Sustainable Phosphate Recovery Using Novel Ca–Mg Bimetallic Modified Biogas Residue-Based Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Batch Adsorption Experiments
2.3. Characterization and Analytical Method
3. Results and Discussion
3.1. Structure–Activity Relationship of Phosphate Adsorption by Ca–Mg/BC
3.2. Optimization of Synthesis Conditions for Sustainable Biochar Production
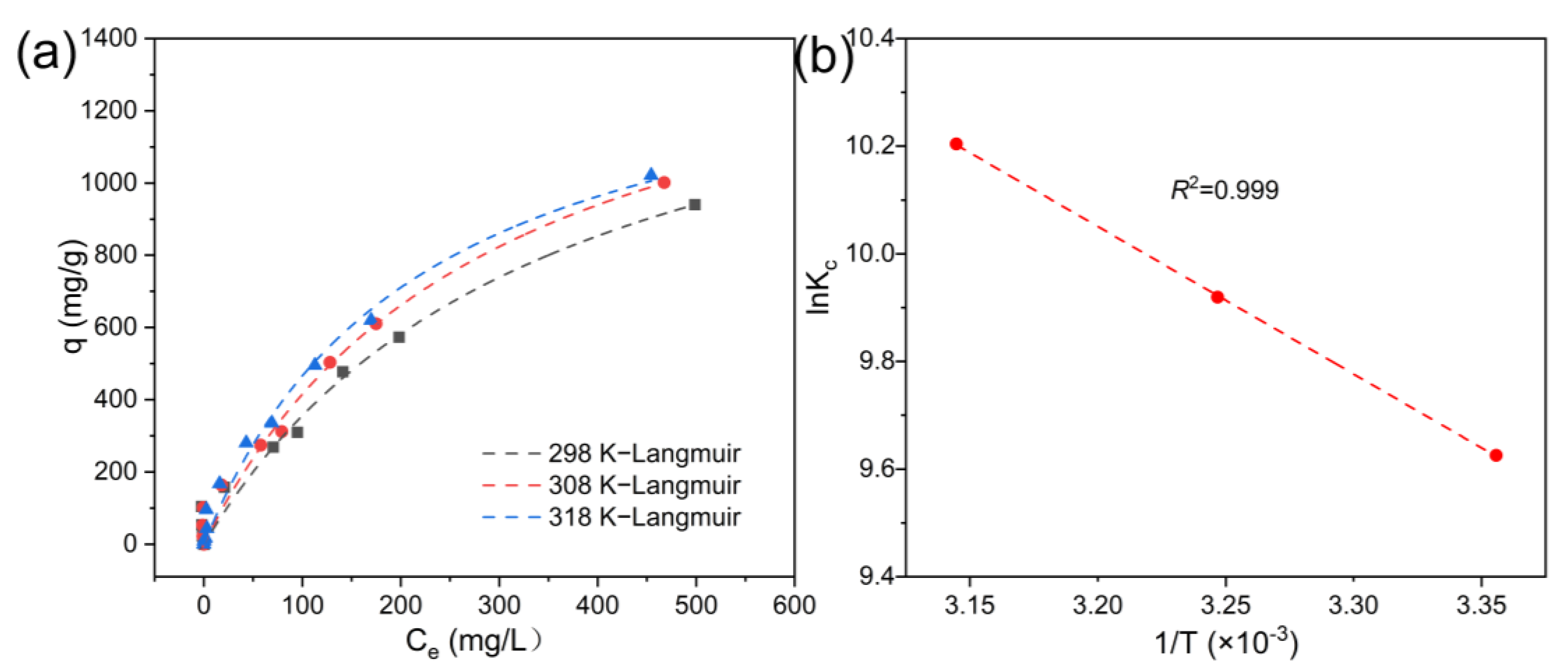
3.3. Adsorption Isotherm, Kinetics, and Thermodynamics
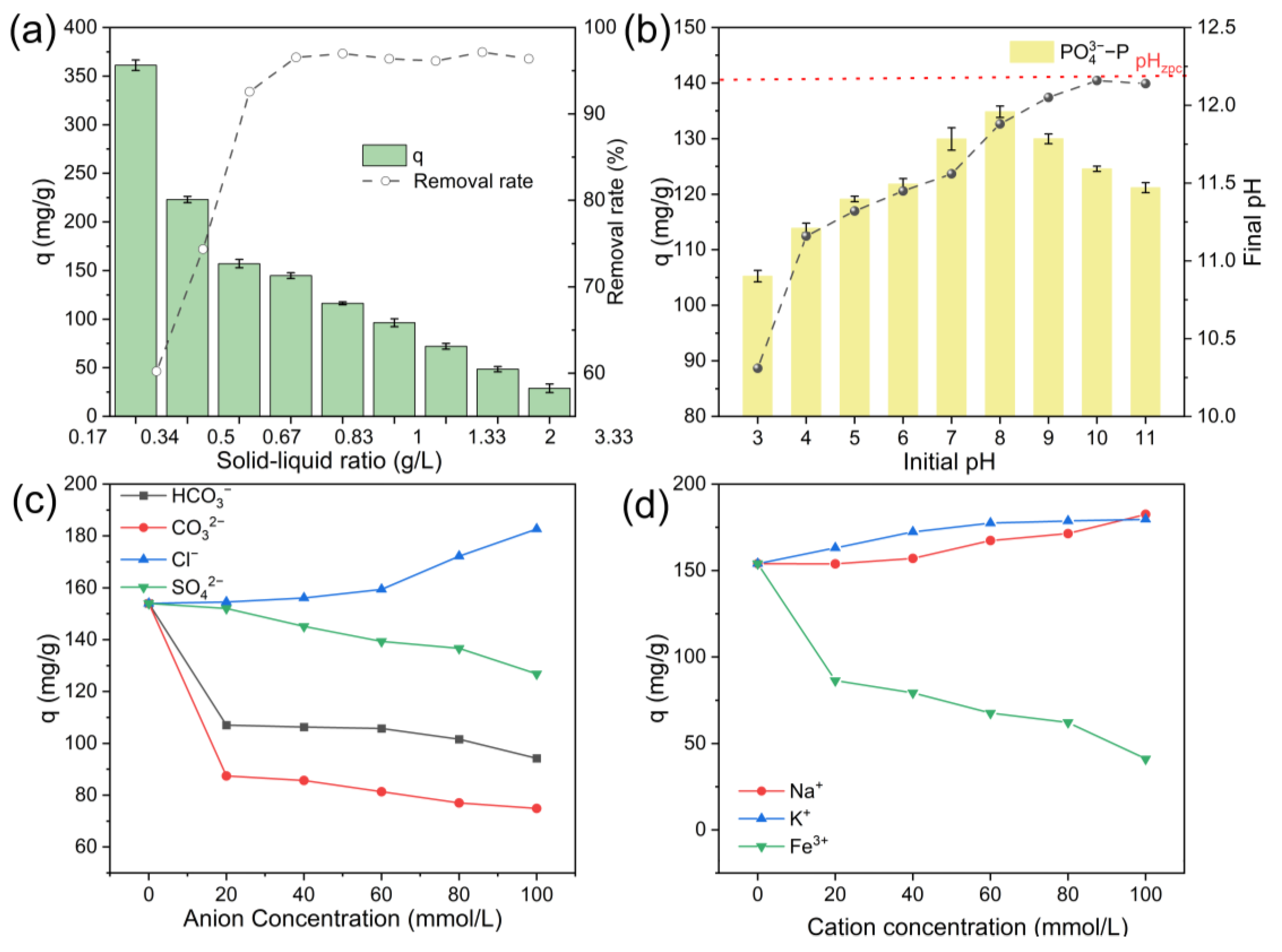
3.4. Analysis of Factors Affecting Phosphate Recovery by Biochar
3.4.1. Optimizing the Biochar Dosage for Sustainable Phosphate Recovery
3.4.2. Effect of pH on Phosphate Adsorption
3.4.3. Effect of Coexisting Ions on Phosphate Adsorption
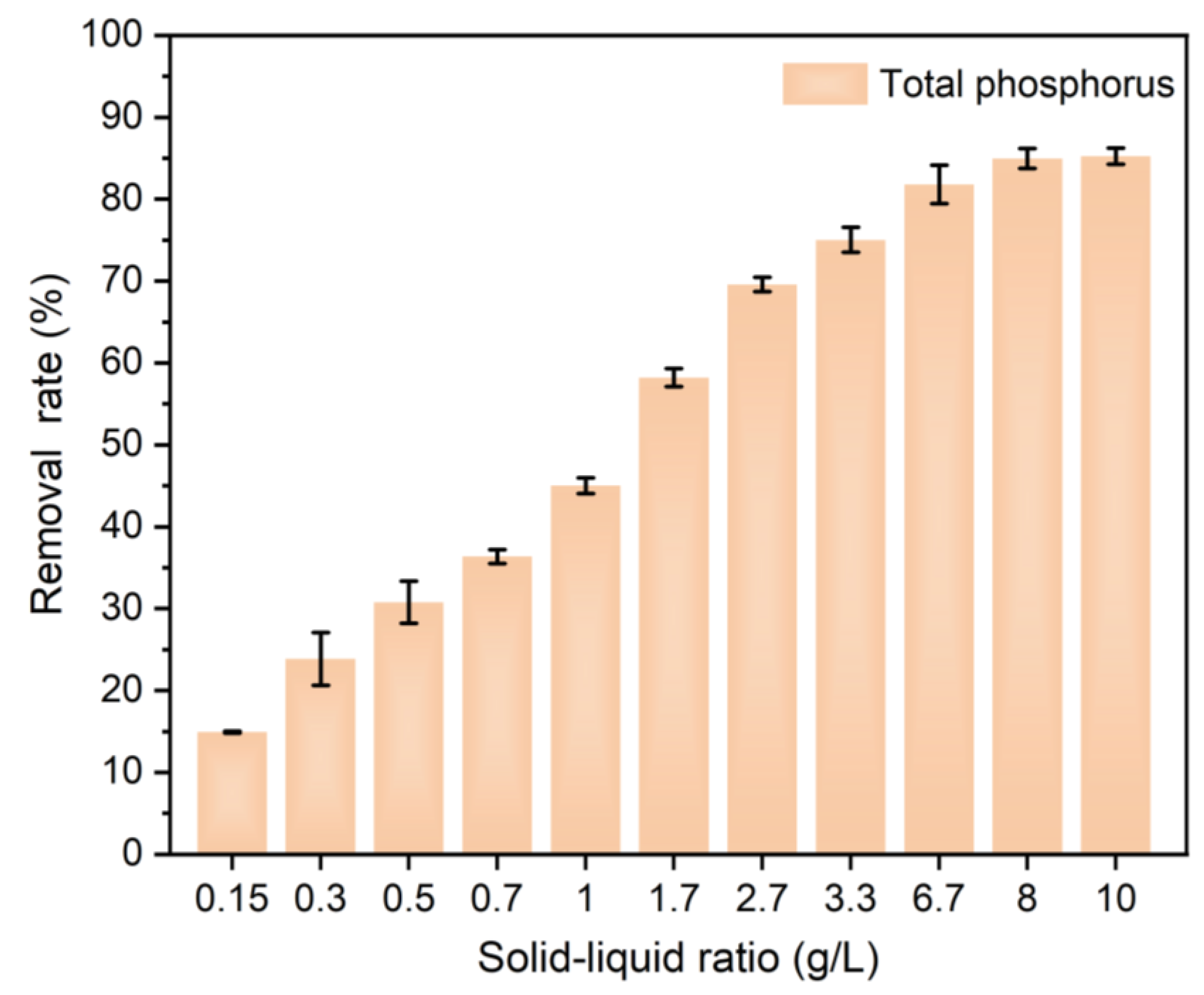
3.4.4. Sustainable Phosphorus Recovery from Actual Biogas Slurry Using Ca–Mg/BC
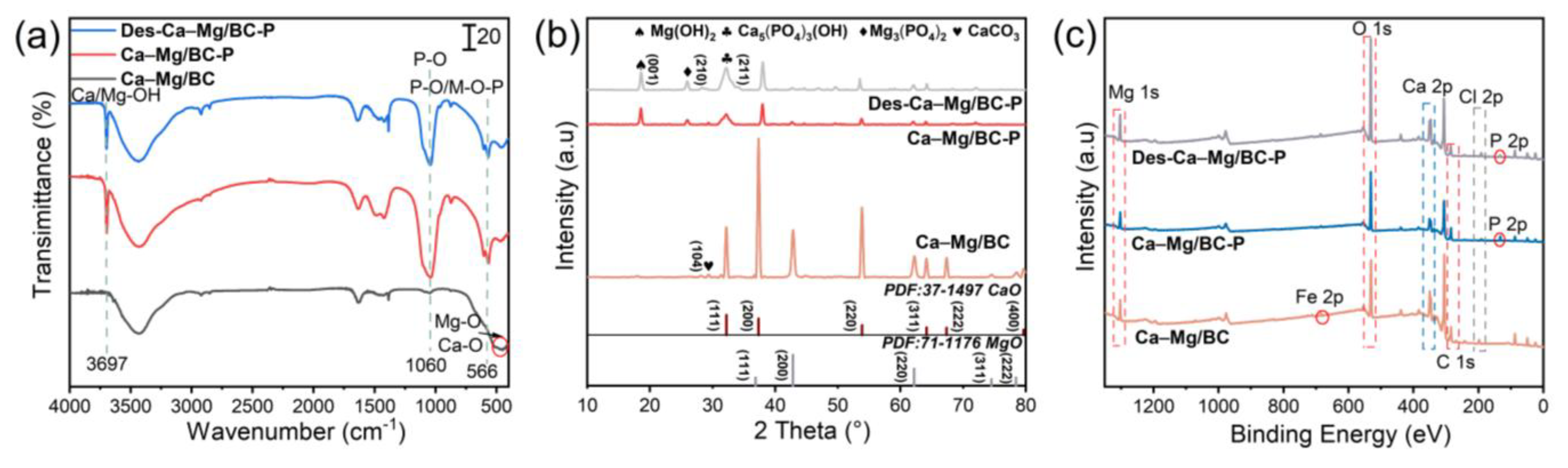
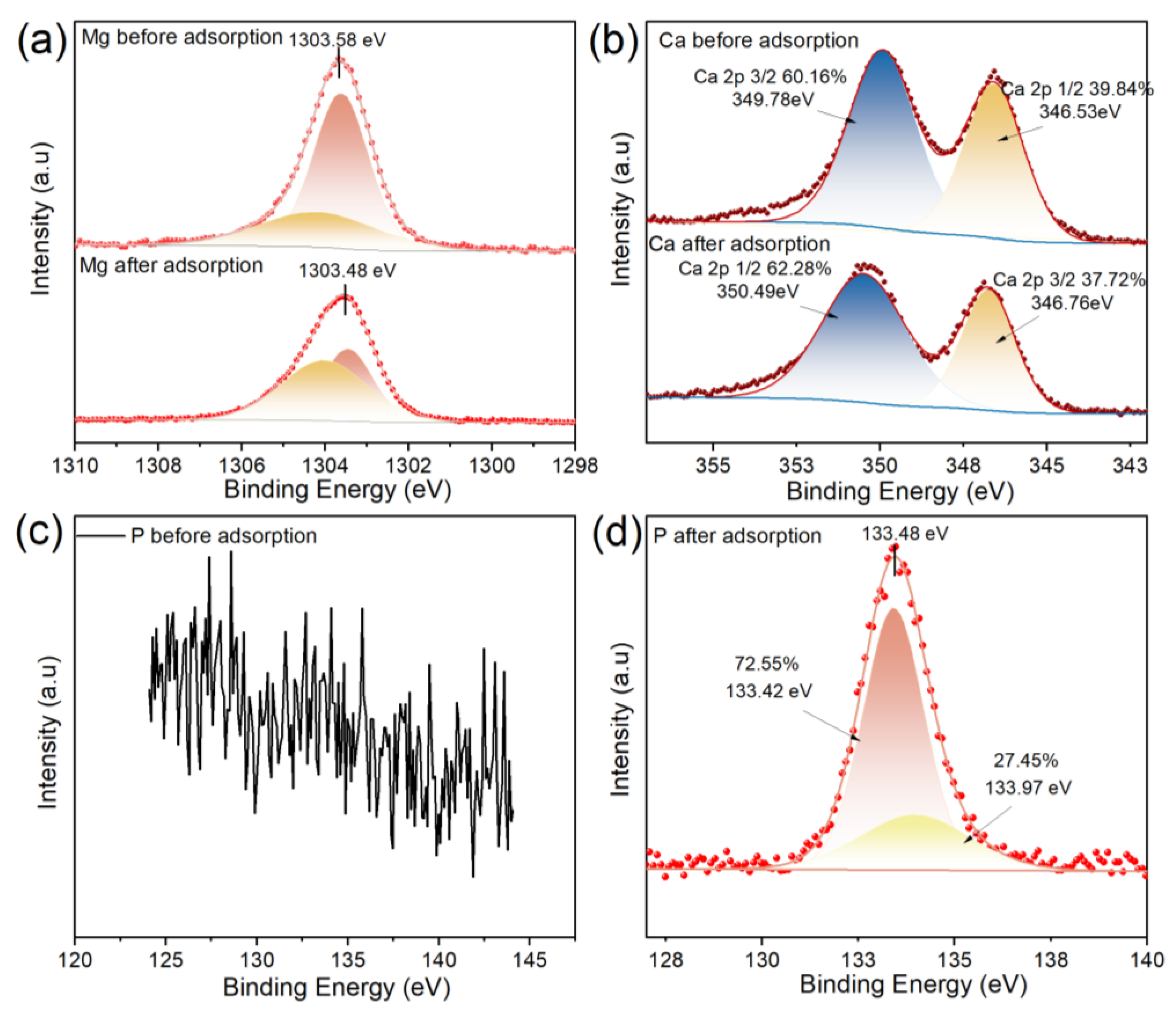
3.5. Phosphate Adsorption Mechanism Investigation
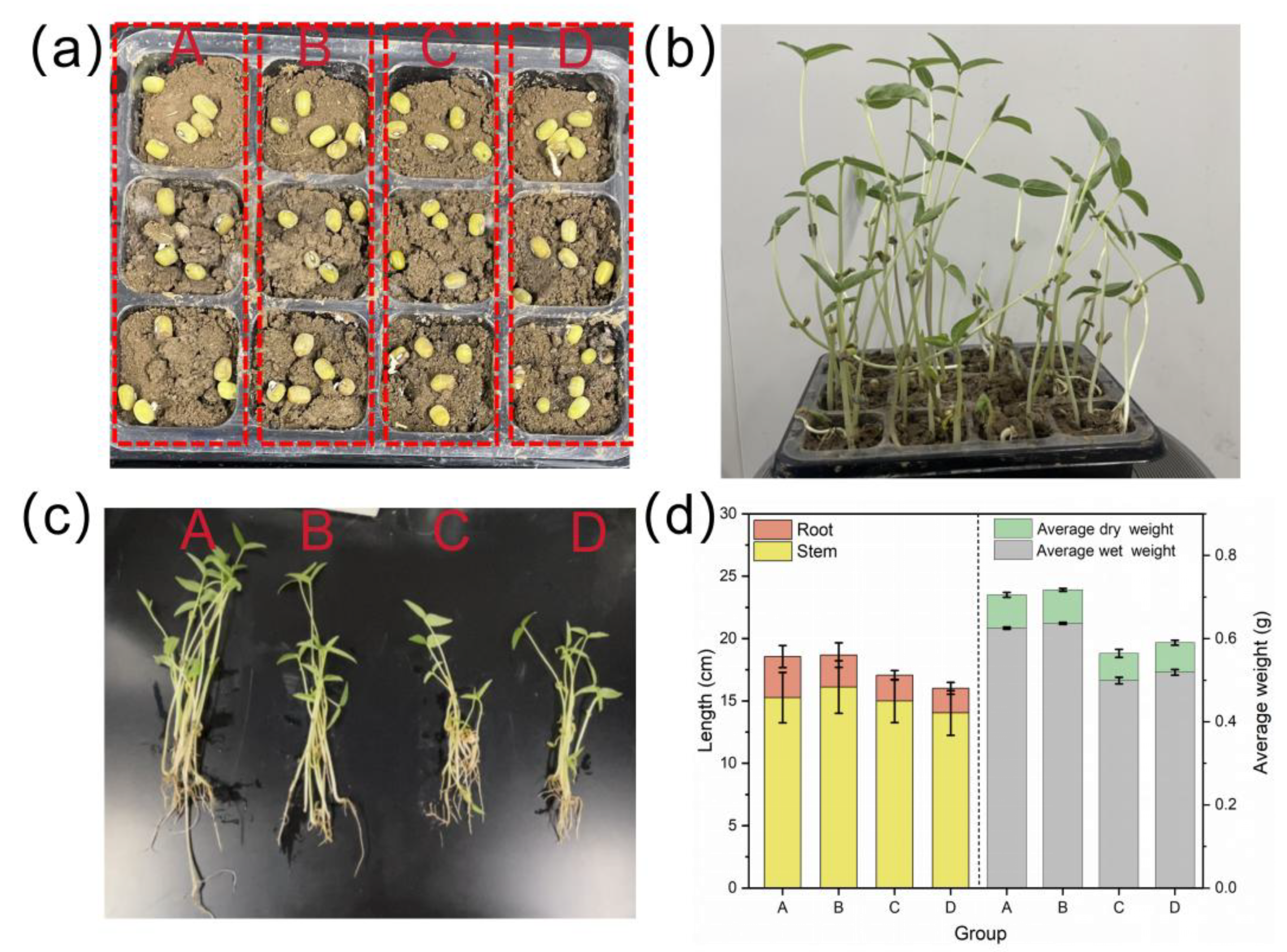
3.6. Sustainable Crop Growth Validation Test
4. Conclusions
- (1)
- Circular wastewater treatment potential was evidenced by 92.56% phosphate removal from a 100 mg/L solution and >80% removal from an actual biogas slurry (8 g/L dosage, 15.51 mg/L).
- (2)
- The multilayer adsorption properties were confirmed through a kinetic analysis, which revealed that the adsorption process followed a pseudo-second-order kinetic model (R2 = 0.9858). The isothermal adsorption data also showed a high degree of agreement with the Freundlich model; 1596.59 mg/g was the maximum adsorption capacity determined using the Langmuir model at 298 K, which is 7.04 times higher than that of BC The thermodynamic parameters ΔG < 0 and ΔH = +1.63 kJ/mol confirmed that the process was a spontaneous heat absorption reaction.
- (3)
- Mechanistic characterization revealed a dual-pathway sustainable phosphorus-management system. Surface-hydrolyzed Ca2+ and Mg2+ initially capture phosphate through electrostatic attraction, forming Ca5(PO4)3(OH) and Mg3(PO4)2 precipitates for long-term nutrient recycling. The results of the pot experiment demonstrated circular economy benefits. The phosphate-saturated material as a soil conditioner could increase the stem length, root length by 15%, and fresh weight by 51%, confirming that it combines the functions of nutrient slow-release and a soil conditioner.
- (4)
- This study established a sustainable technical pathway of “Waste Recycling-Pollution Control-Agricultural Application”, offering an eco-friendly solution for biogas residue and slurry utilization. The bimetallic modification strategy significantly improved the phosphate-adsorption capacity of biochar, demonstrating great potential for sustainable phosphate removal from wastewater and nutrient recycling in agriculture.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.X.; Yu, R.C.; Zhou, M.J. Evolution of harmful algal blooms in the East China Sea under eutrophication and warming scenarios. Water Res. 2022, 221, 118807–118816. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8–24. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-clay, biochar-microorganism and biochar-enzyme composites for environmental remediation: A review. Environ. Chem. Lett. 2023, 21, 1837–1862. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Zhang, C.; Chen, Z. Quantitative evaluation on phosphate adsorption by modified biochar: A meta-analysis. Process Saf. Environ. Prot. 2023, 177, 42–51. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Kunhikrishnan, A.; Rahman, M.M. Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars. Water 2021, 13, 3320. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Kumar, S. Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total Environ. 2022, 806, 150444. [Google Scholar] [CrossRef]
- Cheng, S.; Meng, W.; Xing, B.; Shi, C.; Wang, Q.; Xia, D.; Nie, Y.; Yi, G.; Zhang, C.; Xia, H. Efficient removal of heavy metals from aqueous solutions by Mg/Fe bimetallic oxide-modified biochar in monometallic and bimetallic systems: Experiments and DFT investigations. J. Clean. Prod. 2023, 403, 136821. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Hu, X.; Zhang, Y.; Zhang, D.; Zhang, H.; Xu, H.; Sun, Y.; Gu, X.; Luo, J.; et al. Reclaiming selenium from water using aluminum-modified biochar: Adsorption behaviors, mechanisms, and effects on growth of wheat seedlings. Environ. Pollut. 2024, 361, 124835–124844. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Huo, J.; Zhang, X.; Wen, H.; Zhang, D.; Zhao, Y.; Kang, D.; Guo, W.; Ngo, H.H. Adsorption recovery of phosphorus in contaminated water by calcium modified biochar derived from spent coffee grounds. Sci. Total Environ. 2023, 909, 168426–168435. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, B.; Huang, H.; Yu, W.; Sun, C.; Long, K.; Young, B. Utilization of activated sludge and shell wastes for the preparation of Ca-loaded biochar for phosphate removal and recovery. J. Clean. Prod. 2022, 382, 135395–135404. [Google Scholar] [CrossRef]
- Liu, H.; Shan, J.; Chen, Z.; Lichtfouse, E. Efficient recovery of phosphate from simulated urine by mg/Fe bimetallic oxide modified biochar as a potential resource. Sci. Total Environ. 2021, 784, 147546. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, Y.; Zeng, C.; Yang, L.; Cui, S.; Zhi, S.; Yang, F.; Ding, Y.; Zhang, K.; Zhang, Z. Fe-Al bimetallic oxides functionalized-biochar via ball milling for enhanced adsorption of tetracycline in water. Bioresour. Technol. 2023, 369, 128385–128393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kong, L.; Long, J.; Su, M.; Diao, Z.; Chang, X.; Chen, D.; Song, G.; Shih, K. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef]
- Biswas, B.; Adhikari, S.; Jahromi, H.; Ammar, M.; Baltrusaitis, J.; Torbert, A.; Linhoss, J.; Lamba, J. Magnesium doped biochar for simultaneous adsorption of phosphate and nitrogen ions from aqueous solution. Chemosphere 2024, 358, 142130–142138. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Kuang, X.; Li, J.; Ouyang, Z.; Huang, H.; Chen, J.; Chen, X.; Li, L. Ca–Mg modified attapulgite for phosphate removal and its potential as phosphate-based fertilizer. J. Environ. Manag. 2024, 357, 120727–120735. [Google Scholar] [CrossRef]
- Samaraweera, H.; Sharp, A.; Edwards, J.; Pittman, C.U.; Zhang, X.; Hassan, E.B.; Thirumalai, R.V.K.G.; Warren, S.; Reid, C.; Mlsna, T. Lignite, thermally-modified and Ca/Mg-modified lignite for phosphate remediation. Sci. Total Environ. 2021, 773, 145631–145644. [Google Scholar] [CrossRef]
- Dan Luo, N.; Nan, H.; Zhang, Y.; Sher, F.; Wang, C. Phosphorus recovery from wastewater by Ca-Al layered double hydroxide/biochar as potential agricultural phosphorus for closed-loop phosphorus recycling. Process Saf. Environ. Prot. 2024, 194, 1538–1548. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Wu, M.; Lin, K.; Xu, L.; Zeng, T.; Shi, H.; Zhang, M. Synergistic role of inherent calcium and iron minerals in paper mill sludge biochar for phosphate adsorption. Sci. Total Environ. 2022, 834, 155193–155203. [Google Scholar] [CrossRef]
- Güngör, Ç.; Şakir Ece, M. Competitive adsorption of VOCs (benzene, xylene and ethylbenzene) with Fe3O4@SiO2-NH@BENZOPHENONE magnetic nanoadsorbents. Chem. Eng. J. 2023, 475, 146034–146048. [Google Scholar] [CrossRef]
- Govindan, V.; Raja, A.; Das, G.M.; Joseph Daniel, D.; Ramesh Kumar, R.; Sankaranarayanan, K. Synthesis and luminescence properties of Ca3Ga4O9:Eu3+: An efficient red-emitter for WLEDs. Ceram. Int. 2023, 49, 17566–17576. [Google Scholar] [CrossRef]
- Tarbi, A.; Chtouki, T.; Sellam, M.A.; Benahmed, A.; El Kouari, Y.; Erguig, H.; Migalska-Zalas, A.; Goncharova, I.; Taboukhat, S. The discovery of the effect of compositional disorder on the opto-electronic properties of the deformed InGaAsP quaternary. Mater. Today Commun. 2023, 35, 105678–105682. [Google Scholar] [CrossRef]
- Guo, X.; Liang, S.; Zou, Z.; Xu, X.; Yang, F.; Quan, J.; Li, X.; Duan, H.; Yu, W.; Yang, J. Enhanced phosphorus bioavailability of biochar derived from sewage sludge co-pyrolyzed with K, Ca-rich biomass ash. Water Res. 2025, 271, 122901–122910. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhao, Y.; Wang, L.; Feng, J.; Sun, F. Efficient simultaneous phosphate and ammonia adsorption using magnesium-modified biochar beads and their recovery performance. J. Environ. Chem. Eng. 2023, 11, 110875–110889. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207–112221. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca-Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef]
- Choi, Y.K.; Jang, H.M.; Kan, E.; Wallace, A.R.; Sun, W. Adsorption of phosphate in water on a novel calcium hydroxide-coated dairy manure-derived biochar. Environ. Eng. Res. 2018, 24, 434–442. [Google Scholar] [CrossRef]
- Xang, S.L.; Chu, M.; Gong, C.; Fang, B. Adsorption performance and mechanism of phosphate in water by magnesium oxide-corncob biochar. J. Environ. 2023, 149, 04023053. [Google Scholar] [CrossRef]
- Ou, W.; Lan, X.; Guo, J.; Cai, A.; Liu, P.; Liu, N.; Liu, Y.; Lei, Y. Preparation of iron/calcium-modified biochar for phosphate removal from industrial wastewater. J. Clean. Prod. 2023, 383, 135468–135476. [Google Scholar] [CrossRef]
- Yi, M.; Chen, Y. Enhanced phosphate adsorption on Ca-Mg-loaded biochar derived from tobacco stems. Water Sci. Technol. 2018, 78, 2427–2436. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Issaoui, M.; Kwapinska, M.; Jeguirim, M.; Leahy, J.J.; Kwapinski, W. Poultry manure conversion into eco-friendly materials: Synthesis of Mg-/Al-based biochars, characterization and application for phosphorus recovery from aqueous solutions. Biomass Convers. Bioref. 2024, 14, 25379–25393. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Chen, S.; Sun, Y.; Li, P.; Tan, J.; Jiang, X. Enhanced soil P immobilization and microbial biomass P by application of biochar modified with eggshell. J. Environ. Manag. 2023, 345, 118568–118578. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303–116312. [Google Scholar] [CrossRef]
- Yin, H.; Kong, M.; Fan, C. Batch investigations on P immobilization from wastewaters and sediment using natural calcium rich sepiolite as a reactive material. Water Res. 2013, 47, 4247–4258. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Muryanto, S.; Schmahl, W.W.; Jamari, J.; Bayuseno, A.P. A kinetic and structural analysis of the effects of Ca-and Fe ions on struvite crystal growth. Solid State Sci. 2022, 134, 107062–107068. [Google Scholar] [CrossRef]
- Karapınar, N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J. Hazard. Mater. 2009, 170, 1186–1191. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Chauhan, C.K.; Joshi, M.J. Growth and characterization of struvite-Na crystals. J. Cryst. Growth. 2014, 401, 221–226. [Google Scholar] [CrossRef]
- He, L.; Wang, D.; Zhu, T.; Lv, Y.; Li, S. Pyrolysis recycling of pig manure biochar adsorption material for decreasing ammonia nitrogen in biogas slurry. Sci. Total Environ. 2023, 881, 163315–163323. [Google Scholar] [CrossRef]
- Shashkova, I.; Kitikova, N.; Sycheva, O.; Dzikaya, A.; Nurbekova, M.; Hosseini-Bandegharaei, A.; Ivanets, A. Efficient immobilization of Sr (II) ions in ceramic matrices using thermal transformation of Ti–Ca–Mg phosphate adsorbents. Ceram. Int. 2024, 50, 22836–22847. [Google Scholar] [CrossRef]
- Hashmi, M.U.; Shah, S.A. Dissolution behavior of bioactive glass ceramics with different CaO/MgO ratios in SBF-K9 and r-SBF. Prog. Nat. Sci. Mater. Int. 2014, 24, 354–363. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Cheng, P.; Arbid, Y.; Liu, H.; Zou, X.; Chen, T. Utilization of Waste Adsorbent Generated after Ca/Al-LDH Adsorption of High-Concentration Phosphate: Fluorine Removal. J. Environ. Eng. 2023, 149, 04022090. [Google Scholar] [CrossRef]
- Massit, A.; El Yacoubi, A.; Kholtei, A.; El Idrissi, B.C. XRD and FTIR Analysis of Magnesium Substituted Tricalcium Calcium Phosphate Using a Wet Precipitation Method. Biointerface Res. Appl. Chem. 2021, 11, 8034–8042. [Google Scholar]
- Zhang, P.; Shi, H.; Ruan, X.; Qian, G.; Frost, R.L. Na-dodecylsulfate modification of hydrocalumite and subsequent effect on the structure and thermal decomposition. J. Therm. Anal. Calorim. 2011, 104, 743–747. [Google Scholar] [CrossRef]
- Shan, L.L.; Wang, R.S.; Lai, H.T.; Zhu, Z.B.; Chen, Y.; Ni, Z.Y.; Pang, C.L.; Zhang, Q.Z. Treating waste with waste: Adsorption behavior and mechanism of phosphate in water by modified phosphogypsum biochar. Environ. Sci. Pollut. Res. 2024, 31, 50411–50426. [Google Scholar] [CrossRef]
- Jetsrisuparb, K.; Jeejaila, T.; Saengthip, C.; Kasemsiri, P.; Ngernyen, Y.; Chindaprasirt, P.; Knijnenburg, J.T.N. Tailoring the phosphorus release from biochar-based fertilizers: Role of magnesium or calcium addition during co-pyrolysis. RSC Adv. 2022, 12, 30539–30548. [Google Scholar] [CrossRef]
- Mahajan, R.; Prakash, R.; Kumar, S.; Kumar, V.; Choudhary, R.J.; Phase, D.M. Surface and luminescent properties of Mg3(PO4)2:Dy3+ phosphors. Optik 2021, 225, 165717–165730. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Xu, Y.; Liu, J. Different performances and mechanisms of phosphate adsorption onto metal oxides and metal hydroxides: A comparative study. J. Chem. Technol. Biotechnol. 2015, 91, 1232–1239. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Zhu, R.; Zhang, X.; Yan, L. Phosphate adsorption performance and mechanisms by nanoporous biochar-iron oxides from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 28132–28145. [Google Scholar] [CrossRef] [PubMed]
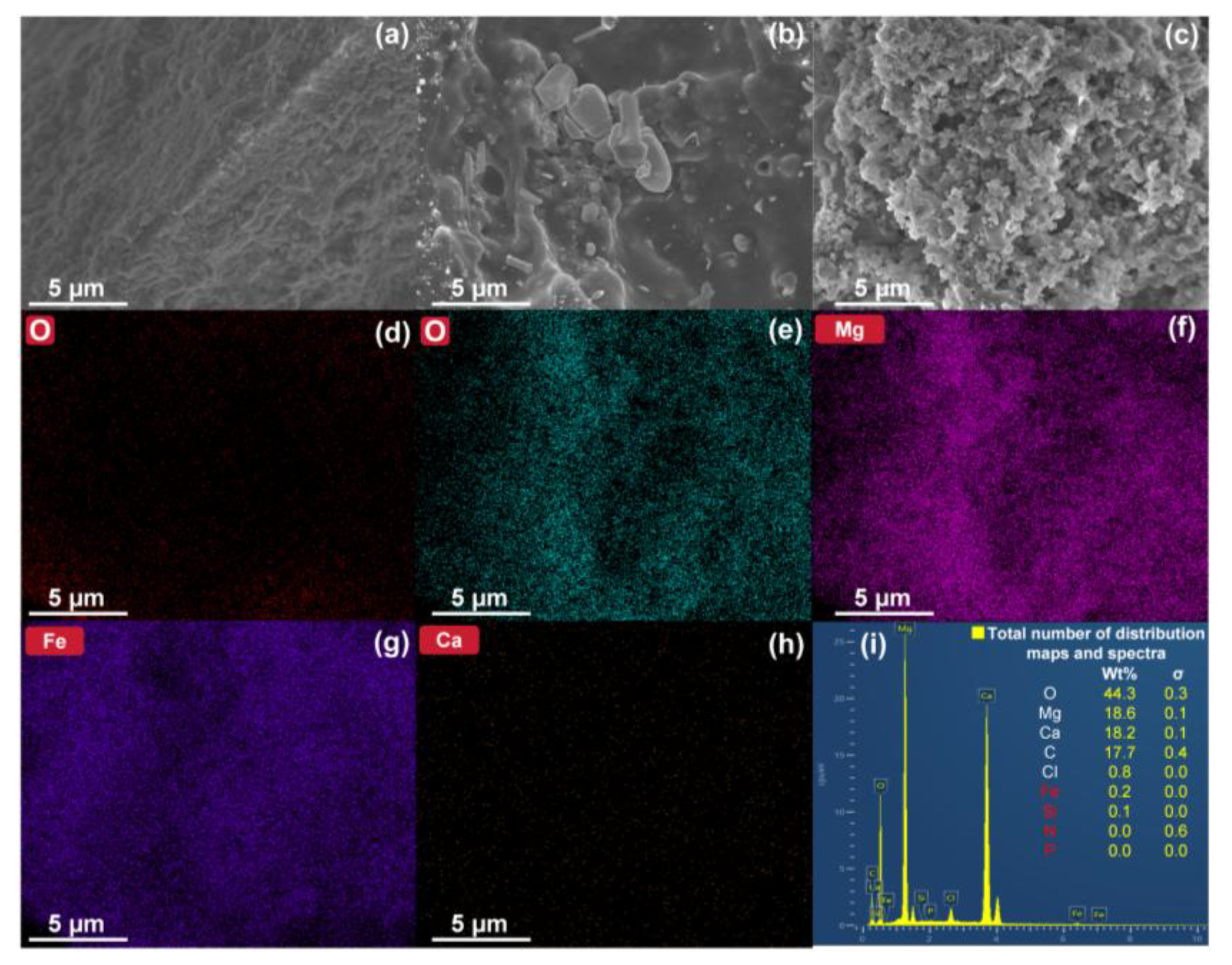
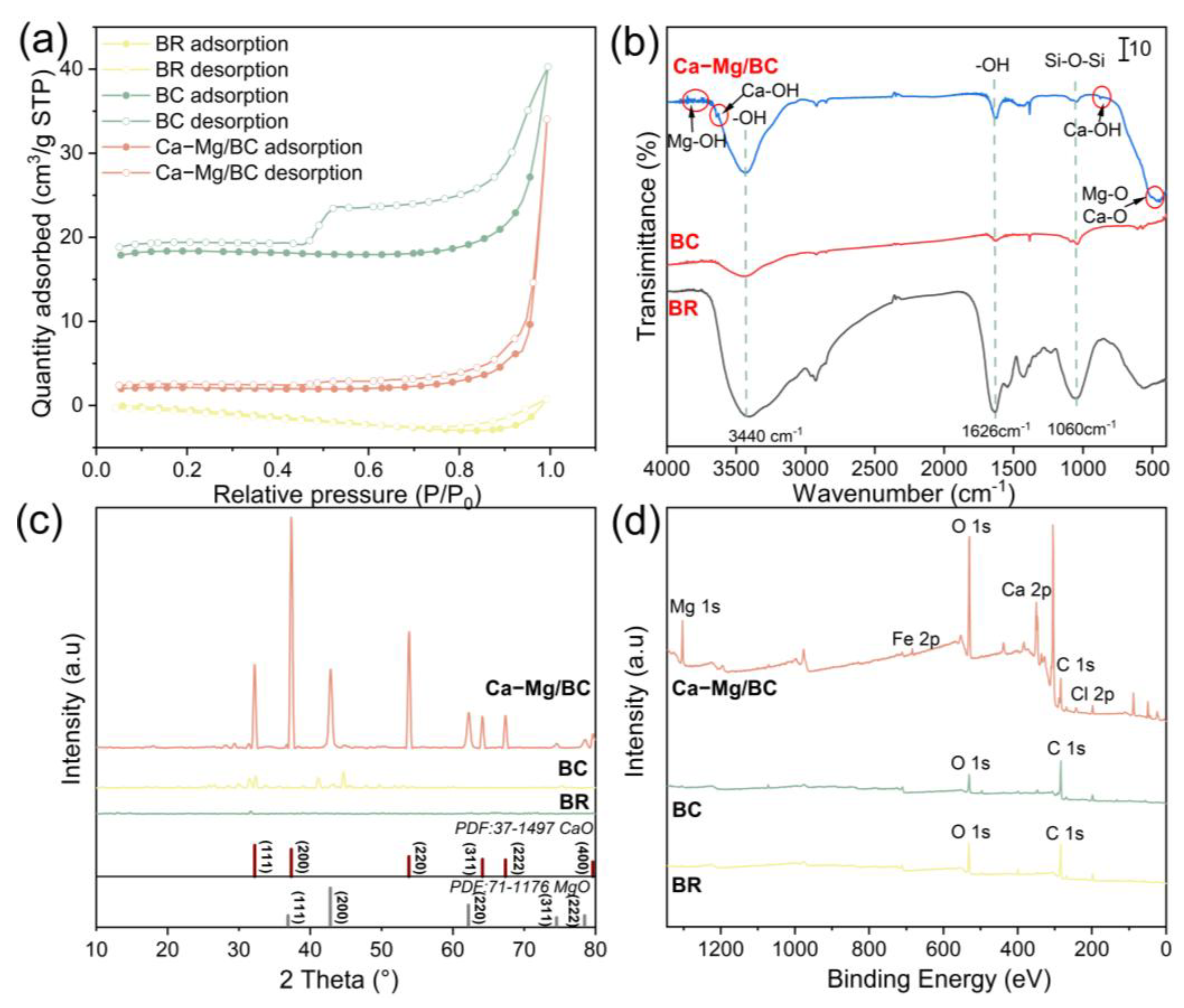
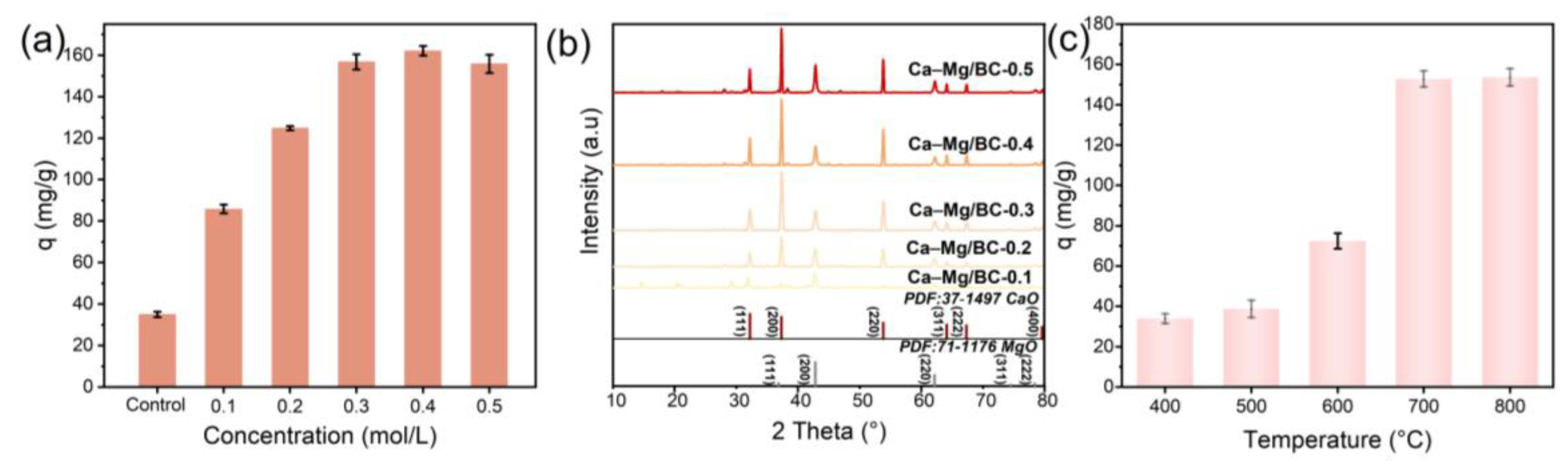
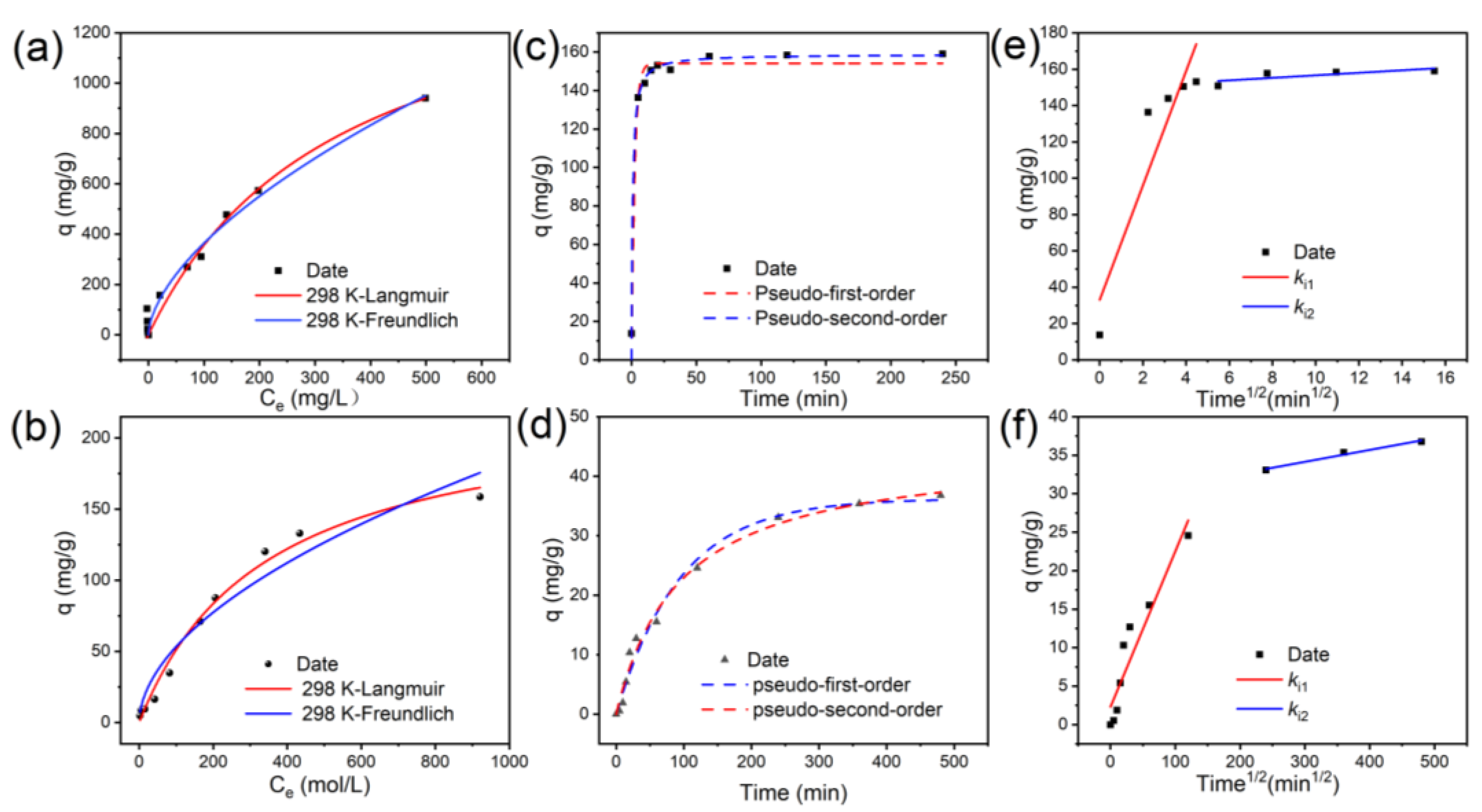
| Parameters | Adsorbents | ||
|---|---|---|---|
| BR | BC | Ca-Mg/BC | |
| SBET (m2·g−1) | 0.20 | 7.97 | 72.33 |
| Total pore volume (cm3·g−1) | 0.0012 | 0.061 | 0.12 |
| APD (nm) | 23.66 | 3.62 | 25.28 |
| Adsorbent | C | O | Ca | Mg | Fe | Si | N | P | Cl |
|---|---|---|---|---|---|---|---|---|---|
| BR | 65.68 | 15.21 | 1.54 | 0.99 | 2.78 | 1.86 | 4.14 | 2.37 | 5.43 |
| BC | 56.67 | 22.08 | 2.05 | 1.21 | 2.1 | 2.9 | 5.49 | 1.9 | 5.6 |
| Ca-Mg/BC | 24.68 | 41.46 | 16.44 | 8.76 | 1.24 | 0.67 | 2.95 | 0.78 | 3.02 |
| Model | Parameter | Ca-Mg/BC | BC |
|---|---|---|---|
| Langmuir | qe (mg·g−1) | 1596.59 | 226.81 |
| KL (L·mg−1) | 0.0029 | 0.0029 | |
| R2 | 0.9683 | 0.9875 | |
| Freundlich | KF | 23.17 | 4.50 |
| 1/n | 0.5879 | 0.5370 | |
| R2 | 0.9757 | 0.9443 |
| Model | Parameter | Ca-Mg/BC | BC |
|---|---|---|---|
| Pseudo-first-order | qe (mg·g−1) | 154.14 | 36.22 |
| k1 (min−1) | 0.41 | 0.01 | |
| R2 | 0.9778 | 0.9837 | |
| Pseudo-second-order kinetic | qe (mg·g−1) | 158.87 | 44.63 |
| k2 (g·mg−1·min−1) | 0.00718 | 2.36 × 10−4 | |
| R2 | 0.9858 | 0.9869 | |
| Intraparticle diffusion | ki1 (mg·g−1·min−0.5) | 31.49 | 0.2021 |
| R2 | 0.8565 | 0.8869 | |
| ki2 (mg·g−1·min−0.5) | 0.69 | 0.01546 | |
| R2 | 0.8045 | 0.9817 |
| Temperature (K) | KC | ΔH (kJ·mol−1) | ΔS (kJ·mol−1·K−1) | ΔG (kJ·mol−1) |
|---|---|---|---|---|
| 298 | 15,145.71 | 2.48 | 0.072 | −23.85 |
| 308 | 20,317.41 | −24.58 | ||
| 318 | 27,019.52 | −25.28 |
| Group | Stem Length (cm) | Root Length (cm) | Average Wet Weight (g) | Average Dry Weight (g) | Phosphorus (g/kg) |
|---|---|---|---|---|---|
| A (Commercial phosphate fertilizer) | 17.44 | 3.52 | 0.65 | 0.084 | 3.86 |
| B (Ca-Mg/BC-P) | 15.98 | 3.24 | 0.59 | 0.069 | 3.79 |
| C (BC-P) | 11.08 | 3.10 | 0.39 | 0.055 | 2.13 |
| D (No additives) | 10.13 | 3.18 | 0.39 | 0.058 | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhuo, G.; Xue, D.; Zhu, G.; Wang, C.-Y. Sustainable Phosphate Recovery Using Novel Ca–Mg Bimetallic Modified Biogas Residue-Based Biochar. Sustainability 2025, 17, 5049. https://doi.org/10.3390/su17115049
Wang Q, Zhuo G, Xue D, Zhu G, Wang C-Y. Sustainable Phosphate Recovery Using Novel Ca–Mg Bimetallic Modified Biogas Residue-Based Biochar. Sustainability. 2025; 17(11):5049. https://doi.org/10.3390/su17115049
Chicago/Turabian StyleWang, Qi, Guanghui Zhuo, Dongxin Xue, Guangcan Zhu, and Chu-Ya Wang. 2025. "Sustainable Phosphate Recovery Using Novel Ca–Mg Bimetallic Modified Biogas Residue-Based Biochar" Sustainability 17, no. 11: 5049. https://doi.org/10.3390/su17115049
APA StyleWang, Q., Zhuo, G., Xue, D., Zhu, G., & Wang, C.-Y. (2025). Sustainable Phosphate Recovery Using Novel Ca–Mg Bimetallic Modified Biogas Residue-Based Biochar. Sustainability, 17(11), 5049. https://doi.org/10.3390/su17115049







