Development of Geopolymeric Mortar from Metakaolin and Ignimbrite from the Añashuayco Quarries, Peru, for Civil Construction
Abstract
:1. Introduction
1.1. Research History
1.2. Literature Review
2. Materials and Methods
2.1. Materials
2.2. Collection and Treatment of Ignimbrite
2.3. Characterization
2.4. Geopolymer Manufacturing Methodology
2.5. Porosity Test
3. Results and Discussion
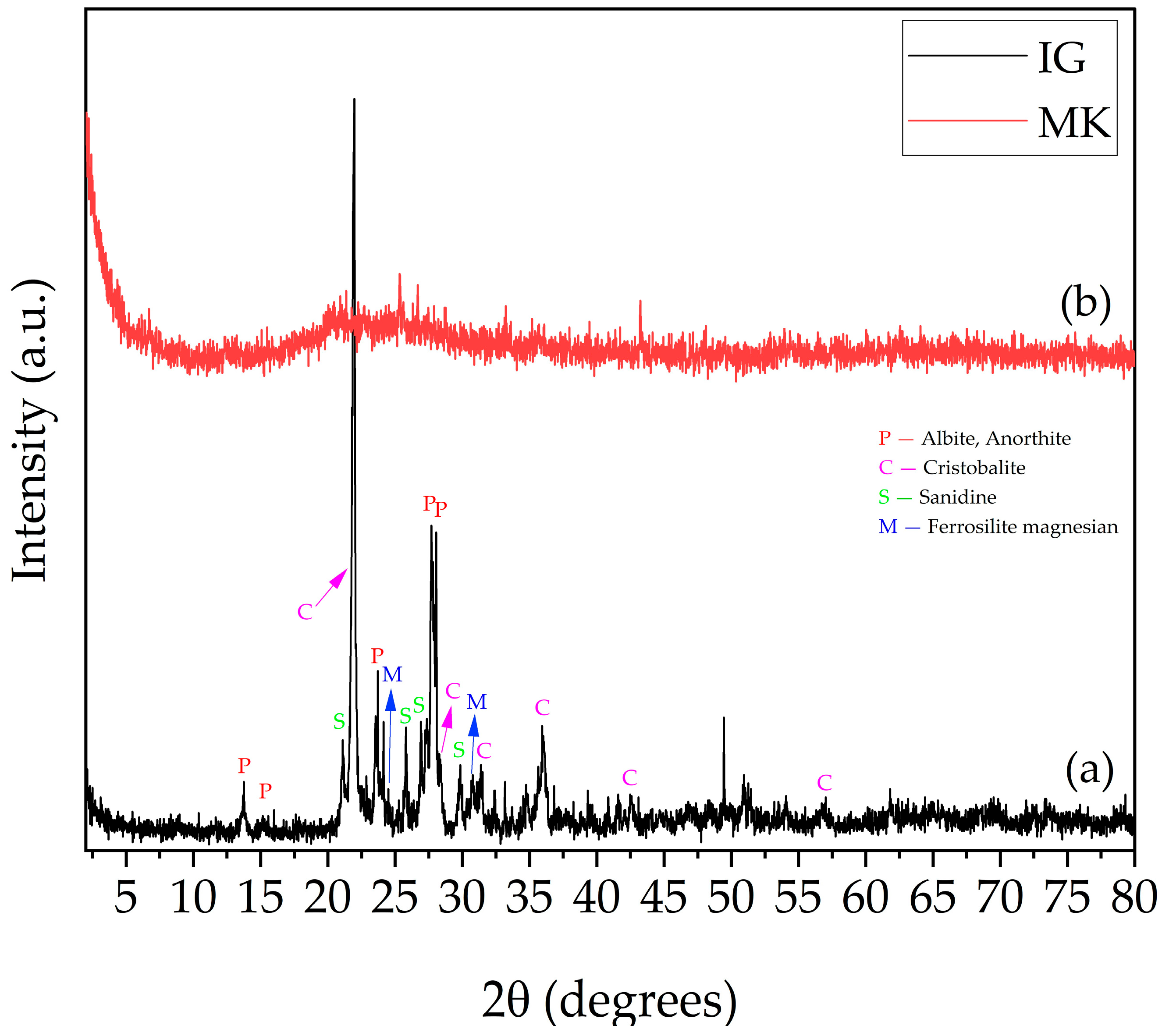
3.1. X-Ray Diffraction Analysis (XRD)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Scanning Electron Microscopy (SEM)
3.4. Chemical Composition of Raw Materials
3.5. Compressive Strength Result
3.5.1. Geopolymer Mortar—Group 1
3.5.2. Geopolymer Mortar—Group 2
3.5.3. Geopolymer Mortar—Group 3
3.5.4. Geopolymer Mortar—Group 4
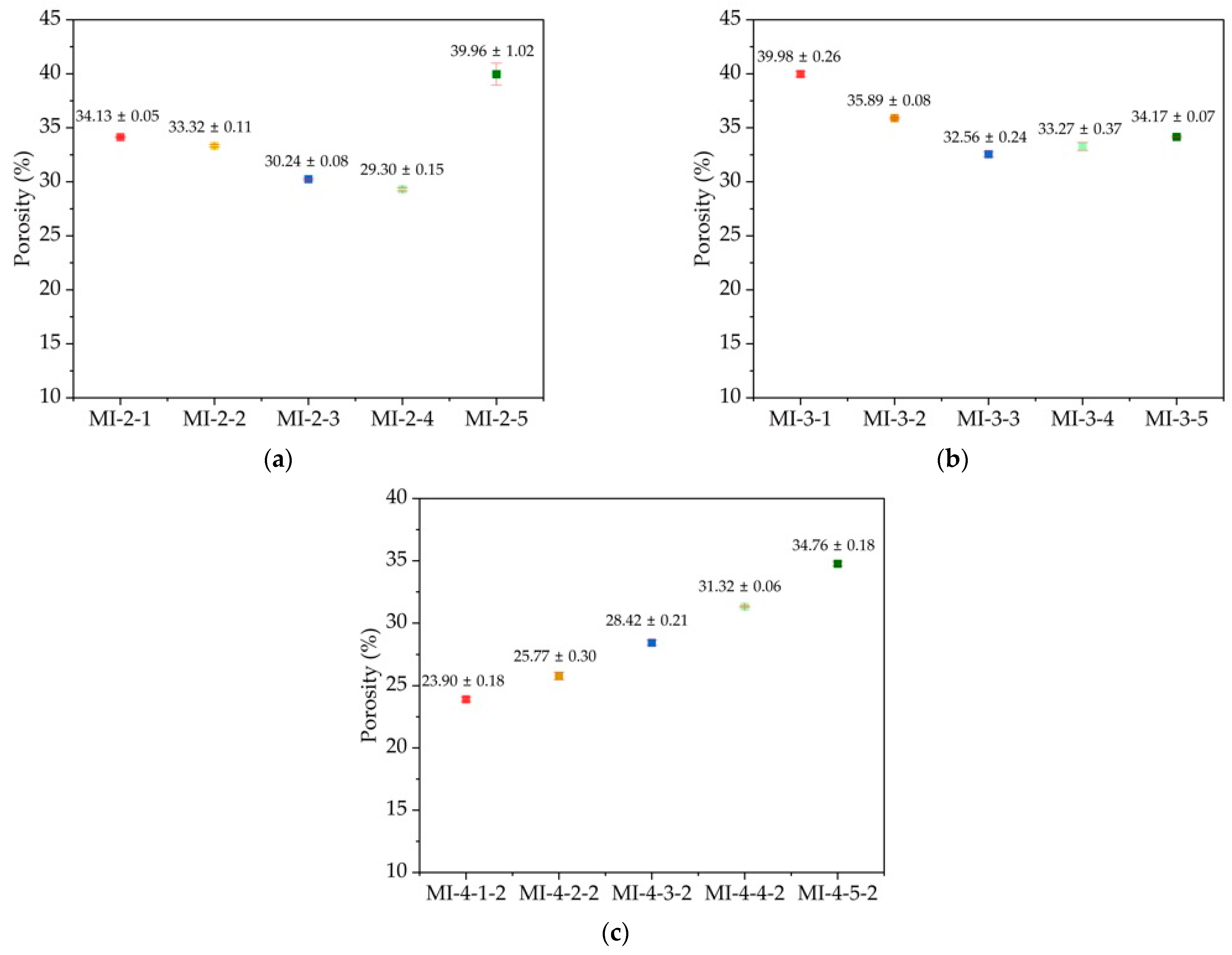
3.6. Porosity Results
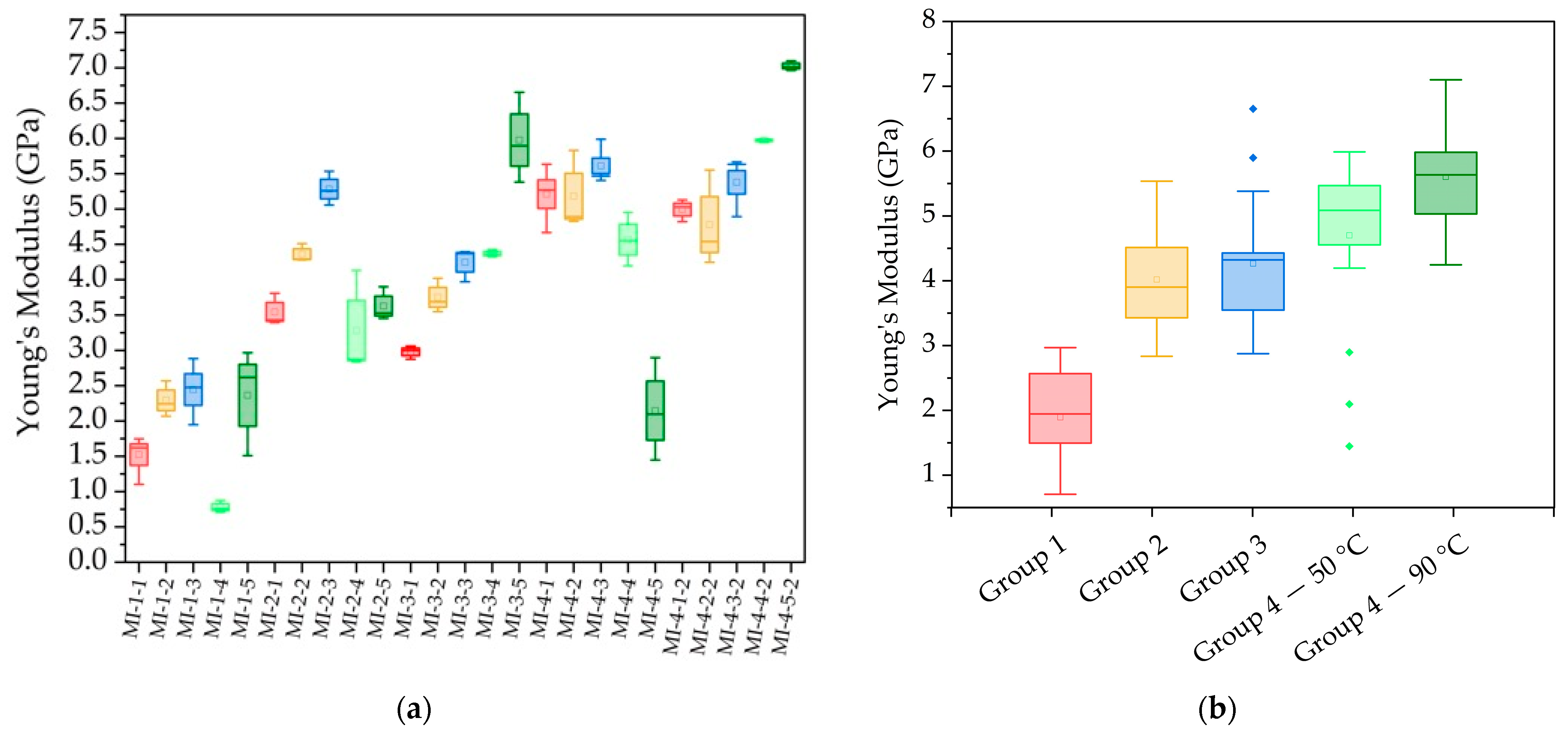
3.7. Young’s Modulus and the Influence of the SiO2/Al2O3 Molar Ratio
3.8. Fourier Transform Infrared Spectroscopy (FTIR) of Geopolymeric Mortars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capasso, I.; Liguori, B.; Ferone, C.; Caputo, D.; Cioffi, R. Strategies for the Valorization of Soil Waste by Geopolymer Production: An Overview. J. Clean Prod. 2021, 288, 125646. [Google Scholar] [CrossRef]
- Purchase, C.K.; Al Zulayq, D.M.; O’Brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Materials 2021, 15, 76. [Google Scholar] [CrossRef]
- Islam, N.; Sandanayake, M.; Muthukumaran, S.; Navaratna, D. Review on Sustainable Construction and Demolition Waste Management—Challenges and Research Prospects. Sustainability 2024, 16, 3289. [Google Scholar] [CrossRef]
- Rondinel-Oviedo, D.R. Construction and Demolition Waste Management in Developing Countries: A Diagnosis from 265 Construction Sites in the Lima Metropolitan Area. Int. J. Constr. Manag. 2023, 23, 371–382. [Google Scholar] [CrossRef]
- Ccanccapa Puma, J.; Hidalgo Valdivia, A.V.; Espinoza Vigil, A.J.; Booker, J. Preserving Heritage Riverine Bridges: A Hydrological Approach to the Case Study of the Grau Bridge in Peru. Heritage 2024, 7, 3350–3371. [Google Scholar] [CrossRef]
- de Silva, S.; Alzueta, J.; Salas, G. The Socioeconomic Consequences of the A.D. 1600 Eruption of Huaynaputina, Southern Peru. In Volcanic Hazards and Disasters in Human Antiquity; Geological Society of America: Boulder, CO, USA, 2000. [Google Scholar]
- Ramos-Guivar, J.A.; Alca-Ramos, Y.V.; Manrique-Castillo, E.V.; Mendoza-Villa, F.; Checca-Huaman, N.-R.; Rueda-Vellasmin, R.; Passamani, E.C. Pyroclastic Dust from Arequipa-Peru Decorated with Iron Oxide Nanoparticles and Their Ecotoxicological Properties in Water Flea D. Magna. Nanomaterials 2024, 14, 785. [Google Scholar] [CrossRef]
- Gałaś, A.; Panajew, P.; Cuber, P. Stratovolcanoes in the Western Cordillera—Polish Scientifi c Expedition to Peru 2003–2012 Reconnaissance Research. Geotourism/Geoturystyka 2014, 37, 61. [Google Scholar] [CrossRef]
- Cas, R.A.F.; Wright, J.V. Ignimbrites and Ignimbrite-Forming Eruptions. In Volcanic Successions Modern and Ancient; Springer: Dordrecht, The Netherlands, 1988; pp. 222–266. [Google Scholar]
- Barbhuiya, S.; Kanavaris, F.; Das, B.B.; Idrees, M. Decarbonising Cement and Concrete Production: Strategies, Challenges and Pathways for Sustainable Development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Shivaprasad, K.N.; Yang, H.-M.; Singh, J.K. A Path to Carbon Neutrality in Construction: An Overview of Recent Progress in Recycled Cement Usage. J. CO2 Util. 2024, 83, 102816. [Google Scholar] [CrossRef]
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting Future Carbon Emissions from Cement Production in Developing Countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Davidovits, J. (Ed.) Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère 16 rue Galilée F-02100: San Quintín, France, 2020; ISBN 9782954453118. [Google Scholar]
- Pradhan, P.; Dwibedy, S.; Pradhan, M.; Panda, S.; Panigrahi, S.K. Durability Characteristics of Geopolymer Concrete—Progress and Perspectives. J. Build. Eng. 2022, 59, 105100. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of Curing Temperature on the Development of Hard Structure of Metakaolin-Based Geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Alawi, A.; Milad, A.; Barbieri, D.; Alosta, M.; Alaneme, G.U.; Imran Latif, Q.B.a. Eco-Friendly Geopolymer Composites Prepared from Agro-Industrial Wastes: A State-of-the-Art Review. CivilEng 2023, 4, 433–453. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Onchiri, R.O.; Gathimba, N.; Sabuni, B. Mix Design Approaches of Eco-Friendly Geopolymer Concrete: A Critical Review. Hybrid Adv. 2024, 7, 100290. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; Machado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunelli, D.D. Effect of Cure Temperature on the Formation of Metakaolinite-Based Geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Sambucci, M.; Sibai, A.; Valente, M. Recent Advances in Geopolymer Technology. A Potential Eco-Friendly Solution in the Construction Materials Industry: A Review. J. Compos. Sci. 2021, 5, 109. [Google Scholar] [CrossRef]
- Al-Fakih, A.; Mahamood, M.A.A.; Al-Osta, M.A.; Ahmad, S. Performance and Efficiency of Self-Healing Geopolymer Technologies: A Review. Constr. Build. Mater. 2023, 386, 131571. [Google Scholar] [CrossRef]
- Özen, S.; Uzal, B. Effect of Characteristics of Natural Zeolites on Their Geopolymerization. Case Stud. Constr. Mater. 2021, 15, e00715. [Google Scholar] [CrossRef]
- Mohammed, Z.M.; Abdulhameed, A.A.; Kazim, H.K. Effect of Alkali—Activated Natural Pozzolan on Mechanical Properties of Geopolymer Concrete. Civ. Environ. Eng. 2022, 18, 312–320. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Volcanic Ash-Based Geopolymer Cements/Concretes: The Current State of the Art and Perspectives. Environ. Sci. Pollut. Res. 2017, 24, 4433–4446. [Google Scholar] [CrossRef] [PubMed]
- Iyigundogdu, Z.; Ürünveren, H.; Beycioğlu, A.; Ibadov, N. Antimicrobial Activity of Eco-Friendly Fly-Ash-Based Geopolymer Mortar. Materials 2025, 18, 1735. [Google Scholar] [CrossRef] [PubMed]
- Poltue, T.; Suddeepong, A.; Horpibulsuk, S.; Samingthong, W.; Arulrajah, A.; Rashid, A.S.A. Strength Development of Recycled Concrete Aggregate Stabilized with Fly Ash-Rice Husk Ash Based Geopolymer as Pavement Base Material. Road Mater. Pavement Des. 2020, 21, 2344–2355. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Alahmari, T.S.; Onchiri, R.O.; Gathimba, N.; Sabuni, B. Impact of Alkaline Concentration on the Mechanical Properties of Geopolymer Concrete Made up of Fly Ash and Sugarcane Bagasse Ash. Sustainability 2024, 16, 2841. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Onchiri, R.O.; Gathimba, N.; Sabuni, B. Effect of Sugarcane Bagasse Ash Addition and Curing Temperature on the Mechanical Properties and Microstructure of Fly Ash-Based Geopolymer Concrete. Open Ceram. 2024, 19, 100616. [Google Scholar] [CrossRef]
- Boumaza, A.; Khouadjia, M.L.K.; Isleem, H.F.; Hamdi, O.M.; Khishe, M. Effect of Blast Furnace Slag on the Fresh and Hardened Properties of Volcanic Tuff-Based Geopolymer Mortars. Sci. Rep. 2025, 15, 13651. [Google Scholar] [CrossRef]
- Muhammad, T.; Ahmad, W.; Ahmad, I.; Yaseen, M.; Ahmad, T. Investigating the Optimization of Activation Parameters and Comparative Mechanical Strength of the Waste Brick Dust-Based Geopolymer Cement. Int. J. Geotech. Eng. 2025, 19, 441–456. [Google Scholar] [CrossRef]
- Neves, R.; de Brito, J.; Bravo, M. Performance of Concrete Produced with Binders Obtained through Alkali-Activation of Different Construction and Demolition Wastes. Constr. Build. Mater. 2025, 473, 141060. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Şahmaran, M. Development and Fracture Characterization of Enhanced Ductile Engineered Geopolymer Composites Utilizing Construction and Demolition Waste-Based Recycled Binders and Aggregates. Constr. Build. Mater. 2025, 472, 140927. [Google Scholar] [CrossRef]
- Altawil, H.; Olgun, M. Optimization of Mechanical Properties of Geopolymer Mortar Based on Class C Fly Ash and Silica Fume: A Taguchi Method Approach. Case Stud. Constr. Mater. 2025, 22, e04332. [Google Scholar] [CrossRef]
- Lakshmi, R.; Kathirvel, P.; Sakthivel, E.; Suburam, R.M. Influence of Nano Silica and Metakaolin on the Strength and Durability Properties of Geopolymer Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2025. [Google Scholar] [CrossRef]
- Doan, T.; Arulrajah, A.; Horpibulsuk, S.; Zhou, A. Low-Carbon Metakaolin Precursor for One-Part and Two-Part Geopolymer Activation of Demolition Wastes. Constr. Build. Mater. 2024, 442, 137663. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of Metakaolin on the Properties of Mortar and Concrete: A Review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Khatib, J.M.; Baalbaki, O.; ElKordi, A.A. Metakaolin. In Waste and Supplementary Cementitious Materials in Concrete; Elsevier: Amsterdam, The Netherlands, 2018; pp. 493–511. [Google Scholar]
- Toprak, S.M.U.; Polat, R.; Levet, A.; Toprak, Ş.N. Effect of Stone Color, Dosage and Alkali Type on Ahlat Stone (Volcanic Origin) Based Geopolymer Concretes. J. Build. Eng. 2023, 67, 106059. [Google Scholar] [CrossRef]
- Huamán-Mamani, F.A.; Palomino-Ñaupa, C.K.; Orta Cuevas, M.d.M.; Medina-Carrasco, S. Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru. Geosciences 2024, 14, 80. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Palmeira, D.K.O.; Osajima, J.A.; Garcia, R.R.P.; Huamán-Mamani, F.A. Fabrication of Metakaolin/Ignimbrite Geopolymer from the Añashuayco Quarry in Arequipa. Mater. Sci. Forum 2024, 1137, 93–99. [Google Scholar] [CrossRef]
- Mohajerani, A.; Suter, D.; Jeffrey-Bailey, T.; Song, T.; Arulrajah, A.; Horpibulsuk, S.; Law, D. Recycling Waste Materials in Geopolymer Concrete. Clean Technol Env. Policy 2019, 21, 493–515. [Google Scholar] [CrossRef]
- INACAL NTP 334.051:2022; Cements. Determining Compressive Strength of Hydraulic Cement Mortars Using 50 Mm Cubic Specimens Sidewides. Test Method. National Institute of Quality—INACAL: Lima, Peru, 2022.
- Mo, B.; Zhu, H.; Cui, X.; He, Y.; Gong, S. Effect of Curing Temperature on Geopolymerization of Metakaolin-Based Geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Qu, C.; Qin, Y.; Wang, T. From Cement to Geopolymers: Performances and Sustainability Advantages of Ambient Curing. J. Build. Eng. 2024, 91, 109555. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of Geopolymer Mortar under Ambient Temperature for in Situ Applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Zulkarnain, F.; Wibowo, S.; Sulieman, M.Z. Evaluating the Elastic Modulus of Geopolymer Concrete: The Impact of Fly Ash as a Cement Substitute Using a No-Soaking Method for Sustainable Construction. J. Adv. Res. Des. 2025, 126, 53–68. [Google Scholar] [CrossRef]
- Alomayri, T.; Raza, A.; Elhadi, K.M.; Shaikh, F. Mechanical, Microstructural, and Thermal Characterization of Geopolymer Composites with Nano-alumina Particles and Micro Steel Fibers. Struct. Concr. 2025, 26, 1540–1559. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An Open-Access Collection of Crystal Structures and Platform for World-Wide Collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- PDF-2 Powder Diffraction File Database; Release 2003; International Centre for Diffraction Data: Newtown Square, PA, USA, 2003.
- Wang, H.; Li, H.; Wang, Y.; Yan, F. Preparation of Macroporous Ceramic from Metakaolinite-Based Geopolymer by Calcination. Ceram. Int. 2015, 41, 11177–11183. [Google Scholar] [CrossRef]
- Menshaz, A.M.; Johari, M.A.M.; Ahmad, Z.A. Characterization of Metakaolin Treated at Different Calcination Temperatures. AIP Conf. Proc. 2017, 1892, 020028. [Google Scholar]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; da Silva, E.F.; Velosa, A.; Rocha, F. The Effect of Natural Zeolite on Microstructure, Mechanical and Heavy Metals Adsorption Properties of Metakaolin Based Geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Abiodun, Y.O.; Olanrewaju, O.A.; Gbenebor, O.P.; Ochulor, E.F.; Obasa, D.V.; Adeosun, S.O. Cutting Cement Industry CO2 Emissions through Metakaolin Use in Construction. Atmosphere 2022, 13, 1494. [Google Scholar] [CrossRef]
- Ertekin, B.; Cimen, Z.; Yilmaz, H.; Yilmaz, U. Synthesis and Characterization of Polyaniline/Ignimbrite Nano-Composite Material. J. Mater. Sci. Eng. 2016, 5, 1000237. [Google Scholar] [CrossRef]
- Provis, J.L.; Yong, S.L.; van Deventer, J.S.J. Characterising the Reaction of Metakaolin in an Alkaline Environment by XPS, and Time- and Spatially-Resolved FTIR Spectroscopy. In Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2015; pp. 299–304. [Google Scholar]
- Kamarudin, N.H.; Harun, Z.; Othman, M.H.D.; Abdullahi, T.; Syamsul Bahri, S.; Kamarudin, N.H.; Yunos, M.Z.; Wan Salleh, W.N. Waste Environmental Sources of Metakaolin and Corn Cob Ash for Preparation and Characterisation of Green Ceramic Hollow Fibre Membrane (h-MCa) for Oil-Water Separation. Ceram. Int. 2020, 46, 1512–1525. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of Geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Ghaebi Panah, N. Effects of Initial SiO2/Al2O3 Molar Ratio and Slag on Fly Ash-Based Ambient Cured Geopolymer Properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Bowen, F.; Jiesheng, L.; Jing, W.; Yaohua, C.; Tongtong, Z.; Xiaoming, T.; Zhengguang, S. Investigation on the Impact of Different Activator to Solid Ratio on Properties and Micro-Structure of Metakaolin Geopolymer. Case Stud. Constr. Mater. 2022, 16, e01127. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer Concrete with Metakaolin for Sustainability: A Comprehensive Review on Raw Material’s Properties, Synthesis, Performance, and Potential Application. Environ. Sci. Pollut. Res. 2022, 30, 25299–25324. [Google Scholar] [CrossRef]
- Abuowda, E.; El-Hassan, H.; El-Maaddawy, T. Characterization of Geopolymer Masonry Mortars Incorporating Recycled Fine Aggregates. Sustainability 2024, 16, 8147. [Google Scholar] [CrossRef]
- Dihaji, H.; Azerkane, D.; Bih, L.; Essaddek, A.; Haily, E.M. Comparative Study of Geopolymers Synthesized with Alkaline and Acid Reactants at Various Liquid-to-Solid Ratios Using Moroccan Kaolin Clay. Constr. Build. Mater. 2025, 468, 140453. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The Effect of Temperature on the Geopolymerization Process of a Metakaolin-Based Geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Migunthanna, J.; Rajeev, P.; Sanjayan, J. Investigation of Waste Clay Brick as Partial Replacement in Geopolymer Binder. Constr. Build. Mater. 2023, 365, 130107. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Azevedo, A.G.d.S.; Strecker, K.; Lombardi, C.T. Metakaolin and Red Ceramic Based Geopolymers Production. Cerâmica 2018, 64, 371. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M. Carbonation in the Pore Solution of Metakaolin-Based Geopolymer. Cem. Concr. Res. 2016, 88, 227–235. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Gharzouni, A.; Hyvernaud, E.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Influence of the Nature and Amount of Carbonate Additions on the Thermal Behaviour of Geopolymers: A Model for Prediction of Shrinkage. Constr. Build. Mater. 2021, 296, 123752. [Google Scholar] [CrossRef]
- Aboulayt, A.; Riahi, M.; Ouazzani Touhami, M.; Hannache, H.; Gomina, M.; Moussa, R. Properties of Metakaolin Based Geopolymer Incorporating Calcium Carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]






| Group | Mixture ID | Proportion % | MK (g) | IG (g) | Fine Sand (Sieved 2 mm) | Liquid to Solid Ratio (L/S) | NaOH 9.0 mol/L (mL) | Drying |
|---|---|---|---|---|---|---|---|---|
| 1 | MI-1-1 | 100–0 | 500 | 0 | 1375 | 0.343 | 171.64 | 28 days * |
| MI-1-2 | 80–20 | 400 | 100 | 1375 | 0.343 | 171.64 | 28 days * | |
| MI-1-3 | 50–50 | 250 | 250 | 1375 | 0.343 | 171.64 | 28 days * | |
| MI-1-4 | 20–80 | 100 | 400 | 1375 | 0.343 | 171.64 | 28 days * | |
| MI-1-5 | 0–100 | 0 | 500 | 1375 | 0.343 | 171.64 | 28 days * | |
| 2 | MI-2-1 | 100–0 | 500 | 0 | 1375 | 0.484 | 242 | 28 days * |
| MI-2-2 | 80–20 | 400 | 100 | 1375 | 0.484 | 242 | 28 days * | |
| MI-2-3 | 50–50 | 250 | 250 | 1375 | 0.484 | 242 | 28 days * | |
| MI-2-4 | 20–80 | 100 | 400 | 1375 | 0.484 | 242 | 28 days * | |
| MI-2-5 | 0–100 | 0 | 500 | 1375 | 0.484 | 242 | 28 days * | |
| 3 | MI-3-1 | 100–0 | 500 | 0 | 1375 | 0.484 | 242 | T = 25 °C—24 h; T = 50 °C—48 h |
| MI-3-2 | 80–20 | 400 | 100 | 1375 | 0.484 | 242 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-3-3 | 50–50 | 250 | 250 | 1375 | 0.484 | 242 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-3-4 | 20–80 | 100 | 400 | 1375 | 0.484 | 242 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-3-5 | 0–100 | 0 | 500 | 1375 | 0.484 | 242 | T = 25 °C—24 h; T = 50 °C—48 h | |
| 4 | MI-4-1 | 100–0 | 500 | 0 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h |
| MI-4-1-2 | 100–0 | 500 | 0 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h, and 90 °C—12 h | |
| MI-4-2 | 80–20 | 400 | 100 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-4-2-2 | 80–20 | 400 | 100 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h, and 90 °C—12 h | |
| MI-4-3 | 50–50 | 250 | 250 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-4-3-2 | 50–50 | 250 | 250 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h, and 90 °C—12 h | |
| MI-4-4 | 20–80 | 100 | 400 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-4-4-2 | 20–80 | 100 | 400 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h, and 90 °C—12 h | |
| MI-4-5 | 0–100 | 0 | 500 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h | |
| MI-4-5-2 | 0–100 | 0 | 500 | 1375 | 0.6 | 300 | T = 25 °C—24 h; T = 50 °C—48 h, and 90 °C—12 h |
| Element | IG | MK | Fine Sand |
|---|---|---|---|
| % | % | % | |
| SiO2 | 73.56 | 47.68 | 57.78 |
| Al2O3 | 13.31 | 43.02 | 17.3 |
| Na2O | 4.27 | 0.07 | 4.17 |
| K2O | 4.1 | 0.26 | 1.9 |
| Fe2O3 | 1.54 | 3.29 | 6.89 |
| CaO | 1.02 | 0.56 | 6.08 |
| TiO2 | 0.2 | 1.91 | 0.85 |
| MgO | 0.25 | 0.2 | 3.32 |
| Mn3O4 | 0.08 | <L.C. | 0.11 |
| P2O5 | 0.05 | 0.06 | 0.21 |
| SO3 | 0.02 | 0.27 | 0.07 |
| ZrO2 | 0.02 | 0.09 | 0.03 |
| V2O5 | <L.C. | 0.04 | 0.03 |
| Cr2O3 | <L.C. | <L.C. | <L.C. |
| SrO | <L.C. | <L.C. | 0.09 |
| P.C. | 1.42 | 2.49 | 1.05 |
| TOTAL | 100 | 100 | 100 |
| Proportion (MK/IG) % | SiO2/Al2O3 |
|---|---|
| 100–0 | 3.87 |
| 80–20 | 4.25 |
| 50–50 | 4.92 |
| 20–80 | 5.77 |
| 0–100 | 6.48 |
| Group | Mixture ID | Proportion % | Compression Strength |
|---|---|---|---|
| 1 | MI-1-1 | 100–0 | 1.47 ± 0.33 |
| MI-1-2 | 80–20 | 2.82 ± 0.26 | |
| MI-1-3 | 50–50 | 4.66 ± 0.48 | |
| MI-1-4 | 20–80 | 0.59 ± 0.13 | |
| MI-1-5 | 0–100 | 2.09 ± 0.34 | |
| 2 | MI-2-1 | 100–0 | 5.90 ± 1.92 |
| MI-2-2 | 80–20 | 8.66 ± 1.12 | |
| MI-2-3 | 50–50 | 11.73 ± 1.41 | |
| MI-2-4 | 20–80 | 6.73 ± 1.37 | |
| MI-2-5 | 0–100 | 4.80 ± 0.33 | |
| 3 | MI-3-1 | 100–0 | 5.14 ± 0.78 |
| MI-3-2 | 80–20 | 8.03 ± 0.29 | |
| MI-3-3 | 50–50 | 9.76 ± 0.16 | |
| MI-3-4 | 20–80 | 10.78 ± 0.12 | |
| MI-3-5 | 0–100 | 17.67 ± 0.66 | |
| 4 | MI-4-1 | 100–0 | 12.13 ± 0.56 |
| MI-4-1-2 | 100–0 | 13.91 ± 0.42 | |
| MI-4-2 | 80–20 | 11.83 ± 0.04 | |
| MI-4-2-2 | 80–20 | 12.11 ± 0.37 | |
| MI-4-3 | 50–50 | 12.43 ± 0.57 | |
| MI-4-3-2 | 50–50 | 10.83 ± 0.39 | |
| MI-4-4 | 20–80 | 11.89 ±1.29 | |
| MI-4-4-2 | 20–80 | 14.62 ± 1.29 | |
| MI-4-5 | 0–100 | 6.46 ± 0.88 | |
| MI-4-5-2 | 0–100 | 18.68 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, A.Í.S.; Palmeira, D.K.O.; Nascimento, A.M.D.S.S.; Osajima, J.A.; Garcia, R.R.P.; Huamán-Mamani, F.A. Development of Geopolymeric Mortar from Metakaolin and Ignimbrite from the Añashuayco Quarries, Peru, for Civil Construction. Sustainability 2025, 17, 5714. https://doi.org/10.3390/su17135714
Morais AÍS, Palmeira DKO, Nascimento AMDSS, Osajima JA, Garcia RRP, Huamán-Mamani FA. Development of Geopolymeric Mortar from Metakaolin and Ignimbrite from the Añashuayco Quarries, Peru, for Civil Construction. Sustainability. 2025; 17(13):5714. https://doi.org/10.3390/su17135714
Chicago/Turabian StyleMorais, Alan Ícaro Sousa, Daniela Krisbéll Ortega Palmeira, Ariane Maria Da Silva Santos Nascimento, Josy Anteveli Osajima, Ramón Raudel Peña Garcia, and Fredy Alberto Huamán-Mamani. 2025. "Development of Geopolymeric Mortar from Metakaolin and Ignimbrite from the Añashuayco Quarries, Peru, for Civil Construction" Sustainability 17, no. 13: 5714. https://doi.org/10.3390/su17135714
APA StyleMorais, A. Í. S., Palmeira, D. K. O., Nascimento, A. M. D. S. S., Osajima, J. A., Garcia, R. R. P., & Huamán-Mamani, F. A. (2025). Development of Geopolymeric Mortar from Metakaolin and Ignimbrite from the Añashuayco Quarries, Peru, for Civil Construction. Sustainability, 17(13), 5714. https://doi.org/10.3390/su17135714









