Functionalized Polyethyleneimine Adsorbent for Efficient and Selective Uranium Extraction from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
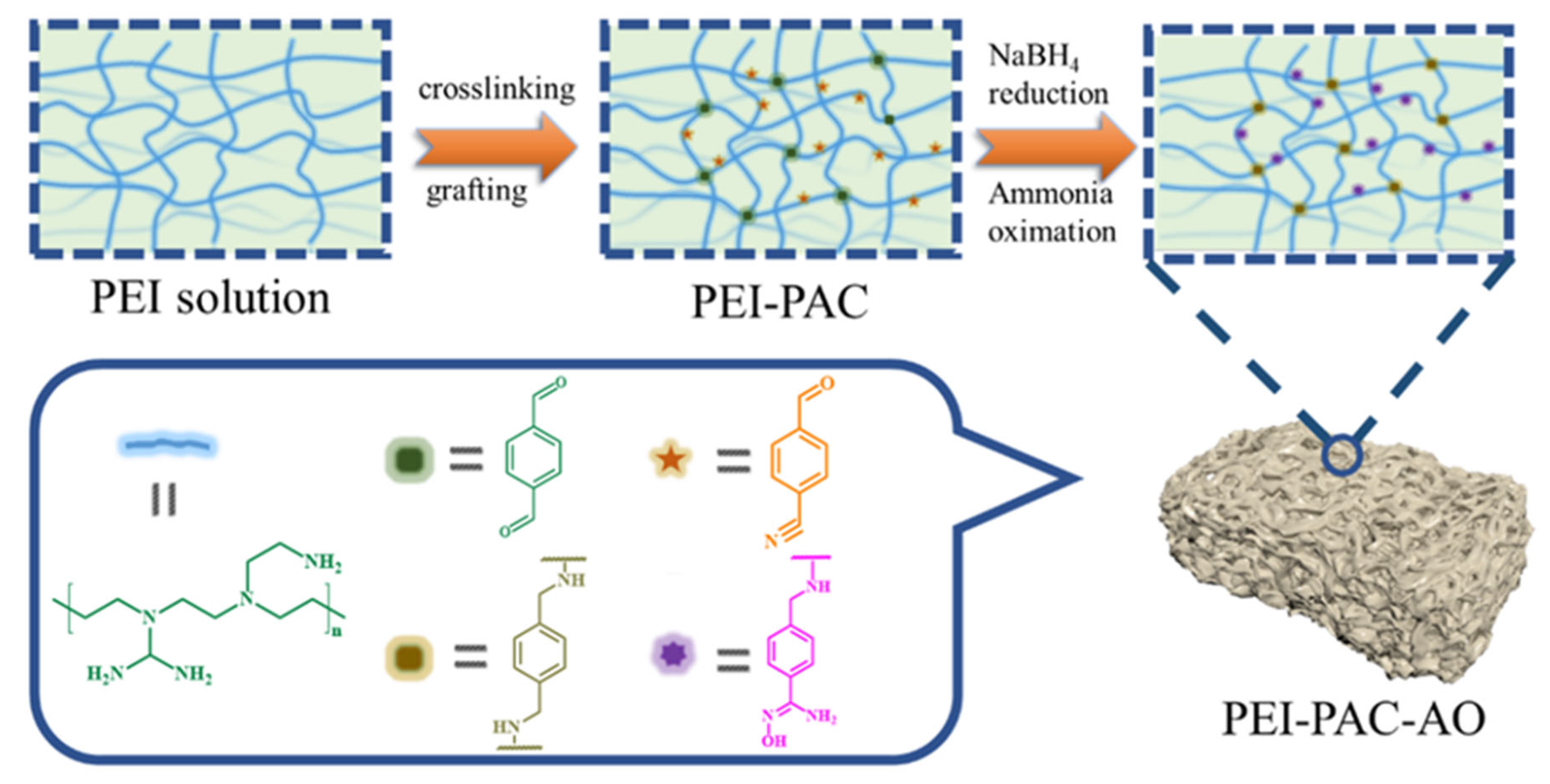
2.2. Preparation of PEI-PAC
2.3. Preparation of PEI-PAC-AO
3. Results and Discussion
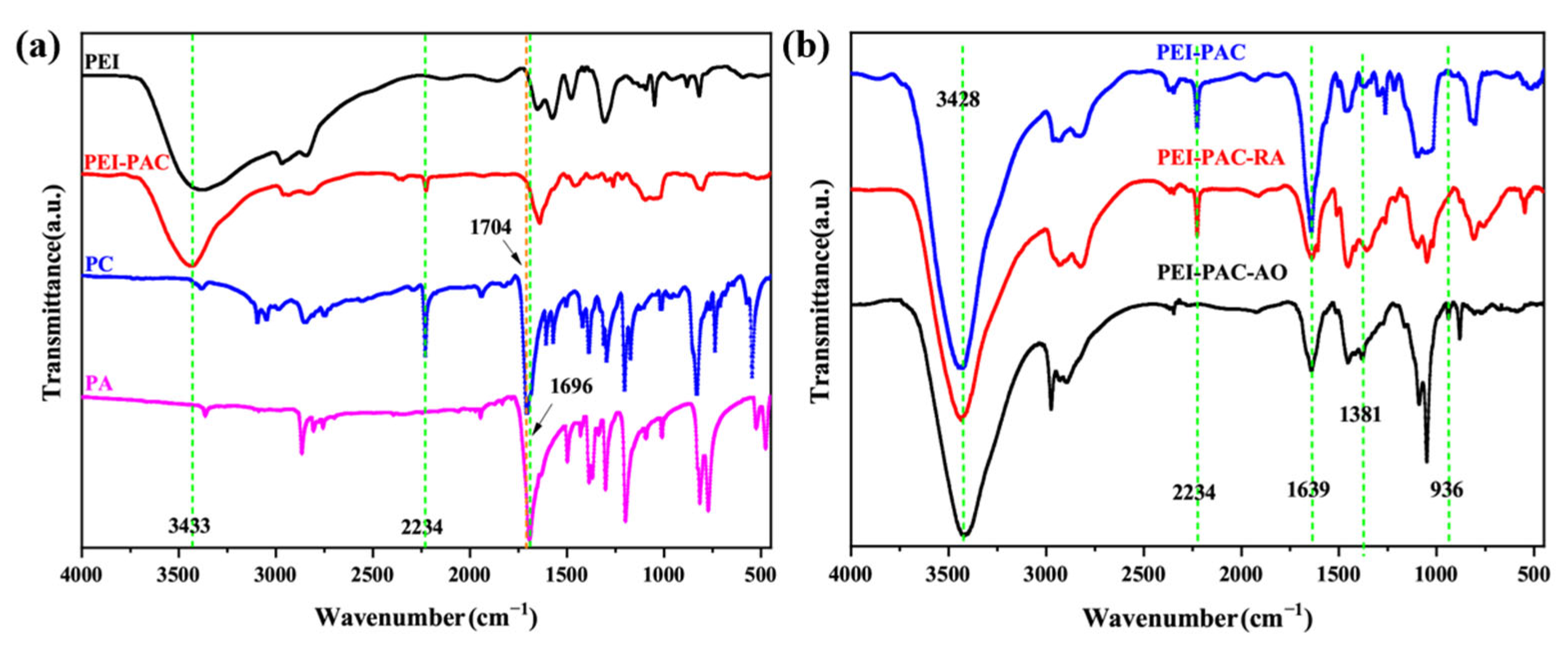
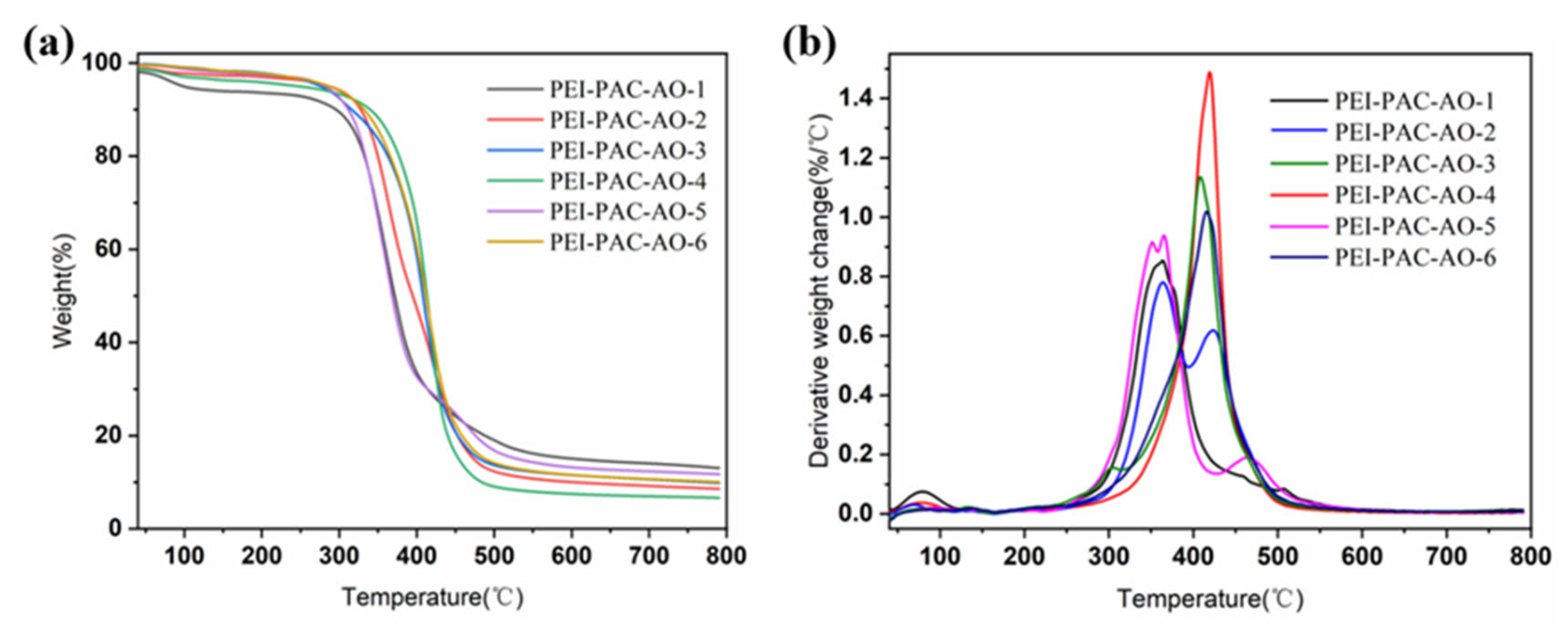
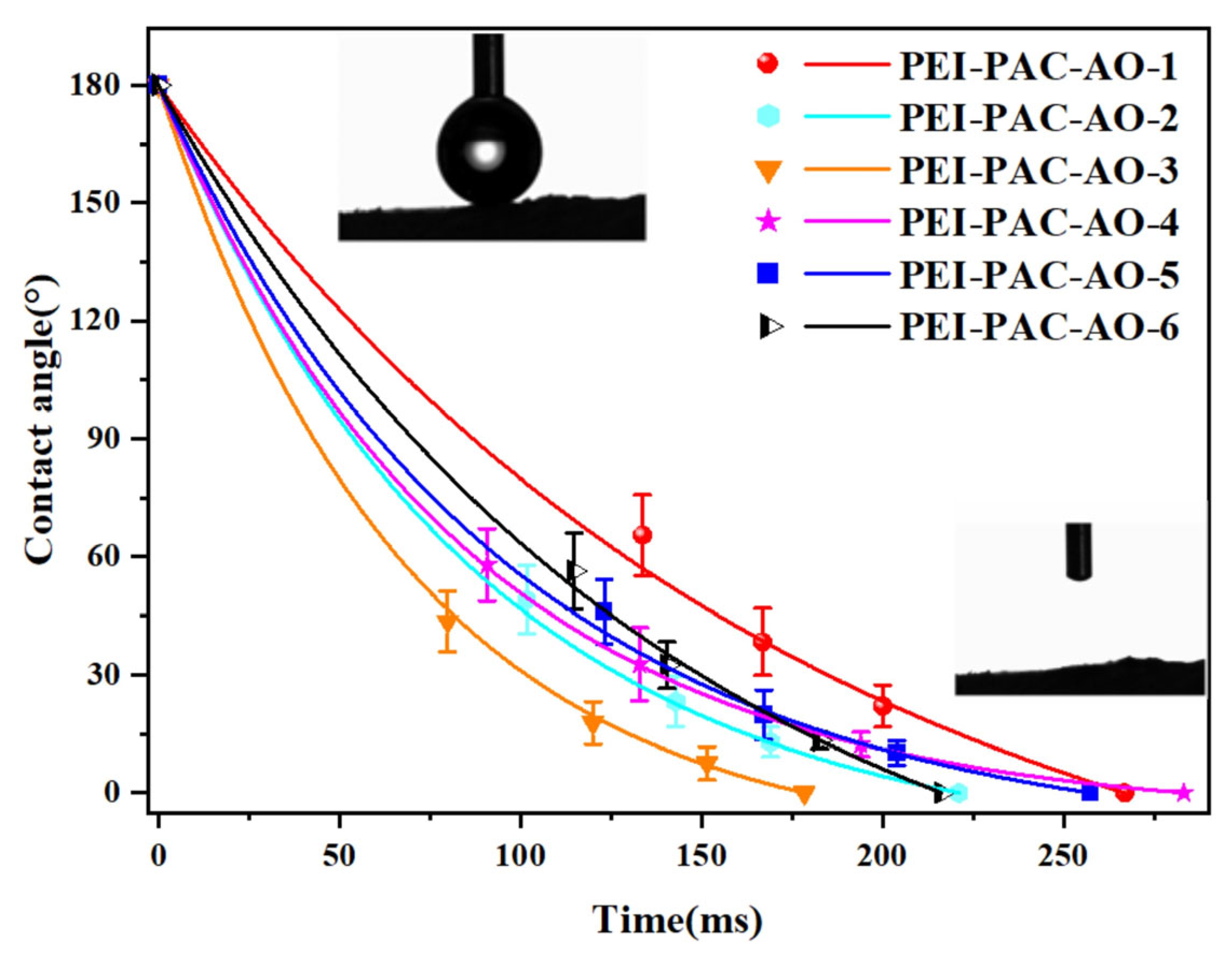
3.1. Characterization of Adsorbents
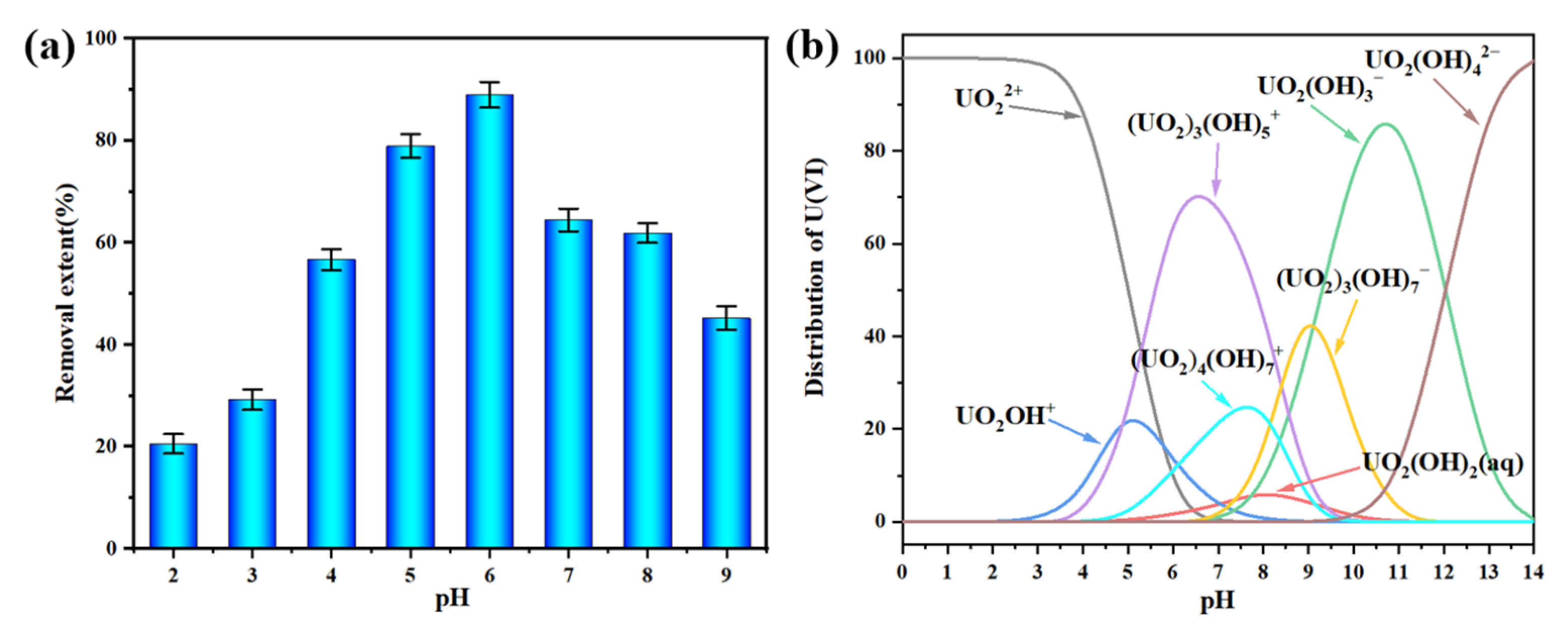
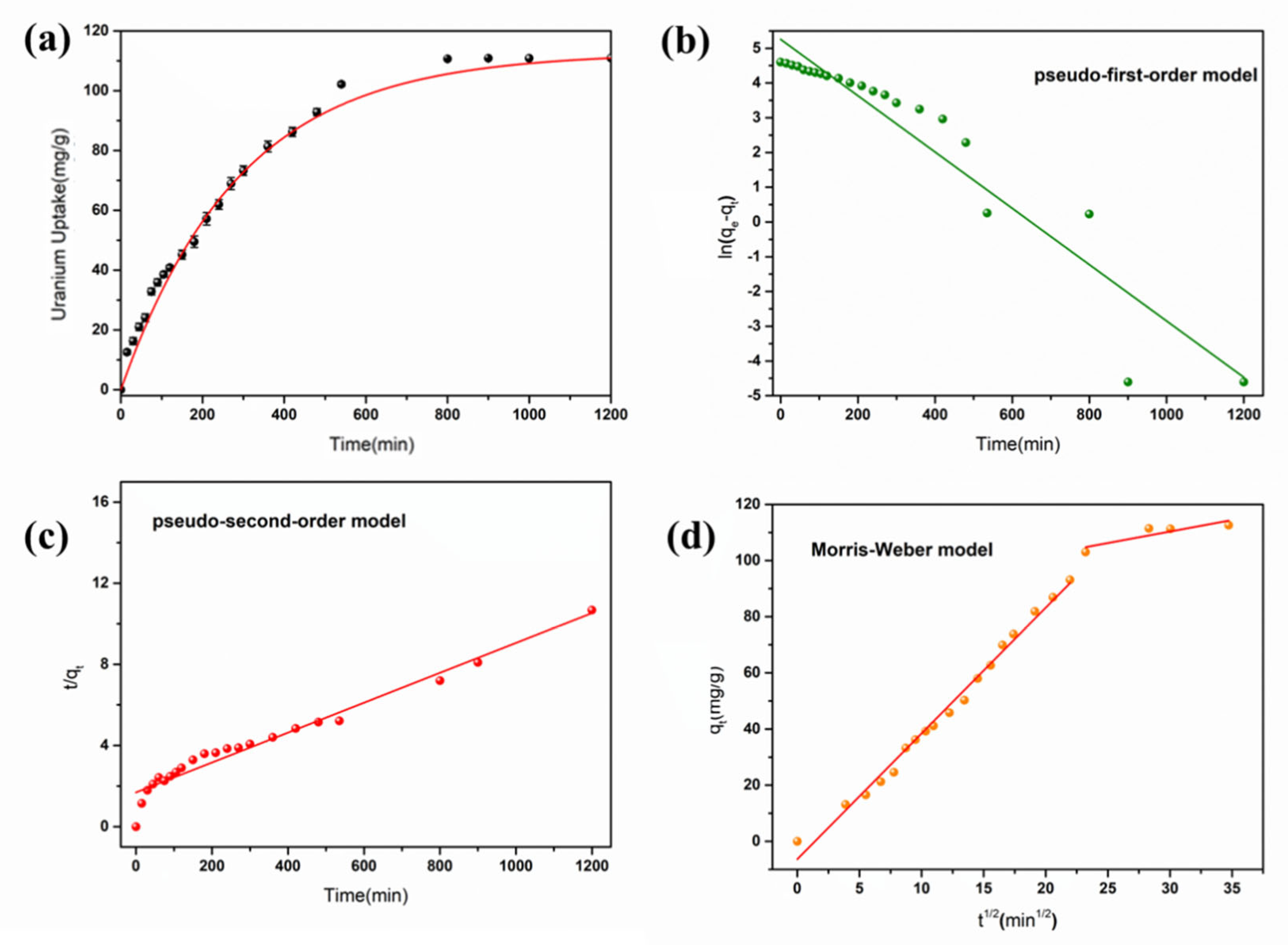
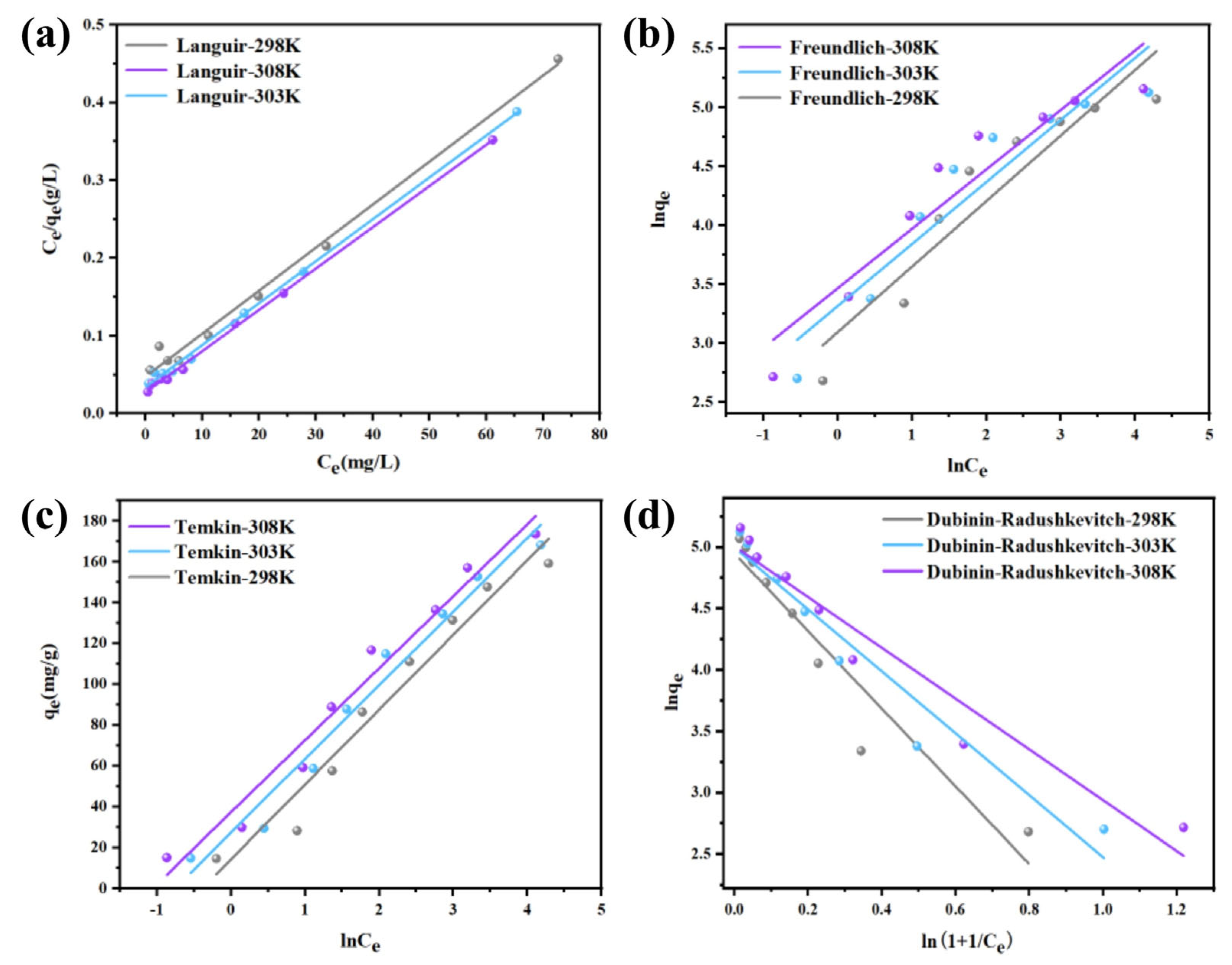
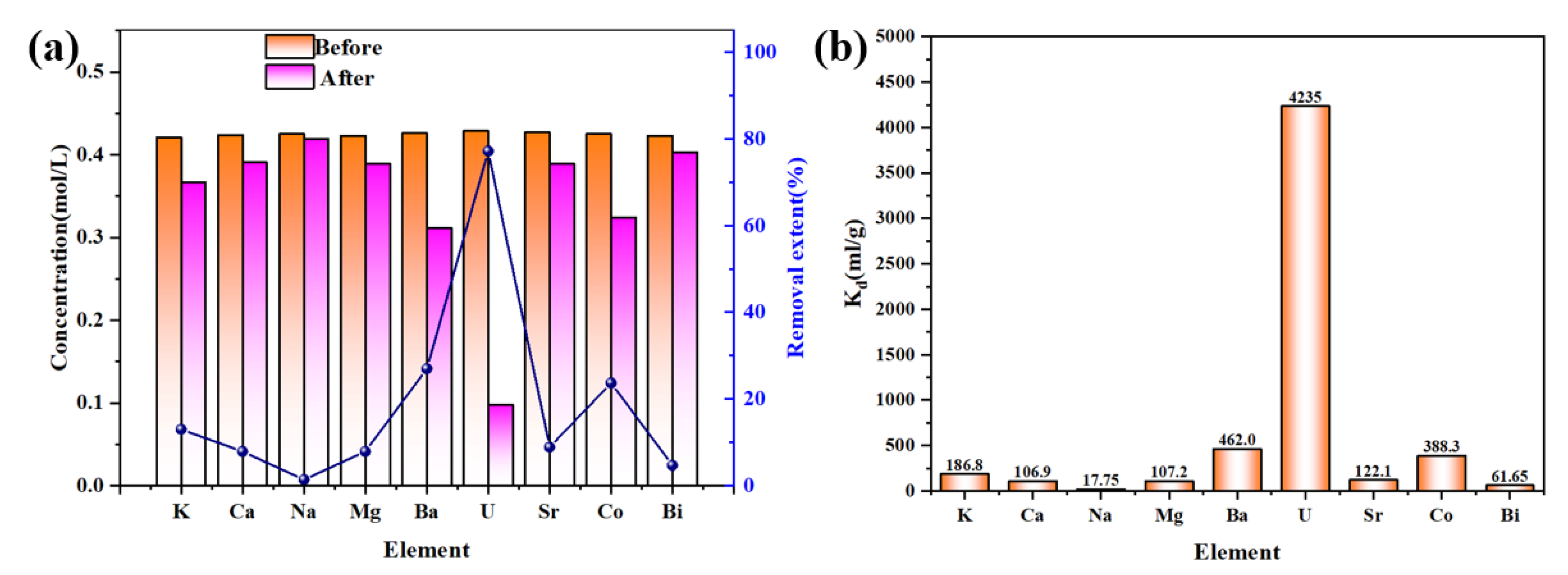
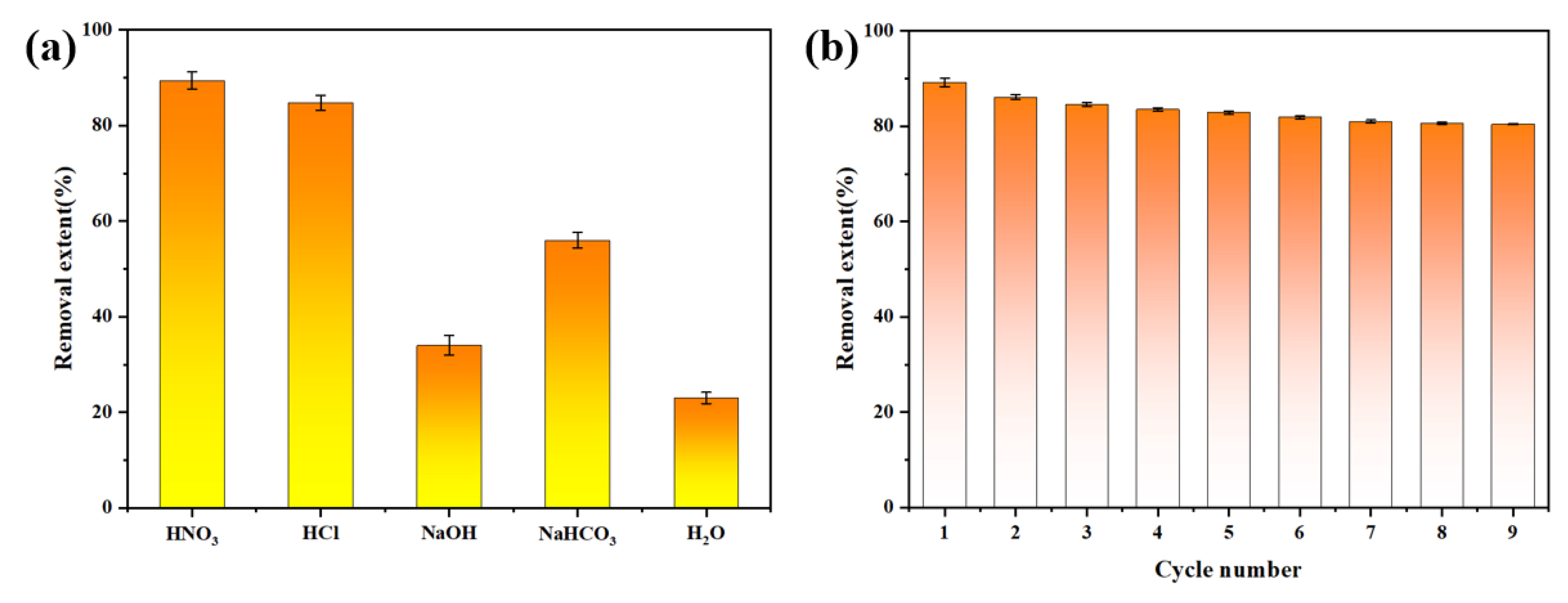
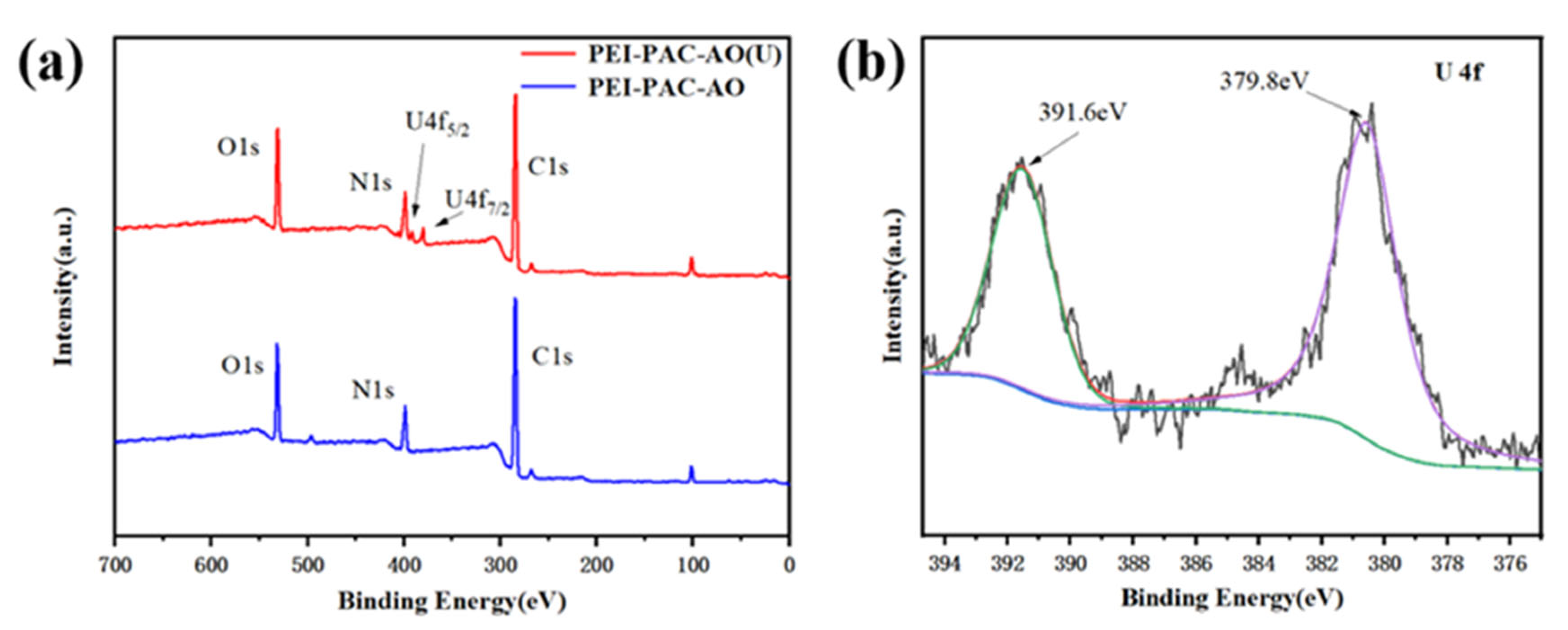
3.2. Characterization of Uranium Adsorption Properties
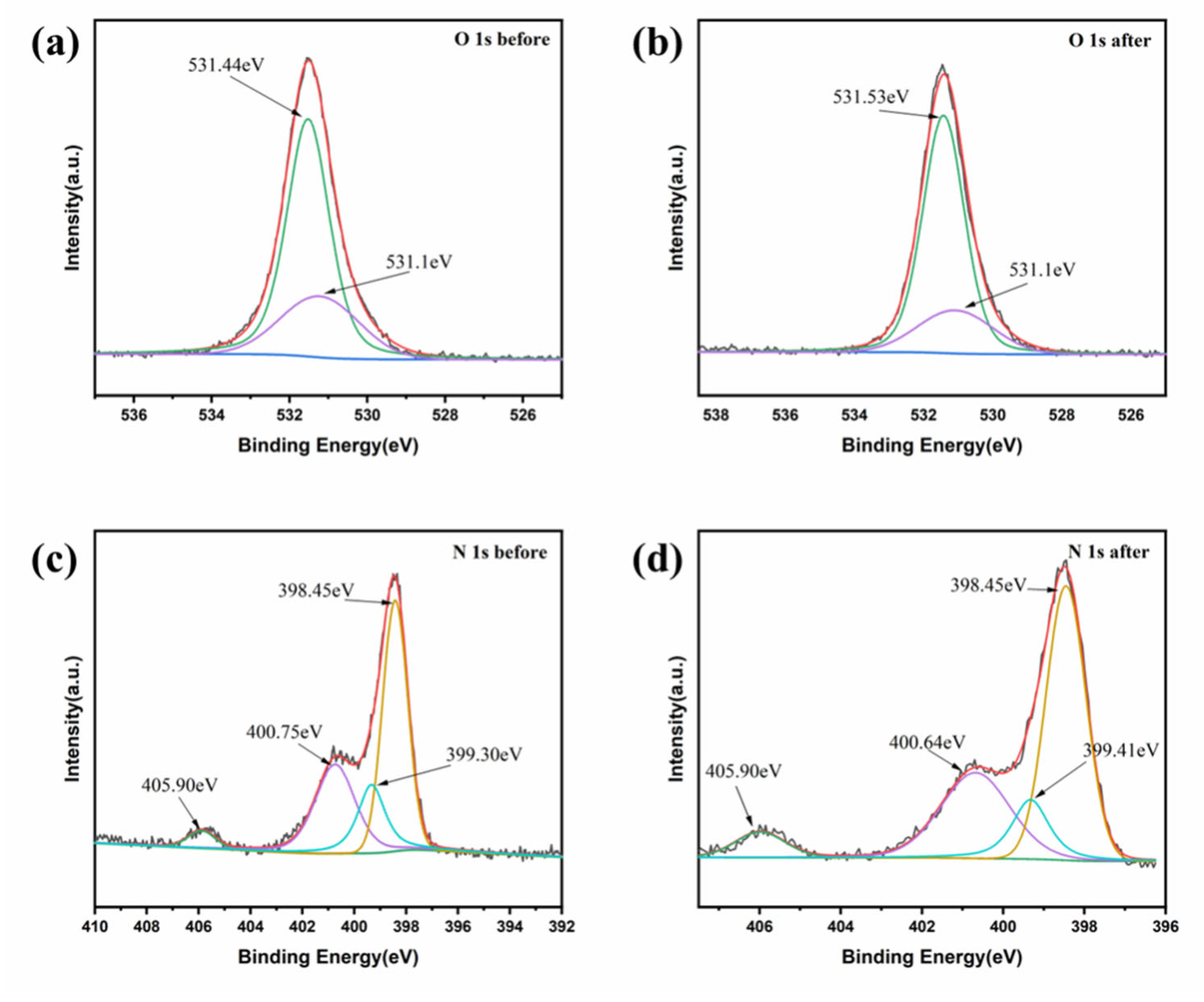
3.3. Possible Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, K.; Cao, X.M.; Zhang, Y.C. System Modeling and Performance Simulation of a Full-Spectrum Solar-Biomass Combined Electricity-Heating-Cooling Multi-Generation System. Sustainability 2025, 17, 4675. [Google Scholar] [CrossRef]
- Ma, C.; Gao, J.; Wang, D.; Yuan, Y.; Wen, J.; Yan, B.; Zhao, S.; Zhao, X.; Sun, Y.; Wang, X.; et al. Sunlight Polymerization of Poly(amidoxime) Hydrogel Membrane for Enhanced Uranium Extraction from Seawater. Adv. Sci. 2019, 6, 1900085. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Salvatores, M.; Schwenk-Ferrero, A.; Gabrielli, F.; Vezzoni, B.; Rineiski, A.; Fazio, C. Sustainable Nuclear Fuel Cycles and World Regional Issues. Sustainability 2012, 4, 1214–1238. [Google Scholar] [CrossRef]
- Gabriel, S.; Baschwitz, A.; Mathonnière, G.; Eleouet, T.; Fizaine, F. A critical assessment of global uranium resources, including uranium in phosphate rocks, and the possible impact of uranium shortages on nuclear power fleets. Ann. Nucl. Energy 2013, 58, 213–220. [Google Scholar] [CrossRef]
- Feng, S.; Feng, L.; Wang, M.; Yuan, Y.; Yu, Q.; Feng, T.; Cao, M.; Wang, N.; Peng, Q. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chem. Eng. J. 2022, 430, 133038. [Google Scholar] [CrossRef]
- Boyarintsev, A.V.; Stepanov, S.I.; Kostikova, G.V.; Zhilov, V.I.; Safiulina, A.M.; Tsivadze, A.Y. Separation and purification of elements from alkaline and carbonate nuclear waste solutions. Nucl. Eng. Technol. 2023, 55, 391–407. [Google Scholar] [CrossRef]
- Zhong, X.; Liang, W.; Lu, Z.; Hu, B. Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent organic frameworks with intramolecular hydrogen-bonding from solutions. Appl. Surf. Sci. 2020, 504, 144403. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Zhang, X.; Tan, H.; Chen, Z.; Wu, J.; Chen, J.; Qiu, H. Metal–Organic Framework-Intercalated Graphene Oxide Membranes for Selective Separation of Uranium. Anal. Chem. 2021, 93, 16175–16183. [Google Scholar] [CrossRef]
- Torkabad, M.G.; Keshtkar, A.R.; Safdari, S.J. Uranium membrane separation from binary aqueous solutions of UO22+-K+ and UO22+-Ca2+ by the nanofiltration process. Sep. Sci. Technol. 2017, 52, 1095–1105. [Google Scholar] [CrossRef]
- Singh, D.K.; Hareendran, K.N.; Sreenivas, T.; Kain, V.; Dey, G.K. Development of a phosphate precipitation method for the recovery of uranium from lean tenor alkaline leach liquor. Hydrometallurgy 2017, 171, 228–235. [Google Scholar] [CrossRef]
- Fox, A.R.; Bart, S.C.; Meyer, K.; Cummins, C.C. Towards uranium catalysts. Nature 2008, 455, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, P.; Dong, F.; Nie, X.; Ding, C.; Wang, S.; Zhang, Y.; Liu, H.; Zhou, S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 2019, 367, 198–207. [Google Scholar] [CrossRef]
- Chen, F.; Lv, M.; Ye, Y.; Miao, S.; Tang, X.; Liu, Y.; Liang, B.; Qin, Z.; Chen, Y.; He, Z. Wang, Insights on uranium removal by ion exchange columns: The deactivation mechanisms, and an overlooked biological pathway. Chem. Eng. J. 2022, 434, 134708. [Google Scholar] [CrossRef]
- Banala, U.K.; Das, N.P.I.; Toleti, S.R. Microbial interactions with uranium: Towards an effective bioremediation approach, Environ. Technol. Innov. 2021, 21, 101254. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, J.; Peng, Q.; Tian, X.; Zhang, J.; Ai, J.; Jia, Q.; Yuan, Y.; Wang, N. Bacterial spore with high tolerance to concentrated acid and nuclear radiation for uranium recovery from nuclear wastewater. Sep. Purif. Technol. 2025, 360, 131250. [Google Scholar] [CrossRef]
- Zerbini, M.; Solari, P.L.; Orange, F.; Jeanson, A.; Leblanc, C.; Gomari, M.; Auwer, C.D.; Beccia, M.R. Exploring uranium bioaccumulation in the brown alga Ascophyllum nodosum: Insights from multi-scale spectroscopy and imaging. Sci. Rep. 2024, 14, 1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gu, A.; Wei, T.; Chen, M.; Guo, X.; Tang, S.; Yuan, Y.; Wang, N. Ligand-Assistant Iced Photocatalytic Reduction to Synthesize Atomically Dispersed Cu Implanted Metal-Organic Frameworks for Photo-Enhanced Uranium Extraction from Seawater. Small 2023, 19, 2208002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, S.; Luo, X.; Bai, J.; Gao, F.; Yan, H.; Li, M.; Huo, L.; Zhang, C.; Wang, J. Photothermal composite polyamidoxime-graphene oxide/polyacrylamide hydrogel for efficient and selective uranium extraction from seawater. Desalination 2025, 593, 118238. [Google Scholar] [CrossRef]
- Xu, R.; Xuan, K.; Chen, S.; Yan, S.; Wan, J.; Zhao, K.; Guo, Y.; Zheng, M.; Liang, R.; Jia, M.; et al. Polysulfide grafted with hydrophilic group for efficient capture of uranium (VI) from water. Sep. Purif. Technol. 2025, 361, 131281. [Google Scholar] [CrossRef]
- Hassanin, M.A.; Negm, S.H.; Youssef, M.A.; Sakr, A.K.; Mira, H.I.; Mohammaden, T.F.; Al-Otaibi, J.S.; Hanfi, M.Y.; Sayyed, M.I.; Cheira, M.F. Sustainable Remedy Waste to Generate SiO2 Functionalized on Graphene Oxide for Removal of U(VI) Ions. Sustainability 2022, 14, 2699. [Google Scholar] [CrossRef]
- Liu, R.; Wan, Q.; Yu, Y.; Zhang, X.; Liu, L.; Wang, H.; Yue, C. Polyacrylate/phytic acid hydrogel derived phosphate-rich macroporous carbon foam for high-efficiency uranium adsorption. J. Water Process Eng. 2023, 53, 103659. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, R.; Zhang, Y.; Cai, T.; Han, B. Defect controlled MOF-808 for seawater uranium capture with high capacity and selectivity. J. Mol. Liq. 2022, 367, 120514. [Google Scholar] [CrossRef]
- Ma, L.; Huang, C.; Yao, Y.; Fu, M.; Han, F.; Li, Q.; Wu, M.; Zhang, H.; Xu, L.; Ma, H. Self-assembled MOF microspheres with hierarchical porous structure for efficient uranium adsorption. Sep. Purif. Technol. 2023, 314, 123526. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, S.; Wang, J.; Yu, F.; Xu, M.; Xiong, J.; Xiao, S.; Liu, Y.; He, Y.; Xu, J.; et al. Synchronous construction of high sulfonic acid grafting degree and large surface area in conjugated microporous polymer adsorbents for efficient removal of uranium (VI). Sep. Purif. Technol. 2023, 309, 122953. [Google Scholar] [CrossRef]
- Sun, Z.; Meng, C.; Zhang, S.; Na, B.; Zou, S.; He, Y. One-pot hydrolysis/amidoximation and self-assembly to polyamidoxime-based composite hydrogels for high-efficiency uranium capture. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130323. [Google Scholar] [CrossRef]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Amin, N.A.S. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Kapilov-Buchman, Y.; Lellouche, E.; Michaeli, S.; Lellouche, J.-P. Unique Surface Modification of Silica Nanoparticles with Polyethylenimine (PEI) for siRNA Delivery Using Cerium Cation Coordination Chemistry. Bioconjug. Chem. 2015, 26, 880–889. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Malek, N.A.N.N.; Lim, J.W.; Ismail, A.F. Enhancing the performance of porous rice husk silica through branched polyethyleneimine grafting for phosphate adsorption. Arab. J. Chem. 2020, 13, 6682–6695. [Google Scholar] [CrossRef]
- Kassab, H.; Maksoud, M.; Aguado, S.; Pera-Titus, M.; Albela, B.; Bonneviot, L. Polyethylenimine covalently grafted on mesostructured porous silica for CO2 capture. RSC Adv. 2012, 2, 2508–2516. [Google Scholar] [CrossRef]
- Nie, D.; Han, Z.; Yu, Y.; Shi, G. Composites of multiwalled carbon nanotubes/polyethyleneimine (MWCNTs/PEI) and molecularly imprinted polymers for dinitrotoluene recognition. Sens. Actuators B Chem. 2016, 224, 584–591. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Jing, X.; Wang, J. Polyethyleneimine-functionalized Luffa cylindrica for efficient uranium extraction. J. Colloid Interface Sci. 2018, 530, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zhou, L.; Yu, H.; Ouyang, J.; Liu, Z.; Liu, Y.; Adesina, A.A. Polyethyleneimine incorporated chitosan/α-MnO2 nanorod honeycomb-like composite foams with remarkable elasticity and ultralight property for the effective removal of U(VI) from aqueous solution. Int. J. Biol. Macromol. 2022, 218, 190–201. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Zhang, L.; Chen, W.; Chu, D.; Tan, L. Recovery of uranium (VI) from aqueous solutions by the polyethyleneimine-functionalized reduced graphene oxide/molybdenum disulfide composition aerogels. J. Taiwan Inst. Chem. Eng. 2020, 106, 198–205. [Google Scholar] [CrossRef]
- Liu, X.; You, Y.; Yang, W.; Yang, L.; Zhang, X.; Qin, Z.; Yin, X. Temperature-sensitive amidoxime-based hydrogels for fast and efficient adsorption of uranium ions. Sep. Purif. Technol. 2024, 340, 126630. [Google Scholar] [CrossRef]
- Gao, F.; Bai, J.; Yan, H.; Zheng, S.; Su, S.; Li, J.; Zhang, C.; Wang, J. Gelatin-polyacrylamide double network enhanced polyamidoxime magnetic photothermal composite hydrogel for highly efficient and selective uranium extraction from seawater. Chem. Eng. J. 2024, 485, 149822. [Google Scholar] [CrossRef]
- Gao, F.; Bai, J.; Li, J.; Yan, H.; Su, S.; Zhang, C.; Wang, J. Artemisia gum reinforced amidoxime gel membrane promotes rapid extraction of uranium from seawater. Sep. Purif. Technol. 2024, 330, 125548. [Google Scholar] [CrossRef]
- Lin, K.; Sun, W.; Feng, L.; Wang, H.; Feng, T.; Zhang, J.; Cao, M.; Zhao, S.; Yuan, Y.; Wang, N. Kelp inspired bio-hydrogel with high antibiofouling activity and super-toughness for ultrafast uranium extraction from seawater. Chem. Eng. J. 2022, 430, 133121. [Google Scholar] [CrossRef]
- Yang, X.; Liew, S.R.; Bai, R. Simultaneous alkaline hydrolysis and non-solvent induced phase separation method for polyacrylonitrile (PAN) membrane with highly hydrophilic and enhanced anti-fouling performance. J.Memb. Sci. 2021, 635, 119499. [Google Scholar] [CrossRef]
- Tan, H.; Shan, T.; Deng, B.; Sha, J.; Liu, C.; Yang, P.; Li, S.; Xie, Z.; Liu, Z. Bioinspired surface engineering of amidoxime functionalized polyvinylidene fluoride membrane for efficient uranium extraction from seawater and wastewater. Sep. Purif. Technol. 2025, 360, 131194. [Google Scholar] [CrossRef]
- Bai, J.; Li, S.; Yan, H.; Jin, K.; Gao, F.; Zhang, C.; Wang, J. Processable amidoxime functionalized porous hyper-crosslinked polymer with highly efficient regeneration for uranium extraction. J. Mol. Liq. 2022, 362, 119745. [Google Scholar] [CrossRef]
- Li, R.; Che, R.; Liu, Q.; Su, S.; Li, Z.; Zhang, H.; Liu, J.; Liu, L.; Wang, J. Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J. Hazard. Mater. 2017, 338, 167–176. [Google Scholar] [CrossRef]
- Bi, C.; Nian, J.; Zhang, C.; Liu, L.; Zhu, L.; Zhu, R.; Qi, Q.; Ma, F.; Dong, H.; Wang, C. Efficient uranium adsorbent prepared by grafting amidoxime groups on dopamine modified graphene oxide. Prog. Nucl. Energy 2023, 155, 104515. [Google Scholar] [CrossRef]
- Chaithanya, M.S.; Das, B.; Vidya, R. Distribution, chemical speciation and human health risk assessment of metals in soil particle size fractions from an industrial area. J. Hazard. Mater. Adv. 2023, 9, 100237. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Q.; Hua, R.; Xia, H.T.; Li, X.X.; Wang, Z.Y.; Fu, X.; Du, Y.J.; Yan, Z.Y.; Wang, Y. Synergistic strategy design of (malonamide-amidoxime) bifunctional branching network crosslinked membrane and application in uranium (VI) resource recovery. Chem. Eng. J. 2023, 461, 142013. [Google Scholar] [CrossRef]
- Song, W.C.; Wang, X.X.; Sun, Y.B.; Hayat, T.; Wang, X.K. Bioaccumulation and transformation of U(VI) by sporangiospores of mucor circinelloides. Chem. Eng. J. 2019, 362, 81–88. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liu, Q.; Li, Z.S.; Liu, J.Y.; Zhang, H.S.; Li, R.M.; Wang, J.; Emelchenko, G.A. Recovery of uranium(VI) from aqueous solutions using a modified honeycomb-like porous carbon material. Dalton Trans. 2017, 46, 420–429. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Huo, L.; Gao, H.; Li, X.; Bai, J. Functionalized Polyethyleneimine Adsorbent for Efficient and Selective Uranium Extraction from Aqueous Solution. Sustainability 2025, 17, 5953. https://doi.org/10.3390/su17135953
Yan H, Huo L, Gao H, Li X, Bai J. Functionalized Polyethyleneimine Adsorbent for Efficient and Selective Uranium Extraction from Aqueous Solution. Sustainability. 2025; 17(13):5953. https://doi.org/10.3390/su17135953
Chicago/Turabian StyleYan, Huijun, Long Huo, Hong Gao, Xuanyi Li, and Jianwei Bai. 2025. "Functionalized Polyethyleneimine Adsorbent for Efficient and Selective Uranium Extraction from Aqueous Solution" Sustainability 17, no. 13: 5953. https://doi.org/10.3390/su17135953
APA StyleYan, H., Huo, L., Gao, H., Li, X., & Bai, J. (2025). Functionalized Polyethyleneimine Adsorbent for Efficient and Selective Uranium Extraction from Aqueous Solution. Sustainability, 17(13), 5953. https://doi.org/10.3390/su17135953




