The Influence of Water Conditions on Heavy Metal Tolerance Mechanisms in Hybrid Poplar (Populus nigra × Populus maximowiczii) in the Light of Sustainable Development Goals
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Experimental Materials
2.2. Experiment Design
2.3. Sample Preparation and Determination of LMWOAs and Phenolic Compounds
2.4. Determination of Salicylic Acid
2.5. Methodology of Metals Analysis
2.5.1. Gases and Reagents
2.5.2. Sample Preparation
2.5.3. Instrumentation
2.6. Statistical Analysis and Calculations
3. Results and Discussion
3.1. Plant Growth and Biomass Production
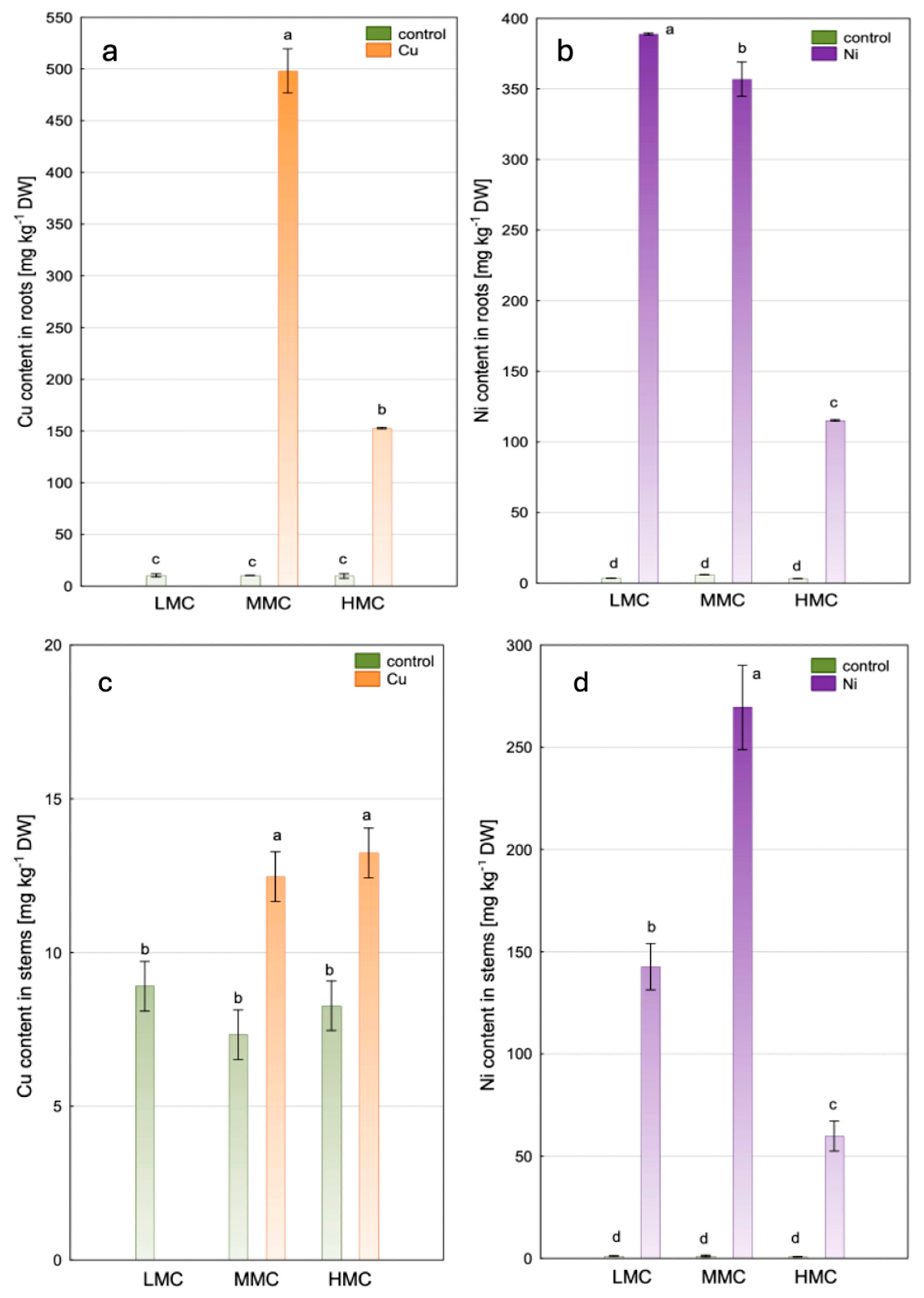
3.2. Copper and Nickel Accumulation
3.3. Physiological Processes Related to Soil Moisture and Cu and Ni Tolerance
3.3.1. LMWOAs Content in Rhizosphere and Roots
3.3.2. LMWOAs and Phenolic Compounds Content in Stems
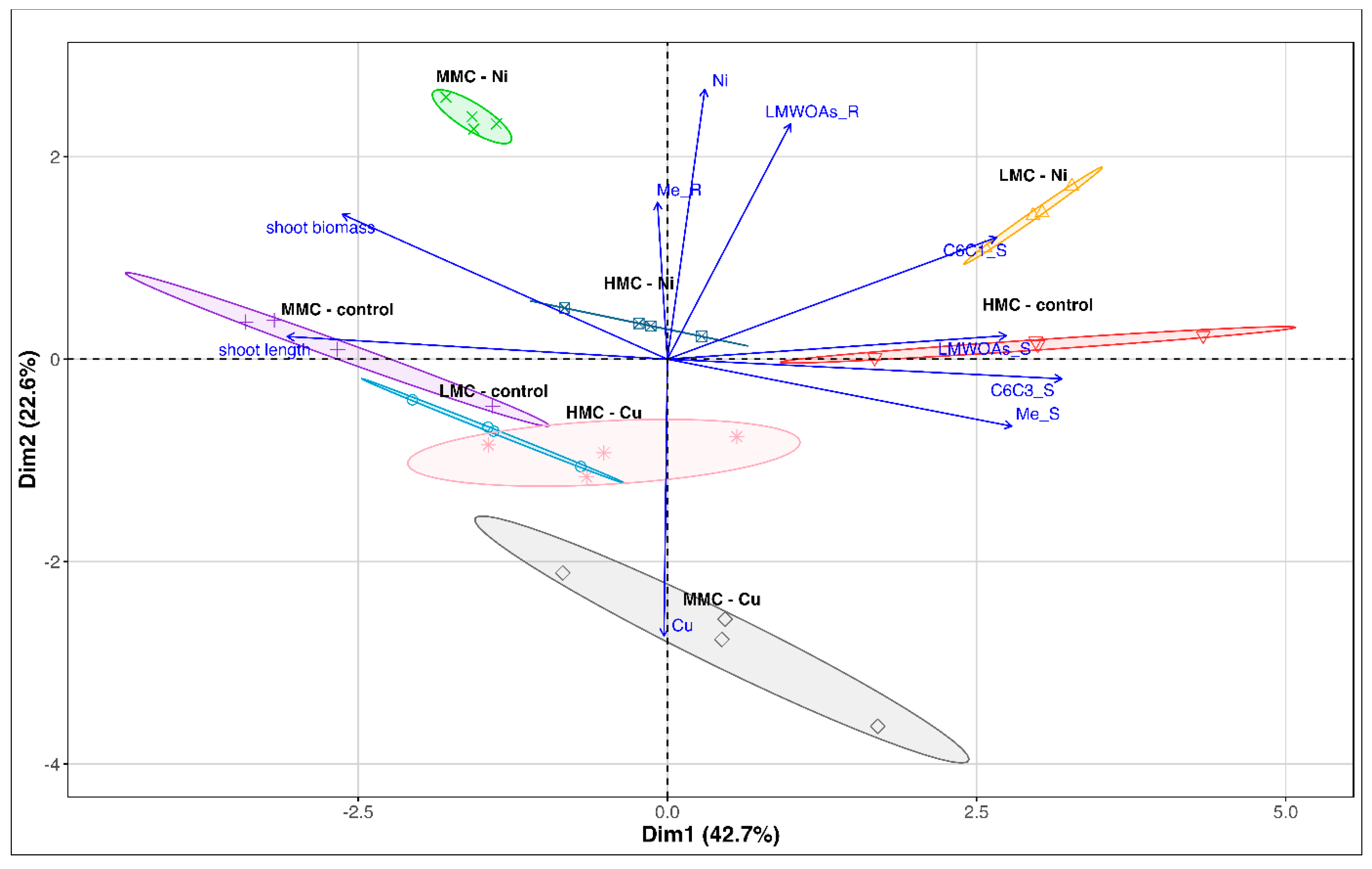
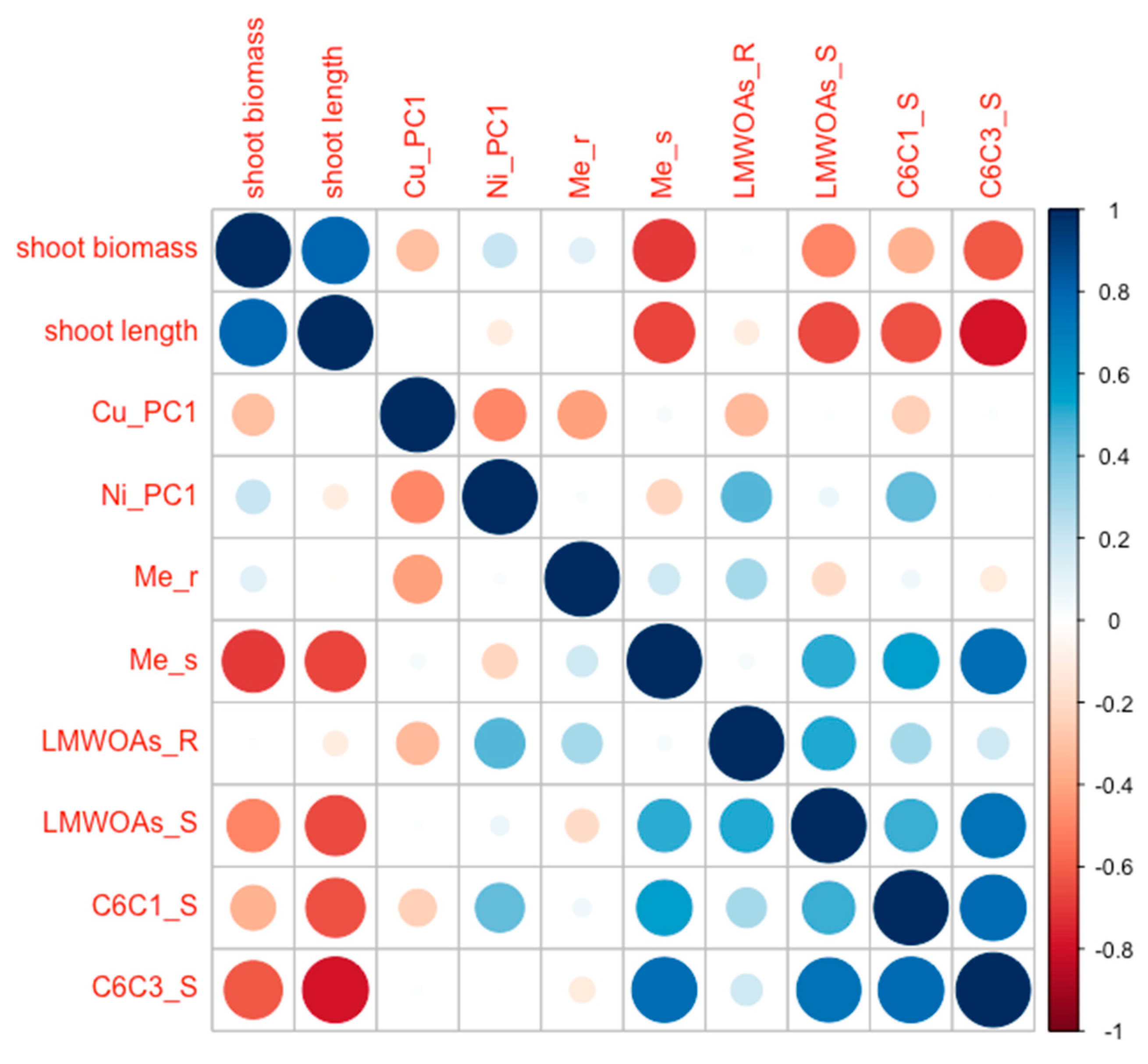
3.4. Principal Component Analysis of Investigated Trails
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kożuchowski, K.M. Stowarzyszenie Klimatologów Polskich. Opady Atmosferyczne w Polsce. 2015. Available online: https://klimatolodzy.pl/images/attachements/Kozuchowski_opady.pdf (accessed on 31 March 2025).
- Kundzewicz, Z.W.; Piniewski, M.; Mezghani, A.; Okruszko, T.; Pińskwar, I.; Kardel, I.; Hov, Ø.; Szcześniak, M.; Szwed, M.; Benestad, R.E.; et al. Assessment of Climate Change and Associated Impact on Selected Sectors in Poland. Acta Geophys. 2018, 66, 1509–1523. [Google Scholar] [CrossRef]
- Pińskwar, I.; Choryński, A.; Graczyk, D.; Kundzewicz, Z.W. Observed Changes in Extreme Precipitation in Po-land: 1991–2015 versus 1961–1990. Theor. Appl. Clim. 2019, 135, 773–787. [Google Scholar] [CrossRef]
- Raczyński, K.; Dyer, J. Changes in Streamflow Drought and Flood Distribution over Poland Using Trend De-composition. Acta Geophys. 2024, 72, 2773–2794. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, B.; Li, H.; Huang, J.; Jiang, L.; Zhang, X.; Tan, Z.; Wu, Z.; Qin, X.; Feng, C.; et al. Soil Heavy Metals and Phytoremediation by Populus Deltoides Alter the Structure and Function of Bacterial Community in Mine Ecosystems. Appl. Soil. Ecol. 2022, 172, 104359. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Walsh, M.E. Short-Rotation Woody Crop Systems, Atmospheric Carbon Dioxide and Carbon Management: A U.S. Case Study. For. Chron. 2011, 77, 259–264. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Abraham, P.E.; Jawdy, S.S.; Gunter, L.E.; Martin, M.Z.; Engle, N.L.; Yang, X.; Tuskan, G.A. The Nature of the Progression of Drought Stress Drives Differential Metabolomic Responses in Populus Deltoides. Ann. Bot. 2019, 124, 617–626. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Wiese, A.H.; Bauer, E.O.; Riemenschneider, D.E. Sapflow of Hybrid Poplar (Populus Nigra L.×P. Maximowiczii A. Henry ‘NM6’) during Phytoremediation of Landfill Leachate. Biomass Bioenergy 2006, 30, 784–793. [Google Scholar] [CrossRef]
- Guerra, F.; Duplessis, S.; Kohler, A.; Martin, F.; Tapia, J.; Lebed, P.; Zamudio, F.; González, E. Gene Expression Analysis of Populus Deltoides Roots Subjected to Copper Stress. Environ. Exp. Bot. 2009, 67, 335–344. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Szöllosi, E.; Tóth, B.; Mészáros, I.; Fodor, F.; Szigeti, Z. Stress Hardening under Long-Term Cadmium Treatment Is Correlated with the Activation of Antioxidative Defence and Iron Acquisition of Chloro-plasts in Populus. Z. Naturforsch. Sect. C J. Biosci. 2016, 71, 323–334. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; Mckenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; Glover, C.J.; et al. In Situ Distribution and Speciation of Toxic Copper, Nickel, and Zinc in Hydrated Roots of Cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic Acids: Versatile Stress-Response Roles in Plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants. Molecules 2022, 28, 241. [Google Scholar] [CrossRef]
- European Council. Council Directive 1999/105/EC of 22 December 1999 on the Marketing of Forest Reproductive Material. Off. J. Eur. Commun. 2000, L11, 17–40. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; pp. 1–236. [Google Scholar]
- Novák, V.; Havrila, J. Method to Estimate the Critical Soil Water Content of Limited Availability for Plants. Biologia 2006, 61, S289–S293. [Google Scholar] [CrossRef]
- Trzecki, S. Możliwość wyznaczania wilgotności trwałego więdnięcia roślin na podstawie maksymalnej higroskopijności i zawartości części spławialnych w glebach mineralnych. Rocz. Glebozn. 1976, 27, 11–18. [Google Scholar]
- Rzasa, S.; Owczarzak, W.; Spychalski, W. Methodological Advances Used to Analyse Maximal Hygroscopic Water in Soils of Different Structure. Int. Agrophys. 1993, 7, 213–220. [Google Scholar]
- Magdziak, Z.; Gąsecka, M.; Budka, A.; Goliński, P.; Mleczek, M. Profile and Concentration of the Low Molecular Weight Organic Acids and Phenolic Compounds Created by Two-Year-Old Acer Platanoides Seedlings Growing under Different As Forms. J. Hazard. Mater. 2020, 392, 122280. [Google Scholar] [CrossRef]
- Baziramakenga, R.; Simard, R.R.; Leroux, G.D. Determination of Organic Acids in Soil Extracts by Ion Chroma-tography. Soil. Biol. Biochem. 1995, 27, 349–356. [Google Scholar] [CrossRef]
- Gąsecka, M.; Krzymińska-Bródka, A.; Magdziak, Z.; Czuchaj, P.; Bykowska, J. Phenolic Compounds and Organic Acid Composition of Syringa Vulgaris L. Flowers Infusions. Molecules 2023, 28, 5159. [Google Scholar] [CrossRef]
- Raskin, I.; Turner, I.M.; Melander, W.R. Regulation of Heat Production in the Inflorescences of an Arum Lily by Endogenous Salicylic Acid. Proc. Natl. Acad. Sci. USA 1989, 86, 2214–2218. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and Excluders-strategies in the Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, K.; Gawrysiak, P.; Woźniak, M.; Rybak, M. Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions. Sustainability 2023, 15, 13084. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr “Ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 31 March 2025).
- Li, Z.; Wang, X.; Liu, Y.; Zhou, Y.; Qian, Z.; Yu, Z.; Wu, N.; Bian, Z. Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus Simonii under PEG-Induced Drought Stress. Forests 2022, 13, 907. [Google Scholar] [CrossRef]
- Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Influence of Drought and Salt Stress on the Growth of Young Populus nigra ‘Italica’ Plants and Associated Mycorrhizal Fungi and Non-Mycorrhizal Fungal Endo-phytes. New For. 2022, 53, 679–694. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, F.; Wang, Y.; Ding, Z.; Yang, X.; Zhu, Z. Differences in Uptake and Accumulation of Copper and Zinc by Salix Clones under Flooded versus Non-Flooded Conditions. Chemosphere 2020, 241, 125059. [Google Scholar] [CrossRef]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Bio Chim. 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Trudić, B.; Kebert, M.; Popović, B.M.; Štajner, D.; Orlović, S.; Galović, V.; Pilipović, A. The Effect of Heavy Metal Pollution in Soil on Serbian Poplar Clones. Sumar. List 2013, 137, 287–295. [Google Scholar]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of Risk Elements by Willow and Poplar Trees. Int. J. Phytore Mediat. 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustos, P.; Szakova, J. Chromium, Nickel, Cadmium, and Lead Accumulation in Maize, Sunflower, Willow, and Poplar. Pol. J. Environ. Stud. 2014, 23, 753–761. [Google Scholar]
- Migeon, A.; Richaud, P.; Guinet, F.; Blaudez, D.; Chalo, M. Hydroponic Screening of Poplar for Trace Element Tolerance and Accumulation. Int. J. Phytoremediat. 2012, 14, 350–361. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, A.; Courbet, G.; Billiot, B.; Jing, L.; Pluchon, S.; Arkoun, M.; Maillard, A.; Roux, C.P.L.; Trouverie, J.; Etienne, P.; et al. Drought Specifically Downregulates Mineral Nutrition: Plant Ionomic Content and Associated Gene Expression. Plant Direct 2022, 6, e402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, L.; Huang, X.; Zou, Z.; Zhang, M.; Guo, W.; Addo-Danso, S.D.; Zhou, L. Mineral Nutrient Uptake, Accumulation, and Distribution in Cunninghamia Lanceolata in Response to Drought Stress. Plants 2023, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhou, J.; Li, J.; Ma, C.; Zhang, Y.; Deng, S.; Yu, W.; Luo, Z. Bin Lead Exposure-Induced Defense Responses Result in Low Lead Translocation from the Roots to Aerial Tissues of Two Contrasting Poplar Species. Environ. Ment. Pollut. 2021, 271, 116346. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Kan, D.; Yu, W.; Chen, X.; Zhang, Y.; Ma, C.; Deng, S.; Zhou, J.; Fayyaz, P.; et al. Sulfur Metabolism, Organic Acid Accumulation and Phytohormone Regulation Are Crucial Physiological Processes Modulating the Different Tolerance to Pb Stress of Two Contrasting Poplars. Tree Physiol. 2022, 42, 1799–1811. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail; Shahid, M.A.; Babar, A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Chen, H.; Zhang, J.; White, J.C.; Chen, G.; Xing, B. Xylem-Based Long-Distance Transport and Phloem Remobilization of Copper in Salix Integra Thunb. J. Hazard. Mater. 2020, 392, 122428. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Tian, S.K.; Yang, X.E.; Wang, X.C.; Brown, P.; Li, T.Q.; He, Z.L. Enhanced Root-to-Shoot Translocation of Cadmium in the Hyperaccumulating Ecotype of Sedum Alfredii. J. Exp. Bot. 2008, 59, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yu, H.; Li, T.; Wu, Y. Effect of Cadmium Stress on Inorganic and Organic Components in Xylem Sap of High Cadmium Accumulating Rice Line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2019, 168, 330–337. [Google Scholar] [CrossRef]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Merlos Rodrigo, M.A.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of Metal/Metalloid Chelation Trade in Plants—An Overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Dung, V.V.; Kuchaeva, L. The Role of Organic Acids in Heavy Metal Tolerance in Plants. Biol. Commun. 2018, 63, 9–16. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Cai, L.; Liu, A.; Ma, X.; Zhou, C.; Wang, G.; Meng, F. Pb Stress Effects on Leaf Chlorophyll Fluo-rescence, Antioxidative Enzyme Activities, and Organic Acid Contents of Pogonatherum Crinitum Seedlings. Flora 2018, 240, 82–88. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Gąsecka, M.; Magdziak, Z.; Rybak, M.; Budzyńska, S.; Rutkowski, P.; Niedzielski, P.; Mleczek, M. Drought Differently Modifies Tolerance and Metal Uptake in Zn- or Cu-Treated Male and Female Salix × Fragilis L. Forests 2024, 15, 562. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic Acid Metabolism in Plants: From Adaptive Physiology to Transgenic Varieties for Cultivation in Extreme Soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Ma, W.; Gao, S.; Jin, Z.; Yue, Q.; Yao, Y. Transcriptomic and Phosphoproteomic Profiling and Metabo-lite Analyses Reveal the Mechanism of NaHCO3-Induced Organic Acid Secretion in Grapevine Roots. BMC Plant Biol. 2019, 19, 389. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics Response for Drought Stress Tolerance in Chinese Wheat Genotypes (Triticum Aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Alekseeva-Popova, N.V.; Drozdova, I.V.; Belyaeva, A.I.; Kalimova, I.B.; Pavlova, N.I.; Shavarda, A.L. Effects of Heavy Metals on the Metabolome of Pinus sylvestris (Pinaceae). Dokl. Biol. Sci. 2022, 507, 364–372. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, L.; Du, Z.; Sun, X.; Amombo, E.; Fan, J.; Fu, J. Effects of Cadmium Exposure on Growth and Metabolic Profile of Bermudagrass [Cynodon dactylon (L.) Pers.]. PloS ONE 2014, 29, e115279. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of Drought Stress on Phenolic Accumulation in Greenhouse-Grown Olive Trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic Compounds as Indicators of Drought Resistance in Shrubs from Patagonian Shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Mao, X.F.; Xu, X.B.; Chen, L.L. Effects of Heavy Metal PB and CD Stress on Physiological Characteristics of Japa-nese Honeysuckle. Appl. Ecol. Environ. Res. 2019, 17, 6415–6427. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of Cadmium Stress on Inductive Enzymatic and Nonenzymatic Responses of ROS and Sugar Metabolism in Multiple Shoot Cultures of Ashwa-gandha (Withania Somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Toler-ance Mechanism in Kandelia Obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic Acid to Decrease Plant Stress. Environ. Chem. Lett. 2016, 15, 101–123. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Borowiak, K.; Bandurska, H.; Golinski, P. Salicylic Acid—A Potential Biomarker of Tobacco Bel-W3 Cell Death Developed as a Response to Ground Level Ozone under Ambient Conditions. Acta Biol. Hung. 2012, 63, 231–249. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and Nickel Co-Treatment Alters Metal Uptake and Stress Parameters of Salix Purpurea × Viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M. Salicylic Acid Accumulation as a Result of Cu, Zn, Cd and Pb Interactions in Com-mon Reed (Phragmites australis) Growing in Natural Ecosystems. Acta Physiol. Plant 2017, 39, 182. [Google Scholar] [CrossRef]

| Variable | Mean ± SE | Median | Min | Max |
|---|---|---|---|---|
| MH (% wt.) | 2.7 ± 0.2 | 2.6 | 2.5 | 3.5 |
| PWP (% wt.) | 5.4 ± 0.3 | 5.2 | 5.0 | 7.0 |
| FC (% wt.) | 38.8 ± 0.4 | 38.8 | 37.6 | 41.4 |
| Bioconcentration Factor (Cu) | Bioconcentration Factor (Ni) | |||
| Control | Treatment | Control | Treatment | |
| LMC | 3.04 b | - | 1.51 b | 4.56 a |
| MMC | 3.24 b | 5.53 a | 2.43 b | 4.99 a |
| HMC | 2.27 b | 1.61 b | 1.18 b | 1.47 b |
| Translocation Factor (Cu) | Translocation Factor (Ni) | |||
| Control | Treatment | Control | Treatment | |
| LMC | 0.87 a | - | 0.32 bc | 0.37 abc |
| MMC | 0.71 a | 0.03 b | 0.21 c | 0.63 a |
| HMC | 0.94 a | 0.09 b | 0.26 bc | 0.52 ab |
| Organic Acid | Water Regimes | Treatment | ||
|---|---|---|---|---|
| Control | Ni | Cu | ||
| Lactic | LMC | bDL | bDL | - |
| MMC | 0.20 ± 0.01 b | 0.40 ± 0.06 a | bDL | |
| HMC | 0.48 ± 0.04 a | 0.46 ± 0.03 a | bDL | |
| Citric | LMC | 9.62 ± 0.52 e | 56.00 ± 3.60 c | - |
| MMC | 25.50 ± 2.99 d | 104 ± 2.27 a | 10.60 ± 1.38 e | |
| HMC | 73.80 ± 1.13 b | 16.60 ± 1.73 e | 50.50 ± 1.62 c | |
| Succinic | LMC | 24.10 ± 2.20 e | 149 ± 13.00 ab | - |
| MMC | 70.20 ± 7.45 d | 116 ± 2.91 c | 16.40 ± 2.26 e | |
| HMC | 145 ± 14.40 bc | 28.80 ± 0.49 e | 178 ± 6.38 a | |
| Fumaric | LMC | bDL | bDL | - |
| MMC | 0.50 ± 0.02 c | bDL | bDL | |
| HMC | 0.77 ± 0.015 b | 1.83 ± 0.038 a | 0.89 ± 0.05 b | |
| Sum | LMC | 33.72 ± 2.72 c | 205 ± 16.40 a | - |
| MMC | 96.20 ± 6.50 b | 220 ± 2.20 a | 27.00 ± 1.05 c | |
| HMC | 220 ± 13.30 a | 47.70 ± 1.23 c | 230 ± 4.63 a | |
| Organic Acid | Water Regimes | Treatment | ||
|---|---|---|---|---|
| Control | Ni | Cu | ||
| Quinic | LMC | bDL | bDL | - |
| MMC | 58.00 ± 3.66 b | bDL | bDL | |
| HMC | 196 ± 16.60 a | 67.09 ± 7.64 b | 35.64 ± 4.50 b | |
| Oxalic | LMC | 2.34 ± 0.05 a | 1.48 ± 0.37 ab | - |
| MMC | 1.22 ± 0.28 b | bDL | bDL | |
| HMC | 1.21 ± 0.25 b | bDL | 2.06 ± 0.30 ab | |
| Malonic | LMC | 77.90 ± 4.55 b | 99.91 ± 17.80 b | - |
| MMC | 38.50 ± 4.52 d | 40.05 ± 4.39 cd | 97.52 ± 0.49 b | |
| HMC | 225 ± 1.67 a | 71.44 ± 5.53 b | 70.15 ± 4.94 bc | |
| Lactic | LMC | 12.10 ± 0.68 ab | 18.61 ± 1.82 a | - |
| MMC | 4.94 ± 2.08 c | 16.73 ± 2.52 ab | 10.73 ± 1.81 cb | |
| HMC | 14.81 ± 0.41 ab | 16.51 ± 1.32 ab | 18.11 ± 2.01 a | |
| Citric | LMC | 1893 ± 542 ab | 2145 ± 33.05 ab | - |
| MMC | 1040 ± 44.20 bc | 1862 ± 224 ab | 2509 ± 465 a | |
| HMC | 1577 ± 332 abc | 675 ± 46.16 c | 1217 ± 325 bc | |
| Acetic | LMC | 1.93 ± 0.47 b | 8.05 ± 1.15 a | - |
| MMC | 1.94 ± 0.25 b | 9.39 ± 2.14 a | 7.64 ± 0.49 a | |
| HMC | 10.00 ± 0.21 a | bDL | bDL | |
| Malic | LMC | 9.46 ± 2.59 d | bDL | - |
| MMC | 33.30 ± 7.12 cd | 161 ± 18.02 a | 144 ± 8.19 a | |
| HMC | 75.42 ± 7.93 b | 17.81 ± 1.20 d | 66.33 ± 2.90 bc | |
| Succinic | LMC | 242 ± 28.31 d | 3037 ± 341 b | - |
| MMC | 1667 ± 113 c | 2089 ± 524 bc | 3044 ± 432 b | |
| HMC | 4663 ± 21.19 a | 1471 ± 79.73 c | 2245 ± 157 bc | |
| Fumaric | LMC | 1.18 ± 0.55 bc | 2.12 ± 0.22 ab | - |
| MMC | 0.83 ± 0.17 c | 1.11 ± 0.17 bc | 1.16 ± 0.45 bc | |
| HMC | 2.55 ± 0.18 a | 1.20 ± 0.24 bc | 1.12 ± 0.17 bc | |
| Sum | LMC | 2240 ± 572 e | 5312 ± 331 abc | - |
| MMC | 2845 ± 109 de | 4179 ± 715 bcd | 5814 ± 798 ab | |
| HMC | 6764 ± 324 a | 2319 ± 31.61 e | 3655 ± 269 cde | |
| Phenolic Acid | Water Regimes | Treatment | ||
|---|---|---|---|---|
| Control | Ni | Cu | ||
| Vanillic | LMC | 1.18 ± 0.31 bc | 11.01 ± 2.75 a | - |
| MMC | 1.09 ± 0.23 bc | 1.56 ± 0.62 bc | 1.21 ± 0.32 bc | |
| HMC | 3.43 ± 0.73 b | 1.92 ± 0.27 bc | 1.35 ± 0.36 bc | |
| Syringic | LMC | 2.04 ± 0.46 de | 10.92 ± 1.43 a | - |
| MMC | 1.65 ± 0.16 e | 5.69 ± 0.87 b | 2.66 ± 0.88 d | |
| HMC | 4.94 ± 0.84 bc | 1.75 ± 0.39 de | 2.96 ± 0.61 cd | |
| Chlorogenic | LMC | 21.01 ± 3.14 cd | 144.91 ± 15.76 a | - |
| MMC | 22.03 ± 2.62 d | 27.90 ± 4.22 d | 63.71 ± 8.51 c | |
| HMC | 88.42 ± 10.39 b | 28.72 ± 2.62 d | 22.60 ± 3.46 d | |
| Caffeic | LMC | 3.34 ± 0.63 bc | 14.71 ± 2.69 a | - |
| MMC | 3.37 ± 0.83 bc | 3.57 ± 0.90 bc | 6.69 ± 1.89 b | |
| HMC | 14.01 ± 4.45 a | 4.98 ± 0.22 bc | 6.17 ± 1.29 b | |
| p-Cumaric | LMC | 1.81 ± 0.19 cd | 3.34 ± 0.27 ab | - |
| MMC | 1.75 ± 0.21 cd | 1.06 ± 0.11 de | 3.50 ± 0.6 ab | |
| HMC | 2.47 ± 0.66 bc | 1.13 ± 0.09 de | 4.38 ± 0.71 a | |
| Ferulic | LMC | 3.21 ± 0.86 cd | 9.45 ± 2.04 a | - |
| MMC | 1.66 ± 0.83 de | 1.85 ± 0.67 de | 5.45 ± 1.27 bc | |
| HMC | 8.23 ± 1.07 ab | 2.75 ± 0.27 cde | 1.68 ± 0.68 de | |
| Sinapic | LMC | 4.55 ± 0.87 cd | 6.97 ± 1.06 ab | - |
| MMC | 2.01 ± 0.58 e | 2.67 ± 0.54 de | 6.03 ± 1.03 bc | |
| HMC | 8.29 ± 1.02 a | 3.44 ± 0.66 de | 3.21 ± 0.56 de | |
| Salicylic | LMC | 8.34 ± 1.60 ab | 5.35 ± 0.89 abc | - |
| MMC | 11.02 ± 4.23 a | 6.85 ± 2.85 abc | 4.83 ± 1.39 bc | |
| HMC | 2.47 ± 0.040 c | 1.10 ± 0.24 c | 1.41 ± 1.25 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdziak, Z.; Gąsecka, M.; Drzewiecka, K.; Ilek, A.; Rybak, M.; Proch, J.; Niedzielski, P. The Influence of Water Conditions on Heavy Metal Tolerance Mechanisms in Hybrid Poplar (Populus nigra × Populus maximowiczii) in the Light of Sustainable Development Goals. Sustainability 2025, 17, 4989. https://doi.org/10.3390/su17114989
Magdziak Z, Gąsecka M, Drzewiecka K, Ilek A, Rybak M, Proch J, Niedzielski P. The Influence of Water Conditions on Heavy Metal Tolerance Mechanisms in Hybrid Poplar (Populus nigra × Populus maximowiczii) in the Light of Sustainable Development Goals. Sustainability. 2025; 17(11):4989. https://doi.org/10.3390/su17114989
Chicago/Turabian StyleMagdziak, Zuzanna, Monika Gąsecka, Kinga Drzewiecka, Anna Ilek, Michał Rybak, Jędrzej Proch, and Przemysław Niedzielski. 2025. "The Influence of Water Conditions on Heavy Metal Tolerance Mechanisms in Hybrid Poplar (Populus nigra × Populus maximowiczii) in the Light of Sustainable Development Goals" Sustainability 17, no. 11: 4989. https://doi.org/10.3390/su17114989
APA StyleMagdziak, Z., Gąsecka, M., Drzewiecka, K., Ilek, A., Rybak, M., Proch, J., & Niedzielski, P. (2025). The Influence of Water Conditions on Heavy Metal Tolerance Mechanisms in Hybrid Poplar (Populus nigra × Populus maximowiczii) in the Light of Sustainable Development Goals. Sustainability, 17(11), 4989. https://doi.org/10.3390/su17114989












