Evaluation of Phosphate-Solubilizing Bacteria (PSB) on Phosphorus Availability in Agricultural Soils and the Growth of Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
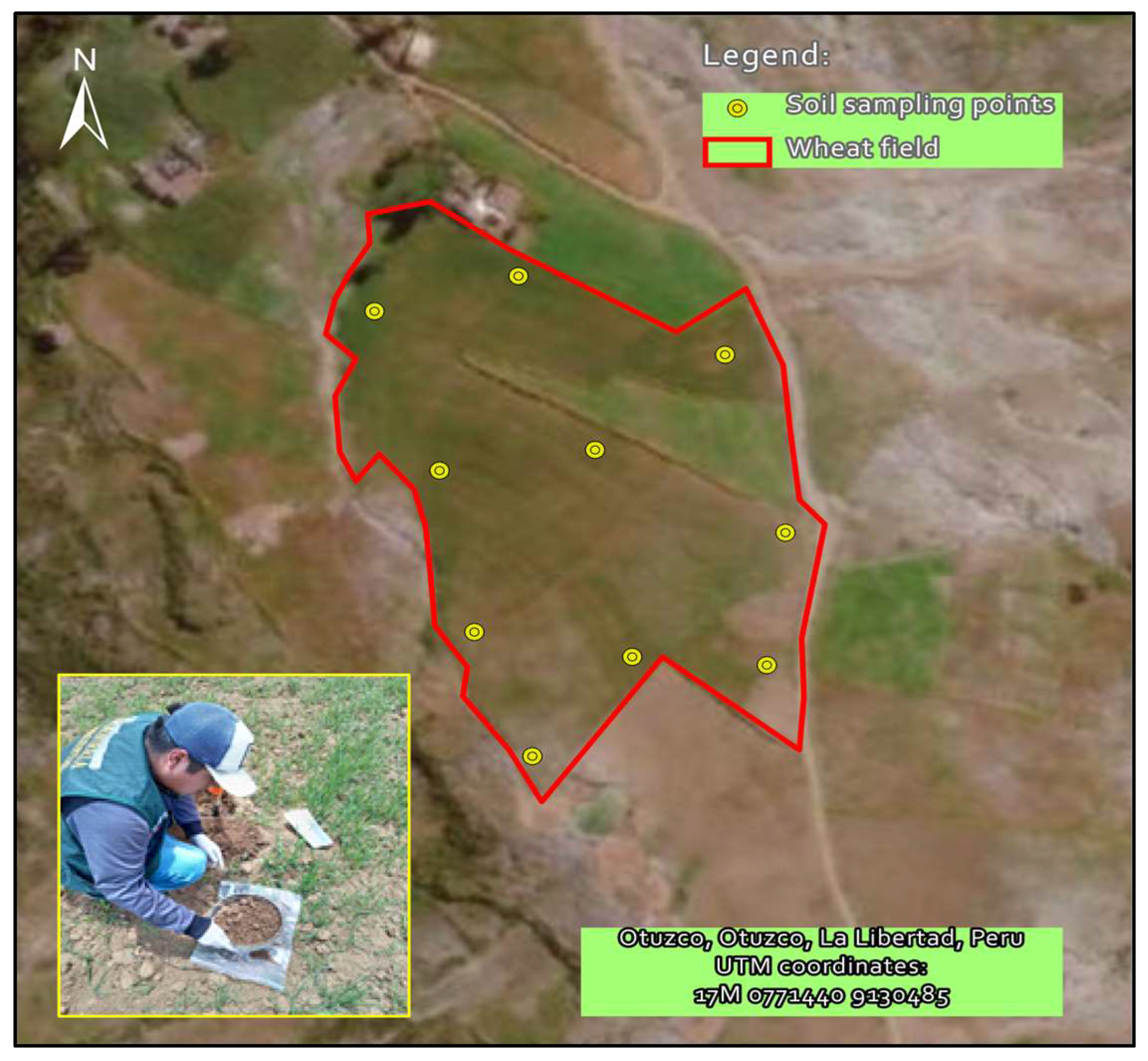
2.1. Agricultural Soil Sampling
2.2. Characterization of Agricultural Soil for Wheat Cultivation
2.3. Isolation and Selection of Phosphate-Solubilizing Bacteria (PSB)
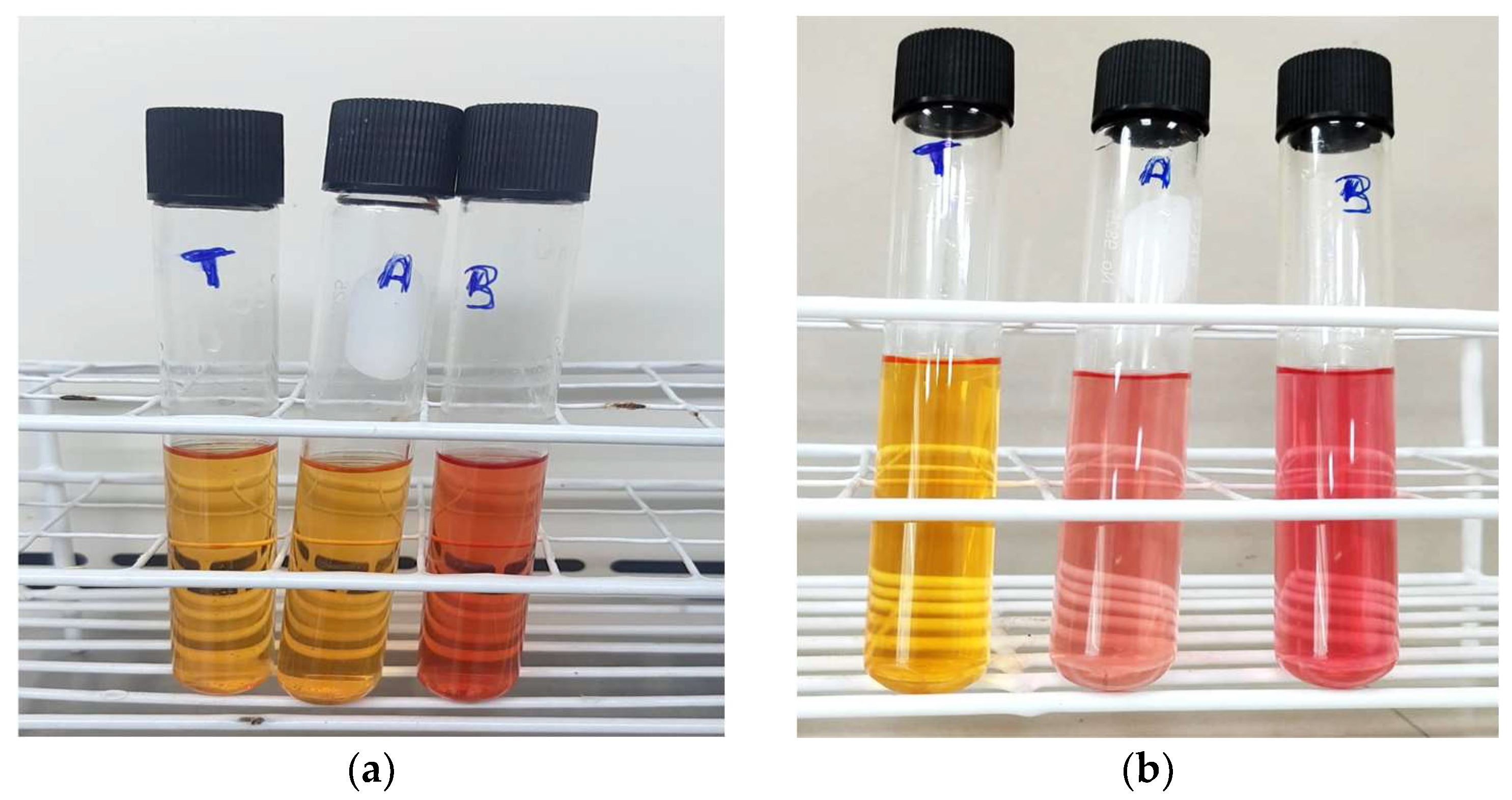
2.4. Phosphate Solubilization Test In Vitro
2.5. Biochemical Identification of Strains MT-13 and MT-8
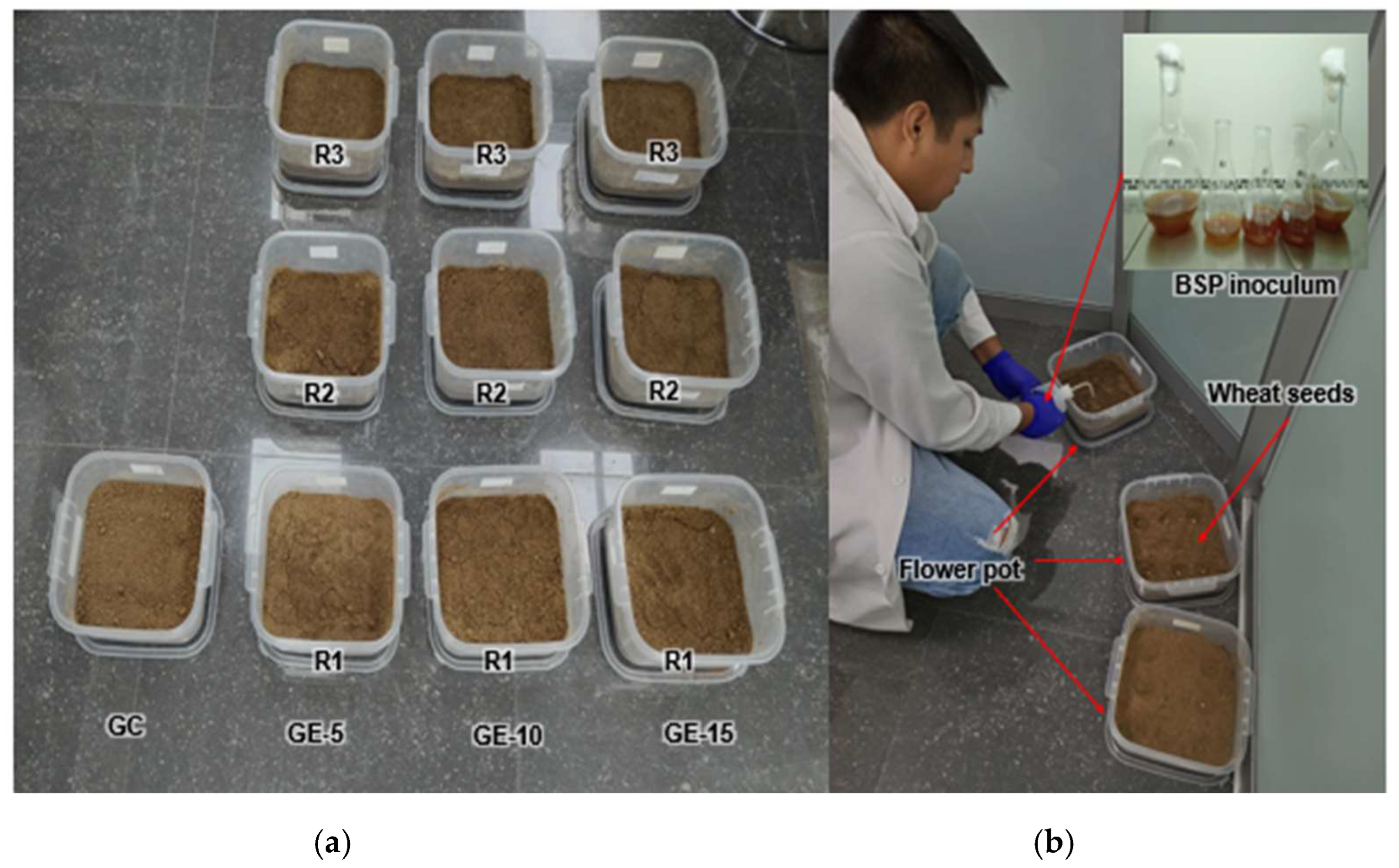
2.6. Preparation of Pots and Inoculation of Wheat Seeds with PSB
2.7. Evaluation of Plant Indicators
2.8. Data Analysis Method
3. Results and Discussion
3.1. Isolation and Identification of Phosphate-Solubilizing Bacteria (PSB)
3.1.1. Isolation of PSB and In Vitro Phosphorus Solubilization Test
3.1.2. Biochemical Identification of MT-8 and MT-13 Strains
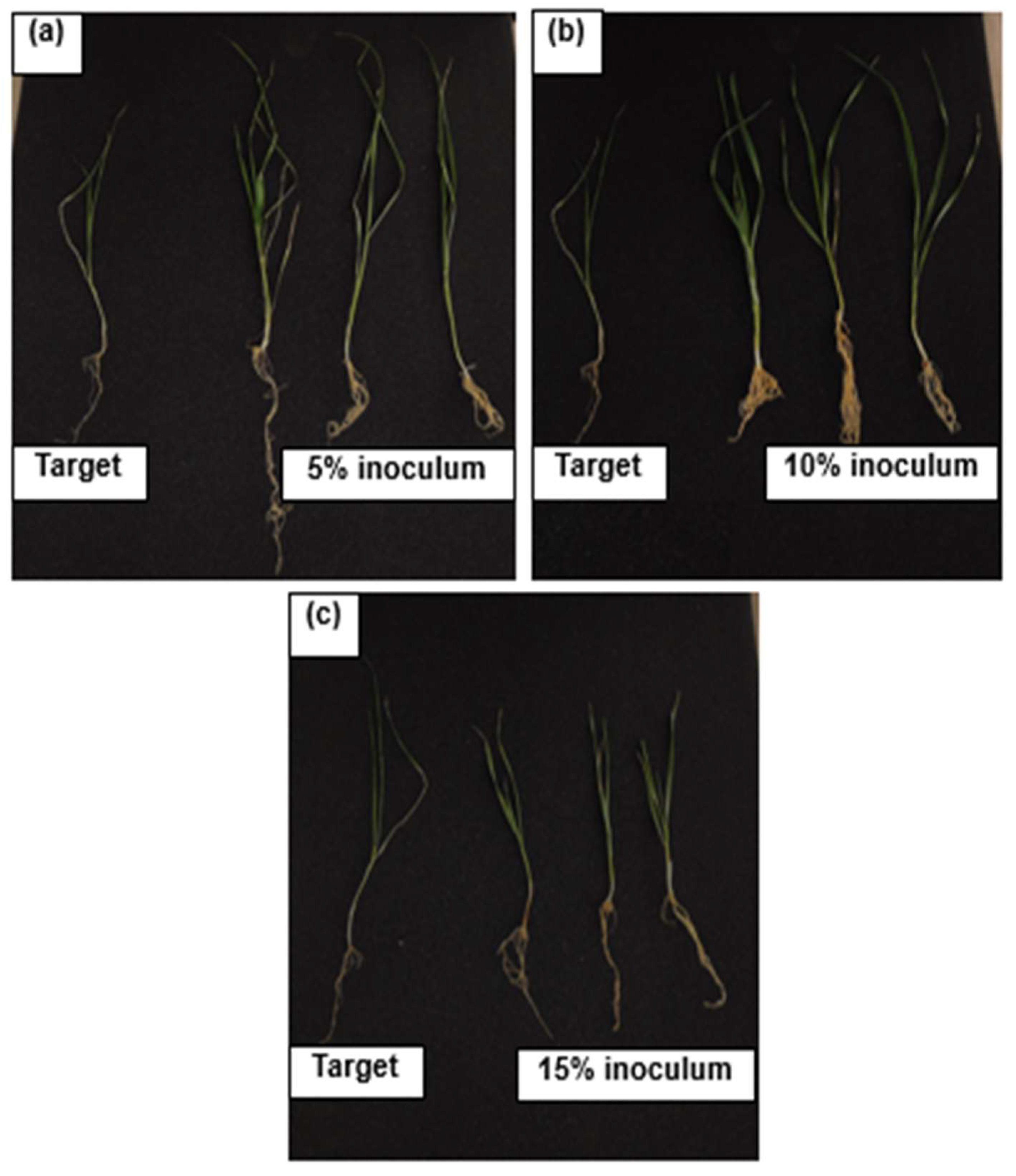
3.2. Effect of PSB on Wheat (Triticum aestivum L.) Growth
3.3. Effect of PSB on Soil P Availability
3.4. Effect of PSB on Agricultural Soil Fertility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; He, F.; Huang, W.; Zhou, Y.; Zhou, J.; Chen, Q.; Wang, F.; Cong, X.; He, B.; Wang, Y. Dicranopteris dichotoma Rhizosphere-Derived Bacillus sp. MQB12 Acts as an Enhancer of Plant Growth via Increasing Phosphorus Utilization, Hormone Synthesis, and Rhizosphere Microbial Abundance. Chem. Biol. Technol. Agric. 2024, 11, 116. [Google Scholar] [CrossRef]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Phosphate-Solubilizing Microorganisms and Their Emerging Role in Sustainable Agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–233. ISBN 9780128163283. [Google Scholar]
- Wei, W.; Yang, M.; Liu, Y.; Huang, H.; Ye, C.; Zheng, J.; Guo, C.; Hao, M.; He, X.; Zhu, S. Fertilizer N Application Rate Impacts Plant-Soil Feedback in a Sanqi Production System. Sci. Total Environ. 2018, 633, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhiyong, S.; Yaxuan, G.; Yuanyuan, W.; Xiang, Y.; Xu, G.; Zhenhong, L.; Jingping, N.; Jianping, L.; Zhenyu, L. Nitrogen-Fixing Bacteria Promote Growth and Bioactive Components Accumulation of Astragalus mongholicus by Regulating Plant Metabolism and Rhizosphere Microbiota. BMC Microbiol. 2024, 24, 261. [Google Scholar] [CrossRef]
- Verma, R.C.; Singh, N.K.; Gangavati, A.R.; Ashoka, P.; Kesarwani, A.; Ali, I.; Pandey, S.K.; Singh, B.V. A Review of Long-Term Effects of Mineral Fertilizers on Soil Microorganisms. Int. J. Plant Soil Sci. 2023, 35, 1145–1155. [Google Scholar] [CrossRef]
- Watson, C.D.; Gardner, M.G.; Hodgson, R.J.; Liddicoat, C.; Peddle, S.D.; Breed, M.F. Global Meta-Analysis Shows Progress towards Recovery of Soil Microbiota Following Revegetation. Biol. Conserv. 2022, 272, 109592. [Google Scholar] [CrossRef]
- Trejo, D.; Sangabriel-Conde, W.; Gavito-Pardo, M.E.; Banuelos, J. Mycorrhizal Inoculation and Chemical Fertilizer Interactions in Pineapple under Field Conditions. Agriculture 2021, 11, 934. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The Role of Beneficial Microorganisms in Soil Quality and Plant Health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Programa de las Naciones Unidas para el Medio Ambiente. Juventud en Acción: Un Llamado a la Participación de los Jóvenes en la Gobernanza Ambiental; PNUMA: Nairobi, Kenia, 2020; Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/34463/JSUNEPPF_Sp.pdf (accessed on 16 July 2024).
- Zhou, Z.; Yrjälä, K.; Chen, J.; Yu, C.; Shi, W.; Qin, H.; Yu, W.; Dai, W.; Hu, Y.; Wu, J. Organic Amendments Combined with Biochar for Improving Soil and Plant Quality in a Torreya Grandis Plantation. J. Soils Sediments 2022, 22, 1080–1094. [Google Scholar] [CrossRef]
- Banco Central de Reserva del Perú. Impacto del Conflicto Rusia-Ucrania en la Economía Peruana. Reporte de Inflación: Panorama Actual y Proyecciones Macroeconómicas 2022–2023, Marzo 2022. Available online: https://www.bcrp.gob.pe/docs/Publicaciones/Reporte-Inflacion/2022/marzo/ri-marzo-2022-recuadro-7.pdf (accessed on 16 July 2024).
- Mary Isabella Sonali, J.; Kavitha, R.; Kumar, P.S.; Rajagopal, R.; Gayathri, K.V.; Ghfar, A.A.; Govindaraju, S. Application of a Novel Nanocomposite Containing Micro-Nutrient Solubilizing Bacterial Strains and CeO2 Nanocomposite as Bio-Fertilizer. Chemosphere 2022, 286, 131800. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Fustamante Cabrera, N.G.; Tarazona Gomez, Y.C. Análisis Bibliométrico Sobre el uso de Biofertilizantes a Base de Microorganismos para el Crecimiento y Rendimiento de Cultivos Agrícolas; Universidad César Vallejo: Trujillo, Peru, 2021. [Google Scholar]
- United States Department of Agriculture. Grain: World Markets and Trade. Foreign Agricultural Service, Septiembre 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/grain.pdf (accessed on 16 July 2024).
- Ministerio de Desarrollo Agrario y Riego del Perú. Abastecimiento de Trigo en el Perú. Dirección General de Seguimiento y Evaluación de Políticas, Diciembre 2022. Available online: https://cdn.www.gob.pe/uploads/document/file/3872810/N.%C2%B0011%7C%20Abastecimiento%20de%20trigo%20en%20el%20Per%C3%BA.pdf?v=1671831710 (accessed on 16 July 2024).
- Shahid, M.; Zeyad, M.T.; Syed, A.; Singh, U.B.; Mohamed, A.; Bahkali, A.H.; Elgorban, A.M.; Pichtel, J. Stress-Tolerant Endophytic Isolate Priestia aryabhattai BPR-9 Modulates Physio-Biochemical Mechanisms in Wheat (Triticum aestivum L.) for Enhanced Salt Tolerance. Int. J. Environ. Res. Public Health 2022, 19, 10883. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Awad-Allah, M.M.A.; Alshallash, K.S.; Alatawi, A.; Alshegaihi, R.M.; Alsalmi, R.A. Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice. Agriculture 2022, 12, 1596. [Google Scholar] [CrossRef]
- Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.R.; Asses, N.; Pinho, H.J.O.; Abbes, C. Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils. Microorganisms 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Jokkaew, S.; Jantharadej, K.; Pokhum, C.; Chawengkijwanich, C.; Suwannasilp, B.B. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability 2022, 14, 12627. [Google Scholar] [CrossRef]
- Amy, C.; Avice, J.-C.; Laval, K.; Bressan, M. Are Native Phosphate-Solubilizing Bacteria a Relevant Alternative to Mineral Fertilizations for Crops? Part II: PSB Inoculation Enables a Halving of P Input and Improves the Microbial Community in the Rapeseed Rhizosphere. Rhizosphere 2022, 21, 100480. [Google Scholar] [CrossRef]
- Li, H.-P.; Han, Q.-Q.; Liu, Q.-M.; Gan, Y.-N.; Rensing, C.; Rivera, W.L.; Zhao, Q.; Zhang, J.-L. Roles of Phosphate-Solubilizing Bacteria in Mediating Soil Legacy Phosphorus Availability. Microbiol. Res. 2023, 272, 127375. [Google Scholar] [CrossRef]
- Rawat, P.; Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Improvement of Phosphorus Uptake, Phosphorus Use Efficiency, and Grain Yield of Upland Rice (Oryza sativa L.) in Response to Phosphate-Solubilizing Bacteria Blended with Phosphorus Fertilizer. Pedosphere 2022, 32, 752–763. [Google Scholar] [CrossRef]
- Khan, H.; Akbar, W.A.; Shah, Z.; Rahim, H.U.; Taj, A.; Alatalo, J.M. Coupling Phosphate-Solubilizing Bacteria (PSB) with Inorganic Phosphorus Fertilizer Improves Mungbean (Vigna radiata) Phosphorus Acquisition, Nitrogen Fixation, and Yield in Alkaline-Calcareous Soil. Heliyon 2022, 8, e09081. [Google Scholar] [CrossRef] [PubMed]
- Osorio, N.W.; Habte, M. Synergistic Influence of an Arbuscular Mycorrhizal Fungus and a P Solubilizing Fungus on Growth and P Uptake of Leucaena leucocephalain an Oxisol. Arid Land Res. Manag. 2001, 15, 263–274. [Google Scholar] [CrossRef]
- Solanki, M.; Kundu, B.S.; Nehra, K. Molecular Diversity of Phosphate Solubilizing Bacteria Isolated from the Rhizosphere of Chickpea, Mustard and Wheat. Ann. Agrar. Sci. 2018, 16, 458–463. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Nessim, M.G.; Abou-el-seoud, I.I.; Darwish, K.M.; Shamseldin, A. Isolation and Selection of Highly Effective Phosphate Solubilizing Bacterial Strains to Promote Wheat Growth in Egyptian Calcareous Soils. Bull. Natl. Res. Cent. 2019, 43, 203. [Google Scholar] [CrossRef]
- Vargas Barrantes, P.; Castro Barquero, L. Aislamiento y Evaluación de Microorganismos Solubilizadores de Fósforo de Andisoles de Costa Rica. Agron. Costarric. 2018, 43, 47–68. [Google Scholar] [CrossRef]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and Genotypic Characterization of Phosphate Solubilizing Bacteria and Their Efficiency on the Growth of Maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Osei, R.; Yang, C.; Cui, L.; Ma, T.; Li, Z.; Boamah, S. Isolation, Identification, and Pathogenicity of Lelliottia amnigena Causing Soft Rot of Potato Tuber in China. Microb. Pathog. 2022, 164, 105441. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, J.; Li, D.; Jiang, Y.; Zhang, Y.; Wang, H.; Zhang, J.; Ahmed, T.; Li, B. Isolation and Molecular Characterization of Plant-Growth-Promoting Bacteria and Their Effect on Eggplant (Solanum melongena) Growth. Agriculture 2021, 11, 1258. [Google Scholar] [CrossRef]
- Scattareggia, J.P. Manejo Sustentable de Residuos Orgánicos Agroindustriales: Producción de Compost a Escala Regional en Mendoza, Argentina. Bachelor’s Thesis, Universidad Nacional de Cuyo, Mendoza, Argentina, 2016. Available online: https://bdigital.uncu.edu.ar/objetos_digitales/8408/tesis-irnr-scattareggia-juan-pablo-2016.pdf (accessed on 14 September 2024).
- Pilotto, L.; Zuluaga, M.Y.A.; Scalera, F.; Piccirillo, C.; Marchiol, L.; Civilini, M.; Pii, Y.; Cesco, S.; Fellet, G. Sustainable Crop Fertilization by Combining Biogenic Nano-Hydroxyapatite and P Solubilizing Bacteria: Observations on Barley. Plant Nano Biol. 2024, 9, 100091. [Google Scholar] [CrossRef]
- Da Costa, P.B.; van Elsas, J.D.; Mallon, C.; dos Anjos Borges, L.G.; Pereira Passaglia, L.M. Efficiency of Probiotic Traits in Plant Inoculation Is Determined by Environmental Constrains. Soil Biol. Biochem. 2020, 148, 107893. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Qamar, R.; ur Rehman, A.; Ali, M.; Rehman, A.; Farooq, M.; Zamir, S.I.; Nadeem, M.; Cheema, M.A.; Shehzad, M.; et al. Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability 2019, 11, 7049. [Google Scholar] [CrossRef]
- Ibnyasser, A.; Elhaissoufi, W.; Haddine, M.; Saidi, R.; Khourchi, S.; Zeroual, Y.; Barakat, A.; Bargaz, A. Phosphate Bio-Solubilization and Cadmium Tolerance Interplay in the Root-Microbe Interface and Consequences on Root P Absorption in Wheat. Environ. Exp. Bot. 2024, 222, 105738. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Khan, A.; Fahad, S.; Nawaz, S.; Ahmed, N.; Arif Ali, M.; Adnan, M.; Dawar, K.; Saud, S.; Hassan, S.; et al. Phosphate Solubilizing Bacteria Optimize Wheat Yield in Mineral Phosphorus Applied Alkaline Soil. J. Saudi Soc. Agric. Sci. 2022, 21, 339–348. [Google Scholar] [CrossRef]
- He, D.; Wan, W. Distribution of Culturable Phosphate-Solubilizing Bacteria in Soil Aggregates and Their Potential for Phosphorus Acquisition. Microbiol. Spectr. 2022, 10, e00290-22. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Saha, N.; Sarkar, A.; Poddar, R.; Pramanik, K.; Kundu, R. Enhancing Phosphorus Availability and Growth of Green Gram (Vigna radiata) in Acidic Red and Laterite Soil through Liquid Formulations of Native Phosphate Solubilizing Bacteria. Biocatal. Agric. Biotechnol. 2024, 61, 103413. [Google Scholar] [CrossRef]
- Wu, B.; Luo, Z.; Yang, P.; Lei, N.; Pei, X.; Guo, S.; Du, J. Phosphorus Solubilizing Bacteria Combined with Sticky Glutinous Rice Paste Accelerated the Vegetation Recovery of Spoil Material under Drought Conditions. Appl. Soil Ecol. 2024, 203, 105647. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Yi, S.; Zheng, F.; Zhang, Z.; Alharbi, S.A.; Filimonenko, E.; Wang, Z.; Kuzyakov, Y. Microbial Composition in Saline and Alkaline Soils Regulates Plant Growth with P-Solubilizing Bacteria. Appl. Soil Ecol. 2024, 203, 105653. [Google Scholar] [CrossRef]
- Wahid, F.; Sharif, M.; Fahad, S.; Ali, A.; Adnan, M.; Rafiullah; Saud, S.; Danish, S.; Arif Ali, M.; Ahmed, N.; et al. Mycorrhiza and Phosphate Solubilizing Bacteria: Potential Bioagents for Sustainable Phosphorus Management in Agriculture. Phyton (B. Aires) 2022, 91, 257–278. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Motasharezadeh, B.; Sarmadian, F. Consortium of Endophyte and Rhizosphere Phosphate Solubilizing Bacteria Improves Phosphorous Use Efficiency in Wheat Cultivars in Phosphorus Deficient Soils. Rhizosphere 2020, 14, 100196. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissoufi, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z.; Delaplace, P.; et al. Phosphate Solubilizing Bacteria Can Significantly Contribute to Enhance P Availability from Polyphosphates and Their Use Efficiency in Wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef]
- Kouas, S.; Djedidi, S.; Ben Slimene Debez, I.; Sbissi, I.; Alyami, N.M.; Hirsch, A.M. Halotolerant Phosphate Solubilizing Bacteria Isolated from Arid Area in Tunisia Improve P Status and Photosynthetic Activity of Cultivated Barley under P Shortage. Heliyon 2024, 10, e38653. [Google Scholar] [CrossRef]
- Liu, Y.; Nessa, A.; Zheng, Q.; Hu, D.; Zhang, W.; Zhang, M. Inoculations of Phosphate-Solubilizing Bacteria Alter Soil Microbial Community and Improve Phosphorus Bioavailability for Moso Bamboo (Phyllostachys edulis) Growth. Appl. Soil Ecol. 2023, 189, 104911. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Xiao, J.; Zhao, W.; Zou, P.; Liao, R.; Xie, K.; Liao, H. Root Metabolites Regulated by FERONIA Promote Phosphorus-Solubilizing Rhizobacteria Enrichment Induced by Arabidopsis thaliana Coping with Phosphorus Deficiency. Microbiol. Res. 2025, 292, 128030. [Google Scholar] [CrossRef]
- Tang, Y.; Che, Y.-J.; Bai, X.-Y.; Wang, Z.-Y.; Gu, S.-Y. Effects of Application of Phosphate and Phosphate-Solubilizing Bacteria on Bacterial Diversity and Phosphorus Fractions in a Phaeozems. Heliyon 2023, 9, e22937. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-Solubilizing Bacteria: Their Agroecological Function and Optimistic Application for Enhancing Agro-Productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Hipólito-Romero, E.; Carcaño-Montiel, M.G.; Ramos-Prado, J.M.; Vázquez-Cabañas, E.A.; López-Reyes, L.; Ricaño-Rodríguez, J. Efecto de inoculantes bacterianos edáficos mixtos en el desarrollo temprano de cultivares mejorados de cacao (Theobroma cacao L.) en un sistema agroforestal tradicional del norte de Oaxaca, México. Rev. Argent. Microbiol. 2017, 49, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Xu, S.; Hou, Z.; Gao, X.; Su, J.; Peng, B.; Zhao, J.; Wang, Z.; Cheng, M.; Zhang, A.; et al. Co-Inoculation of Phosphate-Solubilizing Bacteria and Phosphate Accumulating Bacteria in Phosphorus-Enriched Composting Regulates Phosphorus Transformation by Facilitating Polyphosphate Formation. Bioresour. Technol. 2023, 390, 129870. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, S.; Umbreen, S.; Zhou, C. Soil Phosphorus Fractionation and Its Association with Soil Phosphate-Solubilizing Bacteria in a Chronosequence of Vegetation Restoration. Ecol. Eng. 2021, 164, 106208. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Wang, J.; Zou, S.; Long, X. Phosphorus Solubilizing Bacteria Bacillus thuringiensis and Pantoea ananatis Simultaneously Promote Soil Inorganic Phosphate Dissolution and Soil Pb Immobilization. Rhizosphere 2021, 20, 100448. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, Z.; Han, F.; Chen, S.; Zhou, W. Integrated Application of Phosphorus-Accumulating Bacteria and Phosphorus-Solubilizing Bacteria to Achieve Sustainable Phosphorus Management in Saline Soils. Sci. Total Environ. 2023, 885, 163971. [Google Scholar] [CrossRef]
- Xu, K.; Lv, X.; Yue, F.; Zhang, L.; Wang, P.; Amoah, I.D.; Tang, K.H.D.; Yao, Y.; Li, R. Effects of Phosphate-Solubilizing Fungus Aspergillus Flavus AF-LRH1 on Promoting Phosphorus Solubilization, Wheat Growth and Soil Heavy Metal Remediation. J. Environ. Chem. Eng. 2024, 12, 114357. [Google Scholar] [CrossRef]
- Biswas, S.S.; Biswas, D.R.; Ghosh, A.; Sarkar, A.; Das, A.; Roy, T. Phosphate Solubilizing Bacteria Inoculated Low-Grade Rock Phosphate Can Supplement P Fertilizer to Grow Wheat in Sub-Tropical Inceptisol. Rhizosphere 2022, 23, 100556. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J.B.N. Use of Compost and Phosphate-Solubilizing Bacteria Affect Sugarcane Mineral Nutrition, Phosphorus Availability, and the Soil Bacterial Community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.C.; Zhang, S.; Fu, Y.; Fan, X.; Patel, J.S.; Zhang, M. Characterization of Phosphate-Solubilizing Bacteria Isolated from Calcareous Soils. Appl. Soil Ecol. 2015, 96, 217–224. [Google Scholar] [CrossRef]

| Parameters | Unit | Values |
|---|---|---|
| pH | - | 6.52 ± 0.01 |
| Electrical Conductivity (EC) | (mS/cm) | 0.464 ± 0.01 |
| Available Phosphorus (P) | (ppm) | 33.67 ± 0.10 |
| Organic Matter (OM) | (%) | 1.05 ± 0.01 |
| Potassium Concentration (K) | (ppm) | 442.17 ± 0.01 |
| Saturations | (%) | 34.00 ± 1 |
| Calcium Carbonate Content (CaCO3) | (%) | 0.00 ± 0.00 |
| Biochemical Identification Information | Isolation Code | |
|---|---|---|
| MT-8 | MT-13 | |
| Organism Identified Card Analysis Time Probability Confidence Level Bionumber | Pantoea spp. Gram-negative 5.82 h 97% Excellent 4625730551130010 | Lelliottia amnigena 2 Gram-negative 4.83 h 99% Excellent 4627734543572010 |
| Characteristics | Description | Microscopic Observation |
|---|---|---|
| Isolated Strain Incubation Time Color Morphology Quantity Magnification Bacteria Type | Code MT-8 48 h Red Bacillus Abundant 100× Gram-negative | 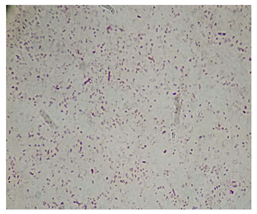 |
| Macroscopic Characteristics | Elongated and cylindrical |
| Characteristics | Description | Microscopic Observation |
|---|---|---|
| Isolated Strain Incubation Time Color Morphology Quantity Magnification Bacteria Type | Code MT-13 48 h Pink Bacillus Abundant 100× Gram-negative | 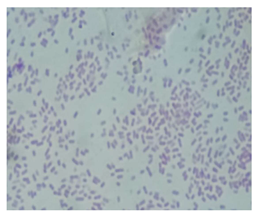 |
| Macroscopic Characteristics | Elongated and cylindrical |
| PSB Inoculum Dose | Height (cm) | Leaf Area (cm2) | Fresh Aerial Biomass (g) | Dry Aerial Biomass (g) | Fresh Radical Biomass (g) | Fresh Radical Biomass (g) | Stem Diameter (mm) |
|---|---|---|---|---|---|---|---|
| No inoculum | 19.20 ± 0.62 B | 4.47 ± 2.3 A | 0.05 ± 0.05 A | 0.30 ± 0.13 A | 0.01 ± 0.02 A | 0.01 ± 0.01 A | 0.10 ± 0.06 A |
| 5% inoculum | 28.17 ± 0.62 D | 13.87 ± 2.3 B | 0.34 ± 0.05 B | 0.38 ± 0.13 A | 0.04 ± 0.02 A | 0.02 ± 0.01 A | 0.50 ± 0.06 B |
| 10% inoculum | 23.77 ± 0.62 C | 14.43 ± 2.3 B | 0.49 ± 0.05 C | 0.12 ± 0.13 A | 0.12 ± 0.02 B | 0.05 ± 0.01 B | 1.17 ± 0.06 C |
| 15% inoculum | 12.13 ± 0.62 A | 3.70 ± 2.3 A | 0.12 ± 0.05 A | 0.03 ± 0.13 A | 0.03 ± 0.02 A | 0.02 ± 0.01 A | 0.37 ± 0.06 B |
| PSB Inoculum Dose | Height (cm) | Leaf Area (cm2) | Fresh Aerial Biomass (g) | Dry Aerial Biomass (g) | Fresh Radical Biomass (g) | Fresh Radical Biomass (g) | Stem Diameter (mm) |
|---|---|---|---|---|---|---|---|
| No inoculum | 27.8 ± 1.29 B | 12.14 ± 1.34 A | 0.10 ± 0.11 A | 0.06 ± 0.01 A | 0.01 ± 0.02 A | 0.01 ± 0.01 A | 0.58 ± 0.07 A |
| 5% inoculum | 36.27 ± 1.29 C | 21.08 ± 1.34 B | 0.40 ± 0.11 A | 0.18 ± 0.01 B | 0.06 ± 0.02 A | 0.03 ± 0.01 B | 1.02 ± 0.07 B |
| 10% inoculum | 39.00 ± 1.29 C | 17.51 ± 1.34 B | 1.01 ± 0.11 B | 0.54 ± 0.01 C | 0.13 ± 0.02 B | 0.11 ± 0.01 C | 1.57 ± 0.07 C |
| 15% inoculum | 18.15 ± 1.29 A | 7.97 ± 1.34 A | 0.17 ± 0.11 A | 0.08 ± 0.01 A | 0.05 ± 0.02 A | 0.05 ± 0.01 B | 0.43 ± 0.07 A |
| Parameters | 10% Inoculum | No Inoculum | Rate of Change (%) |
|---|---|---|---|
| Height (cm) | 39.00 ± 1.29 | 27.8 ± 1.29 | 40.29 |
| Leaf Area (cm2) | 17.51 ± 1.34 | 12.14 ± 1.34 | 44.23 |
| Fresh Aerial Biomass (g) | 1.01 ± 0.11 | 0.10 ± 0.11 | 910 |
| Dry Aerial Biomass (g) | 0.54 ± 0.01 | 0.06 ± 0.01 | 800 |
| Fresh Radical Biomass (g) | 0.13 ± 0.02 | 0.01 ± 0.02 | 1200 |
| Fresh Radical Biomass (g) | 0.11 ± 0.01 | 0.01 ± 0.01 | 1000 |
| Stem Diameter (mm) | 1.57 ± 0.07 | 0.58 ± 0.07 | 170.69 |
| P (ppm) | Height (cm) | Leaf Area (cm2) | Fresh Aerial Biomass (g) | Dry Aerial Biomass (g) | Fresh Radical Biomass (g) | Fresh Radical Biomass (g) | Stem Diameter (mm) | |
|---|---|---|---|---|---|---|---|---|
| P (ppm) | 1 | 0.80 | 0.15 | 0.29 | 0.77 | 0.05 | 0.77 | 0.57 |
| Height (cm) | −0.31 | 1 | 0.94 | 0.50 | 0.02 | 0.75 | 0.03 | 0.63 |
| Leaf Area (cm2) | −0.97 | 0.09 | 1 | 0.44 | 0.92 | 0.2 | 0.92 | 0.43 |
| Fresh Aerial Biomass (g) | −0.89 | 0.7 | 0.77 | 1 | 0.48 | 0.25 | 0.47 | 0.87 |
| Dry Aerial Biomass (g) | −0.35 | 1 | 0.13 | 0.73 | 1 | 0.72 | 4.40 × 10−3 | 0.65 |
| Fresh Radical Biomass (g) | −1 | 0.38 | 0.95 | 0.93 | 0.42 | 1 | 0.72 | 0.62 |
| Fresh Radical Biomass (g) | 0.36 | −1 | −0.13 | −0.74 | −1 | −0.43 | 1 | 0.66 |
| Stem Diameter (mm) | −0.62 | −0.55 | 0.78 | 0.2 | −0.52 | 0.56 | 0.51 | 1 |
| F.V. | SC | gl | CM | F | p-Value |
|---|---|---|---|---|---|
| Model | 1976.84 | 3 | 658.95 | 8.67 | <0.0068 |
| PSB dose | 1976.84 | 3 | 658.95 | 8.67 | <0.0068 |
| Error | 607.83 | 8 | 75.98 | ||
| Total | 2584.67 | 11 |
| PSB Dose | Mean de P (ppm) | n | E.E. | |
|---|---|---|---|---|
| No inoculum | 72.77 | 3 | 5.03 | A |
| 10% inoculum | 96.68 | 3 | 5.03 | B |
| 5% inoculum | 99.94 | 3 | 5.03 | B |
| 15% inoculum | 106.88 | 3 | 5.03 | B |
| Variable | n | Mean | S.D. | W* | P (One-Tailed D) |
|---|---|---|---|---|---|
| P (ppm) | 12 | 94.07 | 15.33 | 0.84 | 0.0499 |
| Treatments | 12 | 2.50 | 1.17 | 0.83 | 0.0340 |
| Variable | P (ppm) | Treatments |
|---|---|---|
| P (ppm) | 1.00 | 0.02 |
| Treatments | 0.65 | 1.00 |
| PSB Doses | pH | O.M. (%) | P (ppm) | K (ppm) | Satur. (%) | C.E. (mS/cm) | CaCO3 (%) |
|---|---|---|---|---|---|---|---|
| No inoculum | 6.25 ± 0.02 | 1.62 ± 0.04 | 72.77 ± 0.13 | 709.85 ± 0.20 | 27.00 ± 0.09 | 3.942 ± 0.15 | 2.10 ± 0.07 |
| 5% inoculum | 6.47 ± 0.09 | 1.61 ± 0.02 | 99.94 ± 0.82 | 720.91 ± 0.60 | 27.67 ± 0.28 | 7.98 ± 0.11 | 2.35 ± 0.05 |
| 10% inoculum | 6.52 ± 0.07 | 1.70 ± 0.16 | 96.68 ± 0.58 | 616.94 ± 0.52 | 28.00 ± 0.10 | 9.83 ± 0.57 | 2.40 ± 0.10 |
| 15% inoculum | 6.61 ± 0.01 | 1.59 ± 0.03 | 106.88 ± 0.13 | 715.38 ± 0.56 | 29.00 ± 0.10 | 12.16 ± 0.08 | 2.80 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enriquez-León, R.; De la Cruz-Mantilla, J.; Huerta-Chombo, G.L. Evaluation of Phosphate-Solubilizing Bacteria (PSB) on Phosphorus Availability in Agricultural Soils and the Growth of Wheat (Triticum aestivum L.). Sustainability 2025, 17, 4545. https://doi.org/10.3390/su17104545
Enriquez-León R, De la Cruz-Mantilla J, Huerta-Chombo GL. Evaluation of Phosphate-Solubilizing Bacteria (PSB) on Phosphorus Availability in Agricultural Soils and the Growth of Wheat (Triticum aestivum L.). Sustainability. 2025; 17(10):4545. https://doi.org/10.3390/su17104545
Chicago/Turabian StyleEnriquez-León, Renzo, Jeffrey De la Cruz-Mantilla, and German Luis Huerta-Chombo. 2025. "Evaluation of Phosphate-Solubilizing Bacteria (PSB) on Phosphorus Availability in Agricultural Soils and the Growth of Wheat (Triticum aestivum L.)" Sustainability 17, no. 10: 4545. https://doi.org/10.3390/su17104545
APA StyleEnriquez-León, R., De la Cruz-Mantilla, J., & Huerta-Chombo, G. L. (2025). Evaluation of Phosphate-Solubilizing Bacteria (PSB) on Phosphorus Availability in Agricultural Soils and the Growth of Wheat (Triticum aestivum L.). Sustainability, 17(10), 4545. https://doi.org/10.3390/su17104545






