Toward Sustainable Soil Remediation: Progress and Perspectives on Biochar-Activated Persulfate Oxidation
Abstract
1. Introduction
2. Soil Remediation by PS
2.1. Progress of PMS in Soil Remediation
2.2. Progress of PDS in Soil Remediation
2.3. Opinions on the Potential of PMS and PDS in Soil Remediation
3. Soil Remediation by PS Activated by Biochar
3.1. Pristine Biochar
3.2. Biochar Loaded with Fe
3.3. Biochar Loaded with Other Metals
| Biochar Descriptions | Pollutant | Pollutant Concentration (mg/kg) | Activator | Removal (%) | Activation Mechanism | Reference |
|---|---|---|---|---|---|---|
| Hydrochar from excess sludge | ATZ | 101.8 | PDS | 95.3 | The synergistic effect of hydrochar and PDS in soil remediation was observed. | Xue et al. [46] |
| Pyrochar from wheat straw | HCH | 10.0 | PDS | 99.0 | External LMWOAs addition enhanced the performance of the biochar-activated PDS system. | Dou et al. [47] |
| Pyrochar from the lychee branch | BPA | 31.9 | PDS | 98.4 | Biochar can activate PDS to generate SO4•− for BPA degradation and alleviate pH drop during soil remediation. | Liu et al. [48] |
| Pyrochar from peanut shells | SMX | 20.0 | PDS | 68.4–90.7 | SO4•− and •OH were the predominant reactive species. Iron minerals in the soil exert a facilitating effect, whereas organic matter exists as an inhibitor. | Chen et al. [49] |
| Pyrochar from seaweed with a ball-milled modification | CIP | 126.0 | PMS | 96.1 | 1O2 is the more dominant ROS, and the non-radical pathway is dominant. | Masud et al. [50] |
| Pyrochar from wheat straw with microwave assistance | PTH | 60.0 | PDS | 88.8 | In biochar and MW systems, the activation of PDS into SO4•−, •OH, O2•−, and 1O2 contributed to the removal of PTH. | Zhao et al. [51] |
| Pyrochar from corn straw loaded with Fe (nZVI@BC) | PAHs | 27.0 | PDS | 71.8 | nZVI@BC activated PDS and enhanced non-radical pathways (1O2). Biochar can also act as an electron shuttle and accelerate electron transfer from BaP to PDS. | Qu et al. [44] |
| Pyrochar from peanut shells supported nanoscale nZVI (nZVI/p-BC) | NCB | 13.0–15.2 | PDS | 64.0–82.4 | The cooperation of the non-free radical (1O2) and the free radical (SO4•− and •OH) contributed to the high degradation, owing to nZVI and p-BC collaboratively activating PDS. | Wan et al. [9] |
| Pyrochar from bamboo waste supported nano iron (BC-nZVI) | TPHs | 13,259 | PDS | 62.6 | The degradation of TPHs was potentially related to the redox action of Fe2+ and Fe3+. | Zhang et al. [53] |
| Pyrochar from corn stalks supported Fe nanoparticle (FeNPs@BC) | ATZ | 20.6 | PDS | 100 | The participation of SO4•−, •OH, and 1O2 degraded ATZ. | Li et al. [55] |
| Pyrochar from rice straw loaded with Fe (B-nZVI/BC) | PCA | 3.6 | PDS | 95.9 | SO4•−, •OH, and O2•− radicals were responsible for PCA degradation. | Guo et al. [56] |
| Pyrochar from wood pulp N-doped biochar-modified ZVI (NBC-ZVI) | PAHs | 98.3 | PDS | 95.5 | NBC induced direct electron transfer from ZVI to NBC to activate PDS for SO4•− generation. | Wang et al. [57] |
| Biochar-supported nZVI (nZVI/BC) | 2-ethylnitrobenzene Biphenyl 4-(methylsulfonyl) toluene 4-phenylphenol | 1.5–1.6 0.02–0.2 0.3–0.4 1.7–2.5 | PDS | 88.6–99.9 | In situ pilot-scale study. | Zeng et al. [58] |
| Pyrochar from corn stalks loaded with Fe0 and FeS (Fe0-FeS@BC) | SMX | 20 | PDS | 97.5 | SMX was efficiently removed with the participation of 1O2, O2•−, •OH, and SO4•−. | Liu and Yang [54] |
| Pyrochar loaded with FeS (FeS@BC) | TPHs | 4186.4 | PDS | 61.8 | The reduction of Fe2+/Fe3+ and the activation by biochar acted as an electron transfer mediator to promote the generation of SO4•−. | Xia et al. [59] |
| Pyrochar from corn straw and pyrite (pyrite-biochar composites) | SMX | 10.0 | PMS | 76.0 | The introduction of pyrite into biochar significantly increased the release of Fe(II), further enhancing the activation of PMS and the generation of ROS. •OH, SO4•−, and 1O2 (dominant ROS) were produced. | Zhao et al. [60] |
| Pyrochar from lignin loaded with Cu and Fe (Fe–Cu@BC-GM) | PAHs | 20.0 | PMS | 68.0 | Fe–Cu@BC-GM activated PMS to generate a lot of free radicals, such as O2•−, SO4•−, •HO, and 1O2, through electron transfer. | Zhu et al. [63] |
| Pyrochar from wheat straw loaded with Fe and Mn (FeMn@BC) | THI | 5.0 | PDS | 92.5 | FeMn@BC produced more HO• and SO4•−. | Li et al. [62] |
| Pyrochar from sludge and rice straw loaded with ZnCl2 (SSBC) | Crude oil | 10 | PDS | 34.2 | The SSBC is rich in surface -COOH groups (1.03 mM/g) and -OH groups (2.77 mM/g), which were responsible for PS activation to generate SO4•− and •OH. | Liu et al. [64] |
4. Biochar Modification Enhanced Activation of PS
4.1. Metal-Modified Biochar
4.1.1. Iron
4.1.2. Copper
4.1.3. Cobalt
4.1.4. Manganese
4.1.5. Zinc
4.1.6. Lanthanum
4.1.7. Multiple Metals
4.2. Non-Metallic-Modified Biochar Enhanced Activation of PS
4.2.1. Nitrogen Doping
4.2.2. Sulfur Doping
4.2.3. Boron Doping
4.2.4. Phosphorus Doping
4.2.5. Multiple Elements
4.3. Functional Group Modification
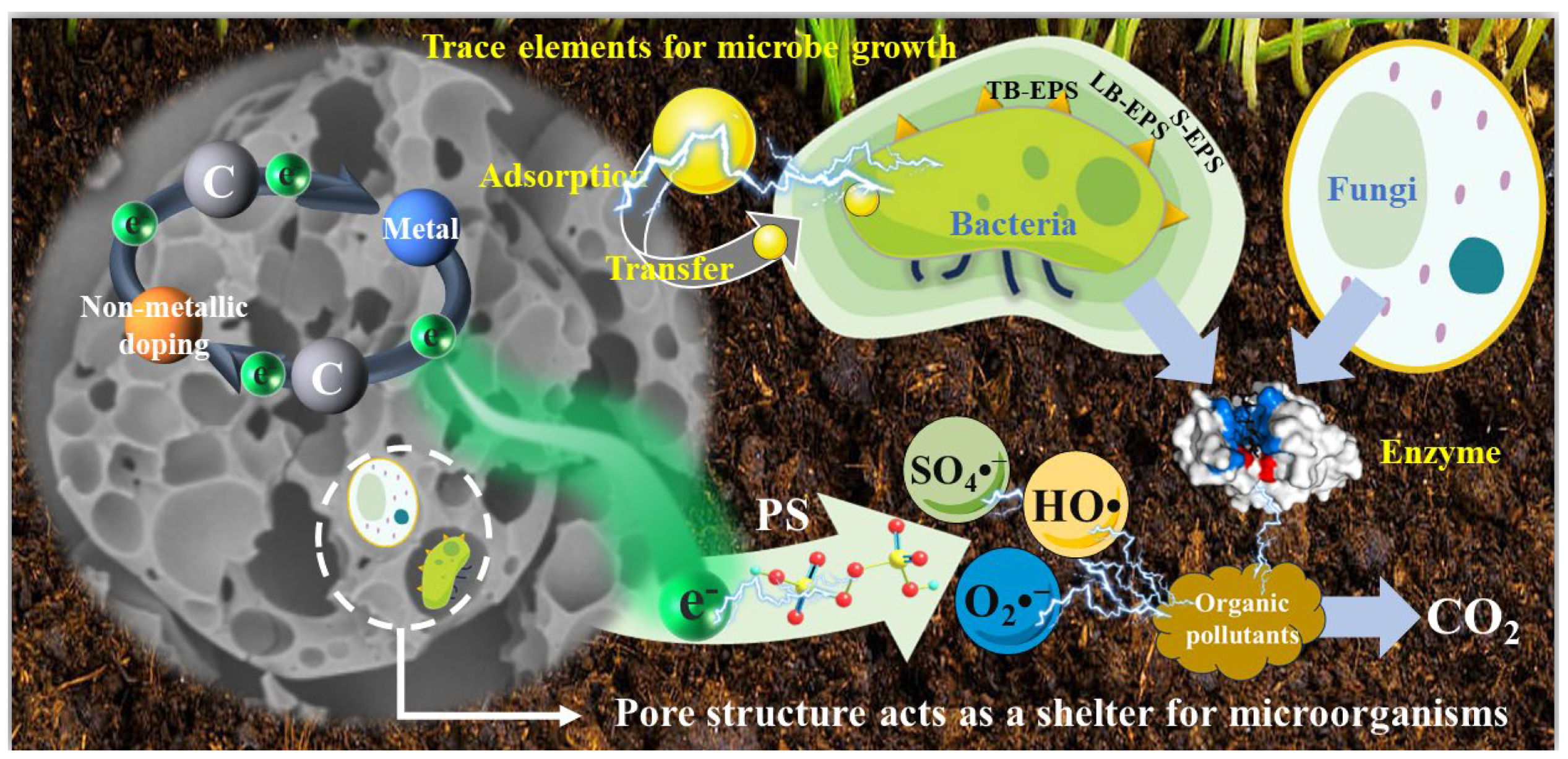
5. Impact of PS/Biochar Technology on Functional Microorganisms
6. Perspectives and Outlooks
6.1. Sustainable Application: Cost, Scalability, and Environmental Safety
6.2. Toward Smart, Tunable, and Regenerable Biochar Catalysts
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation List
| Abbreviation | Full Name |
| PS | Persulfate |
| AOP | Advanced oxidation process |
| PMS | Peroxymonosulfate |
| PDS | Peroxydisulfate |
| nZVI | Nanoscale-zero valent iron |
| NCB | Nitrochlorobenzene |
| HA | Humic acid |
| PAHs | Polycyclic aromatic hydrocarbons |
| ROS | Reactive oxygen species |
| TPH | Total petroleum hydrocarbons |
| TCE | Trichloroethylene |
| TCS | Triclosan |
| PPCP | Pharmaceutical and personal care product |
| DDT | Dichloro-Diphenyl-Trichloroethane |
| CPF | Chlorpyrifos |
| TCP | 3,5,6-trichloro-2-pyridinol |
| FH | Porous iron material |
| EPR | Electron paramagnetic resonance |
| BaP | Benzo[a]pyrene |
| N-CG | N-doped coal gangue |
| STZ | Sulfathiazole |
| TPHP | Triphenyl phosphate |
| CRs | Reducing reagents |
| TP | Tea polyphenols |
| H2A | Ascorbic acid |
| NAP | Naproxen |
| AA | L-ascorbic acid |
| CAT | (+)-catechin hydrate |
| TCPF | O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate |
| MCB | Monochlorobenzene |
| CA | Citric acid |
| ATZ | Atrazine |
| LMWOAs | low-molecular-weight organic acids |
| BPA | Bisphenol A |
| CIP | Ciprofloxacin |
| PTH | Ethyl-parathion |
| BC-nZVI | Biochar-supported nano iron |
| nZVI@BC | Fe-biochar composites |
| NBC-ZVI | N-doped biochar (NBC)-ZVI composite |
| FeMn@BC | Fe and manganese oxides |
| THI | Thiacloprid |
| Fe–Cu@BC-GM | Fe and Cu nanoparticles |
| SSBC | Biochar loaded with ZnCl2 |
| HCH | 2 γ-hexachlorocyclohexanes |
| PCA | p-chloroaniline |
| Co-MBC | Co-loaded magnetic biochar |
| Co-BC | Co-loaded biochar |
| PBDEs | Polybrominated diphenyl ethers |
| EPS | Extracellular polymeric substances |
| LCA | Life cycle assessments |
| ML | Machine learning |
References
- Zhang, P.; Yuan, Y.; Zhang, J.; Wen, T.; Wang, H.; Qu, C.; Tan, W.; Xi, B.; Hui, K.; Tang, J. Specific response of soil properties to microplastics pollution: A review. Environ. Res. 2023, 232, 116427. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent developments and prospects of sustainable remediation treatments for major contaminants in soil: A review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Sharma, P.; Dwivedi, S.; Singh, A.; Gaur, V.K.; Varjani, S.; Srivastava, J.K.; Pandey, A.; Chang, J.-S.; Ngo, H.H. A review on control and abatement of soil pollution by heavy metals: Emphasis on artificial intelligence in recovery of contaminated soil. Environ. Res. 2023, 225, 115592. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-B.; Sherpa, K.; Bui, X.-T.; Nguyen, V.-T.; Vo, T.-D.-H.; Ho, H.-T.-T.; Chen, C.-W.; Dong, C.-D. Biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Wan, J.; Guo, Y.; Zhang, Z.; Deng, R.; Wang, X.; Cao, S.; Zhang, X.; Miao, Y.; Jiang, J.; Song, Z.; et al. Persulfate activation with biochar supported nanoscale zero- valent iron: Engineering application for effective degradation of NCB in soil. Sci. Total Environ. 2024, 933, 173053. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.; Ren, Y.; Zhu, X.; Si, S.; Kou, B.; Zhang, Z.; Wang, J.; Shen, B. Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate: Performance, process and mechanisms. Bioresour. Technol. 2024, 399, 130633. [Google Scholar] [CrossRef]
- Gasim, M.F.; Lim, J.-W.; Low, S.-C.; Lin, K.-Y.A.; Oh, W.-D. Can biochar and hydrochar be used as sustainable catalyst for persulfate activation? Chemosphere 2022, 287, 132458. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Gopinath, A.; Ranjith, N.; Ravindran, V.; Ummar, S.; Nidheesh, P.V.; Kumar, M.S. Carbonaceous materials as effective adsorbents and catalysts for the removal of emerging contaminants from water. J. Clean. Prod. 2022, 350, 131319. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Teel, A.L.; Watts, R.J. Activation of Peroxymonosulfate by Subsurface Minerals. J. Contam. Hydrol. 2016, 191, 33–43. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, C.; Kong, L.; Wu, D.; Liu, Y.; Lee, P.-H.; Shih, K. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process. J. Hazard. Mater. 2018, 354, 63–71. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Lyu, C.; He, D.; Chang, Y.; Zhang, Q.; Wen, F.; Wang, X. Cobalt oxyhydroxide as an efficient heterogeneous catalyst of peroxymonosulfate activation for oil-contaminated soil remediation. Sci. Total Environ. 2019, 680, 61–69. [Google Scholar] [CrossRef]
- Bajagain, R.; Jeong, S.-W. Degradation of petroleum hydrocarbons in soil via advanced oxidation process using peroxymonosulfate activated by nanoscale zero-valent iron. Chemosphere 2021, 270, 128627. [Google Scholar] [CrossRef]
- Oba, B.T.; Zheng, X.; Aborisade, M.A.; Liu, J.; Yohannes, A.; Kavwenje, S.; Sun, P.; Yang, Y.; Zhao, L. Remediation of trichloroethylene contaminated soil by unactivated peroxymonosulfate: Implication on selected soil characteristics. J. Environ. Manag. 2021, 285, 112063. [Google Scholar] [CrossRef]
- Yuan, C.; Dai, Y.-D.; Chen, Y.-C. Analysis of electric field efficacy and remediation performance of triclosan contaminated soil by Co–Fe/al oxidation electrodes coupled with peroxymonosulfate (PMS) in an ECGO system with diversified electrode configurations. Chemosphere 2022, 307, 135841. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Zhang, Z.; Zhao, X.; Lin, C.; He, M.; Ouyang, W. Peroxymonosulfate assisted mechanochemical remediation of high concentration DDTs contaminated soil. Chemosphere 2023, 339, 139651. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Liu, X.; Ma, X.; Zhang, Z.; Lin, C.; He, M.; Ouyang, W. Efficient degradation of chlorpyrifos and intermediate in soil by a novel microwave induced advanced oxidation process: A two-stage reaction. J. Hazard. Mater. 2024, 464, 133001. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, J.; Gao, Y.; Zhen, K.; Sun, H.; Wang, C. Efficient remediation of the field soil contaminated with PAHs by amorphous porous iron material activated peroxymonosulfate. Chemosphere 2023, 327, 138516. [Google Scholar] [CrossRef]
- Liang, C.; Wang, J.; Li, C.; Han, W.; Niu, Y.; Li, B.; Yin, S.; Sun, Z. Chemical inertness conversion of carbon fraction in coal gangue via N-doping for efficient benzo(a)pyrene degradation. J. Colloid Interface Sci. 2024, 666, 547–559. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, S.; Nie, M.; Yan, C.; Qiu, L.; Wu, L.; Ding, M. Remediation of sulfathiazole contaminated soil by peroxymonosulfate: Performance, mechanism and phytotoxicity. Sci. Total Environ. 2022, 830, 154839. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Q.; Wang, X.; Huang, Q.; Cang, L. Electrokinetic combined peroxymonosulfate (PMS) remediation of PAH contaminated soil under different enhance methods. Chemosphere 2022, 286, 131595. [Google Scholar] [CrossRef]
- Zeng, G.; Yang, R.; Zhou, Z.; Huang, J.; Danish, M.; Lyu, S. Insights into naphthalene degradation in aqueous solution and soil slurry medium: Performance and mechanisms. Chemosphere 2022, 291, 132761. [Google Scholar] [CrossRef]
- Li, C.; Yin, S.; Yan, Y.; Liang, C.; Ma, Q.; Guo, R.; Zhang, Y.; Deng, J.; Sun, Z. Efficient benzo(a)pyrene degradation by coal gangue-based catalytic material for peroxymonosulfate activation. J. Environ. Manag. 2024, 351, 119645. [Google Scholar] [CrossRef]
- Dong, X.; Feng, R.; Yang, X.; Jiang, Y.; Chen, L.; Chen, L.; Jiang, C.; Cai, T. Complexation and reduction of soil iron minerals by natural polyphenols enhance persulfate activation for the remediation of triphenyl phosphate (TPHP)-contaminated soil. Chem. Eng. J. 2022, 435, 134610. [Google Scholar] [CrossRef]
- Dong, X.; Dai, M.; Yang, T.; Chen, L.; Yu, H.; Chen, L.; Zhao, R.; Jiang, C. Mechanism of interaction between ascorbic acid and soil iron-containing minerals for peroxydisulfate activation and organophosphorus flame retardant degradation. Environ. Res. 2024, 244, 117883. [Google Scholar] [CrossRef]
- Feng, R.; Chen, L.; Li, W.; Cai, T.; Jiang, C. Activation of persulfate with natural organic acids (ascorbic acid/catechin hydrate) for naproxen degradation in water and soil: Mechanism, pathway, and toxicity assessment. J. Hazard. Mater. 2023, 459, 132152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, J.; Liu, X.; Lin, C.; Sun, K.; Zhang, H.; Li, X.; Fan, G. Activation of peroxydisulfate by nanoscale zero-valent iron for sulfamethoxazole removal in agricultural soil: Effect, mechanism and ecotoxicity. Chemosphere 2019, 223, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Liu, X.; Ren, W.; Huang, J.; Zhou, Z.; Lin, C.; He, M.; Ouyang, W. Comparison of peroxodisulfate and peroxymonosulfate activated by microwave for degradation of chlorpyrifos in soil: Effects of microwaves, reaction mechanisms and degradation products. Sep. Purif. Technol. 2023, 306, 122682. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, P.; Zhang, Z.; Wang, C.; Wang, Q.; Rončević, S.D.; Sun, H. The interface mechanism of ball-milled natural pyrite activating persulfate to degrade monochlorobenzene in soil: Intrinsic synergism of S and Fe species. Sep. Purif. Technol. 2024, 341, 126946. [Google Scholar] [CrossRef]
- Fan, G.; Liu, X.; Li, X.; Lin, C.; He, M.; Ouyang, W. Mechanochemical treatment with CaO-activated PDS of HCB contaminated soils. Chemosphere 2020, 257, 127207. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, X.; Sun, W.; Wang, J.; Li, C.; Zhao, Q.; Li, Y.; Tian, S. Persulfate-based remediation of organic-contaminated soil: Insight into the impacts of natural iron ions and humic acids with complexation/redox functionality. Sci. Total Environ. 2023, 905, 167177. [Google Scholar] [CrossRef]
- Song, Y.; Fang, G.; Zhu, C.; Zhu, F.; Wu, S.; Chen, N.; Wu, T.; Wang, Y.; Gao, J.; Zhou, D. Zero-valent iron activated persulfate remediation of polycyclic aromatic hydrocarbon-contaminated soils: An in situ pilot-scale study. Chem. Eng. J. 2019, 355, 65–75. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Pan, Z.; Li, F.; Gao, Z.; Li, X. Degradation of carbamazepine in piezo-activation of persulfate systems: Comparison of PDS and PMS. Chem. Eng. J. 2024, 500, 156570. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of persulfates by carbonaceous materials: A review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Hung, C.-M. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, J.; Silvestri, S.; Carissimi, E. New composite of pecan nutshells biochar-ZnO for sequential removal of acid red 97 by adsorption and photocatalysis. Biomass Bioenergy 2020, 140, 105648. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Zhong, Q.; Xu, Z.; Zhao, L.; Qiu, H.; Cao, X. Roles of the mineral constituents in sludge-derived biochar in persulfate activation for phenol degradation. J. Hazard. Mater. 2020, 398, 122861. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Xue, J.; Sun, M.; Li, K.; Wang, J.; Zhang, G.; Wang, L.; Jiang, Z.; Zhang, Y. Superefficient non-radical degradation of benzo[a]pyrene in soil by Fe-biochar composites activating persulfate. Chem. Eng. J. 2024, 481, 148585. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Chen, H.; He, Z.; Cao, G.; Wang, K.; Li, F.; Ren, N.; Xing, D.; Ho, S.-H. Enhancing Biochar-Based Nonradical Persulfate Activation Using Data-Driven Techniques. Environ. Sci. Technol. 2023, 57, 4050–4059. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, L.; Fan, X.; Luo, K.; Guo, S.; Chen, H.; Li, X.; Jian, Q. Responses of soil fertility and microbiomes of atrazine contaminated soil to remediation by hydrochar and persulfate. J. Hazard. Mater. 2022, 435, 128944. [Google Scholar] [CrossRef]
- Dou, J.; Su, X.; Wu, J.; Li, S.; Dai, H.; Liu, M.; Tang, Y.; Lu, Z.; Xu, J.; He, Y. Peroxydisulfate-Driven Reductive Dechlorination as Affected by Soil Constituents: Free Radical Formation and Conversion. Environ. Sci. Technol. 2024, 58, 8065–8075. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Chen, K.; Liang, J.; Xu, X.; Zhao, L.; Qiu, H.; Wang, X.; Cao, X. Roles of soil active constituents in the degradation of sulfamethoxazole by biochar/persulfate: Contrasting effects of iron minerals and organic matter. Sci. Total Environ. 2022, 853, 158532. [Google Scholar] [CrossRef]
- Masud, M.A.A.; Annamalai, S.; Shin, W.S. Remediation of ciprofloxacin in soil using peroxymonosulfate activated by ball-milled seaweed kelp biochar: Performance, mechanism, and phytotoxicity. Chem. Eng. J. 2023, 465, 142908. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Miao, D.; Sun, H.; Zhang, P.; Jia, H. Activation of persulfate for the degradation of ethyl-parathion in soil: Combined effects of microwave with biochar. J. Environ. Manag. 2024, 351, 119930. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, Q.; Di, L.; Zhou, Y.; Zhou, Y. Iron-based biochar as efficient persulfate activation catalyst for emerging pollutants removal: A review. Chin. Chem. Lett. 2023, 34, 108357. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Huo, J.; Xie, H.; Xu, C.; Liang, S. Combining chemical oxidation and bioremediation for petroleum polluted soil remediation by BC-nZVI activated persulfate. Chem. Eng. J. 2020, 382, 123055. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.-Y. Enhanced removal of sulfamethoxazole in soil by ball-milled Fe0-FeS@BC activated persulfate process. J. Environ. Chem. Eng. 2023, 11, 110747. [Google Scholar] [CrossRef]
- Li, R.; Shen, X.; Zhang, J.; Jiang, Q.; Wang, L.; Zhang, Y. Tailoring biochar supported iron nanoparticles to activate persulfate for atrazine degradation in soil. J. Environ. Chem. Eng. 2024, 12, 111967. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, D.; Yan, Z.; Qian, L.; Yang, L.; Yan, J.; Chen, M. Efficient Remediation of p-chloroaniline Contaminated Soil by Activated Persulfate Using Ball Milling Nanosized Zero Valent Iron/Biochar Composite: Performance and Mechanisms. Nanomaterials 2023, 13, 1517. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Zhang, P.; Wang, C.; Jia, H.; Sun, H. Incorporation of N-doped biochar into submicron zero-valent iron for efficient peroxydisulfate activation in soil remediation: Performance and mechanism. Chem. Eng. J. 2024, 482, 148832. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, T.; Ding, Y.; Fang, G.; Wang, X.; Ye, B.; Ge, L.; Gao, J.; Wang, Y.; Zhou, D. Biochar-supported nano-scale zerovalent iron activated persulfate for remediation of aromatic hydrocarbon-contaminated soil: An in-situ pilot-scale study. Biochar 2022, 4, 64. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Q.; Zhao, L.; Wang, L.; Tang, J. Enhanced degradation of petroleum hydrocarbons in soil by FeS@BC activated persulfate and its mechanism. Sep. Purif. Technol. 2022, 282, 120060. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, T.; Wang, Z.; Cheng, W.; Li, L.; Wang, Y.; Xie, X. Activation of peroxymonosulfate with natural pyrite-biochar composite for sulfamethoxazole degradation in soil: Organic matter effects and free radical conversion. J. Hazard. Mater. 2024, 469, 133895. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, Q.; Qiu, Y.; Meng, L.; Wei, X.; Sang, W.; Tao, J. Insight into the degradation of Orange G by persulfate activated with biochar modified by iron and manganese oxides: Synergism between Fe and Mn. J. Water Process Eng. 2020, 37, 101470. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Cui, K.; Chen, X.; Yang, X.; Dong, D.; Xi, S.; Wu, Z.; Wu, F. Remediating thiacloprid-contaminated soil utilizing straw biochar-loaded iron and manganese oxides activated persulfate: Removal effects and soil environment changes. J. Hazard. Mater. 2023, 459, 132066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, F.; Sun, S.; Wang, Y.; Su, Y.; Zhao, C.; Zhang, X.; Gu, Y.; Li, L. Enhanced crude oil degradation and reshaped microbial community structure using straw-sludge biochar-persulfate oxidative system in oil-contaminated soil. J. Environ. Chem. Eng. 2023, 11, 109690. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Zhao, G.; Su, P.; Wang, J.; Li, Y.; Zhou, W.; Mu, Y.; Zhang, J.; Liu, W. A critical review on antibiotics removal by persulfate-based oxidation: Activation methods, catalysts, oxidative species, and degradation routes. Process Saf. Environ. Prot. 2024, 187, 622–643. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Hu, L.; Shi, L.; Dawolo, E.H.; Ding, N.; Liu, H. Cobalt-Modified Biochar from Rape Straw as Persulfate Activator for Degradation of Antibiotic Metronidazole. Processes 2024, 12, 1596. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, X.; Zhao, Y.; Wang, H. In-situ soil remediation via heterogeneous iron-based catalysts activated persulfate process: A review. Chem. Eng. J. 2022, 431, 133833. [Google Scholar] [CrossRef]
- Zhou, T.; Han, Y.; Xiang, W.; Wang, C.; Wu, X.; Mao, J.; Huang, M. Revealing the heterogeneous activation mechanism of peroxydisulfate by CuO: The critical role of surface-binding organic substrates. Sci. Total Environ. 2022, 802, 149833. [Google Scholar] [CrossRef]
- Qian, L.; Liu, P.; Shao, S.; Wang, M.; Zhan, X.; Gao, S. An efficient graphene supported copper salen catalyst for the activation of persulfate to remove chlorophenols in aqueous solution. Chem. Eng. J. 2019, 360, 54–63. [Google Scholar] [CrossRef]
- Wang, M.; Ren, D.; Wang, Z.; Li, Y.; Zhang, S.; Gong, X.; Zhang, X. A novel Cu/HAP@sBC composite as a persulfate activator for the removal of 2,4,6-trichlorophenol. Catal. Commun. 2023, 184, 106793. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Q.; Xu, J.; Guo, H.; Wang, L. Application of the persulfate activated by molten anhydrous CuCl2 modified biochar to degrade antibiotics: Performance and the role of C-O-Cu structure. Sep. Purif. Technol. 2023, 326, 124767. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Guo, C.; Lian, F.; Li, Z.; Wang, M.; Sun, B.; Wu, W.; Sun, H. A novel cobalt-iron bimetallic hydrochar for the degradation of triclosan in the aqueous solution: Performance, reusability, and synergistic degradation mechanism. Environ. Pollut. 2024, 358, 124487. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Fu, Y.; Wang, Y.; Cai, Y.; Liu, Y.; Xu, Z.; Diao, Z. Persulfate oxidation of norfloxacin by cobalt doped water hyacinth biochar composite: The key role of cobalt and singlet oxygen. J. Water Process Eng. 2024, 59, 104967. [Google Scholar] [CrossRef]
- Gao, S.; Pan, J.; Zhang, Y.; Zhao, Z.; Cui, J. Mn-NSC co-doped modified biochar/permonosulfate system for degradation of ciprofloxacin in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132640. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Fang, Z. Effect of Mn-based magnetic biochar /PS reaction system on oxidation of metronidazole. Chemosphere 2023, 332, 138747. [Google Scholar] [CrossRef]
- Yan, C.; Yu, C.; Ti, X.; Bao, K.; Wan, J. Preparation of Mn-doped sludge biochar and its catalytic activity to persulfate for phenol removal. Environ. Sci. Pollut. Res. 2024, 31, 18737–18749. [Google Scholar] [CrossRef]
- Liu, D.; Guo, A.; Qi, Y.; Ji, Z.; Li, H.; Cao, X.; Zhang, Z.; Zhang, X.; Wu, K.; Cai, A. Activation of persulfate by magnetic Mg/Mn–layered double oxide–doped biochar composite for ciprofloxacin removal and bacterial inactivation. Sep. Purif. Technol. 2024, 329, 125322. [Google Scholar] [CrossRef]
- Yang, H.; Choi, G.-R.; Jae Jeong, Y.; Cho, I.S.; Park, S.-J.; Lee, C.-G. Enhancing acetaminophen removal through persulfate activation with ZnCl2-SPI biochar: A study on reactive oxygen species contribution according to acetaminophen concentration. Chem. Eng. J. 2024, 496, 154065. [Google Scholar] [CrossRef]
- Demarema, S.; Nasr, M.; Ookawara, S.; Abdelhaleem, A. Enhanced synergistic system for the persulfate activation under visible light using novel N–ZnO photocatalyst supported on Lantana camara-based biochar. Chemosphere 2024, 349, 140840. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhang, X.; Sun, Y.; Fan, G.; Song, G.; Chai, B. Porous pie-like nitrogen-doped biochar as a metal-free peroxymonosulfate activator for sulfamethoxazole degradation: Performance, DFT calculation and mechanism. Appl. Surf. Sci. 2024, 647, 158965. [Google Scholar] [CrossRef]
- Peng, Y.; Xue, C.; Luo, J.; Zheng, B.; Fang, Z. Lanthanum-doped magnetic biochar activating persulfate in the degradation of florfenicol. Sci. Total Environ. 2024, 916, 170312. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-M.; Elanchezhiyan, S.S.; Yoon, Y.; Wang, D.; Kim, S.; Muthu Prabhu, S.; Park, C.M. Accelerated photocatalytic degradation of organic pollutants over carbonate-rich lanthanum-substituted zinc spinel ferrite assembled reduced graphene oxide by ultraviolet (UV)-activated persulfate. Chem. Eng. J. 2020, 393, 124733. [Google Scholar] [CrossRef]
- Razmi, R.; Ardjmand, M.; Ramavandi, B.; Heydarinasab, A. Optimization of phenol removal from wastewater by activation of persulfate and ultrasonic waves in the presence of biochar catalyst modified by lanthanum chloride. Water Environ. J. 2019, 33, 499–507. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, Z.; Zhou, L.; Lan, Y.; Chen, C. Simultaneous oxidation of 4-aminophenylarsonic acid and adsorption of the produced inorganic arsenic by a combination of Co3O4-La2CO5@RSBC with peroxymonosulfate. Chem. Eng. J. 2021, 413, 127417. [Google Scholar] [CrossRef]
- Koba-Ucuna, O.; Arslan-Alaton, I.; Sora, I.N.; Bekbölet, M. Persulfate-enhanced lanthanum iron oxide-mediated photocatalysis can effectively degrade an aqueous industrial dye and mineralize water and wastewater. Desalination Water Treat. 2022, 267, 215–230. [Google Scholar] [CrossRef]
- Ge, L.; Shao, B.; Liang, Q.; Huang, D.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; et al. Layered double hydroxide based materials applied in persulfate based advanced oxidation processes: Property, mechanism, application and perspectives. J. Hazard. Mater. 2022, 424, 127612. [Google Scholar] [CrossRef]
- Yue, D.; Yan, X.; Guo, C.; Qian, X.; Zhao, Y. NiFe Layered Double Hydroxide (LDH) Nanosheet Catalysts with Fe as Electron Transfer Mediator for Enhanced Persulfate Activation. J. Phys. Chem. Lett. 2020, 11, 968–973. [Google Scholar] [CrossRef]
- Wu, L.; Hong, J.; Zhang, Q.; Chen, B.-Y.; Wang, J.; Dong, Z. Deciphering highly resistant characteristics to different pHs of oxygen vacancy-rich Fe2Co1-LDH/PS system for bisphenol A degradation. Chem. Eng. J. 2020, 385, 123620. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F.; Yang, Q.; Zhong, Y.; Shu, X.; Yao, F.; Xie, T.; Li, X.; Wang, D.; Zeng, G. Sulfate radical induced degradation of Methyl Violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate. J. Environ. Manag. 2018, 227, 406–414. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Nengzi, L.-c.; Chen, Y.; Li, Y.; Zhang, X.; Cheng, X. Efficient degradation of sulfamethoxazole by CuCo LDH and LDH@fibers composite membrane activating peroxymonosulfate. Chem. Eng. J. 2020, 398, 125676. [Google Scholar] [CrossRef]
- He, S.; Yin, R.; Chen, Y.; Lai, T.; Guo, W.; Zeng, L.; Zhu, M. Consolidated 3D Co3Mn-layered double hydroxide aerogel for photo-assisted peroxymonosulfate activation in metronidazole degradation. Chem. Eng. J. 2021, 423, 130172. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H.; Deng, L.; Shi, Z. Peroxymonosulfate-assisted photocatalytic degradation of sulfadiazine using self-assembled multi-layered CoAl-LDH/g-C3N4 heterostructures: Performance, mechanism and eco-toxicity evaluation. J. Water Process Eng. 2020, 33, 101084. [Google Scholar] [CrossRef]
- Lu, H.; Sui, M.; Yuan, B.; Wang, J.; Lv, Y. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals. Chem. Eng. J. 2019, 357, 140–149. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Y.; Qian, L.; Gao, W.; Ouyang, D.; Chen, M. Heterogeneously catalyzed persulfate with a CuMgFe layered double hydroxide for the degradation of ethylbenzene. J. Hazard. Mater. 2017, 338, 372–380. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, H.; Xiong, Z.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2020, 391, 123604. [Google Scholar] [CrossRef]
- Zhang, H.; Nengzi, L.-c.; Wang, Z.; Zhang, X.; Li, B.; Cheng, X. Construction of Bi2O3/CuNiFe LDHs composite and its enhanced photocatalytic degradation of lomefloxacin with persulfate under simulated sunlight. J. Hazard. Mater. 2020, 383, 121236. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, W.; Deng, L.; Luo, J.; Zhou, S.; Liu, X.; Pei, Y.; Shi, Z.; Crittenden, J. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling. J. Colloid Interface Sci. 2018, 515, 92–100. [Google Scholar] [CrossRef]
- Ali, J.; Wenli, L.; Shahzad, A.; Ifthikar, J.; Aregay, G.G.; Shahib, I.I.; Elkhlifi, Z.; Chen, Z.; Chen, Z. Regulating the redox centers of Fe through the enrichment of Mo moiety for persulfate activation: A new strategy to achieve maximum persulfate utilization efficiency. Water Res. 2020, 181, 115862. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Hong, Y.; Peng, J.; Zhao, X.; Yan, Y.; Lai, B.; Yao, G. Efficient degradation of atrazine by CoMgAl layered double oxides catalyzed peroxymonosulfate: Optimization, degradation pathways and mechanism. Chem. Eng. J. 2019, 370, 354–363. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Han, S.; Zhou, J.; Qian, G.; Gao, N. Cycle of Ni(II)-Ni(III)-Ni(II) in Ni-doped layered double hydroxides for activation of intercalated peroxydisulfate. Chem. Eng. J. 2020, 386, 123937. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Zhu, N.; Dang, Z.; Bi, Y.; Pei, F. Re-utilization of spent Cu2+-immobilized MgMn-layered double hydroxide for efficient sulfamethoxazole degradation: Performance and metals synergy. Chem. Eng. J. 2020, 392, 123709. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, M.; Chen, Y.; Jia, W.; Wu, Y.; Liang, Y.; Du, J.; Wu, Q.; Feng, X.; Wang, H.; et al. Constructing nanoconfined spaces in persulfate oxidation processes for efficient degradation of emerging contaminants: Structures, mechanisms, and challenges. Sep. Purif. Technol. 2025, 354, 128689. [Google Scholar] [CrossRef]
- Xia, X.; Zeng, S.; Li, K.; Zeng, L.; Miao, S. Unraveling the outstanding catalytic efficiency of unprocessed bone-derived biochar: A deep dive into the mechanisms of native organic encapsulation and defective nitrogen doping in boosting persulfate activation for tetracycline degradation. Sep. Purif. Technol. 2025, 353, 128571. [Google Scholar] [CrossRef]
- Yang, X.; Siyu, D.; Huo, J.; Naraginti, S.; Zhu, M.; Hong, R.; Zhang, X.; Wei, X.; Xu, X.; Xie, G.; et al. Nitrogen self-doped layered porous Myriophyllum aquaticum biomass–derived biochar activated persulfate to efficiently degrade printing and dyeing wastewater. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Cui, J.; Wei, L.; Ning, C.; Zhang, F.; Cui, J.; Xiangli, P. Highly-efficient persulfate activation by nitrogen-doped biochar derived from dewatered sewage sludge for 2,4-dichlorophenol removal: Process and mechanism. J. Environ. Chem. Eng. 2024, 12, 112561. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, X.; Su, X.; Zhang, S. Efficient degradation of phenol in water by ball-milling modulated nitrogen-doped structured biochar. J. Environ. Chem. Eng. 2024, 12, 112397. [Google Scholar] [CrossRef]
- Zeng, S.; Xia, X.; Miao, S.; Zhang, J.; Li, K. Green synthesis of highly pyrrolic nitrogen-doped biochar for enhanced tetracycline degradation: New insights from endogenous mineral components and organic nitrogen synergy. J. Clean. Prod. 2024, 444, 141177. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Ye, X.; Lv, Y.; Liu, Y.; Liu, M. Enhanced persulfate activation for sulfadiazine degradation by N, S self-doped biochar from sludge and sulfonated lignin: Emphasizing the roles of graphitic nitrogen and thiophene sulfur. J. Environ. Chem. Eng. 2024, 12, 112407. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Peng, J.; Zhang, Q.; Yao, J.; Wu, X.; Li, Y. Sulfur and nitrogen co-doped magnetic biochar coupled with hydroxylamine for high-efficiency of persulfate activation and mechanism study. Environ. Res. 2023, 216, 114745. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, B.; Jin, M.; Chen, L.; Yi, G.; Chen, L.; Wu, Y.; Zhang, Y.; Xing, B. Sulfur and nitrogen co-doped biochar activated persulfate to degrade phenolic wastewater: Changes in impedance. J. Mol. Struct. 2023, 1294, 136344. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Zeng, G.; Lu, Y.; Dong, H.; Wang, J.; Liu, Y.; Feng, C.; et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 2020, 111, 100654. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.-L.; Huang, Y.-N.; Wang, W.-K.; Xu, J. Boron regulates catalytic sites of biochar to enhance the formation of surface-confined complex for improved peroxydisulfate activation. Chemosphere 2022, 301, 134690. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, H.; Han, Z.; Xu, S.; Dong, Z.; Zhou, K.; Zhang, S.; Cheng, Z. Remediation of atrazine in environment by persulfate activation via N/B co-doped Si-rich biochar: Performance, mechanisms, degradation pathways and phytotoxicity. Chem. Eng. J. 2023, 477, 147131. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, T.; Chen, Z.; Long, Y.; Jiang, J.; Zhou, S.; Hu, J.; Ma, S. Mechanisms and influences on the formation of electron-transfer complexes in the activation of N,B co-doped graphitic biochar for peroxydisulfate degradation of oxytetracycline. Appl. Surf. Sci. 2024, 655, 159635. [Google Scholar] [CrossRef]
- Xie, J.; Xu, P.; Liu, M.; Liu, Y.; Zhu, L.; Yu, F.; Zhang, P.; Li, J.; Luo, Y.; Zhou, B. Anchoring phosphorus on in-situ nitrogen-doped biochar by mechanical milling for promoted electron transfer from diclofenac sodium to peroxymonosulfate. Sep. Purif. Technol. 2022, 301, 121964. [Google Scholar] [CrossRef]
- Wang, C.; Holm, P.E.; Andersen, M.L.; Thygesen, L.G.; Nielsen, U.G.; Hansen, H.C.B. Phosphorus doped cyanobacterial biochar catalyzes efficient persulfate oxidation of the antibiotic norfloxacin. Bioresour. Technol. 2023, 388, 129785. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Du, X.; Jia, H.; Sun, H. P-doped biochar regulates nZVI nanocracks formation for superefficient persulfate activation. J. Hazard. Mater. 2023, 450, 130999. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Hu, K.; Nie, L.; Wang, H.; Ma, L.; Du, Q.; Wang, G. Degradation of acetaminophen using persulfate activated with P-doped biochar and thiosulfate. Inorg. Chem. Commun. 2022, 146, 110160. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zhou, Y.; Feng, H.; Ren, X.; Tang, J.; Wang, J.; Deng, L.; Shao, B. Non-radical oxidation by N,S,P co-doped biochar for persulfate activation: Different roles of exogenous P/S doping, and electron transfer path. J. Clean. Prod. 2022, 374, 133995. [Google Scholar] [CrossRef]
- Hou, Y.; Zhou, P.; Liu, F.; Tong, K.; Lu, Y.; Li, Z.; Liang, J.; Tong, M. Rigid covalent organic frameworks with thiazole linkage to boost oxygen activation for photocatalytic water purification. Nat. Commun. 2024, 15, 7350. [Google Scholar] [CrossRef]
- Chen, J.; Ren, R.; Liu, Y.; Li, C.; Wang, Z.; Qi, F. Multi-Heteroatom Doped Fe@CN Activation Peroxomonosulfate for the Removal of Trace Organic Contaminants from Water: Optimizing Fabrication and Performance. Water 2023, 15, 4241. [Google Scholar] [CrossRef]
- Shi, Y.; Yin, M.; Liu, D.; Gao, X.; Liu, X.; Yang, T.; Zhao, Z.; Ji, X.; Zhao, C.; Shao, X. Single-step synthesis of nitrogen and phosphorus co-doped biochar and its application in dye removal: Synergistic effects of adsorption and peroxymonosulfate activation. Environ. Res. 2025, 279, 121866. [Google Scholar] [CrossRef]
- Dou, J.; Cheng, J.; Lu, Z.; Tian, Z.; Xu, J.; He, Y. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Liu, Z.; Sha, X.; Fei, Z.; Yu, L.; Wu, X.; Wen, X.; Luo, R.; Xu, Q.; et al. Efficient activation of persulfate by N, S, Fe co-doped rice husk biochar for degradation of sulfamethazine: Synergistic effect and degradation mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2025, 718, 136958. [Google Scholar] [CrossRef]
- He, X.; Luo, Y.; Yi, Y.; Su, S.; Qin, W. Peroxymonosulfate activation by Fe–Mn Co-doped biochar for carbamazepine degradation. RSC Adv. 2024, 14, 1141–1149. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Logginou, O.; Kouvelis, K.; Petala, A.; Antonopoulou, M.; Mantzavinos, D.; Frontistis, Z. Peroxydisulfate Activation by Biochar from Banana Peel Promoted with Copper Phosphide for Bisphenol S Degradation in Aqueous Media. Catalysts 2024, 14, 789. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.B.; Gao, M.-T.; Zhang, Y.; Li, J.; Hu, J. Effects of functional group loss on biochar activated persulfate in-situ remediation of phenol pollution in groundwater and its countermeasures. J. Environ. Manag. 2023, 341, 118076. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, K.; Zhao, H. The debatable role of singlet oxygen in persulfate-based advanced oxidation processes. Water Res. 2023, 235, 119925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guo, H.; Zhang, Y.; Korshin, G.V.; Yang, B. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: Activation performance and structure-function relationship. Water Res. 2019, 157, 406–414. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, R.; Tang, Y.; Ye, S.; Wu, S.; Qin, F.; Xiang, L.; Tan, X.; Zeng, G.; Yan, M. Carbonyl and defect of metal-free char trigger electron transfer and O2•− in persulfate activation for Aniline aerofloat degradation. Water Res. 2023, 231, 119659. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.-P. SO3H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis. Chem. Rev. 2019, 119, 11576–11630. [Google Scholar] [CrossRef]
- Haider, M.I.S.; Liu, G.; Yousaf, B.; Arif, M.; Aziz, K.; Ashraf, A.; Safeer, R.; Ijaz, S.; Pikon, K. Synergistic interactions and reaction mechanisms of biochar surface functionalities in antibiotics removal from industrial wastewater. Environ. Pollut. 2024, 356, 124365. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, C.; Zhong, D.; Han, Y.; Zhang, Z.; Zhu, H.; Han, H. Synergistic degradation on aromatic cyclic organics of coal pyrolysis wastewater by lignite activated coke-active sludge process. Chem. Eng. J. 2019, 364, 410–419. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Chen, F.; Wang, X.; Liu, K.; Yang, Y.; Tang, M. Influence of biochar remediation on Eisenia fetida in Pb-contaminated soils. Chemosphere 2022, 295, 133954. [Google Scholar] [CrossRef]
- Xie, X.; Gu, S.; Hao, L.; Zhang, T.; Guo, Z. Rhizosphere Microbial Communities and Geochemical Constraining Mechanism of Antimony Mine Waste-Adapted Plants in Southwestern China. Microorganisms 2022, 10, 1507. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Q.; Chen, F.; Zhu, Q.; Wang, Y.; Liu, G. Remediation of PBDEs-metal co-contaminated soil by the combination of metal stabilization, persulfate oxidation and bioremediation. Chemosphere 2020, 252, 126538. [Google Scholar] [CrossRef]
- Song, L.; Niu, X.; Zhou, B.; Xiao, Y.; Zou, H. Application of biochar-immobilized Bacillus sp. KSB7 to enhance the phytoremediation of PAHs and heavy metals in a coking plant. Chemosphere 2022, 307, 136084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, R.; Li, T.; Zhou, R.; Hou, Z.; Zhang, X. Interactions among microorganisms functionally active for electron transfer and pollutant degradation in natural environments. Eco-Environ. Health 2023, 2, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Arrowsmith, S.; Hall, C.A.; Miranda, E.M.; Alam, F.; Dahlen, P.; Sra, K.; Kamath, R.; McMillen, S.J.; Sihota, N.; et al. Biodegradation of petroleum hydrocarbons in a weathered, unsaturated soil is inhibited by peroxide oxidants. J. Hazard. Mater. 2022, 433, 128770. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wu, Z.; Li, Y.; Cao, H.; Su, C. Effect of various chemical oxidation reagents on soil indigenous microbial diversity in remediation of soil contaminated by PAHs. Chemosphere 2019, 226, 483–491. [Google Scholar] [CrossRef]
- Díaz-López, M.; Nicolás, E.; López-Mondéjar, R.; Galera, L.; Garrido, I.; Fenoll, J.; Bastida, F. Combined ozonation and solarization for the removal of pesticides from soil: Effects on soil microbial communities. Sci. Total Environ. 2021, 758, 143950. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The role of extracellular polymeric substances (EPS) in chemical-degradation of persistent organic pollutants in soil: A review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Ma, Y.; Sun, Y. Improved volatile fatty acid production in anaerobic digestion via simultaneous temperature regulation and persulfate activation by biochar: Chemical and biological response mechanisms. Environ. Res. 2025, 264, 120271. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, H.; Xu, P.; Tang, H. Soil bioremediation by Pseudomonas brassicacearum MPDS and its enzyme involved in degrading PAHs. Sci. Total Environ. 2022, 813, 152522. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef]
- Gurumurthy, D.M.; Bharagava, R.N.; Kumar, A.; Singh, B.; Ashfaq, M.; Saratale, G.D.; Mulla, S.I. EPS bound flavins driven mediated electron transfer in thermophilic Geobacillus sp. Microbiol. Res. 2019, 229, 126324. [Google Scholar]
- Dinakarkumar, Y.; Ramakrishnan, G.; Gujjula, K.R.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications and future perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Miao, X.; Chen, X.; Wu, W.; Lin, D.; Yang, K. Intrinsic defects enhanced biochar/peroxydisulfate oxidation capacity through electron-transfer regime. Chem. Eng. J. 2022, 438, 135606. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Qiu, S.; Hu, Z.; Liu, C.; Yue, C.; Zhou, J. Efficient oxidative remediation of polycyclic aromatic hydrocarbons (PAHs)-contaminated soil: A thorough comprehension of Fe-loaded biochar activated persulfate. Chemosphere 2024, 368, 143699. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Tong, J.; Wang, J.; Ruan, C.; Lyu, J.; Shi, J. Research progress on activated persulfate by biochar: Soil and water environment remediation, mechanism exploration and simulation calculation. Chem. Eng. J. 2024, 493, 152718. [Google Scholar] [CrossRef]
- Mitra, S.; Saran, R.K.; Srivastava, S.; Rensing, C. Pesticides in the environment: Degradation routes, pesticide transformation products and ecotoxicological considerations. Sci. Total Environ. 2024, 935, 173026. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Y.; Jeyakumar, P.; Lin, L.; Zhang, D.; Yang, M.; Zhu, J.; Ki Lin, C.S.; Wang, H.; Wang, Z.; et al. Soil pH restricts the ability of biochar to passivate cadmium: A meta-analysis. Environ. Res. 2023, 219, 115110. [Google Scholar] [CrossRef]
- Daraei, E.; Bayat, H.; Gregory, A.S. Impact of natural biochar on soil water retention capacity and quinoa plant growth in different soil textures. Soil Tillage Res. 2024, 244, 106281. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Long, L.-L.; Zhu, Q.-R.; Chen, C.; Xu, M.; Wu, J.; Yang, G. Mechanism and ecological environmental risk assessment of peroxymonosulfate for the treatment of heavy metals in soil. Sci. Total Environ. 2024, 926, 171717. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Rashwan, A.K. Life cycle assessment of biochar as a green sorbent for soil remediation. Curr. Opin. Green Sustain. Chem. 2024, 46, 100882. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Lichtfouse, E.; Maurer, C.; Liu, H. Life cycle assessment of biochar for sustainable agricultural application: A review. Sci. Total Environ. 2024, 951, 175448. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Jia, Y.; Zhang, Y.; Fan, J. Synergistic activation of peroxymonosulfate by highly dispersed iron-based sulfur–nitrogen co-doped porous carbon for bisphenol a removal: Mechanistic insights and selective oxidation. RSC Adv. 2025, 15, 4356–4368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, R.; Li, J.; Huang, T.; Wu, B.; Ma, J.; Wen, Q.; Tan, J.; Huang, W. Synthesis optimization and adsorption modeling of biochar for pollutant removal via machine learning. Biochar 2023, 5, 25. [Google Scholar] [CrossRef]
- Yuan, X.; Suvarna, M.; Lim, J.Y.; Pérez-Ramírez, J.; Wang, X.; Ok, Y.S. Active Learning-Based Guided Synthesis of Engineered Biochar for CO2 Capture. Environ. Sci. Technol. 2024, 58, 6628–6636. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yang, L.; Lei, X.; Zhang, W.; Ai, Z.; Yang, Z.; Zhan, H.; Yang, J.; Yuan, X.; Peng, H.; et al. Machine learning predicting and engineering the yield, N content, and specific surface area of biochar derived from pyrolysis of biomass. Biochar 2022, 4, 63. [Google Scholar] [CrossRef]


| Pollutant | Pollutant Concentration (mg/kg) | Activator | Removal (%) | Process Descriptions | Reference |
|---|---|---|---|---|---|
| Oil | 78–99 | CoOOH | 88.3 | Rapid Co2+/Co3+ redox cycle and CoOH formation improved the continuous generation of ROS (SO4•−, O2•−, and 1O2). | Lyu et al. [17] |
| TPH | 6625 | None | 32.8 | Blank without activator. | Bajagain and Jeong [18] |
| Fe0 | 43.3 | Using reduced iron. | |||
| Fe2+ | 39.7 | Using FeSO4. | |||
| Co2+ | 40.4 | Using CoCl2. | |||
| nZVI | 61.1 | The efficient catalyst of ZVI was attributed to its small size and large surface area, providing more reactive sites for the oxidation reaction. | |||
| nZVI | >96.0 | By five serial treatments of 3% PMS +0.2% nZVI. | |||
| TCE | 100 | None | 95.3 | Unactivated PMS can degrade TCE in soil, possessing a negligible impact on the particle size distribution and soil texture. | Oba et al. [19] |
| TCS | 535 | Trimetallic electrode | 66.0 | Co2+ in trimetallic oxidation electrode activated PMS to produce SO4•− coupled with an electrokinetic geo-oxidation system. | Yuan et al. [20] |
| DDT | 7565 | None | >95.0 | Minerals in the soil participated in the soil remediation during the ball milling process, probably through non-radical ways rather than ROS oxidation. | Xu et al. [21] |
| CPF | 100 | Microwave | >90.0 | The Fe(II)/Fe(III) oxidation–reduction cycle caused SO4•− generation, and reactive metastable heating pad waste-PMS caused electron transfer. | Shang et al. [22] |
| PAHs | 692 | None | 24.5–82.8 | Producing O2•−, SO4•−, •HO, 1O2, and SO5•− for soil remediation. | Zhou et al. [26] |
| 692 | Electrokinetic | 14.7–34.1 | During the electrokinetic-enhanced process, the more rings the PAH had, the more difficult to remove. | ||
| 946.1 | Amorphous FH | 77.8–94.7 | Radicals •OH and SO4•−, as well as non-radicals (1O2 and Fe(IV)=O), participated in the soil remediation. | Tang et al. [23] | |
| 100 | None | 72.5 | Blank without activator. | Zeng et al. [27] | |
| nZVI | 79.9 | HA and HA-like reductive compounds in soil play a vital role during Fe(II) and Fe(III) cycles, affecting the generation of ROS. | |||
| nZVI+CA | 96.8 | CA could promote the desorption of PAH from the soil medium. | |||
| 69.4 | N-CG | 71.3–97.0 | Pyridinic and graphitic N were speculated to be the reactive sites for PMS activation. | Liang et al. [24] | |
| Bap | 79.9 | CG | 17.2 | Using coal gangue to calcine. | Li et al. [28] |
| Ca–N | 21.7 | Using anhydrous calcium chloride and melamine to calcine. | |||
| CG-N | 27.9 | Using coal gangue and melamine to calcine. | |||
| CG-Ca | 60.9 | Using coal gangue and anhydrous calcium chloride to calcine. | |||
| CG-Ca-N1/8 | 100 | Using coal gangue, anhydrous calcium chloride, and melamine to calcine. And CG-Ca-N1/8 induced in the production of •OH, SO4•−, •O2− and 1O2. | |||
| STZ | 50 | None | 96.5 | 1O2 was the predominant ROS that contributed to the soil remediation. | Zhang et al. [25] |
| Pollutant | Pollutant Concentration (mg/kg) | Activator | Removal (%) | Process Descriptions | Reference |
|---|---|---|---|---|---|
| TPHP | 50 | TP | 10.8–58.6 | The interaction between Fe-minerals in soil and TP accelerated ROS generation for TPHP degradation, triggering the activation of PDS by accelerating the Fe(III) ↔ Fe(II)redox cycle. | Dong et al. [29] |
| H2A | 96.80 | High Fe-minerals (e.g., α-Fe2O3) content enhanced PDS activation, while high SOM content inhibits TPHP degradation by consuming ROS. | Dong et al. [30] | ||
| NAP | 48.4 | None | 46.5 | Soil minerals and SOM could activate PDS to generate ROS (SO4•−) to degrade NAP. | Feng et al. [31] |
| CAT | 71.8 | AA/CAT could promote the activation of PDS by Fe. | |||
| AA | 95.3 | AA can activate PDS to produce ascorbate free radicals (AscH•−), which further transfer electrons to PDS and generate SO4•−. | |||
| HA | >42.7 | Modified HA with blocked Ar-OH and/or -COOH groups proved their importance in HA’s complexation and reduction capabilities. | |||
| SMX | 20 | nZVI | 86.5–96.1 | The reactive species during the degradation of SMX was radical •OH. | Zhou et al. [32] |
| CPF | 100 | None | 20 | Blank without activator. | Shang et al. [33] |
| Microwave | 36 | Microwave at 60 °C. | |||
| Microwave | 85 | Microwave at 80 °C. Radicals SO4•− and •OH were produced under microwave irradiation. The collisions between oxidants and soil facilitated the degradation of CPF through the thermal effects and non-thermal effects of microwaves. | |||
| MCB | 95.6 | Ball-milled pyrite | 66.1–93.8 | FeIV, SO4•−, and •OH were the main radicals produced in the ball-milled pyrite and PDS system. Ball milling restrained the formation of the passivation layer and increased the Fe utilization. | Qiu et al. [34] |
| HCB | 200 | CaO | 80.0 | Calculated radical •OH content was almost three times that of SO4•−, suggesting a dominant role of radical •OH. | Fan et al. [35] |
| PAHs | 100 | None | 65.5 | Blank without activator. | Zeng et al. [27] |
| nZVI | 72.6 | HA and HA-like reductive compounds in soil play a vital role during Fe(II) and Fe(III) cycles, affecting the generation of ROS. | |||
| nZVI+CA | 93.5 | CA could promote the desorption of PAH (naphthalene) from the soil medium. | |||
| 692 | None | 32.8–78.5 | Producing O2•−, SO4•−, •HO, 1O2, and SO5•− for soil remediation. | Zhou et al. [26] | |
| 40 | None | 2.5 | Blank without activator. | Wang et al. [36] | |
| Fe3+ | 7.5 | Weak activation of PDS by Fe3+. | |||
| HA-1 | 30.0 | Chemically modified HAs through ethylation (blocking -COOH and Ar-OH). | |||
| HA-2 | 34.0 | Chemically modified HAs through hydrolysis (blocking Ar-OH). | |||
| HA-3 | 42.7 | Chemically modified HAs through amination (blocking -COOH). | |||
| 17.0 | nZVI | 82.2 | Micro/nanostructured ZVI (nZVI). | Song et al. [37] | |
| C-nZVI | 62.8 | Stearic-coated micro/nanostructured ZVI (C-nZVI). | |||
| M-nZVI | 69.1 | Commercial micron-sized ZVI (mZVI). | |||
| DDT | 7565 | HA | >42.7 | Modified HA with blocked Ar-OH and/or -COOH groups proved their importance in HA’s complexation and reduction capabilities. | Xu et al. [21] |
| STZ | 50 | None | 22.9 | PDS is not easily activated. | Zhang et al. [25] |
| Pollutant | Biochar | Microorganisms and Functions | Reference |
|---|---|---|---|
| ATZ | Sludge-derived biochar | Comamonas and Cloacibacterium: The hydrolysis of nitrogenous heterocyclic compounds. Alicyclobacillus and Halomonas: Resistant to PDS stress. Bacillus: Positively correlated with the degradation of ATZ. | Xue et al. [46] |
| PAHs | nZVI@BC | Firmicutes: Main degradable bacteria. Bacillus: PAHs-degrading bacteria. | Zhu et al. [63] |
| THI | FeMn@BC | Actinobacteriota: Possibly converting the intermediate of THI into smaller molecules. | Li et al. [62] |
| Crude oil | SSBC | Bacteroidetes, Alcanivorax, Marinobacter, Aliifodinibius, and Salinisphaera: Crude-oil-degrading bacteria. | Liu et al. [64] |
| TPHs | BC-nZVI | Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria: Related to TPHs degradation. Acinetobacter, Corynebacterium_1, and Staphylococcus: TPHs-degrading bacteria. | Zhang et al. [53] |
| PBDEs Metals | Commercial rice Husk biochar | Gammaproteobacteria, Alphaproteobacteria, Clostridia, and Acidobacteria: Associated with the degradation of PBDEs and their intermediates. | Ma et al. [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, Q.; Xu, X.; Li, X.; Yang, A.; Liu, B.; Yu, B.; Al-Hazmi, H.E.; Hassan, G.K. Toward Sustainable Soil Remediation: Progress and Perspectives on Biochar-Activated Persulfate Oxidation. Sustainability 2025, 17, 5253. https://doi.org/10.3390/su17125253
Jian Q, Xu X, Li X, Yang A, Liu B, Yu B, Al-Hazmi HE, Hassan GK. Toward Sustainable Soil Remediation: Progress and Perspectives on Biochar-Activated Persulfate Oxidation. Sustainability. 2025; 17(12):5253. https://doi.org/10.3390/su17125253
Chicago/Turabian StyleJian, Qiwei, Xianbao Xu, Xiang Li, Aiwu Yang, Bin Liu, Bo Yu, Hussein E. Al-Hazmi, and Gamal Kamel Hassan. 2025. "Toward Sustainable Soil Remediation: Progress and Perspectives on Biochar-Activated Persulfate Oxidation" Sustainability 17, no. 12: 5253. https://doi.org/10.3390/su17125253
APA StyleJian, Q., Xu, X., Li, X., Yang, A., Liu, B., Yu, B., Al-Hazmi, H. E., & Hassan, G. K. (2025). Toward Sustainable Soil Remediation: Progress and Perspectives on Biochar-Activated Persulfate Oxidation. Sustainability, 17(12), 5253. https://doi.org/10.3390/su17125253








