mg/L); and
is the maximum effective release amount (mg/kg).
2.5. Representation of Uncertainty and Risk
The rainfall and other meteorological parameters in the 11 provinces along the Yangtze River, including Qinghai (QH), Tibet (XZ), Sichuan (SC), Yunnan (YN), Chongqing (CQ), Hubei (HB), Hunan (HN), Jiangxi (JX), Anhui (AH), Jiangsu (JS), and Shanghai (SH), were mainly referenced from the China Statistical Yearbook 2022.
The groundwater-related parameters, such as the moisture content of the unsaturated zone, soil bulk density, thickness of the groundwater mixing zone, Darcy groundwater velocity, porosity, and soil permeability, were based on the recommended values in the “Technical Guidelines for Investigation of Soil Pollution Status at Construction Sites” (HJ 25.3-2019) [
15].
For the road-related parameters, the thickness of the road base layer was based on the domestic highway standards and ranged from 0.3 to 1.2 m, and the width of the roads (W) varied between 3.5 and 30 m. The horizontal distance from the road (as an exposure source) to the monitoring well was set at 100 m and followed the guideline in the “Highway Environmental Protection Design Code”, which recommends a minimum distance of 100 m between the central line of highways and water sources.
3. Results and Discussion
3.1. Release of Contaminants from Titanium Gypsum Under Experimentally Simulated Conditions
According to the “Technical guideline on pollution prevention and control for solid waste recycling” (HJ 1091-2020) [
32], the control of harmful substances in road base materials produced from solid waste follows the requirements specified in GB/T 30760 [
33]. GB/T 30760 specifies limits for eight heavy metals: Ni, As, Cd, Cu, Zn, Mn, Cr, and Pb. Leaching test results indicated that Pb, Ni, As, Cd, Zn, and Mn were detected at varying concentrations in the leachate of titanium gypsum. Therefore, these six heavy metals are selected as priority pollutants for further analysis and evaluation.
Contaminant concentrations at exposure points are influenced by both source strength and groundwater transport processes, with the Potential Hazard Index (PHI) serving as an indicator of the likelihood of exceeding environmental quality standards [
29]. The PHI is defined as the ratio of the measured concentration to the corresponding regulatory limit [
29,
34]. As shown in
Figure 1, for the six inorganic contaminants evaluated, Mn exhibited the highest PHI value of 14.8, while Cd had the lowest at 0.35, representing a 42-fold difference and indicating that Mn has a significantly greater potential to exceed regulatory thresholds after groundwater transport compared to Cd. The results showed that Zn and Cd had PHI values below 1, suggesting that their leaching concentrations were below the Class III groundwater quality standards, thus posing negligible risk to the groundwater environment when solid waste is used as road fill material. In contrast, Pb, Ni, As, and Mn had PHI values exceeding 1, specifically 4.6, 1.4, 5.6, and 14.8, respectively. These values indicate that their concentrations surpassed the Class III groundwater standard limits, which are 0.1 mg/L for Mn, 0.01 mg/L for As, 0.01 mg/L for Pb, and 0.02 mg/L for Ni. Based on the PHI values, the decreasing order of contamination potential is Mn > As > Pb > Ni, underscoring the need for targeted management strategies to control the migration of these elements and prevent groundwater contamination. Lin and Li et al. [
35,
36] also found that, in addition to the main component calcium sulfate, titanium gypsum contains certain heavy metals. X-ray fluorescence (XRF) analysis revealed that the magnesium oxide content is approximately 1%.
The leaching rates were calculated using the NEN 7371 and HJ/T 299 standard leaching methods to evaluate their applicability when assessing the utilization of solid waste in road construction, and the results are shown in
Figure 2. Under the HJ/T 299 method, the leaching rates for various heavy metals across the different samples were generally low at around 1%, which indicates insignificant leaching. This low leaching rate requires high precision analytical equipment to detect the leaching concentrations and may lead to larger detection errors under the same conditions. In contrast, the leaching rates for most heavy metals based on the NEN 7371 method ranged from 5% to 17%, which suggests that there was more pronounced leaching and that any potential inaccuracies had been reduced. This is consistent with the findings of Li et al. [
37], who used the NEN 7371 method to test the leaching of heavy metals from cement clinkers under various conditions. Their results showed that nearly all heavy metals were detectable, with most leaching rates exceeding 10%, and the leaching concentrations correlated well with the metal content in the clinkers. This confirms the suitability of the NEN 7371 method for assessing the leaching characteristics of heavy metals in cement clinkers.
The NEN 7371 method is based on continuous titration of the acid solution into the leaching system during its two leaching phases, which maintains a constant pH in the leachate and effectively consumes the acid neutralization capacity of the samples [
18]. This leads to continuous chemical reactions, such as neutralization at the solid–liquid interface, and promotes material exchange between the two phases. Conceptually, it simulates the continuous leaching process shown by weak acidic agents such as acid rain, which aligns well with the potential leaching environments encountered during the practical application of building materials [
38]. In contrast, the HJ/T 299 method uses acidic aqueous solutions as the extractant, which simulates the release of pollutants under acid rain conditions. When solid samples are added, the leaching process is completed in a single step. However, when strongly alkaline solid waste materials are added, even at a solid-to-liquid ratio of 1:10, the extractant is rapidly neutralized, rendering the leaching system alkaline, which deviates from the intended basis of the method, thereby limiting its applicability.
3.2. Exposure Risks and Titanium Gypsum Utilization in Road Construction
A conservative risk assessment approach was adopted in this study by incorporating parameter uncertainties via Monte Carlo simulation, with the 95th percentile concentration selected as the representative exposure value.
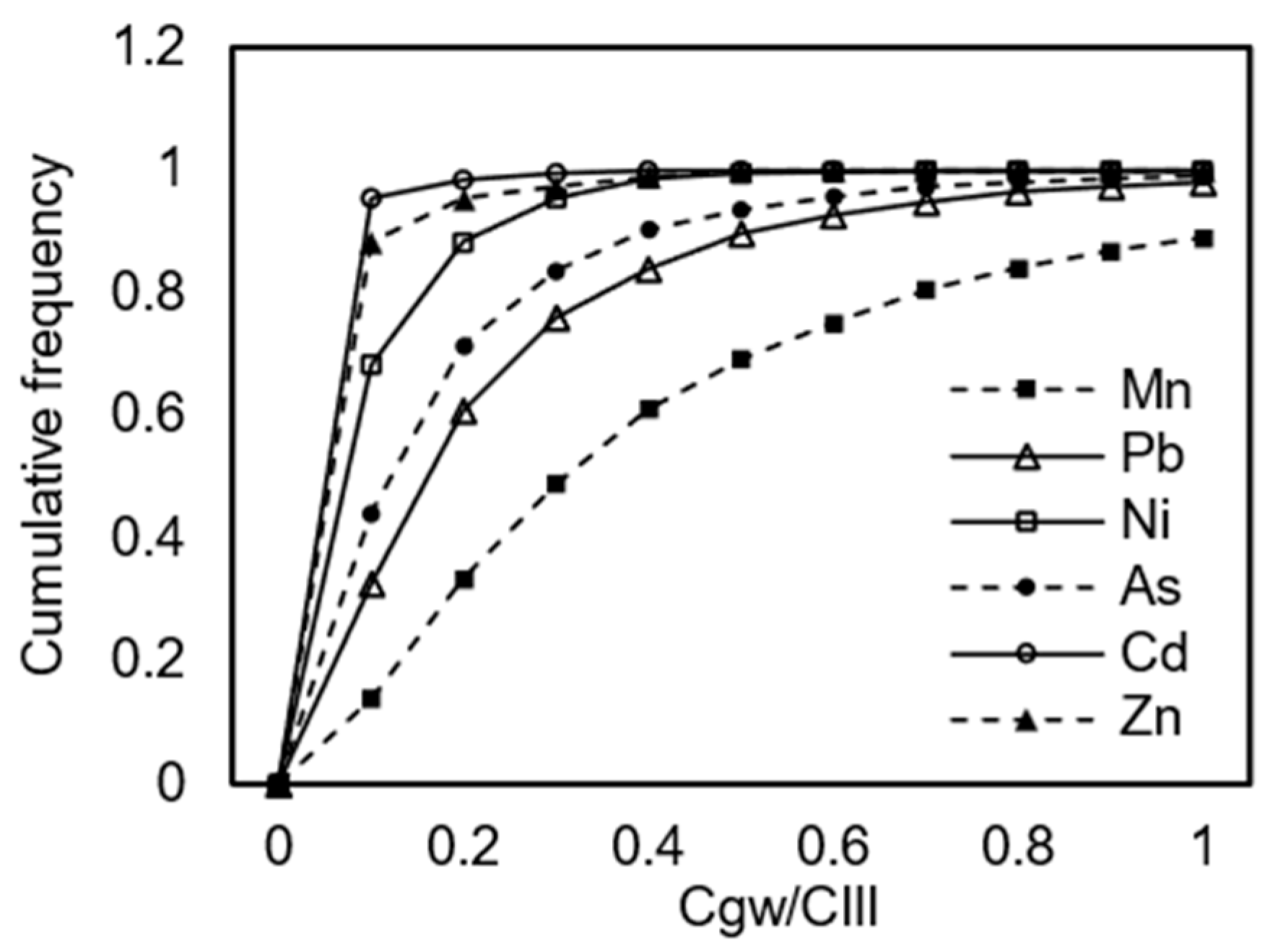
Figure 3 presents the cumulative frequency distributions of pollutant components under uncertain conditions. By comparing these distributions with the Class III groundwater quality limits, the probability and magnitude of exceedance were determined. The predicted probability distribution and exposure concentrations at the exposure point were calculated using an attenuation source model, with initial release concentrations derived from the NEN 7371 leaching test results summarized in
Table 1.
Figure 3 and
Table 1 show that the exposure concentrations for Pb were consistently below its corresponding Class III groundwater limit of 0.01 mg/L, with ratios ranging from 0.13 to 0.71. These are <1, which indicates that the risk is manageable. Similarly, the Ni exposure concentrations were under its Class III limit of 0.02 mg/L, with ratios between 0.03 and 0.19, which suggests that it is a controlled risk; the As exposure concentrations were below its Class III limit of 0.01 mg/L, with ratios of 0.1 to 0.53, which suggests the risk can be contained; the Cd exposure concentrations were below its stricter Class III limit of 0.005 mg/L, with ratios from 0.009 to 0.05, which suggests that it is a controlled risk; and the Zn exposure concentrations were well within its Class III limit of 1 mg/L, with ratios between 0.01 and 0.06, suggesting that it is a manageable risk. However, the Mn concentration results after groundwater migration and transformation suggest that there is an 11% probability that it could exceed its Class III limit, with the exposure concentration potentially reaching 1.5 times the limit.
Pollutants migrate and diffuse outwards through the aquifer after they are leached into groundwater by rainfall [
39].
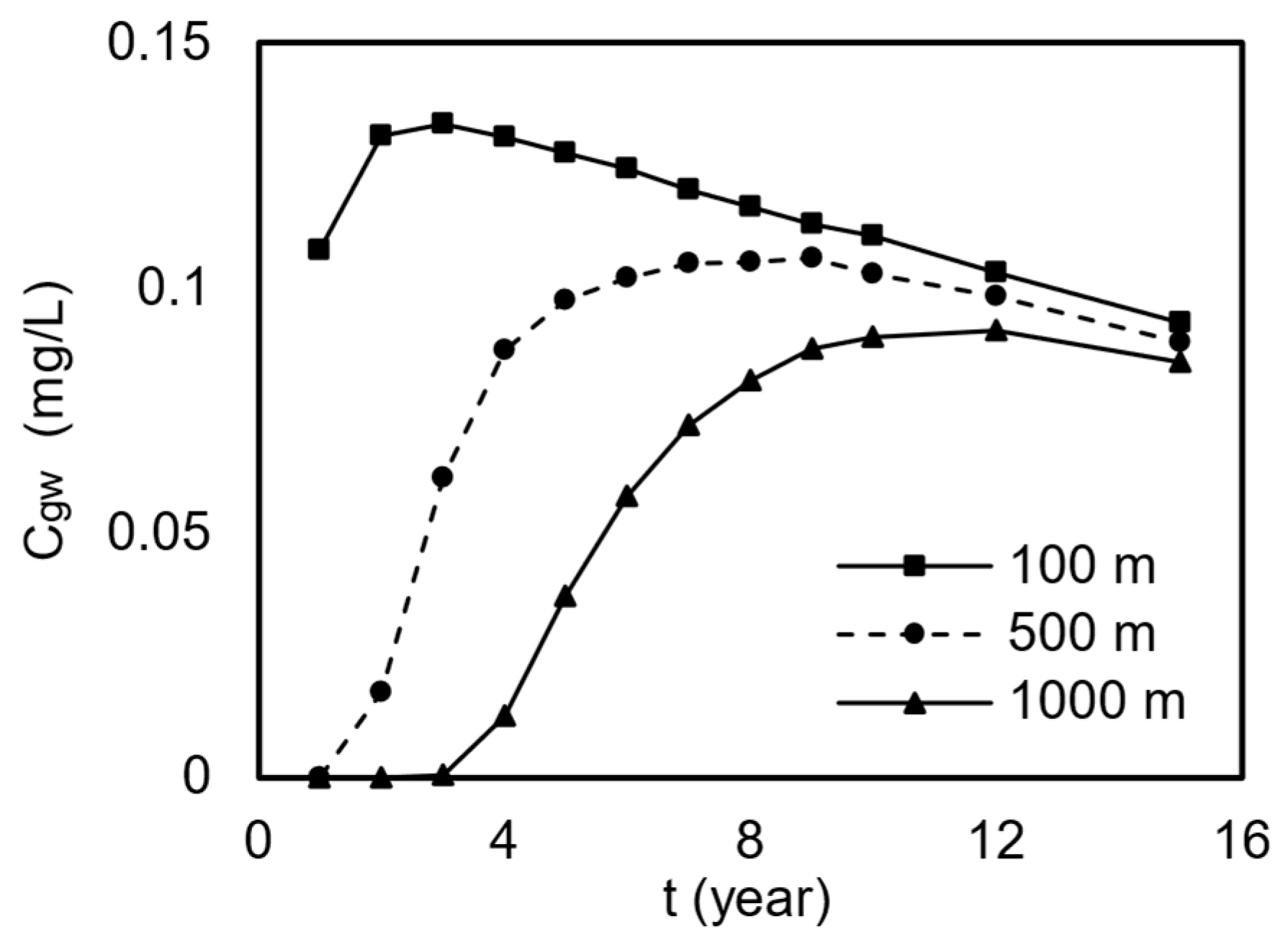
Figure 4 details the temporal variation in Mn concentrations in groundwater at distances of 100 m, 500 m, and 1000 m from a road. The Mn concentrations in groundwater gradually increased at first for all distances over time before reaching a maximum and then slowly declining. Closer distances resulted in the faster attainment of peak concentrations, higher peak values, and longer periods when the limit was exceeded. Taking the 500 m distance as an example, concentrations gradually rose to 0.1 mg/L by the 6th year, exceeding the Class III water limit, peaked in the 9th year, decreased below the limit by the 12th year, and subsequently continued to decrease.
Figure 5 illustrates the variation in peak exposure concentrations with distance. It shows that as the distance increases, the peak exposure concentration decreases. Based on the 95th percentile exposure concentration, the peak value at 100 m was 0.127 mg/L, which exceeded the Class III limit, but it decreased below the limit at 700 m. Thus, the minimum buffer distance for Mn in Jiangsu Province is approximately 700 m. The results also suggest that Mn concentrations remain below the Class III limit when the observation well is located more than 700 m away from the road. At the 50th percentile exposure concentration, the peak value at 100 m from the road is 0.03 mg/L, which is significantly lower than the limit and potentially underestimates the environmental risks. Ji et al. [
40], using Monte Carlo simulations to investigate critical factors affecting buffer distances between hazardous waste landfills and water sources, found that reducing contaminant release using methods such as solidification and stabilization can effectively shorten buffer distances and mitigate post-leakage environmental risks. Therefore, when using titanium gypsum as a road base material, adding a certain proportion of cement or other binders could help reduce the environmental risks.
3.3. Regional Variations in Groundwater Contamination Risk from Titanium Gypsum Used in Road Construction
Variations in hydrogeological and climatic conditions across different regions significantly impact the migration and diffusion processes for pollutant components. Factors such as precipitation and net infiltration directly influence the release of harmful substances from roadbed filling materials [
41]. This study primarily focused on the impact of the variations in precipitation among the 11 provinces along the Yangtze River Basin on the risks from harmful substances.
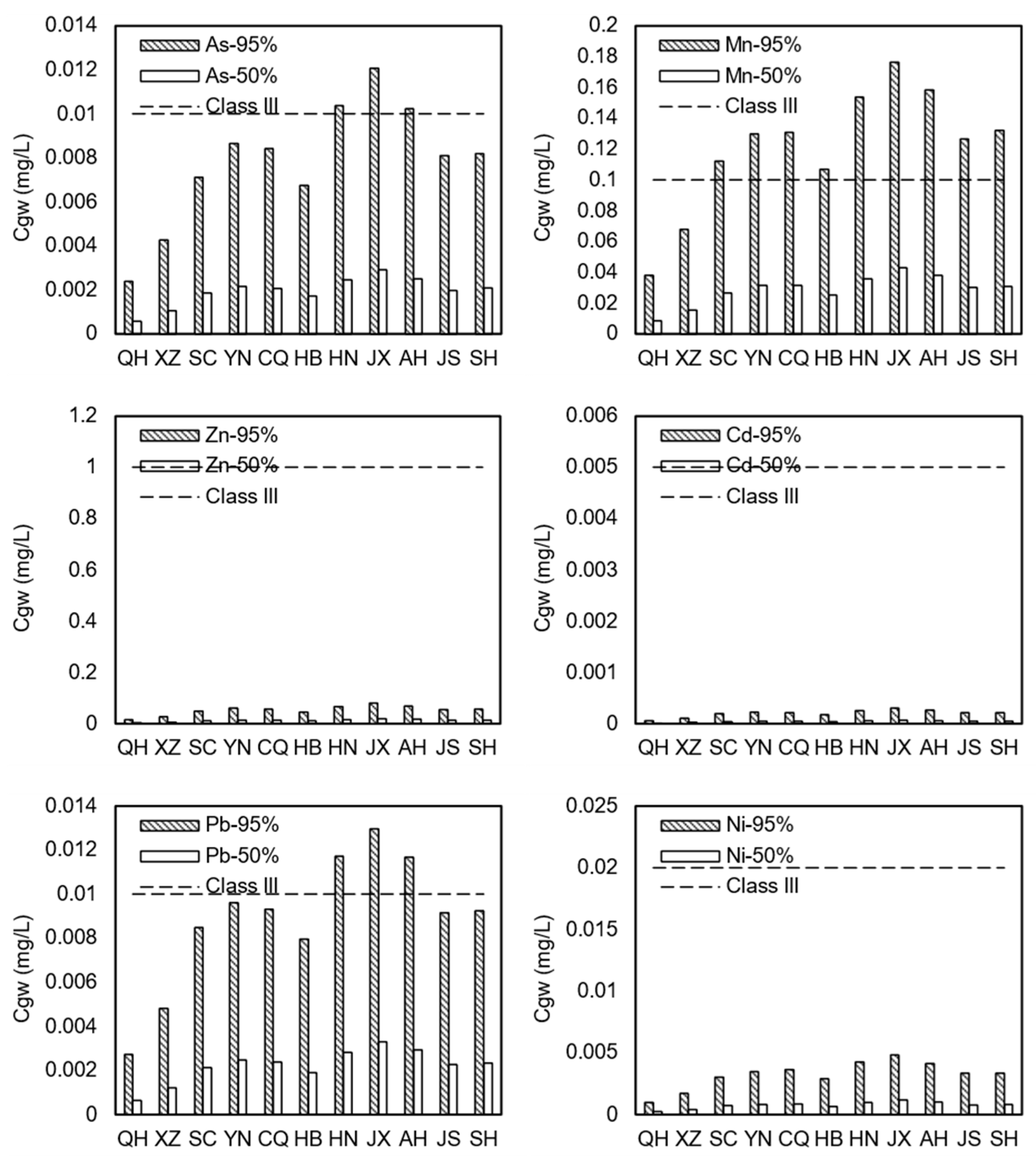
The exposure point concentration differences for the six heavy metals in these 11 provinces, as simulated by the model in
Section 2.3, are illustrated in
Figure 6. The results show that the ratios for the Pb, Ni, As, Cd, and Zn exposure concentrations to the Class III groundwater limits in these provinces ranged from 0.01 to 0.71. These are all below 1, indicating that these metals would not exceed the limits and that the risks were manageable. However, the Mn exposure concentration ratio to the Class III limit varied between 0.5 and 1.5, which suggests that there is a potential risk of exceedance. Except for Qinghai and Tibet, the Mn exposure concentrations in the remaining nine provinces ranged from 0.11 to 0.18 mg/L, which exceeded the Class III limit of 0.1 mg/L. Jiangxi had the highest exposure concentration at 0.18 mg/L, with an 11% exceedance probability. Variations in climatic conditions, such as precipitation, can lead to differences in net infiltration when titanium gypsum is used for road construction. This can cause further regional disparities in pollutant release and shallow aquifer contamination risks.
Figure 6 shows that Qinghai and Tibet have lower exposure concentrations for all six heavy metals, while Jiangxi has the highest. For example, the Mn exposure concentration in Qinghai is the lowest at 0.03 mg/L, whereas it is highest in Jiangxi at 0.18 mg/L, which is a six-fold difference.
The concentration patterns of each metal were consistent across provinces, despite variations in exposure levels of the six heavy metals. This study found that higher precipitation correlates with greater exceedance risks. Ji et al. [
40] noted that environmental risks from heavy metal migration are greater in humid regions than in arid ones. Li et al. [
42] reported that precipitation differences across nine Yellow River provinces significantly affect net infiltration rates during road use of oil and gas extraction waste, impacting pollutant release and shallow groundwater contamination risk. For example, barium exposure concentrations in Sichuan (the highest precipitation) were nearly 41 times those in Ningxia (the lowest precipitation). These findings indicate a positive correlation between precipitation and both exposure concentrations and environmental risks.
3.4. Regulatory Limits for Pollutant Control When Titanium Gypsum Is Used for Road Construction
The proposed regulatory limits for heavy metal contaminants in the 11 provinces along the Yangtze River, using leaching concentrations as the source strength, were calculated using Equations (9) and (10) and are shown in
Figure 7. Manganese and arsenic were chosen to represent low-toxicity and high-toxicity heavy metal pollutants, respectively.
In terms of individual pollutants, the regulatory limits for Mn pollution calculated by this study varied from 0.89 to 4.01 mg/L across the provinces, with the lowest (Jiangxi) and the highest (Qinghai) differing by nearly 4.5 times. The As limits ranged from 0.05 to 0.27 mg/L, which is a difference of approximately 5.4 times between the lowest in Jiangxi and the highest in Qinghai. In general, the control limits for As are stricter than those for Mn, primarily due to the differences in hazard characteristics and dilution/attenuation characteristics of the contaminants in the medium. Increased hazard potential and weaker attenuation capabilities necessitate stricter source controls and lower leaching limits, and vice versa.
Regionally, the regulatory limits for Mn in Jiangxi and Anhui were lower than the leaching concentration limits specified in GB 30760 at approximately 0.8–0.9 times the standard limit. The limits for the remaining provinces exceeded the standard by 1.1 to 4 times. In contrast, the As limits in Qinghai and Tibet were higher than GB 30760 at 2.7 and 1.5 times the standard, respectively, while the limits for other provinces were lower by 0.52–0.89 times. The As limits for all 11 provinces along the Yangtze River were 0.10–0.53 times lower than the Class I solid waste limits, while the Mn limits were 0.49–2.0 times lower. These results suggest that adopting the heavy metal leaching concentration limits in GB 30760 as regulatory limits is more effective than Class I solid waste limits in controlling the environmental risks associated with utilizing titanium gypsum in road construction.
The observed regional variations highlight the limitations of a uniform national standard. GB 30760 was developed based on generalized assumptions without fully considering local hydrological, geochemical, or climatic conditions [
33]. For example, although Qinghai and Tibet have higher As limits than GB 30760, their low rainfall and arid climate reduce metal leaching, lowering actual environmental risk. In contrast, wetter regions like Jiangxi and Anhui require stricter controls due to enhanced leaching potential. This suggests that future revisions of GB 30760 should consider regionally differentiated thresholds—particularly for contaminants like As with high toxicity and variable environmental behavior [
40,
42]. Incorporating site-specific factors into regulatory frameworks can improve both scientific accuracy and practical applicability, supporting the safe and sustainable reuse of industrial byproducts such as titanium gypsum in civil engineering applications.
However, this study has several limitations. First, it relies on laboratory simulations to estimate contaminant release and migration, which may not fully capture real-world environmental complexity. Key factors such as microbial activity, redox changes, and seasonal variations were not fully considered. Second, there is limited research on the long-term environmental impacts of titanium gypsum in road construction, mainly due to a lack of monitoring data. The long-term accumulation and potential effects of contaminants like manganese on human health and ecosystems remain poorly understood. Addressing these gaps is essential for improving risk assessment and management strategies.
4. Conclusions
This study demonstrated that the traditional HJ/T 299-2007 leaching method underestimates the heavy metal leaching rates from titanium gypsum, potentially leading to an underestimation of environmental risks. Specifically, while Pb, Ni, As, Cd, and Zn leach from titanium gypsum road materials to varying extents, Mn presents a significant exceedance risk, with an 11% probability of surpassing regulatory limits and a maximum exceedance factor of 1.8. Significant regional variations in regulatory thresholds for titanium gypsum pollutants were observed among 11 provinces along the Yangtze River, with the highest threshold (Qinghai) being nearly five times greater than the lowest (Jiangxi). These findings highlight the inadequacies of current assessment methods and emphasize the need for enhanced national standards. Future research should focus on frameworks that integrate local climatic and soil conditions to enable targeted risk mitigation measures, as well as explore the application of green additives or natural binding materials in titanium gypsum to reduce heavy metal leaching risks and improve its long-term stability, thereby promoting the concept of a circular economy in road engineering. In addition, long-term monitoring mechanisms, life cycle assessments, and spatial risk modeling are crucial for the safe and sustainable utilization of titanium gypsum and will provide strong support for policy formulation and scientific decision-making.