Oxygenated Nanobubbles as a Sustainable Strategy to Strengthen Plant Health in Controlled Environment Agriculture
Abstract
1. Introduction
2. Methodology
3. Major Root Zone Diseases in Hydroponics and Soilless Substrate-Based Plant Production Systems
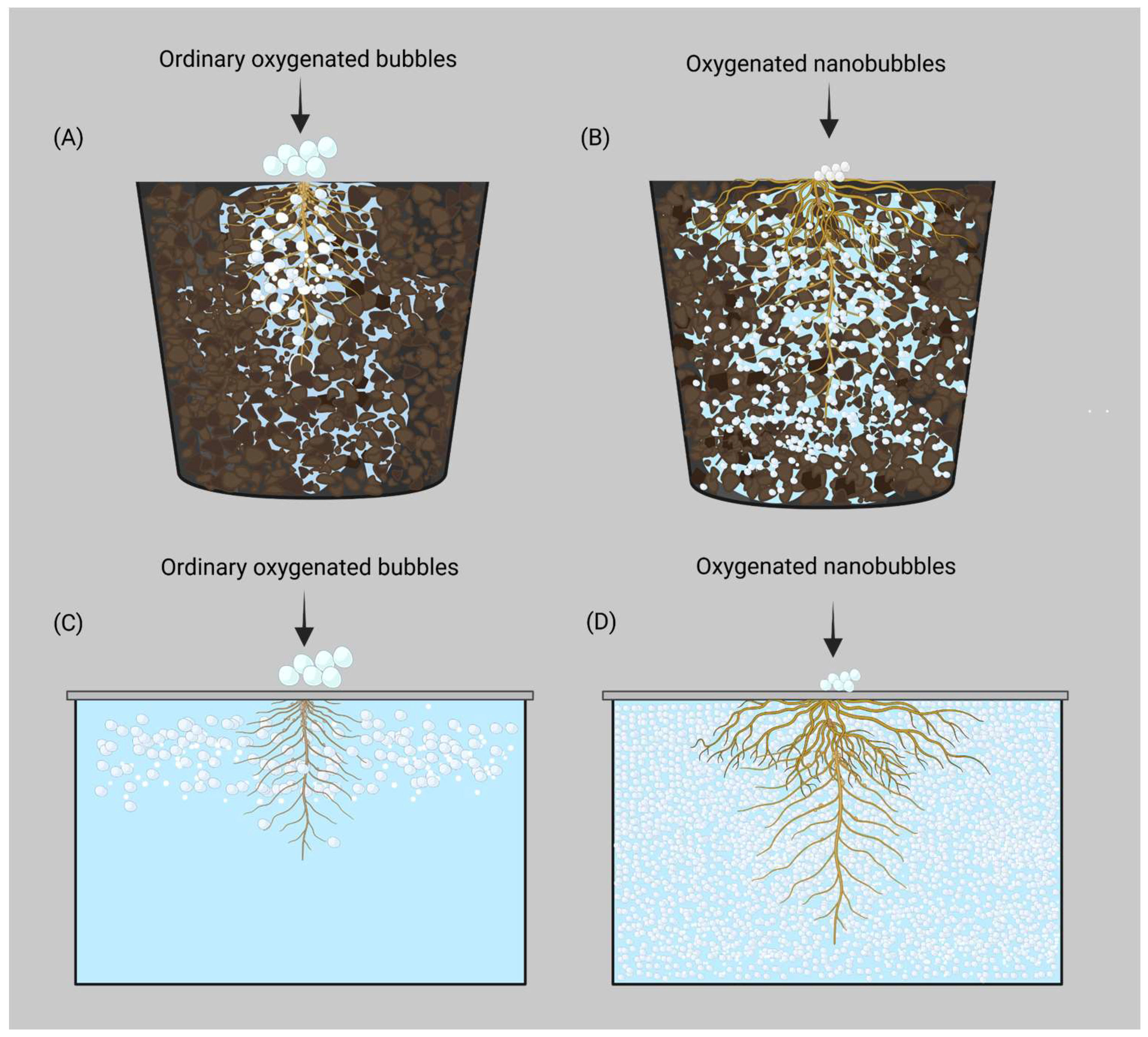
4. ONB Production Techniques and Their Significance in CEA-Based Crop Production
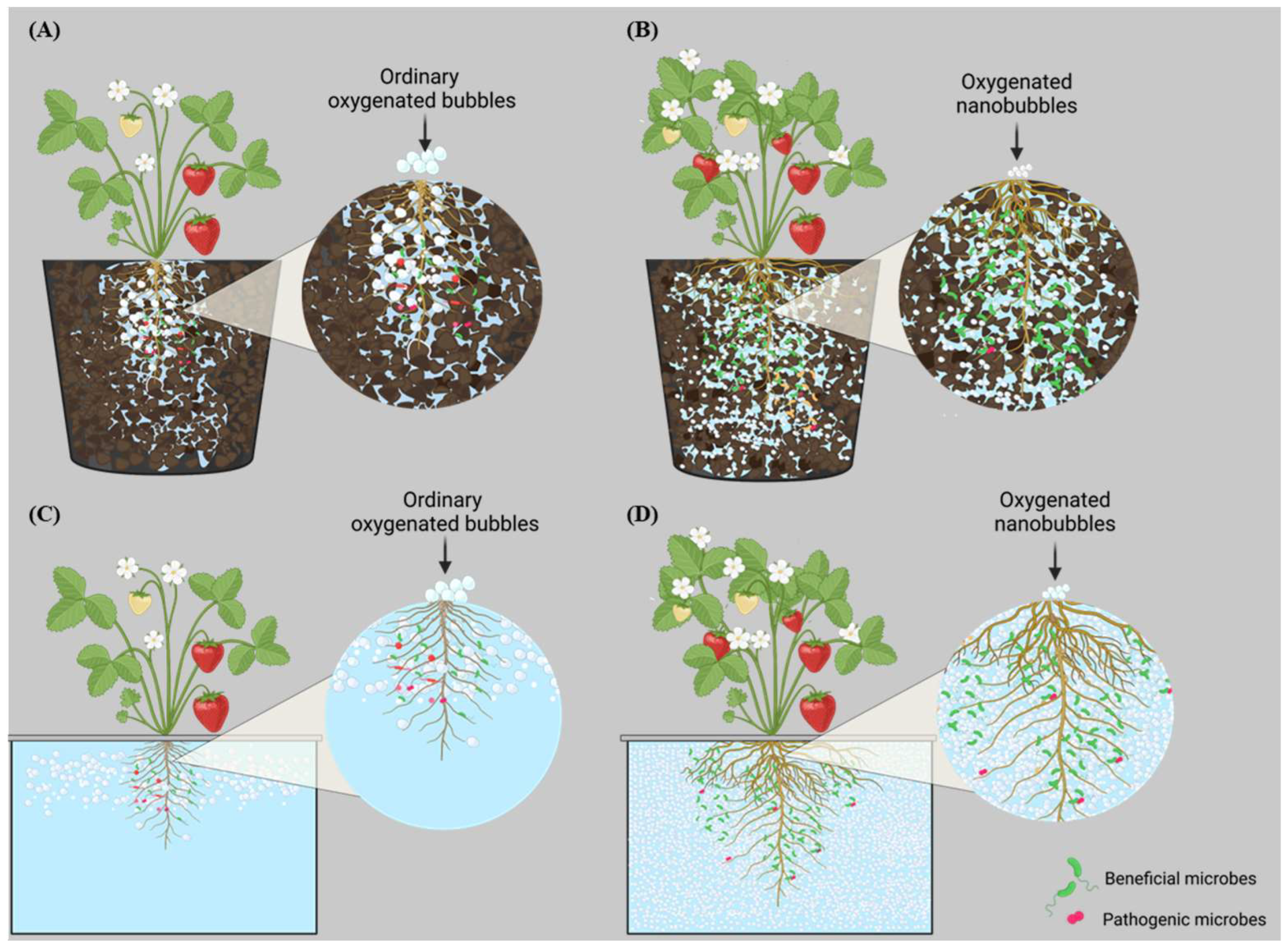
5. The Impact of DO and Beneficial Microbes on Root Zone Diseases
6. Integration of ONBs and Beneficial Microbes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Goodman, W.; Minner, J. Will the Urban Agricultural Revolution Be Vertical and Soilless? A Case Study of Controlled Environment Agriculture in New York City. Land Use Policy 2019, 83, 160–173. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- The State of Food and Agriculture 2021; Food and Agriculture Organization: Rome, Italy, 2021. [CrossRef]
- Dsouza, A.; Newman, L.; Graham, T.; Fraser, E.D.G. Exploring the Landscape of Controlled Environment Agriculture Research: A Systematic Scoping Review of Trends and Topics. Agric. Syst. 2023, 209, 103673. [Google Scholar] [CrossRef]
- Becker, K. Understanding Dissolved Oxygen. Available online: https://www.growertalks.com/Article/?articleid=22058 (accessed on 23 March 2025).
- Bonachela, S.; Vargas, J.A.; Acuña, R.A. Effect of Increasing the Dissolved Oxygen in the Nutrient Solution to Above-Saturation Levels in a Greenhouse Watermelon Crop Grown in Perlite Bags in a Mediterranean Area. Acta Hortic. 2005, 697, 25–32. [Google Scholar] [CrossRef]
- Song, W.; Zhou, L.; Yang, C.; Cao, X.; Zhang, L.; Liu, X. Tomato Fusarium Wilt and Its Chemical Control Strategies in a Hydroponic System. Crop Prot. 2004, 23, 243–247. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Bhatia, S.; Sapkota, S.; Sofkova-Bobchevaa, S. Effect of Oxygen-Nanobubbles on the Growth of Lettuce in Hydroponics. Acta Hortic. 2023, 1377, 703–708. [Google Scholar] [CrossRef]
- Ma, J.; Rukh, G.; Ruan, Z.; Xie, X.; Ye, Z.; Liu, D. Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys Praecox. Life 2022, 12, 808. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Otsubo, K.; Watanabe, H.; Suzuki, M.; Nakayama, K.; Fukuda, T.; Fujinaga, M.; Suga, H.; Kageyama, K. Root and Crown Rot of Strawberry Caused by Pythium Helicoides and Its Distribution in Strawberry Production Areas of Japan. J. Gen. Plant Pathol. 2014, 80, 423–429. [Google Scholar] [CrossRef]
- Koike, S. Pythium Wilt: Main Root Rot Disease of Lettuce|Trical Diagnostics. Available online: https://www.tricaldiagnostics.com/2018/10/26/pythium-wilt-main-root-rot-disease-of-lettuce/ (accessed on 23 March 2025).
- Hao, J.; Wuyun, D.; Xi, X.; Dong, B.; Wang, D.; Quan, W.; Zhang, Z.; Zhou, H. Application of 6-Pentyl-α-Pyrone in the Nutrient Solution Used in Tomato Soilless Cultivation to Inhibit Fusarium Oxysporum HF-26 Growth and Development. Agronomy 2023, 13, 1210. [Google Scholar] [CrossRef]
- Koike, S.T.; Gordon, T.R. Management of Fusarium Wilt of Strawberry. Crop Prot. 2015, 73, 67–72. [Google Scholar] [CrossRef]
- Dijst, G.; Schneider, J.H.M. Flowerbulbs Diseases Incited by Rhizoctonia Species. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Springer: Berlin/Heidelberg, Germany, 1996; pp. 279–288. [Google Scholar] [CrossRef]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding Root Rot Disease in Agricultural Crops. Horticulturae 2021, 7, 33. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.T.; Lamprecht, S.C.; McLeod, A.; Mazzola, M. Characterization of Rhizoctonia spp. Recovered from Crop Plants Used in Rotational Cropping Systems in the Western Cape Province of South Africa. Plant Dis. 2007, 90, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles Promote Nutrient Utilization and Plant Growth in Rice by Upregulating Nutrient Uptake Genes and Stimulating Growth Hormone Production. Sci. Total Environ. 2021, 800, 149627. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The Role of Beneficial Microorganisms in Soil Quality and Plant Health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2011, 28, 1327–1350. [Google Scholar] [CrossRef]
- Misra, V.; Mall, A.K.; Singh, D. Rhizoctonia Root-Rot Diseases in Sugar Beet: Pathogen Diversity, Pathogenesis and Cutting-Edge Advancements in Management Research. Microbe 2023, 1, 100011. [Google Scholar] [CrossRef]
- Lecomte, S.M.; Achouak, W.; Abrouk, D.; Heulin, T.; Nesme, X.; Haichar, F.E.Z. Diversifying Anaerobic Respiration Strategies to Compete in the Rhizosphere. Front. Environ. Sci. 2018, 6, 427325. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Han, Q.; Huang, X.; Jiang, Y.; Sun, H.; Li, H. Influence of Micro/Nanobubbles on Clogging in Drip Irrigation Systems. RSC Adv. 2020, 10, 38912–38922. [Google Scholar] [CrossRef] [PubMed]
- Bokaei, N.; Tabatabaei, S.J. Enhancing Anthurium (Anthurium andraeanum) Growth: The Impact of Oxygen Nanobubbles in Hydroponic Systems. J. Plant Nutr. 2025, 48, 1779–1788. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.; Chen, L.; Zhang, H.; Li, G.; Wang, G.; Cui, J.; Filatova, I.; Liu, Y. Effect of Micro–Nano Bubbles on the Remediation of Saline–Alkali Soil with Microbial Agent. Sci. Total Environ. 2024, 912, 168940. [Google Scholar] [CrossRef]
- Rajendran, S.; Domalachenpa, T.; Arora, H.; Li, P.; Sharma, A.; Rajauria, G. Hydroponics: Exploring Innovative Sustainable Technologies and Applications across Crop Production, with Emphasis on Potato Mini-Tuber Cultivation. Heliyon 2024, 10, e26823. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Cáceres, G.P.; Pérez-Urrestarazu, L.; Avilés, M.; Borrero, C.; Lobillo Eguíbar, J.R.; Fernández-Cabanás, V.M. Susceptibility to Water-Borne Plant Diseases of Hydroponic vs. Aquaponics Systems. Aquaculture 2021, 544, 737093. [Google Scholar] [CrossRef]
- Yan, K.; Ma, Y.; Bao, S.; Li, W.; Wang, Y.; Sun, C.; Lu, X.; Ran, J. Exploring the Impact of Coconut Peat and Vermiculite on the Rhizosphere Microbiome of Pre-Basic Seed Potatoes under Soilless Cultivation Conditions. Microorganisms 2024, 12, 584. [Google Scholar] [CrossRef]
- Lawson, L. Rhizoctonia Root Rot: Symptoms And How To Control|PT Growers and Consumers. Available online: https://www.pthorticulture.com/en-us/training-center/rhizoctonia-root-rot-symptoms-and-how-to-control (accessed on 23 March 2025).
- Hutton, D.G.; Forsberg, L.I. Phytophthora Root Rot in Hydroponically Grown Lettuce. Australas. Plant Pathol. 1991, 20, 76–79. [Google Scholar] [CrossRef]
- Amimoto, K. Selection in Strawberry with Resistance to Phytophthora Root Rot for Hydroponics. Acta Hortic. 1992, 319, 273–278. [Google Scholar] [CrossRef]
- Benti, E.A. Control of Bacterial Wilt (Ralstonia solanacearum) and Reduction of Ginger Yield Loss through Integrated Management Methods in Southwestern Ethiopia. Open Agric. J. 2023, 17, e187433152212300. [Google Scholar] [CrossRef]
- Yuliar; Asi Nion, Y.; Toyota, K. Recent Trends in Control Methods for Bacterial Wilt Diseases Caused by Ralstonia Solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia Solanacearum, a Widespread Bacterial Plant Pathogen in the Post-Genomic Era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.; Ogden, A.B.; Pandey, S.; Sandoya, G.V.; Shi, A.; Nankar, A.N.; Jayakodi, M.; Huo, H.; Jiang, T.; Tripodi, P.; et al. Improvement of Crop Production in Controlled Environment Agriculture through Breeding. Front. Plant Sci. 2025, 15, 1524601. [Google Scholar] [CrossRef]
- Itoh, M.; Iwasaki, Y. Control of Ralstonia Solanacearum in Tomato Hydroponics Using a Polyvinylidene Fluoride Ultrafiltration Membrane. Acta Hortic. 2018, 1227, 299–304. [Google Scholar] [CrossRef]
- Elsayed, T.R.; Jacquiod, S.; Nour, E.H.; Sørensen, S.J.; Smalla, K. Biocontrol of Bacterial Wilt Disease Through Complex Interaction Between Tomato Plant, Antagonists, the Indigenous Rhizosphere Microbiota, and Ralstonia Solanacearum. Front. Microbiol. 2020, 10, 484496. [Google Scholar] [CrossRef] [PubMed]
- Cockerton, H.M.; Li, B.; Vickerstaff, R.J.; Eyre, C.A.; Sargent, D.J.; Armitage, A.D.; Marina-Montes, C.; Garcia-Cruz, A.; Passey, A.J.; Simpson, D.W.; et al. Identifying Verticillium Dahliae Resistance in Strawberry through Disease Screening of Multiple Populations and Image Based Phenotyping. Front. Plant Sci. 2019, 10, 442989. [Google Scholar] [CrossRef]
- Water Quality Solutions Team Nanobubble Aeration Explained—Water Quality Solutions. Available online: https://waterqualitysolutions.com.au/nanobubble-aeration-explained/ (accessed on 23 March 2025).
- Chen, W.; Bastida, F.; Liu, Y.; Zhou, Y.; He, J.; Song, P.; Kuang, N.; Li, Y. Nanobubble Oxygenated Increases Crop Production via Soil Structure Improvement: The Perspective of Microbially Mediated Effects. Agric. Water Manag. 2023, 282, 108263. [Google Scholar] [CrossRef]
- Deboer, E.J.; Richardson, M.D.; Gentimis, T.; McCalla, J.H. Analysis of Nanobubble-Oxygenated Water for Horticultural Applications. Horttechnology 2024, 34, 769–773. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T. Preparation Method and Application of Nanobubbles: A Review. Coatings 2023, 13, 1510. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- del Moral Torres, F.; Hernández Maqueda, R.; Meca Abad, D.E. Enhancing Root Distribution, Nitrogen, and Water Use Efficiency in Greenhouse Tomato Crops Using Nanobubbles. Horticulturae 2024, 10, 463. [Google Scholar] [CrossRef]
- Yafuso, E.J.; Fisher, P.R. Oxygenation of Irrigation Water during Propagation and Container Production of Bedding Plants. HortScience 2017, 52, 1608–1614. [Google Scholar] [CrossRef]
- Yang, S.; Tsai, P.; Kooij, E.S.; Prosperetti, A.; Zandvliet, H.J.W.; Lohse, D. Electrolytically Generated Nanobubbles on Highly Orientated Pyrolytic Graphite Surfaces. Langmuir 2009, 25, 1466–1474. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Size Control of Nanobubbles Generated from Shirasu-Porous-Glass (SPG) Membranes. J. Memb. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Purusothaman, M.; Rameshkumar, C.; Joy, N.; Sachin, S.; Siva Thanigai, K. Generation and Characterization of Nanobubbles for Heat Transfer Applications. Mater. Today Proc. 2021, 43, 3391–3393. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation Using a Multiphase Pump, Properties and Features in Flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Zhang, W.; Marhaba, T. Generation of Nanobubbles by Ceramic Membrane Filters: The Dependence of Bubble Size and Zeta Potential on Surface Coating, Pore Size and Injected Gas Pressure. Chemosphere 2018, 203, 327–335. [Google Scholar] [CrossRef]
- Xu, J.; Salari, A.; Wang, Y.; He, X.; Kerr, L.; Darbandi, A.; de Leon, A.C.; Exner, A.A.; Kolios, M.C.; Yuen, D.; et al. Microfluidic Generation of Monodisperse Nanobubbles by Selective Gas Dissolution. Small 2021, 17, 2100345. [Google Scholar] [CrossRef]
- Alam, H.S.; Sutikno, P.; Soelaiman, T.A.F.; Sugiarto, A.T. Bulk Nanobubbles: Generation Using a Two-Chamber Swirling Flow Nozzle and Long-Term Stability in Water. J. Flow. Chem. 2022, 12, 161–173. [Google Scholar] [CrossRef]
- Wu, M.; Song, H.; Liang, X.; Huang, N.; Li, X. Generation of Micro-Nano Bubbles by Self-Developed Swirl-Type Micro-Nano Bubble Generator. Chem. Eng. Process.-Process Intensif. 2022, 181, 109136. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and Stability of Size-Adjustable Bulk Nanobubbles Based on Periodic Pressure Change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef]
- Cowan, N.; Ferrier, L.; Spears, B.; Drewer, J.; Reay, D.; Skiba, U. CEA Systems: The Means to Achieve Future Food Security and Environmental Sustainability? Front. Sustain. Food Syst. 2022, 6, 891256. [Google Scholar] [CrossRef]
- Craveiro, D. Why Controlled Environment Agriculture (CEA) Is the Future of Farming. Available online: https://www.danthermgroup.com/uk/insights/why-controlled-environment-agriculture-cea-is-the-future-of-farming (accessed on 23 March 2025).
- Ben, P. The Correct Oxygen Content in the Roots of a Plant. Available online: https://royalbrinkman.com/knowledge-center/technical-projects/correct-oxygen-in-roots-plant (accessed on 23 March 2025).
- Moleaer. Oxygen Uptake by Roots Is Critical for Plant Respiration. Available online: https://www.moleaer.com/blog/horticulture/high-plant-yields-oxygen-uptake-by-roots-is-critical-for-plant-respiration (accessed on 23 March 2025).
- Ben-Noah, I.; Friedman, S.P. Review and Evaluation of Root Respiration and of Natural and Agricultural Processes of Soil Aeration. Vadose Zone J. 2018, 17, 1–47. [Google Scholar] [CrossRef]
- Ouyang, Z.; Tian, J.; Yan, X.; Shen, H. Effects of Different Concentrations of Dissolved Oxygen or Temperatures on the Growth, Photosynthesis, Yield and Quality of Lettuce. Agric. Water Manag. 2020, 228, 105896. [Google Scholar] [CrossRef]
- Suyantohadi, A.; Kyoren, T.; Hariadi, M.; Purnomo, M.H.; Morimoto, T. Effect of High Consentrated Dissolved Oxygen on the Plant Growth in a Deep Hydroponic Culture under a Low Temperature. IFAC Proc. Vol. 2010, 43, 251–255. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Mamun, M.A.; Lee, B.R.; Park, S.H.; Muchlas, M.; Bae, D.W.; Kim, T.H. Interactive Regulation of Immune-Related Resistance Genes with Salicylic Acid and Jasmonic Acid Signaling in Systemic Acquired Resistance in the Xanthomonas–Brassica Pathosystem. J. Plant Physiol. 2024, 302, 154323. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Puzanskiy, R.K.; Shishova, M.F. Plant Life with and without Oxygen: A Metabolomics Approach. Int. J. Mol. Sci. 2023, 24, 16222. [Google Scholar] [CrossRef]
- Moleaer. Root Respiration: Why Plants Need Oxygen to Thrive. Available online: https://www.moleaer.com/blog/horticulture/root-respiration (accessed on 23 March 2025).
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated Pest Management: Good Intentions, Hard Realities. A Review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonà, A. Root Architectural Traits of Rooted Cuttings of Two Fig Cultivars: Treatments with Arbuscular Mycorrhizal Fungi Formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Jan, M.; Muhammad, S.; Jin, W.; Zhong, W.; Zhang, S.; Lin, Y.; Zhou, Y.; Liu, J.; Liu, H.; Munir, R.; et al. Modulating Root System Architecture: Cross-Talk between Auxin and Phytohormones. Front. Plant Sci. 2024, 15, 1343928. [Google Scholar] [CrossRef]
- Zhang, T.; Jian, Q.; Yao, X.; Guan, L.; Li, L.; Liu, F.; Zhang, C.; Li, D.; Tang, H.; Lu, L. Plant Growth-Promoting Rhizobacteria (PGPR) Improve the Growth and Quality of Several Crops. Heliyon 2024, 10, e31553. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Dong, Z.; Zhao, Y.; Feng, Y.; Fu, Y.; Wang, Z.; Zhu, J.; Ma, L. Micro-/Nanobubble Oxygenation Irrigation Enhances Soil Phosphorus Availability and Yield by Altering Soil Bacterial Community Abundance and Core Microbial Populations. Front. Plant Sci. 2025, 15, 1497952. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xu, G.; Li, G. Effect of Nanobubble Application on Performance and Structural Characteristics of Microbial Aggregates. Sci. Total Environ. 2021, 765, 142725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dong, J.; Li, S.; Lei, W.; Liu, A. Effects of Micro/Nano-Ozone Bubble Nutrient Solutions on Growth Promotion and Rhizosphere Microbial Community Diversity in Soilless Cultivated Lettuces. Front. Plant Sci. 2024, 15, 1393905. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Spratling, W.T.; Jespersen, D.; Waltz, C.; Martinez-Espinoza, A.D.; Bahri, B.A. Evaluation of Oxygenated and Ozonated Nanobubble Water Treatments for Dollar Spot Suppression in Seashore Paspalum. Agron. J. 2025, 117, e21744. [Google Scholar] [CrossRef]
- Chérif, M.; Tirilly, Y.; Bélanger, R.R. Effect of Oxygen Concentration on Plant Growth, Lipidperoxidation, and Receptivity of Tomato Roots to Pythium F under Hydroponic Conditions. Eur. J. Plant Pathol. 1997, 103, 255–264. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Tainter, F.H. Ecology and Epidemiology Effect of Dissolved Oxygen Concentration on the Relative Susceptibility of Shortleaf and Loblolly Pine Root Tips to Phytophthora Cinnamomi. Phytopathology 1989, 79, 1114–1118. [Google Scholar] [CrossRef]
- Chaurasia, G. Nanobubbles: An Emerging Science in Nanotechnology. MGM J. Med. Sci. 2023, 10, 327–334. [Google Scholar] [CrossRef]
- Wang, J.; He, Q.; Cao, K.; Zhou, B.; Niu, X.; Wang, D.; Chen, R.; Zheng, Z. Micro-Nano Bubble Water with Potassium Fertigation Improves Strawberry Yield and Quality by Changing Soil Bacterial Community. Rhizosphere 2023, 28, 100783. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, J.; Lu, X.; Zhou, C. Development of Plant Systemic Resistance by Beneficial Rhizobacteria: Recognition, Initiation, Elicitation and Regulation. Front. Plant Sci. 2022, 13, 952397. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.F. Physiological Effects of Dissolved Oxygen Tension and Redox Potential on Growing Populations of Micro-Organisms. J. Appl. Chem. Biotechnol. 1972, 22, 417–440. [Google Scholar] [CrossRef]
| Diseases | Relevant Pathogens | Crops | Impacts | References |
|---|---|---|---|---|
| Pythium Root Rot | Pythium spp. | Strawberries; lettuce; basil | Significant yield loss; plant death | [11,12] |
| Fusarium Wilt | Fusarium oxysporum | Strawberries; tomato | Reduced growth; wilting; plant death | [12,13] |
| Rhizoctonia Root Rot | Rhizoctonia solani | Lettuce; tomato; ornamental plants; barley; canola | Root rot; reduced yield | [15,16,17] |
| Phytophthora Root Rot | Phytophthora spp. | Strawberries; lettuce | Severe root rot; plant collapse | [32,33] |
| Bacterial Wilt | Ralstonia solanacearum | Tomato | Rapid wilting; plant death | [38,39] |
| Verticillium Wilt | Verticillium dahliae | Strawberries | Stunted growth; leaf chlorosis; yield loss | [40] |
| Nanobubble Generation Techniques | Diameter of Nanobubbles | Principles of Methods | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Mechanical Stirring | 150–200 nm | Introducing gas into a liquid to generate bubbles | Simple to implement; cost-effective | For a small amount of nanobubble production | [50,51] |
| Nanoscale Pore Membrane | 360–720 nm | Imposing gas flow across nanoporous membranes | Precise control in size and distribution | Membrane clogging | [49,52] |
| Microfluidic Method | Highly controllable <500 nm | Gas and liquid are combined in microchannels to produce controlled bubbles | High precision in size; integrated with other processes | Complex and expensive | [53] |
| Acoustic Cavitation | 200–301 nm | Utilizing ultrasonic waves to generate bubbles by rapid compression and expansion | Rapid production of nanobubbles; energy-efficient | Requires specialized equipment and limited size control | [54] |
| Hydrodynamic Cavitation | <200–301 nm | Changes in pressure inside a fluid induce cavitation, resulting in the formation of bubbles | Simple to implement and low-cost | Flow rate and pressure could impact the production | [54,55] |
| Dissolved Gas Release | Depending on gas solubility | Dissolving gas at elevated pressure, followed by pressure release to generate bubbles | Simple; inexpensive | Limited size control | [44] |
| Periodic Pressure Variation | Size decreases with exposure. | Periodically adjusting pressure to facilitate the dissolution and precipitation of bubbles | Precise control in uniform bubble production | Small-scale production | [56] |
| Hydraulic Air Compression | Increases in outlet pipe height | Gas is hydraulically compressed and combined with liquid to generate bubbles | Cost-effective production of nanobubbles at low cost | Limited control of size and distribution | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, M.A.; Islam, T. Oxygenated Nanobubbles as a Sustainable Strategy to Strengthen Plant Health in Controlled Environment Agriculture. Sustainability 2025, 17, 5275. https://doi.org/10.3390/su17125275
Mamun MA, Islam T. Oxygenated Nanobubbles as a Sustainable Strategy to Strengthen Plant Health in Controlled Environment Agriculture. Sustainability. 2025; 17(12):5275. https://doi.org/10.3390/su17125275
Chicago/Turabian StyleMamun, Md Al, and Tabibul Islam. 2025. "Oxygenated Nanobubbles as a Sustainable Strategy to Strengthen Plant Health in Controlled Environment Agriculture" Sustainability 17, no. 12: 5275. https://doi.org/10.3390/su17125275
APA StyleMamun, M. A., & Islam, T. (2025). Oxygenated Nanobubbles as a Sustainable Strategy to Strengthen Plant Health in Controlled Environment Agriculture. Sustainability, 17(12), 5275. https://doi.org/10.3390/su17125275







