Can the Urea Fatty Fraction Support Sustainable Agriculture in the Improvement of Soil Properties?
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Material Used in the Incubation Experiment
2.2. Incubation Condition
2.3. Soil Chemical Analyses
2.4. Soil Enzyme Activity
2.5. Micromorphological Methods
2.6. Statistical Analyses
3. Results
3.1. Soil Properties
3.1.1. pH Value
3.1.2. Carbon Content
3.1.3. Nitrogen Content
3.2. Enzyme Activity
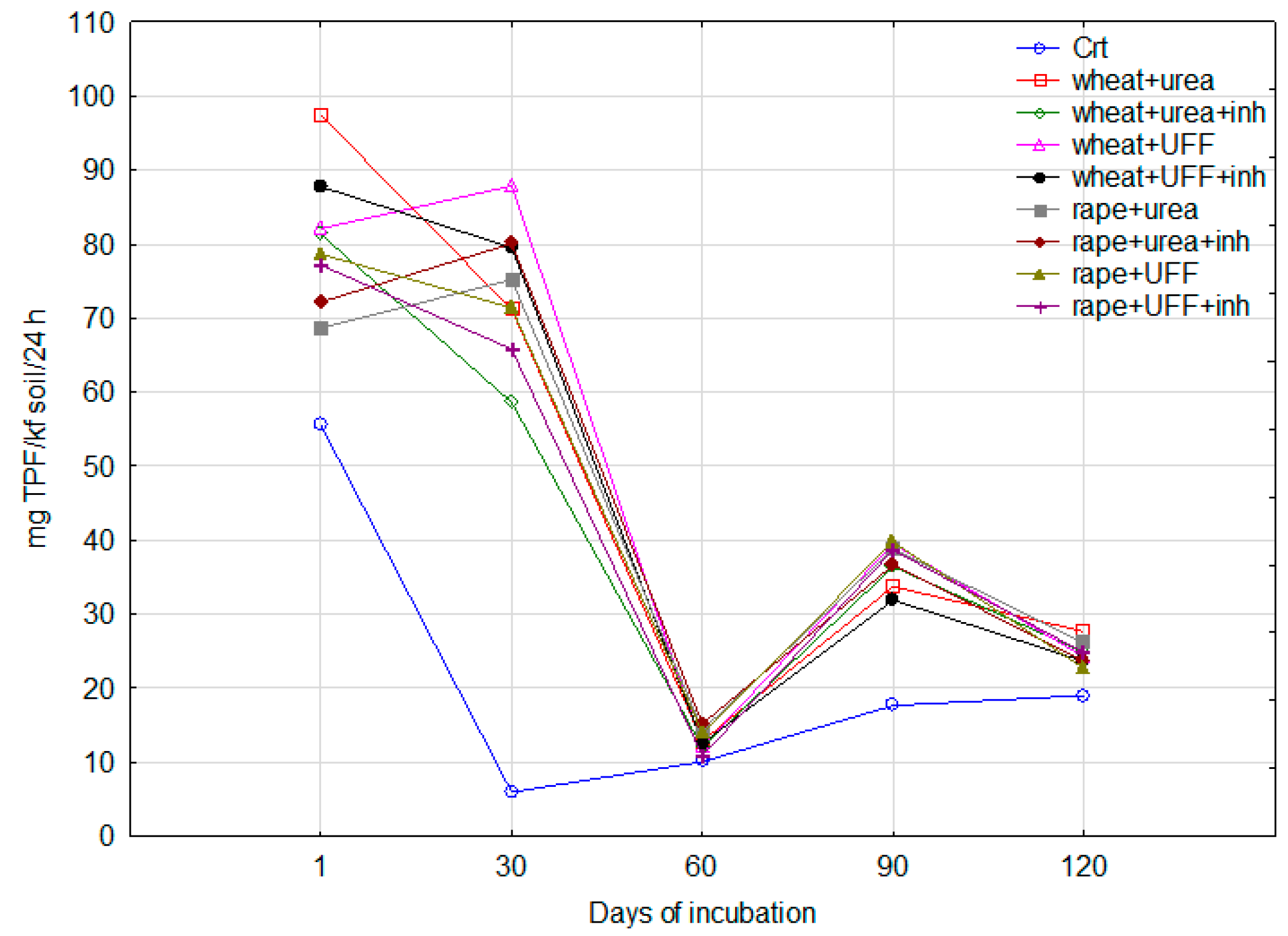
3.2.1. Dehydrogenase Activity
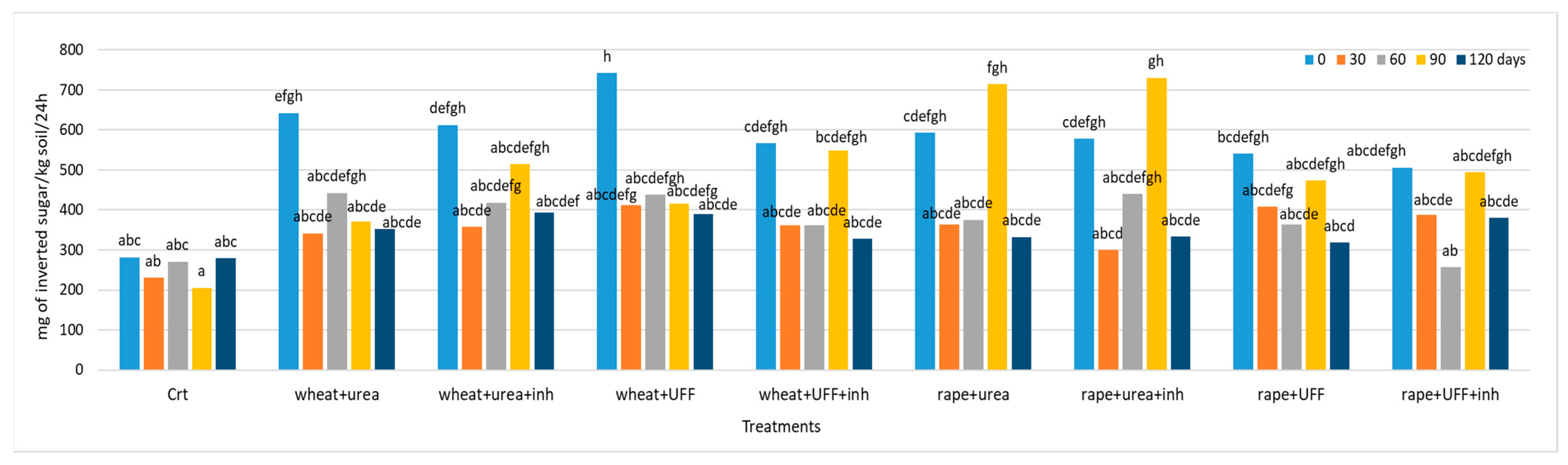
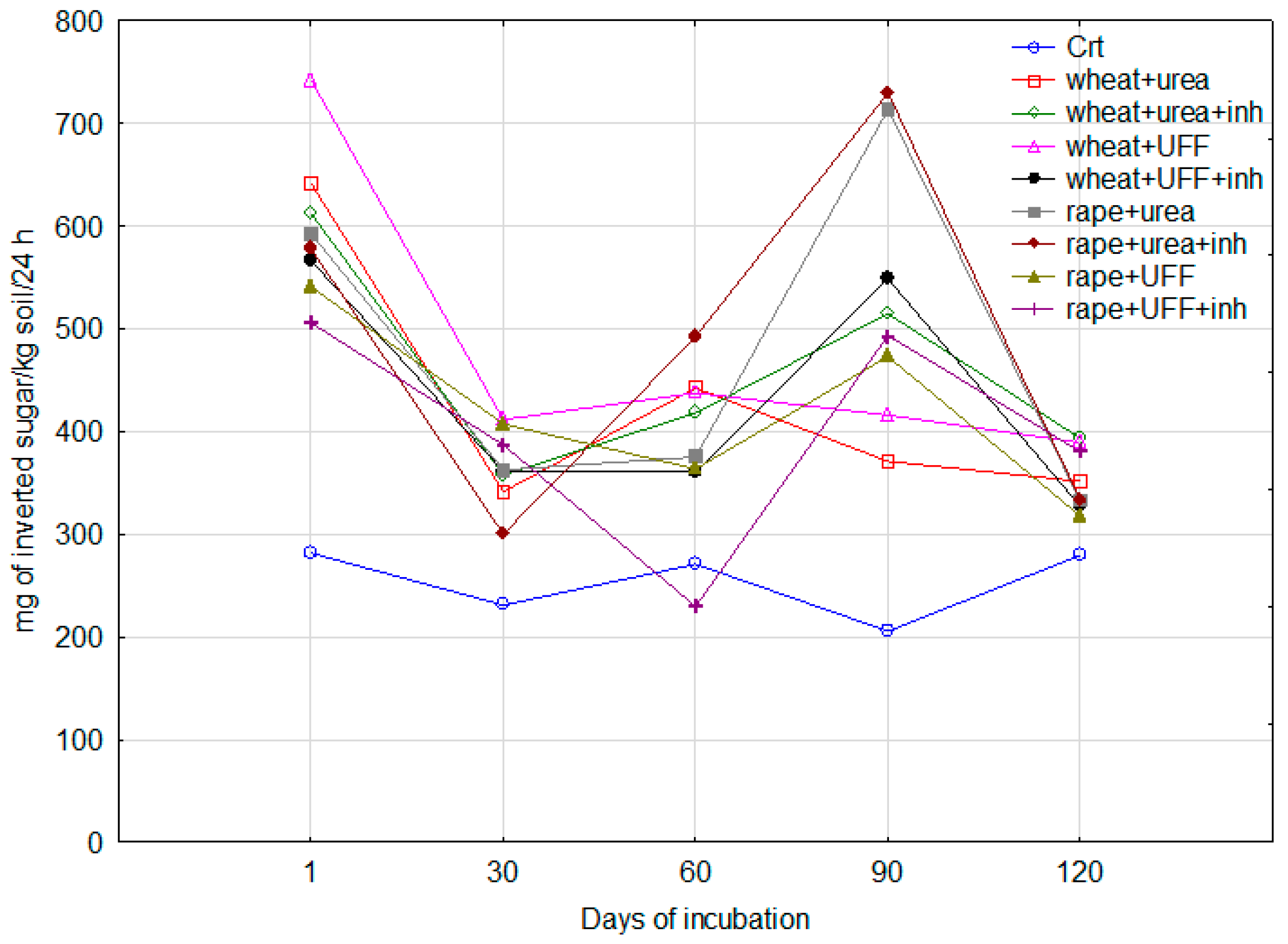
3.2.2. Invertase Activity
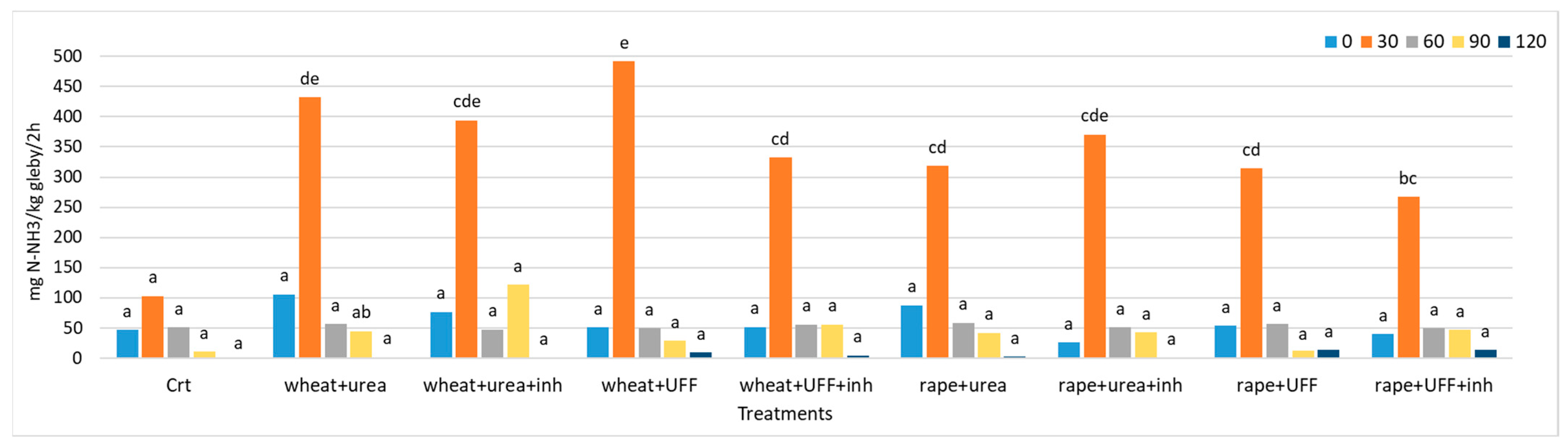
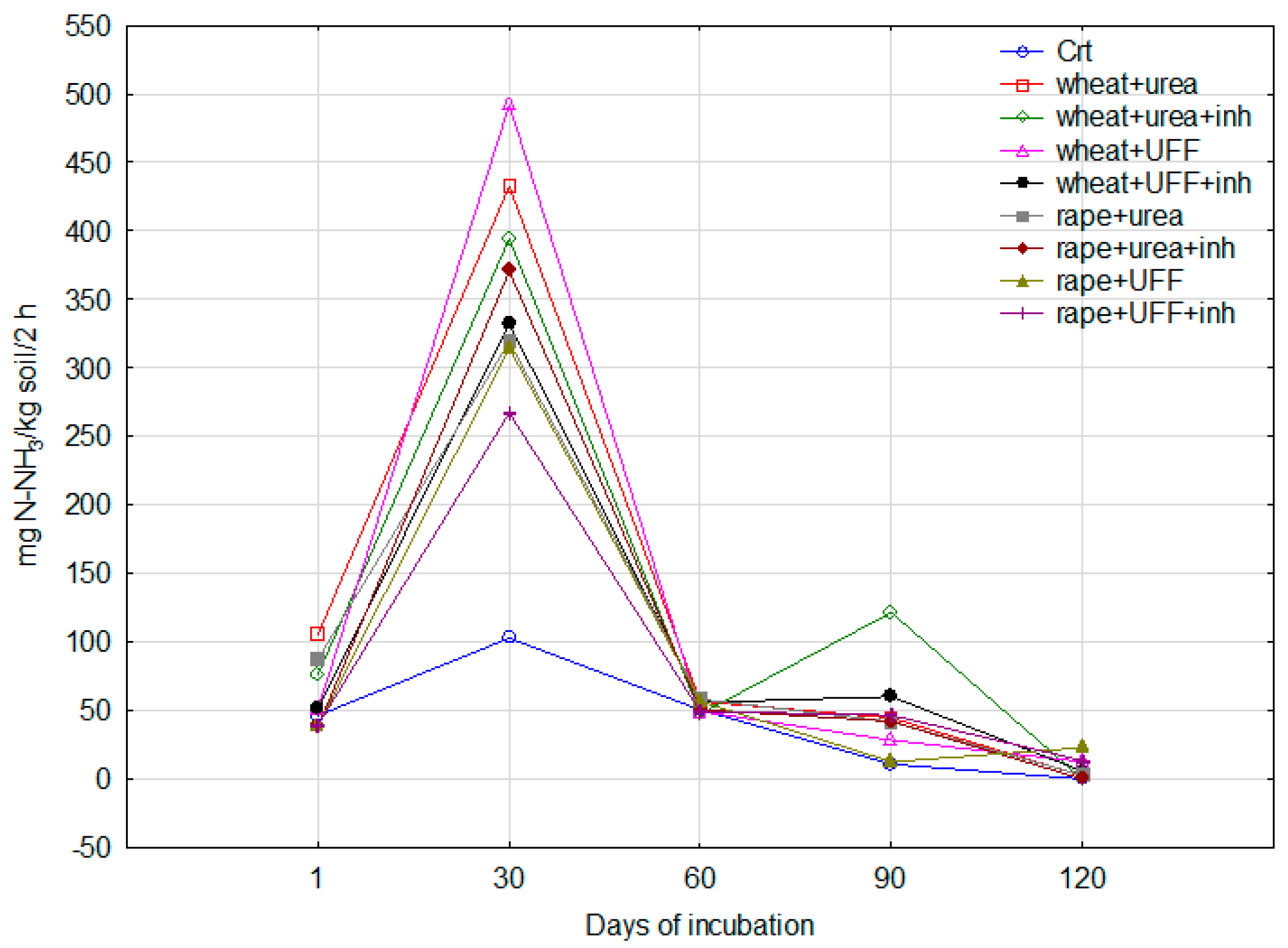
3.2.3. Urease Activity
3.3. Micromorphological Studies
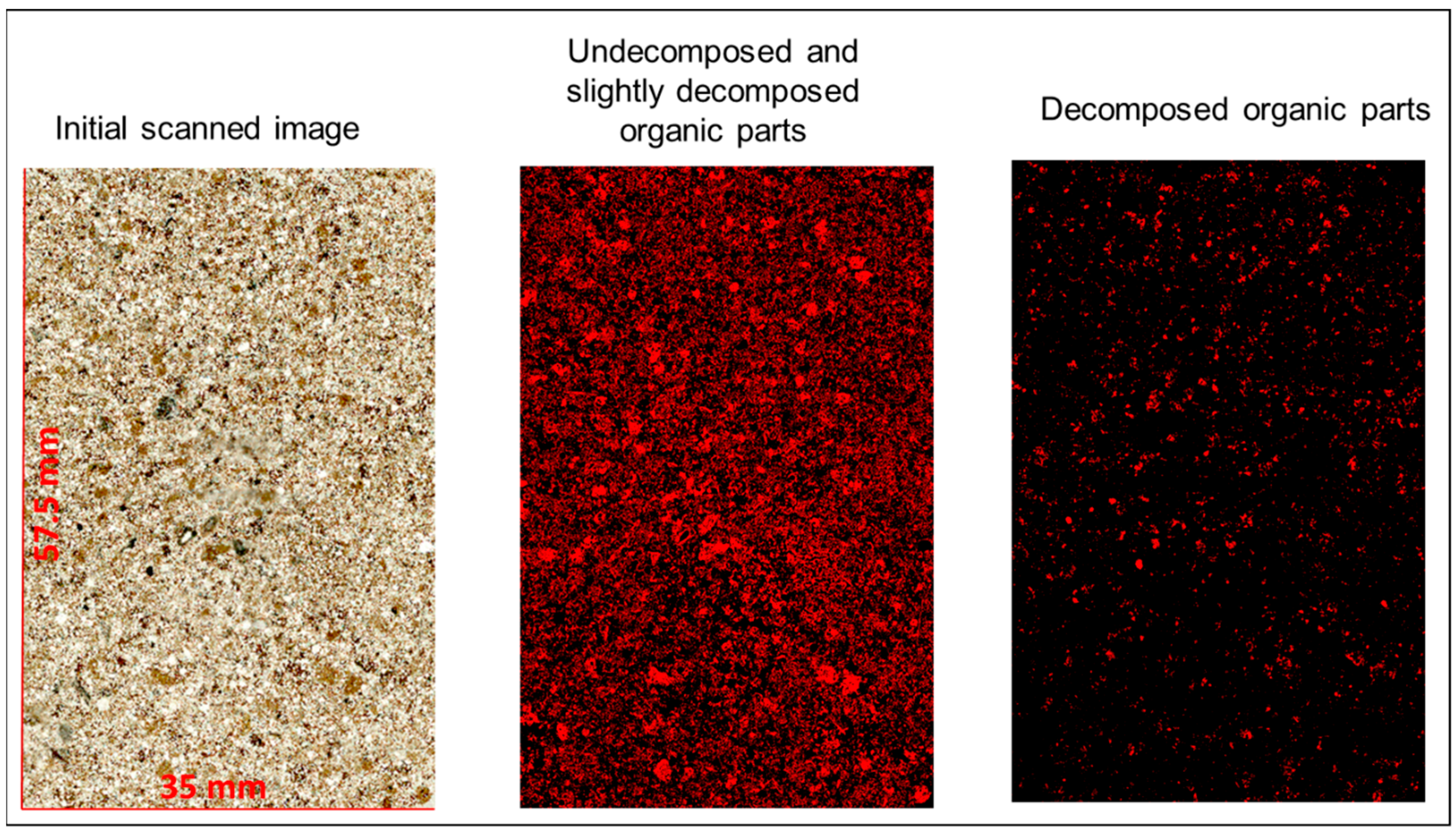
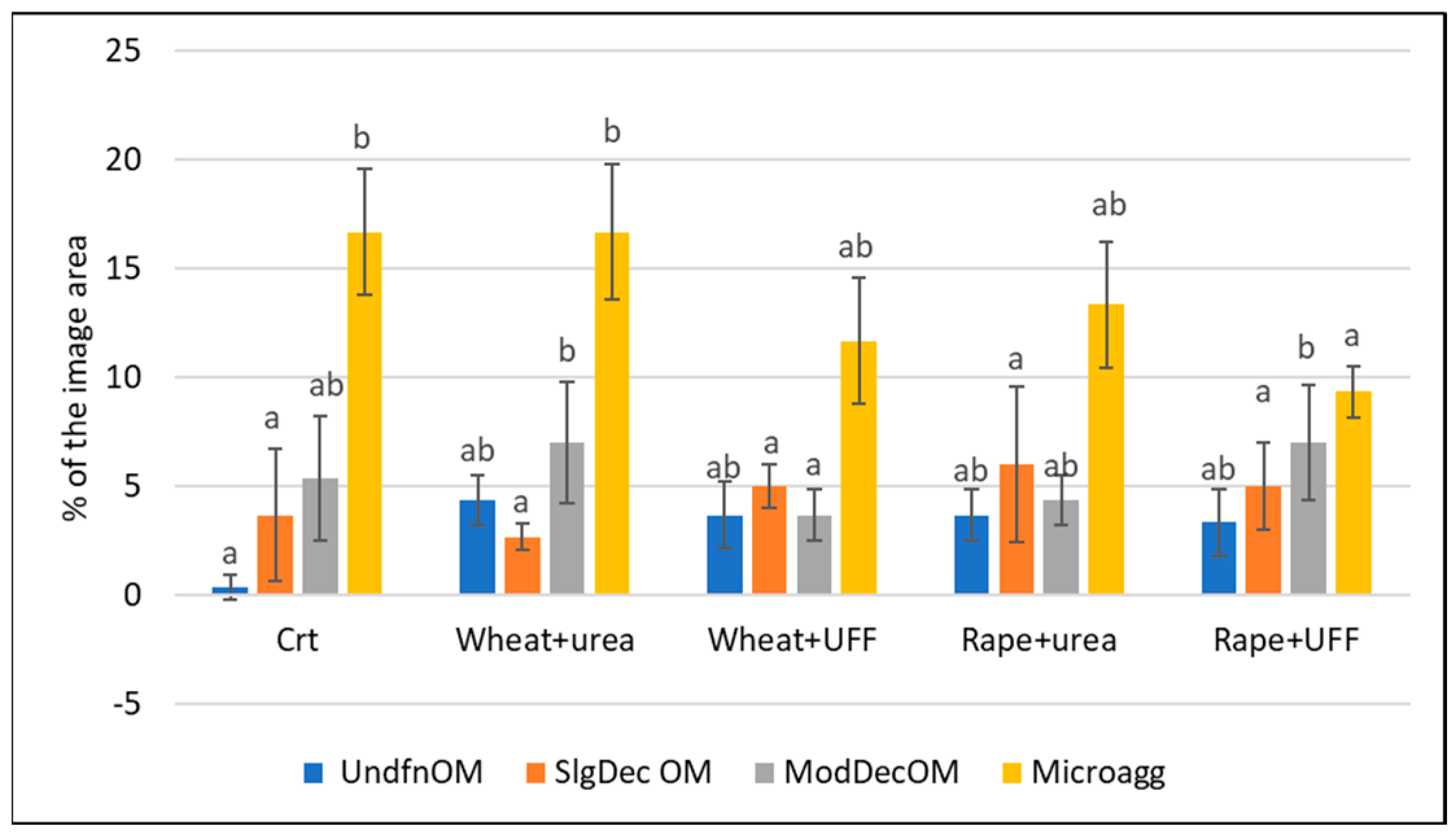
3.3.1. Image Analysis of the Organic Matter
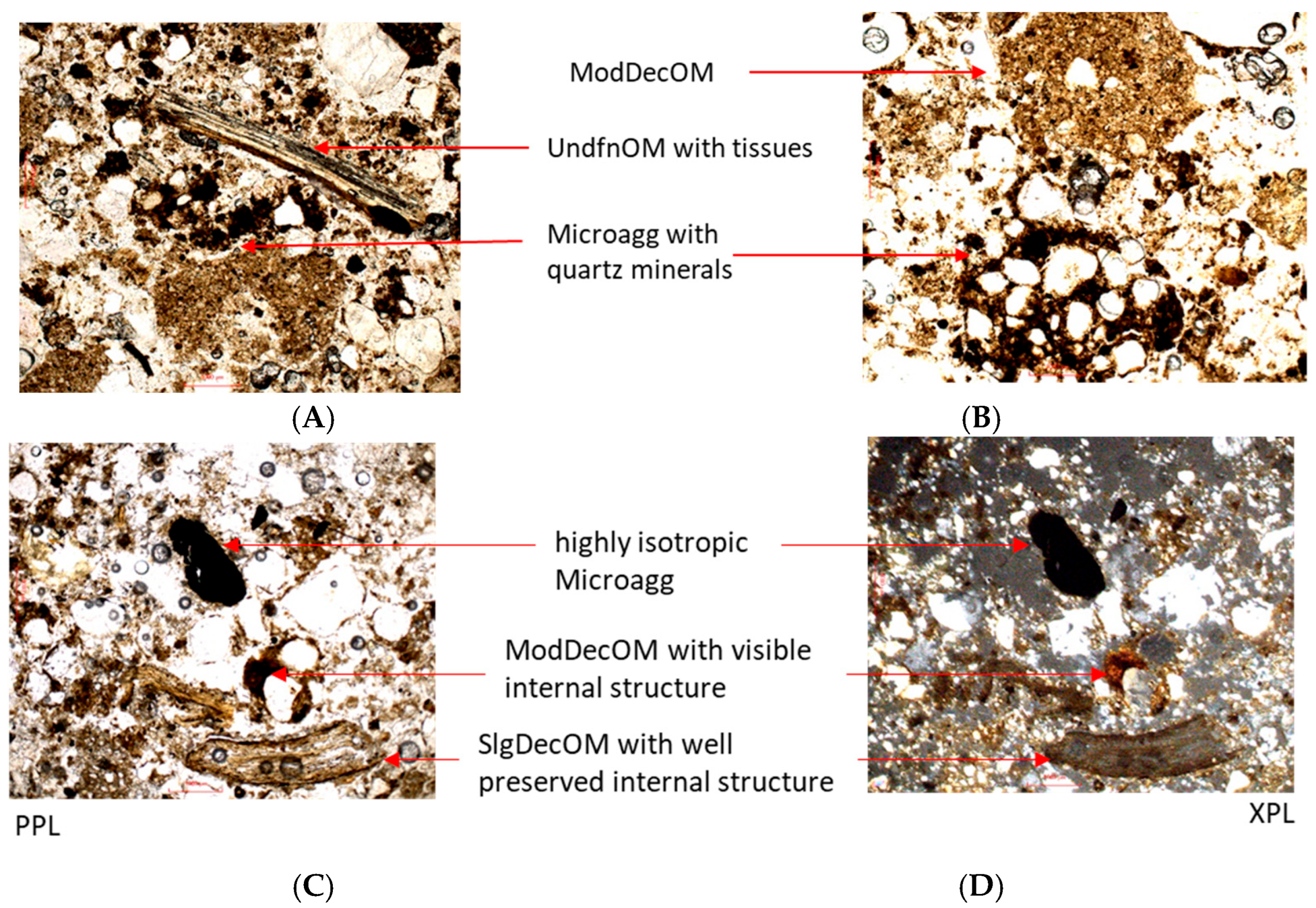
3.3.2. Organic Matter Decomposition Studied Under Optical Microscopy
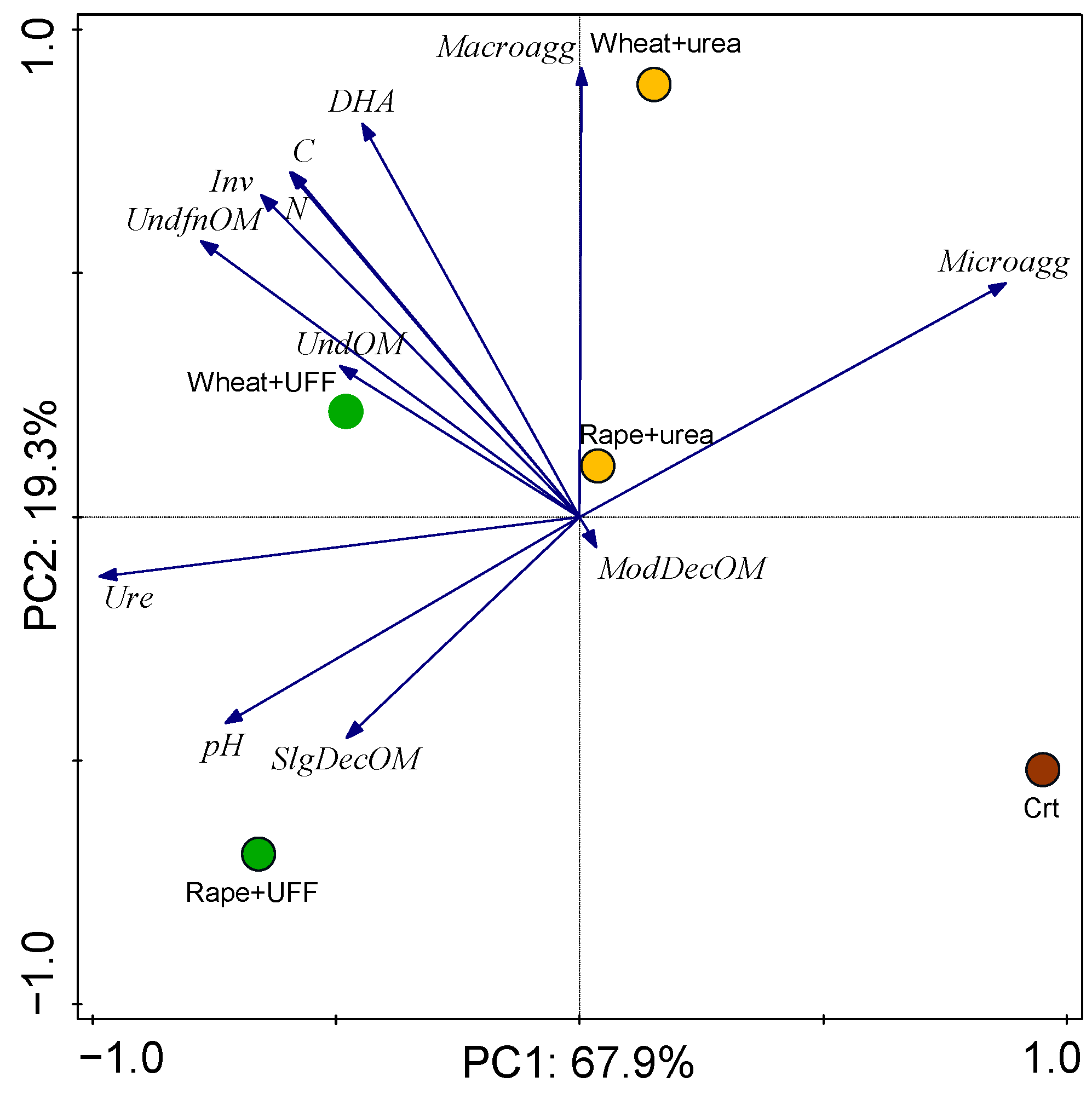
3.3.3. Results of PCA
4. Discussion
4.1. Changes in Soil pH and Carbon and Nitrogen Contents and Their Relationship with Enzyme Activity as a Result of Introducing Fertilizer and Straw into the Soil
4.2. Implications of the Application of Urea and Straw on the Transformation of Soil Organic Matter
4.3. Main Factors Shaping Treated Soil Properties at the End of the Experiment
4.4. The Practical Application Effect and Cost of UFF in the Field
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Jaworski, T.J.; Grochowska, S. Circular Economy—The criteria for achieving and the prospect of implementation in Poland. Arch. Waste Manag. Environ. Prot. 2017, 19, 13–22. (In Polish) [Google Scholar]
- Filipek-Mazur, B.; Pużyńska, K.; Tabak, M.; Pużyński, S. Enzymatic Activity of Soil under Spelt Grown in An Organic Farming System in Poland’s Temperate Climate. Agronomy 2020, 10, 930. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczókowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- ACT of 10 July 2007 on Fertilizers and Fertilization, Journal of Laws of 2024, Item 105; Chancellery of the Parliament of RP: Warszawa, Poland, 2007.
- Wiśniowska-Kielian, B.; Filipek-Mazur, B.; Gambuś, F. Possibility of Using the By-Product of Fatty Acid Extraction from Fish in Fertilization as an Element of the Circular Economy. Agronomy 2024, 14, 127. [Google Scholar] [CrossRef]
- Chen, W.; He, P.; Zhang, H.; Lü, F. Effects of volatile fatty acids on soil properties, microbial communities, and volatile metabolites in wheat rhizosphere of loess. J. Clean. Prod. 2024, 476, 143798. [Google Scholar] [CrossRef]
- Pikuła, D. Wpływ wieloletniego nawożenia słomą na plonowanie roślin i żyzność gleby. Stud. Rap. IUNG-PIB 2015, 45, 85–96. (In Polish) [Google Scholar] [CrossRef]
- Duer, I.; Fotyma, M.; Madej, A. (Eds.) Kodeks Dobrej Praktyki Rolniczej; Ministerstwo Rolnictwa i Rozwoju Wsi, Ministerstwo Środowiska: Warszawa, Poland, 2004; p. 98. (In Polish) [Google Scholar]
- Gardziuk, P. Gospodarcze Znaczenie i Możliwości Wykorzystania Słomy Na Cele Energetyczne W Polsce; Monografie i Rozprawy Naukowe; IUNG-PIB Puławy: Puławy, Poland, 2015; Volume 45, p. 173. [Google Scholar]
- Spöttle, M.; Alberici, S.; Toop, G.; Peters, D.; Gamba, L.; Ping, S.; van Steen, H.; Bellefleur, D. Cereal straw. In Low ILUC Potential of Wastes and Residues for Biofuels; Project no. BIEDE 13386/BIENL12798; ECOFYS Netherlands B.V.: Utrecht, The Netherlands, 2013; pp. 7–48. [Google Scholar]
- Harasim, A. Plony słomy wybranych zbóż w zależności od sposobu jej zbioru. Pol. J. Agron. 2016, 26, 34–38. (In Polish) [Google Scholar]
- Ludwicka, A.; Grzybek, A. Bilans biomasy rolnej (słomy) na potrzeby energetyki. Probl. Inżynierii Rol. 2010, 2, 101–111. (In Polish) [Google Scholar]
- Sun, R.C.; Tomkinson, J. Separation and Characterization of Cellulose from Wheat Straw. Sep. Sci. Technol. 2005, 39, 391–411. [Google Scholar] [CrossRef]
- Kuś, A.; Madej, A. Zagospodarowanie słomy a bilans materii organicznej. Zagadnienia Doradz. Rol. 2017, 90, 40–57. (In Polish) [Google Scholar]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in North-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Pang, D.; Yin, Y.; Han, M.; Li, Y.; Luo, Y.; Xu, X.; Li, Y.; Wang, Z. Straw return and appropriate tillage method improve grain yield and nitrogen efficiency of winter wheat. J. Integr. Agric. 2017, 16, 1708–1719. [Google Scholar] [CrossRef]
- Tian, P.; Lian, H.; Wang, Z.; Jiang, Y.; Li, C.; Sui, P.; Qi, H. Effects of deep and shallow tillage with straw incorporation on soil organic carbon, total nitrogen and enzyme activities in Northeast China. Sustainability 2020, 12, 8679. [Google Scholar] [CrossRef]
- Stoops, G. Guidelines for Analysis and Description of Soil and Regolith Thin Sections, 2nd ed.; SSSA: Madison, WI, USA, 2003. [Google Scholar]
- Ciarkowska, K. Organic matter transformation and porosity development in non-reclaimed mining soils of different ages and vegetation covers: A field study of soils of the zinc and lead ore area in SE Poland. J. Soils Sediments 2017, 17, 2066–2079. [Google Scholar] [CrossRef]
- Zaiets, O.; Poch, R.M. Micromorphology of organic matter and humus in Mediterranean mountain soils. Geoderma 2016, 272, 83–92. [Google Scholar] [CrossRef]
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998.
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for NAMING Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Tan, K.T. Soil Sampling, Preparation and Analysis; Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Elementar Analysensysteme GmbH. Operating Instructions Vario MAX Cube Analyzer; Version 12.11.2013; Elementar Analysensysteme GmbH: Langenselbold, Duitsland, 2013. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Metody Analizy i Oceny Właściwości Gleb i Roślin; Katalog; Instytut Ochrony Środowiska: Warsaw, Poland, 1991. [Google Scholar]
- Gorlach, E.; Mazur, T. Agricultural chemistry. In Basics of Nutrition and Principles of Fertilization; PWN: Warszawa, Poland, 2001; p. 347. (In Polish) [Google Scholar]
- Thalmann, A. Methods of dehydrogenase activity determination with triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Frankenberger, W.T., Jr.; Johanson, J.B. Factors affecting invertase activity in soils. Plant Soil 1983, 74, 313–323. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from grey-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Guidelines for Soil Description; Food and Agriculture Organization: Rome, Italy, 2006; ISBN 92-5-105521-1. [Google Scholar]
- González-Vargas, T.; Gutiérrez-Castorena, M.D.C. Brightness values-based discriminant functions for classification of degrees of organic matter decomposition in soil thin sections. Span. J. Soil Sci. 2022, 12, 10348. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System); StatSoft Inc.: Tulsa, OK, USA, 2019.
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, version 5.1; Wageningen University & Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Ghaly, A.E.; Ramakrishnan, V.V. Nitrification of Urea and Assimilation of Nitrate in Saturated Soils Under Aerobic Conditions. Am. J. Agric. Biol. Sci. 2013, 8, 330–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, S.; Wang, W.; Wu, F.; Pan, K. Effects of decomposed and undecomposed straw of three crops on clubroot disease of Chinese cabbage and soil nutrients. Sci. Rep. 2024, 14, 22990. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; You, B.; Peng, Y.; Gao, C.; Qin, T.; Zhau, Y.; Sun, C.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2020, 13, 986763. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S. Soil pH changes after application of plant shoot materials of faba bean and wheat. Plant Soil 2000, 220, 279–287. [Google Scholar] [CrossRef]
- Karcz, H.; Kantorek, M.; Grabowicz, M.; Wierzbicki, K. Możliwość wykorzystania słomy jako źródła paliwowego w kotłach energetycznych (The feasibility of straw as a fuel source for power generating boilers). Piece Przemysłowe Kotły 2013, XI–XII, 8–15. (In Polish) [Google Scholar]
- Abalos, D.; Sanz-Cobena, A.; Misselbrook, T.; Vallejo, A. Effectiveness of urease inhibition on the abatement of ammonia, nitrous oxide and nitric oxide emissions in a non-irrigated Mediterranean barley field. Chemosphere 2012, 89, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Kira, O.; Shaviv, A.; Dubowski, Y. Concomitant tracking of NH3, N2O and soil mineral-N using steady-state incubation cells to enhance sustainability of urea fertilization approaches. Geoderma 2021, 404, 115305. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Yin, C.; Li, L.; Zhao, J.; Yang, J.; Zhao, H. Impacts of Returning Straw and Nitrogen Application on the Nitrification and Mineralization of Nitrogen in Saline Soil. Water 2023, 15, 564. [Google Scholar] [CrossRef]
- da Fonseca, A.B.; Santos, C.; Nunes, A.P.P.; Oliveira, D.P.; de Melo, M.E.A.; Takayama, T.; Mansur, B.L.; de Jesus Fernandes, T.; do Carmo Alexandrino, G.; Neves Dias, M.A.; et al. Urease inhibitors technologies as strategy to mitigate agricultural ammonia emissions and enhance the use efficiency of urea-based fertilizers. Sci. Rep. 2023, 13, 22739. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, A.R.; Lewu, F. Potential Use of Soil Enzymes as Soil Quality Indicators in Agriculture. In Frontiers in Soil and Environmental Microbiology, 1st ed.; Nayak, S.K., Mishra, B.B., Eds.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2020; Chapter 6. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Koper, J. Kształtowanie aktywności dehydrogenaz w glebie płowej po zmianie nawożenia. Zesz. Probl. Postępów Nauk. Rol. 2006, 512, 521–527. [Google Scholar]
- Gałązka, A.; Gawryjołek, K.; Grządziel, J.; Frąc, M.; Księżak, J. Microbial community diversity and the interaction of soil under maize growth in different cultivation techniques. Plant Soil Environ. 2017, 63, 264–270. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; Mcmullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clement, J.C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Chang, E.H.; Chung, R.S.; Tsai, Y.T. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2007, 53, 132–140. [Google Scholar] [CrossRef]
- Romero, E.; Fernández-Bayo, J.; Díaz, J.M.C.; Nogales, R. Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Appl. Soil Ecol. 2010, 44, 198–204. [Google Scholar] [CrossRef]
- Tabak, M.; Lisowska, A.; Filipek-Mazur, B. Bioavailability of Sulfur from Waste Obtained during Biogas Desulfurization and the Effect of Sulfur on Soil Acidity and Biological Activity. Processes 2020, 8, 863. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ahn, M.-Y. Organo-Mineral–Enzyme Interaction and Soil Enzyme Activity. In Soil Enzymology. Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 22, pp. 271–292. [Google Scholar] [CrossRef]
- Reichel, R.; Wei, J.; Islam, M.S.; Schmid, C.; Wissel, H.; Schlöder, P.; Schloter, M.; Brüggemann, N. Potential of wheat straw, spruce sawdust and lignin as high organic carbon soil amendments to improve agricultural nitrogen capacity: An incubation study. Front. Plant Sci. 2018, 9, 900. [Google Scholar] [CrossRef]
- Jiang, D.; Jiang, N.; Jiang, H.; Chen, L. Urease inhibitors increased soil ureC gene abundance and intracellular urease activity when extracellular urease activity was inhibited. Geoderma 2023, 430, 116295. [Google Scholar] [CrossRef]
- Liu, Y.; He, N.; Xu, L.; Tian, J.; Gao, Y.; Zheng, S.; Wang, Q.; Wen, X.; Xu, X.; Yakov, K. A new incubation and measurement approach to estimate the temperature response of soil organic matter decomposition. Soil Biol. Biochem. 2019, 138, 107596. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Jin, J.; Fu, M. Effects of different nitrogen applications and straw return depth on straw microbial and carbon and nitrogen cycles in paddy fields in the cool zone. Sci. Rep. 2024, 14, 6424. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Skonecki, S.; Potręć, M.; Laskowski, J. Physical and chemical properties of agricultural wastes/Właściwości fizyczne I chemiczne odpadów rolniczych. Acta Agrophysica 2011, 18, 443–455. (In Polish) [Google Scholar]
- Cassity-Duffey, K.; Cabrera, M.; Franklin, D.; Gaskin, J.; Kissel, D. Effect of soil texture on nitrogen mineralization from organic fertilizers in four common southeastern soils. Soil Sci. Soc. Am. J. 2020, 84, 534–542. [Google Scholar] [CrossRef]
- Uddin, S.; Islam, M.R.; Jahangir, M.M.R.; Rahman, M.M.; Hassan, S.; Hassan, M.M.; Abo-Shosha, A.A.; Ahmed, A.F.; Rahman, M.M. Nitrogen Release in Soils Amended with Different Organic and Inorganic Fertilizers under Contrasting Moisture Regimes: A Laboratory Incubation Study. Agronomy 2021, 11, 2163. [Google Scholar] [CrossRef]
- Müller, R.; Zahorka, A.; Holawe, F.; Inselsbacher, E.; Glatzel, S. Incubation of ombrotrophic bog litter and mixtures of Sphagnum, Betula and Calluna results in the formation of single litter-specific decomposition patterns. Geoderma 2023, 440, 116702. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J.; Cooledge, E.C.; Chadwick, D.R.; Jones, D.L. Phosphorus fertilization promotes carbon cycling and negatively affects microbial carbon use efficiency in agricultural soils: Laboratory incubation experiments. Geoderma 2024, 450, 117038. [Google Scholar] [CrossRef]
- Xing, H.; Wang, E.; Smith, C.J.; Rolston, D.; Yu, Q. Modelling nitrous oxide and carbon dioxide emission from soil in an incubation experiment. Geoderma 2011, 167–168, 328–339. [Google Scholar] [CrossRef]
- de Carvalho, M.; Ciarkowska, K.; Wojnar, L. Contribution of the Soil Macro- and Microstructure to Organic Matter Stabilisation in Natural and Post-Mining/Industrial Soils under Temperate Climatic Conditions. Sustainability 2024, 16, 2747. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global Potential bioethanol Production from Wasted Crops and Crop Residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat Straw Autohydrolysis: Process Optimization and Products Characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Luxhøi, J.; Magid, J.; Tscherko, D.; Kandeler, E. Dynamics of invertase, xylanase and coupled quality indices of decomposing green and brown plant residues. Soil Biol. Biochem. 2002, 34, 501–508. [Google Scholar] [CrossRef]
- Witaszek, K.; Kupryaniuk, K.; Kupryaniuk, J.; Panasiewicz, J.; Czekała, W. Optimization of Straw Particle Size for Enhanced Biogas Production: A Comparative Study of Wheat and Rapeseed Straw. Energies 2025, 18, 1794. [Google Scholar] [CrossRef]
- Kabuyah, R.N.T.M.; van Dongen, B.E.; Bewsher, A.D.; Robinson, C.H. Decomposition of lignin in wheat straw in a sand-dune grassland. Soil Biol. Biochem. 2012, 45, 128–131. [Google Scholar] [CrossRef]
- Ling, J.; Dungait, J.A.J.; Delgado-Baquerizo, M.; Ciu, Z.; Zhou, R.; Zhang, W.; Gao, Q.; Chen, Y.; Yue, S.; Kuzyakov, Y.; et al. Soil organic carbon thresholds control fertilizer effects on carbon accrual in croplands worldwide. Nat. Commun. 2025, 16, 3009. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects in soils after combined and repeated substrate additions. Geoderma 2005, 128, 38–51. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Sukor, A.S.A.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Zaman, M.; Zaman, S.; Adhinarayanan, C.; Nguyen, M.L.; Nawaz, S.; Dawar, K.M. Effects of urease and nitrification inhibitors on the efficient use of urea for pastoral systems. Soil Sci. Plant Nutr. 2013, 59, 649–659. [Google Scholar] [CrossRef]
| Treatments | Acronym |
|---|---|
| Control—soil without any additives | Crt |
| Soil + wheat straw + urea | wheat + urea |
| Soil + wheat straw + urea + urease inhibitor | wheat + urea + inh |
| Soil + wheat straw + UFF | wheat + UFF |
| Soil + wheat straw + UFF + urease inhibitor | wheat + UFF + inh |
| Soil + raped straw + urea | rape + urea |
| Soil + raped straw + urea + urease inhibitor | rape + urea + inh |
| Soil + raped straw + UFF | rape + UFF |
| Soil + raped straw + UFF + urease inhibitor | rape + UFF + inh |
| Class | PPL | XPL | |||
|---|---|---|---|---|---|
| Colour | Opacity | Internal Structure (%) | Birefringence | Isotropy | |
| 1. Non-decomposed fine organic parts | light brown | - | 75–100 | high | - |
| (UndfnOM) | |||||
| 2. Slightly decomposed fine organic parts | yellowish brown | - | 50–75 | low | - |
| (SlgDecOM) | |||||
| 3. Moderately decomposed fine organic parts | reddish brown to dark brown | medium | 25–50 | - | high |
| (ModDecOM) | |||||
| 4. Decomposed fine organic parts | dark brown to black | high | - | - | high |
| (Microagg) | |||||
| Treatments | pHH2O | C | N | ||||
|---|---|---|---|---|---|---|---|
| g·kg−1 d.m. | |||||||
| Days of Incubation | |||||||
| 1 | 120 | 1 | 120 | 1 | 120 | ||
| Crt | 6.89 c * | 6.10 b | 13.87 ab | 13.27 a | 1.29 a | 1.30 ab | |
| wheat + urea | 7.27 d | 5.99 ab | 15.13 ab | 14.53 ab | 1.43 ab | 1.42 ab | |
| wheat + urea + inh | 7.24 d | 5.93 a | 15.30 ab | 14.63 ab | 1.43 ab | 1.44 ab | |
| wheat + UFF | 7.20 d | 5.93 a | 15.83 ab | 14.47 ab | 1.48 b | 1.42 ab | |
| wheat + UFF + inh | 7.20 d | 6.00 ab | 15.67 ab | 14.17 ab | 1.44 ab | 1.38 ab | |
| rape + urea | 7.18 d | 6.09 b | 16.10 b | 14.63 ab | 1.43 ab | 1.43 ab | |
| rape + urea + inh | 7.17 d | 6.04 ab | 15.73 ab | 14.00 ab | 1.45 ab | 1.37 ab | |
| rape + UFF | 7.19 d | 6.05 ab | 14.13 ab | 13.93 ab | 1.39 ab | 1.37 ab | |
| rape + UFF + inh | 7.17 d | 6.09 b | 14.80 ab | 14.17 ab | 1.43 ab | 1.40 ab | |
| Univariate tests of significance | |||||||
| time | F | 9441 | 19.48 | 3.50 | |||
| p | 0.0000 | 0.0000 | 0.069 | ||||
| treatment | F | 6 | 2.95 | 4.48 | |||
| p | 0.0001 | 0.0121 | 0.001 | ||||
| Time × treatment | F | 20 | 0.67 | 0.53 | |||
| p | 0.0000 | 0.7166 | 0.829 | ||||
| Squares sum test for the full model vs. squares sum for residuals | |||||||
| R2 corrected | 0.995 | 0.372 | 0.333 | ||||
| F | 567.56 | 2.847 | 2.563 | ||||
| p | 0.000 | 0.004 | 0.009 | ||||
| Effect | DHA | Inv | Ure | |
|---|---|---|---|---|
| time | F | 237.796 | 115.991 | 1050.740 |
| p | 0.000 | 0.000 | 0.000 | |
| treatment | F | 11.866 | 69.753 | 14.299 |
| p | 0.000 | 0.000 | 0.000 | |
| Time × treatment | F | 3.687 | 9.446 | 10.236 |
| p | 0.001 | 0.000 | 0.000 | |
| R values for the full models | ||||
| R2 adjusted | 0.893 | 0.897 | 0.961 | |
| F | 26.457 | 27.405 | 76.029 | |
| p | 0.000 | 0.000 | 0.000 | |
| Treatment | UndOM | Macroagg | Sum |
|---|---|---|---|
| % | |||
| Crt | 11.22 | 5.95 | 17.17 |
| wheat + urea | 23.11 | 16.30 | 39.41 |
| wheat + UFF | 15.56 | 13.57 | 29.13 |
| rape + urea | 30.54 | 6.86 | 37.40 |
| rape + UFF | 23.39 | 4.00 | 27.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipek-Mazur, B.; Wiśniowska-Kielian, B.; Wojnar, L.; Ciarkowska, K. Can the Urea Fatty Fraction Support Sustainable Agriculture in the Improvement of Soil Properties? Sustainability 2025, 17, 5529. https://doi.org/10.3390/su17125529
Filipek-Mazur B, Wiśniowska-Kielian B, Wojnar L, Ciarkowska K. Can the Urea Fatty Fraction Support Sustainable Agriculture in the Improvement of Soil Properties? Sustainability. 2025; 17(12):5529. https://doi.org/10.3390/su17125529
Chicago/Turabian StyleFilipek-Mazur, Barbara, Barbara Wiśniowska-Kielian, Leszek Wojnar, and Krystyna Ciarkowska. 2025. "Can the Urea Fatty Fraction Support Sustainable Agriculture in the Improvement of Soil Properties?" Sustainability 17, no. 12: 5529. https://doi.org/10.3390/su17125529
APA StyleFilipek-Mazur, B., Wiśniowska-Kielian, B., Wojnar, L., & Ciarkowska, K. (2025). Can the Urea Fatty Fraction Support Sustainable Agriculture in the Improvement of Soil Properties? Sustainability, 17(12), 5529. https://doi.org/10.3390/su17125529






