The Impact of Organic Fertilizer Substitution on Microbial Community Structure, Greenhouse Gas Emissions, and Enzyme Activity in Soils with Different Cultivation Durations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Experimental Design
2.3. Measurement Indexes and Methods
2.4. Soil Microbial Analyses
2.5. Statistical Analysis
3. Results
3.1. Physical and Chemical Properties of Soil
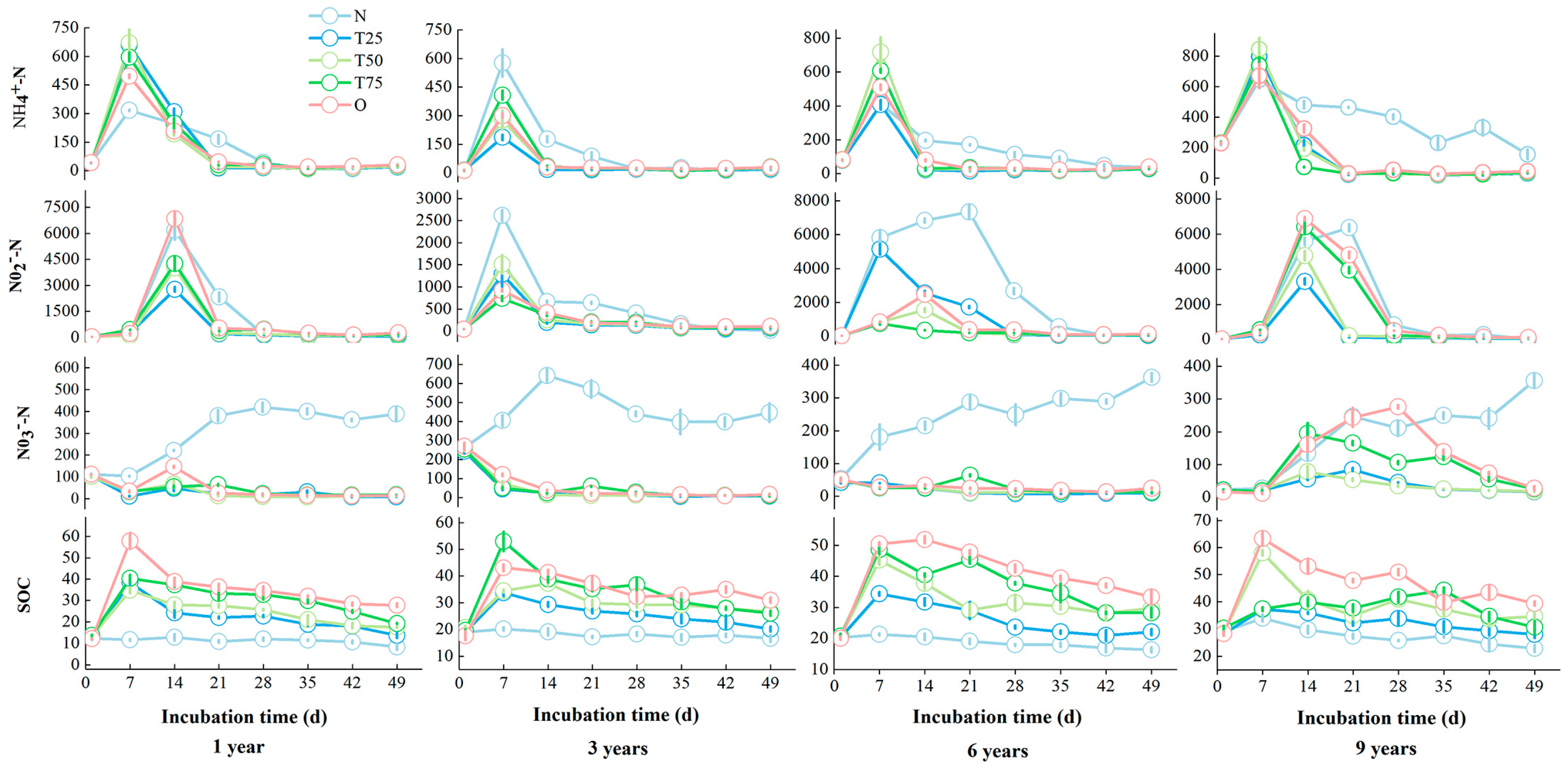
3.1.1. Carbon and Nitrogen Nutrients
3.1.2. Soil Enzyme Activity
3.2. Greenhouse Gases
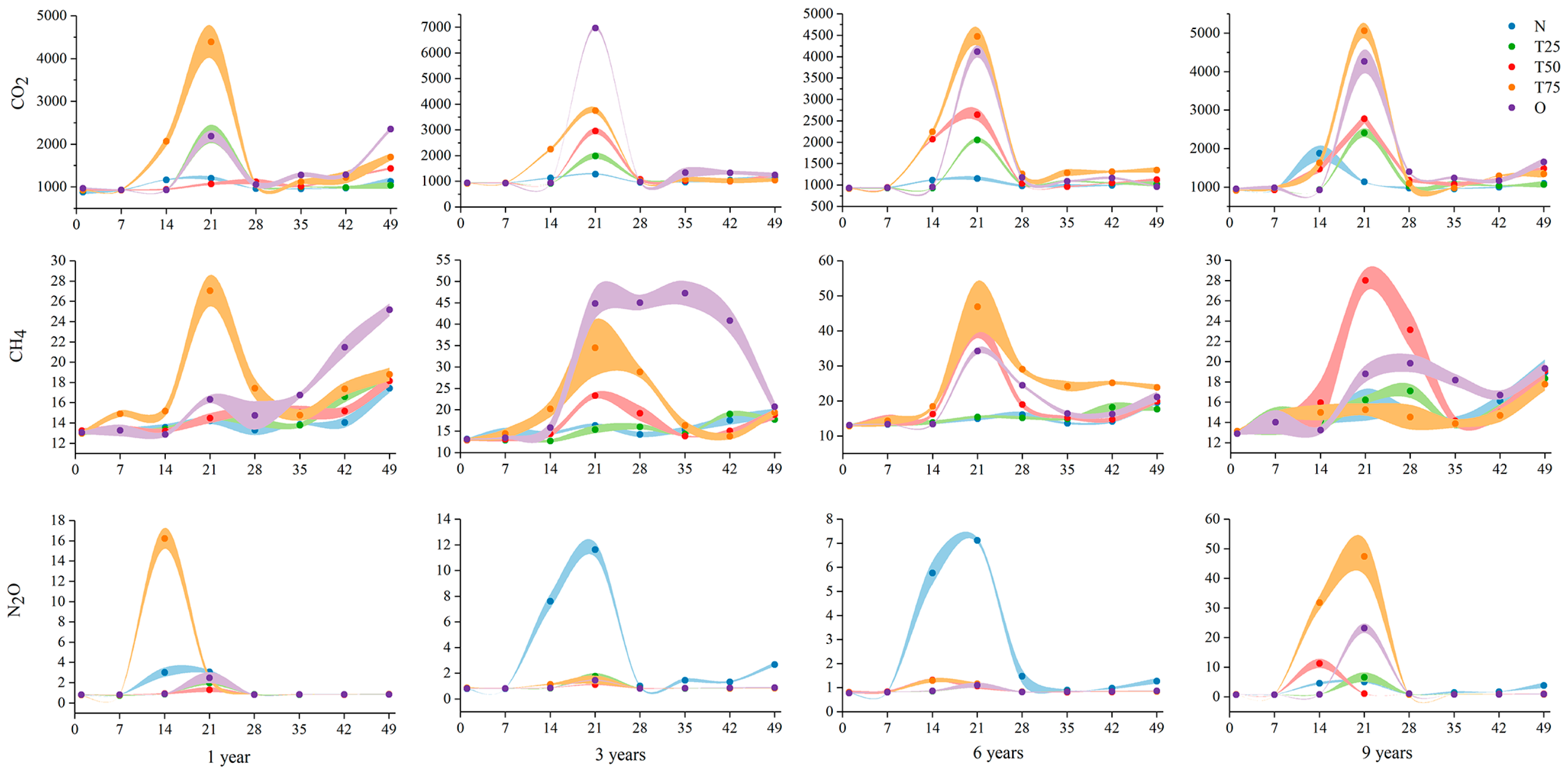
3.2.1. CO2, CH4, and N2O Emissions
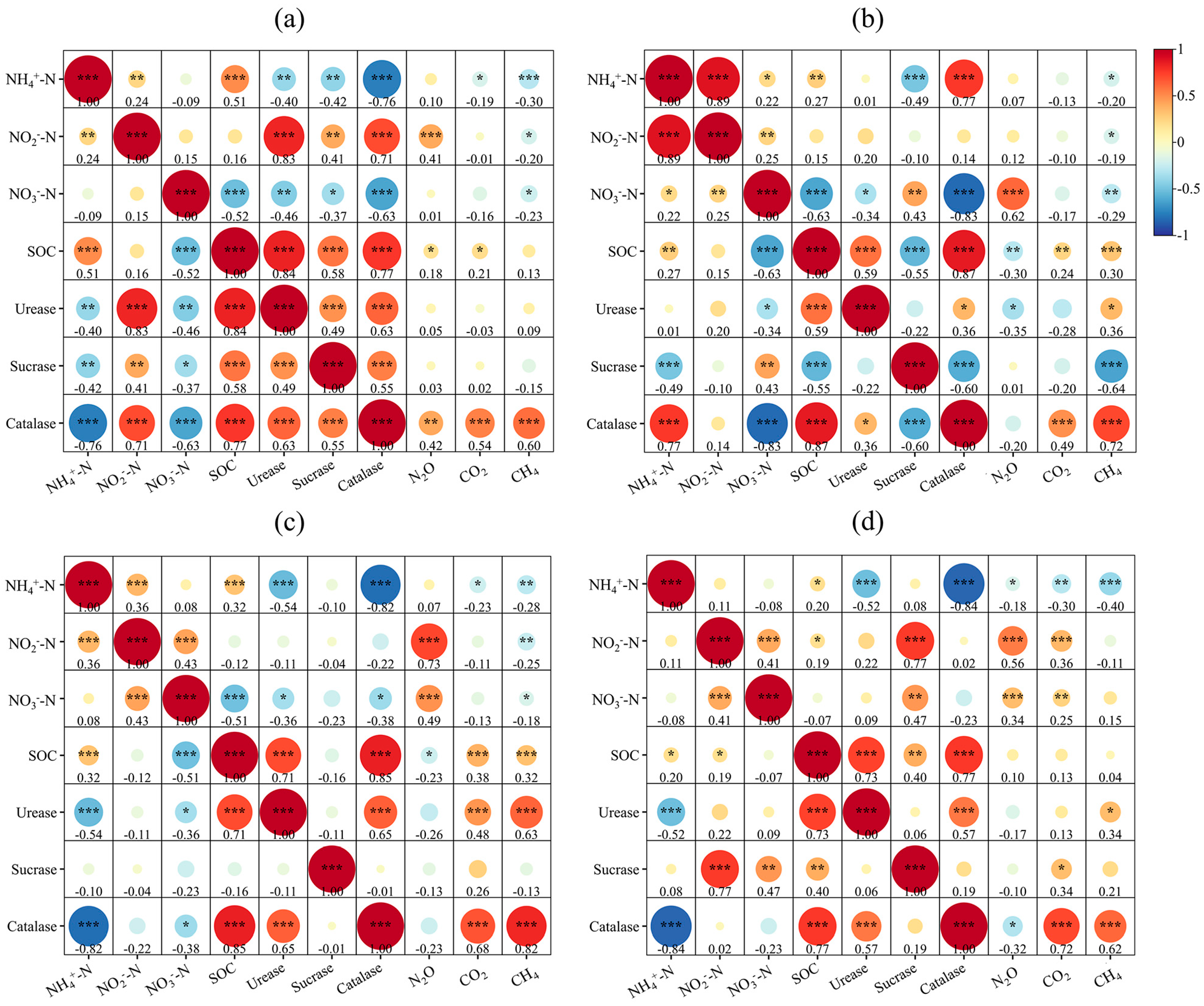
3.2.2. Correlation Analysis Between Soil Properties and Greenhouse Gas Emissions
3.3. Soil Microbial Community Structure
3.3.1. Total Number and Diversity of Species
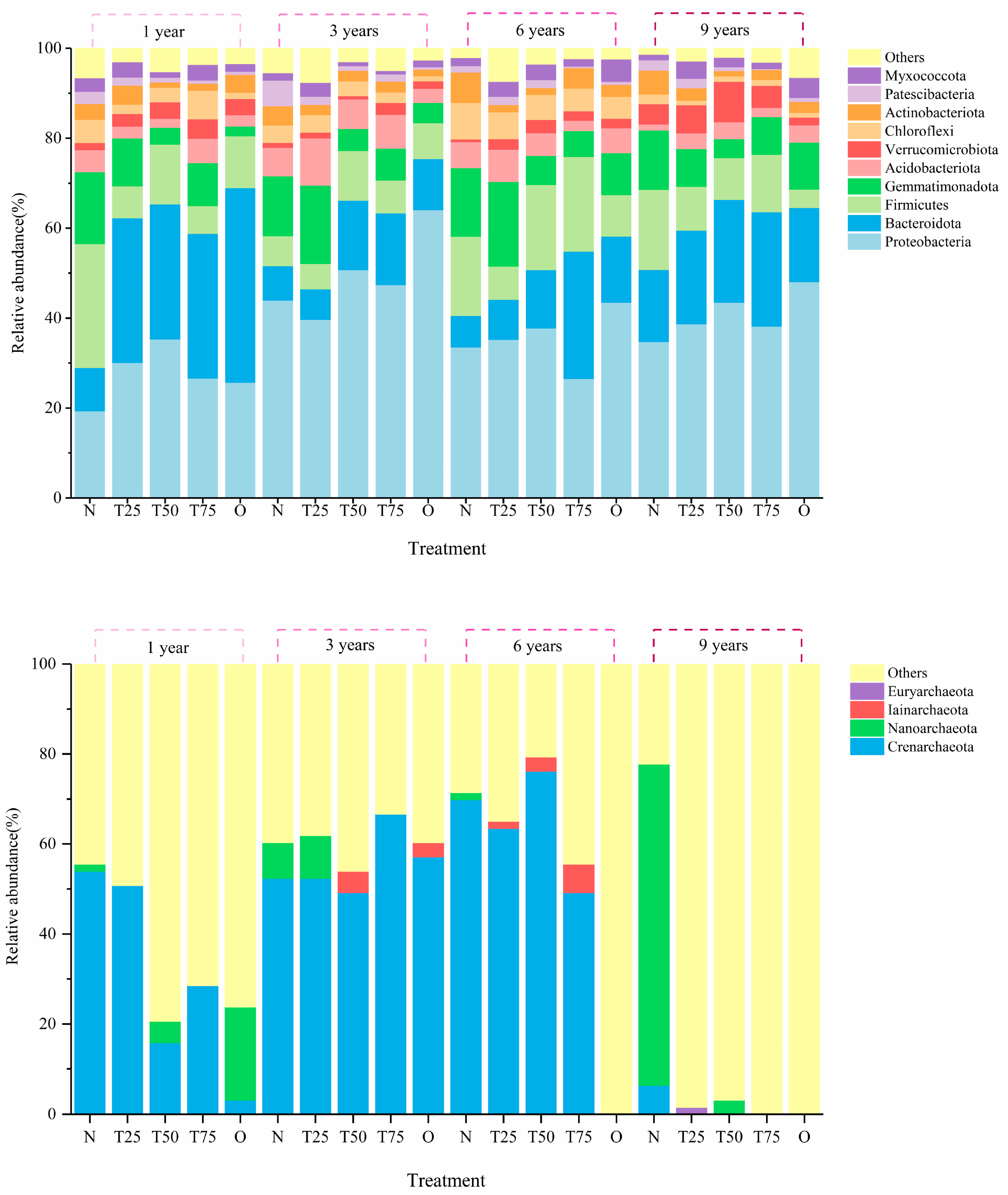
3.3.2. Relative Species Abundance at Phyla and Genus Levels
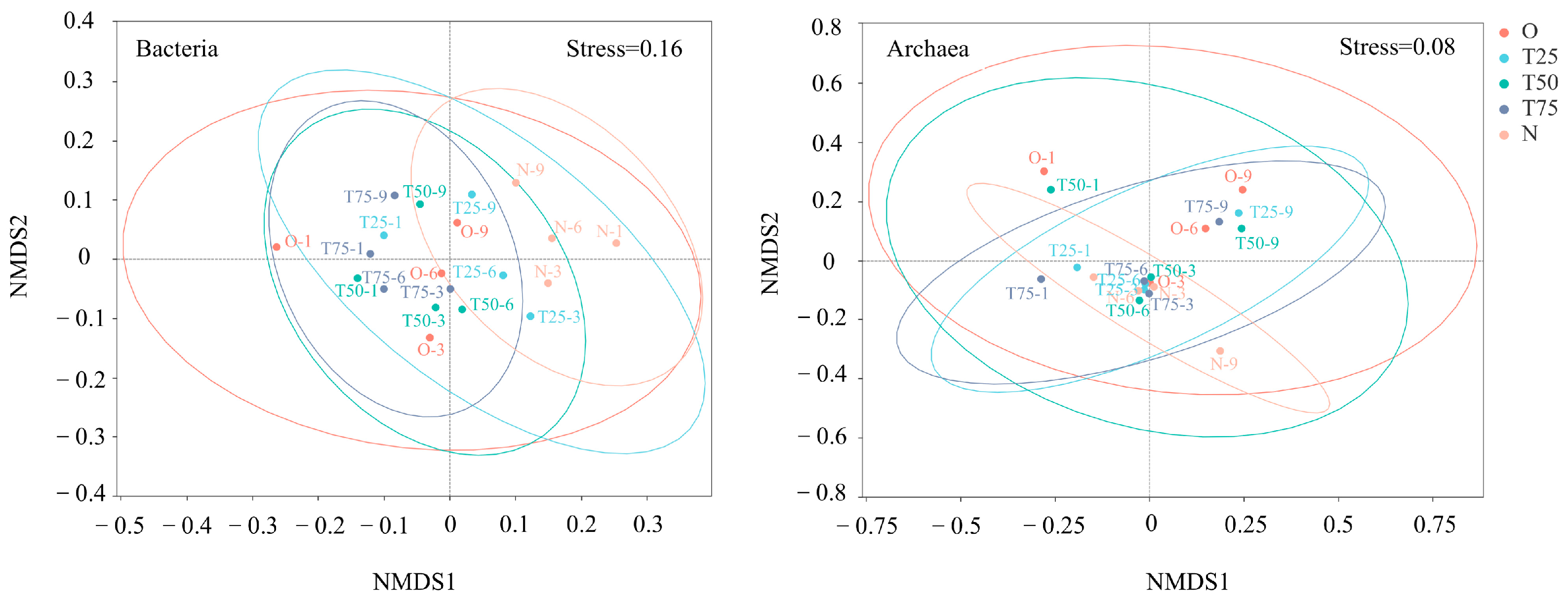
3.3.3. Non-Metric Multi-Dimensional Scaling Analysis
3.3.4. LDA Effect Size Analysis
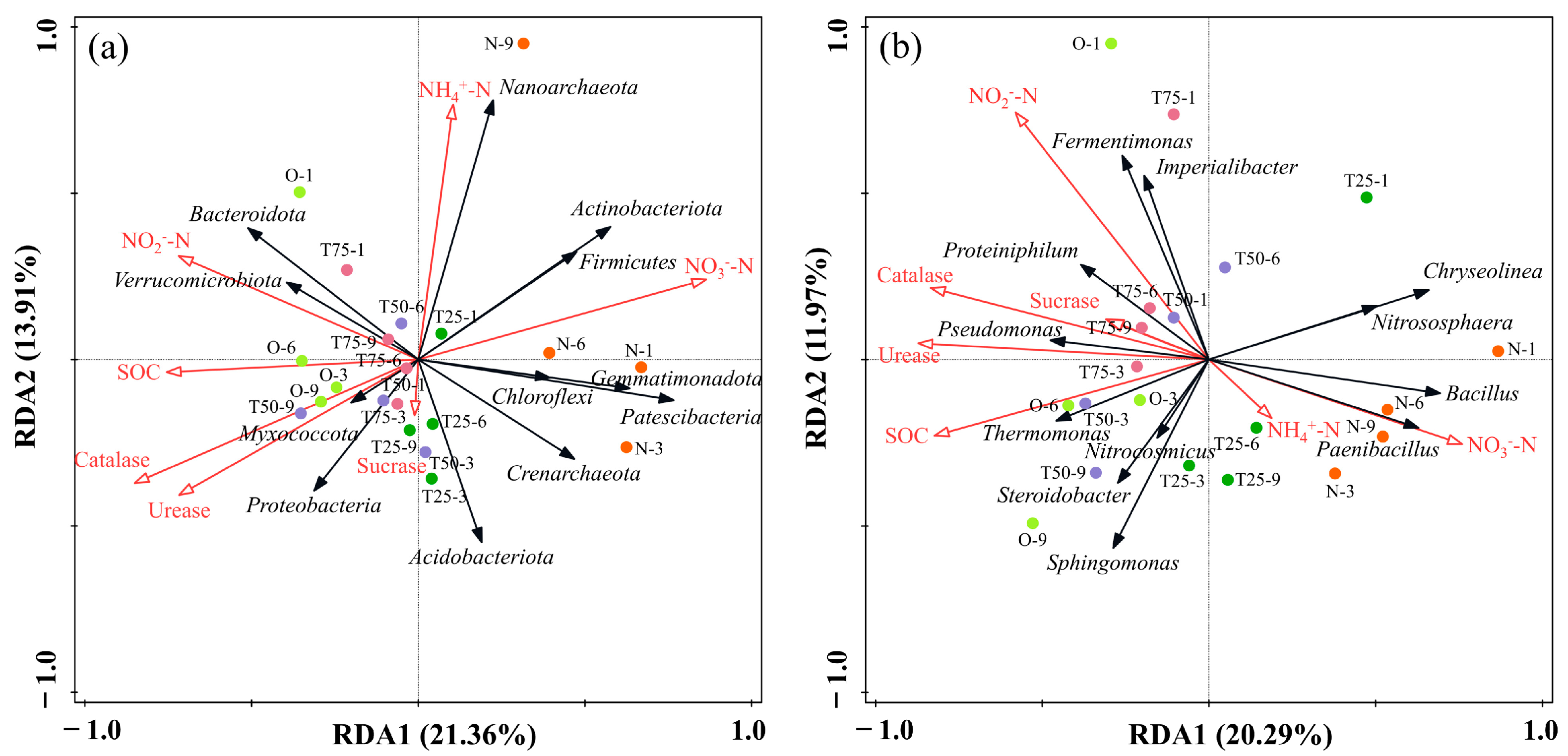
3.3.5. Redundancy Analysis
4. Discussion
4.1. Effects of Different Fertilization Treatments on Soil Physicochemical Properties and Enzyme Activities
4.2. Impact on Soil Greenhouse Gas Emissions
4.3. Effects of Different Fertilization Treatments on Soil Microbial Abundance in Different Years
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Cossu, M. Energy sustainable greenhouse crop cultivation using photovoltaic technologies. Renew. Sustain. Energy Rev. 2019, 109, 116–137. [Google Scholar] [CrossRef]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global greenhouse vegetable production systems are hotspots of soil N2O emissions and nitrogen leaching: A meta-analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Wang, X.; Tan, Y.; Wang, X.; Liu, X.; Guo, B. Effects of nitrification and urease inhibitors on ammonia-oxidizing microorganisms, denitrifying bacteria, and greenhouse gas emissions in greenhouse vegetable fields. Environ. Res. 2023, 237, 116781. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.J.; Frossard, E.; Kabwe, S.; Jayne, T.S. Understanding fertilizer adoption and effectiveness on maize in Zambia. Food Pol. 2019, 86, 101721. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Liu, Y. Cultivated land protection and rational use in China. Land Use Policy 2021, 106, 105454. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y.; Miao, H.; Xue, S.; Chen, Z.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Xie, T.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: A review. Ecotoxicol. Environ. Saf. 2021, 220, 112338. [Google Scholar] [CrossRef]
- Case, S.D.C.; Oelofse, M.; Hou, Y.; Oenema, O.; Jensen, L.S. Farmer perceptions and use of organic waste products as fertilisers—A survey study of potential benefits and barriers. Agric. Syst. 2017, 151, 84–95. [Google Scholar] [CrossRef]
- Lin, W.; Zhen, Z.; Liu, H.; Wang, N.; Guo, L.; Meng, J.; Ding, N.; Wu, G.; Jiang, G. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar] [CrossRef]
- Gai, X.; Liu, H.; Liu, J.; Zhai, L.; Yang, B.; Wu, S.; Ren, T.; Lei, Q.; Wang, H. Long-term benefits of combining chemical fertilizer and manure applications on crop yields and soil carbon and nitrogen stocks in North China Plain. Agric. Water Manag. 2018, 208, 384–392. [Google Scholar] [CrossRef]
- Lv, F.; Song, J.; Giltrap, D.; Feng, Y.; Yang, X.; Zhang, S. Crop yield and N2O emission affected by long-term organic manure substitution fertilizer under winter wheat-summer maize cropping system. Sci. Total Environ. 2020, 732, 139321. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Song, D.; Zhou, W.; Liu, G.; Liang, G.; He, P.; Sun, G.; Yuan, F.; Liu, Z.; Yao, Y.; et al. Partial substitution of chemical nitrogen with organic nitrogen improves rice yield, soil biochemical indictors and microbial composition in a double rice cropping system in south China. Soil Tillage Res. 2021, 205, 104753. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef]
- Tuo, Y.; Wang, Z.; Zheng, Y.; Shi, X.; Liu, X.; Ding, M.; Yang, Q. Effect of water and fertilizer regulation on the soil microbial biomass carbon and nitrogen, enzyme activity, and saponin content of Panax notoginseng. Agric. Water Manag. 2023, 278, 108145. [Google Scholar] [CrossRef]
- Shao, X.; Zheng, J. Soil organic carbon, black carbon, and enzyme activity under long-term fertilization. J. Integr. Agric. 2014, 13, 517–524. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Vatankhah, M.; Kennedy, J.F. Catalase-associated immune responses in plant-microbe interactions: A review. Int. J. Biol. Macromol. 2024, 280, 135859. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, A.; Shen, H. Effects of nitrogen addition on the combined global warming potential of three major soil greenhouse gases: A global meta-analysis. Environ. Pollut. 2023, 334, 121848. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xin, L. Spatial and temporal evolution and greenhouse gas emissions of China’s agricultural plastic greenhouses. Sci. Total Environ. 2023, 863, 160810. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Jiang, C.; Ma, R.; Hao, Q. Effects of plastic film mulching and nitrogen fertilization on the emissions of greenhouse gases from vegetable field in Southwest China. Ecol. Front. 2024, 44, 459–466. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, Y.; Yu, K.; Feng, H. Effects of straw mulching and plastic film mulching on improving soil organic carbon and nitrogen fractions, crop yield and water use efficiency in the Loess Plateau, China. Agric. Water Manag. 2018, 201, 133–143. [Google Scholar] [CrossRef]
- Lin, S.; Wang, W.; Sardans, J.; Lan, X.; Fang, Y.; Singh, B.P.; Xu, X.; Wiesmeier, M.; Tariq, A.; Zeng, F.; et al. Effects of slag and biochar amendments on microorganisms and fractions of soil organic carbon during flooding in a paddy field after two years in southeastern China. Sci. Total Environ. 2022, 824, 153783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Tian, H.; Xiao, Q.; Jiang, H. Pyrosequencing-based assessment of soil microbial community structure and analysis of soil properties with vegetable planted at different years under greenhouse conditions. Soil Tillage Res. 2019, 187, 1–10. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Fang, Y.; Cowie, A.L.; Dougherty, W.J.; Collins, D.; Dalal, R.C.; Singh, B.K. Tillage history and crop residue input enhanced native carbon mineralisation and nutrient supply in contrasting soils under long-term farming systems. Soil Tillage Res. 2019, 193, 71–84. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Chen, X.; Masiliunas, D.; Huang, S. Aggregate-associated changes in nutrient properties, microbial community and functions in a greenhouse vegetable field based on an eight-year fertilization experiment of China. J. Integr. Agric. 2020, 19, 2530–2548. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, J.; Wang, L.; Hou, M.; Chen, D. Effects of fermented organic fertilizer application on soil N2O emission under the vegetable rotation in polyhouse. Environ. Res. 2021, 200, 111491. [Google Scholar] [CrossRef]
- Yuan, S.; Li, R.; Zhang, Y.; Luan, H.; Tang, J.; Wang, L.; Ji, H.; Huang, S. Effects of long-term partial substitution of inorganic fertilizer with pig manure and/or straw on nitrogen fractions and microbiological properties in greenhouse vegetable soils? J. Integr. Agric. 2024, 23, 2083–2098. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Ju, X.; Shen, J.; Cheng, Y.; Deng, N.; Song, X.; Di, H.; Li, P.; Han, L.; et al. Nitrification inhibitor induced microbial NH4+-N immobilization improves maize nitrogen use efficiency in strong ammonia oxidation soil. Soil Biol. Biochem. 2025, 202, 109687. [Google Scholar] [CrossRef]
- Yuan, F.; Yin, S.; Xu, Y.; Xiang, L.; Wang, H.; Li, Z.; Fan, K.; Pan, G. The Richness and Diversity of Catalases in Bacteria. Front. Microbiol. 2021, 12, 645477. [Google Scholar] [CrossRef]
- Touceda-González, M.; Álvarez-López, V.; Prieto-Fernández, Á.; Rodríguez-Garrido, B.; Trasar-Cepeda, C.; Mench, M.; Puschenreiter, M.; Quintela-Sabarís, C.; Macías-García, F.; Kidd, P.S. Aided phytostabilisation reduces metal toxicity, improves soil fertility and enhances microbial activity in Cu-rich mine tailings. J. Environ. Manag. 2017, 186, 301–313. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Li, M.; Gong, C.; Liu, Z.; Yuan, J.; Li, X.; Song, C. Ammonia nitrogen and dissolved organic carbon regulate soil microbial gene abundances and enzyme activities in wetlands under different vegetation types. Appl. Soil Ecol. 2024, 196, 105310. [Google Scholar] [CrossRef]
- Sheoran, S.; Prakash, D.; Yadav, P.K.; Gupta, R.K.; Al-Ansari, N.; El-Hendawy, S.; Mattar, M.A. Long-term application of FYM and fertilizer N improve soil fertility and enzyme activity in 51st wheat cycle under pearl millet-wheat. Sci. Rep. 2024, 14, 21695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Tan, H.; Tuo, Y.; Chang, X.; Liang, J.; Yang, Q.; He, X. Characteristics of forest soil enzyme activities, nutrient restriction, and physicochemical properties at different altitudes and their seasonal dynamics. Eurasian Soil Sci. 2025, 58, 4. [Google Scholar] [CrossRef]
- Ren, K.; Sun, Y.; Zou, H.; Li, D.; Lu, C.; Duan, Y.; Zhang, W. Effect of replacing synthetic nitrogen fertilizer with animal manure on grain yield and nitrogen use efficiency in China: A meta-analysis. Front. Plant Sci. 2023, 14, 1153235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Effects of a decade of organic fertilizer substitution on vegetable yield and soil phosphorus pools, phosphatase activities, and the microbial community in a greenhouse vegetable production system. J. Integr. Agric. 2022, 21, 2119–2133. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, C.; Bi, R.; Jiao, Y.; Wang, B.; Dong, Y.; Xiong, Z. Optimizing organic fertilization towards sustainable vegetable production evaluated by long-term field measurement and multi-level fuzzy comprehensive model. Agric. Ecosyst. Environ. 2024, 368, 109008. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Li, Y.; Li, R.; Huang, S.; Wang, H. Risk assessment of heavy metal accumulation in cucumber fruits and soil in a greenhouse system with long-term application of organic fertilizer and chemical fertilizer. Agriculture 2024, 14, 1870. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agric. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Du, Y.; Guo, X.; Li, J.; Liu, Y.; Luo, J.; Liang, Y.; Li, T. Elevated carbon dioxide stimulates nitrous oxide emission in agricultural soils: A global meta-analysis. Pedosphere 2022, 32, 3–14. [Google Scholar] [CrossRef]
- Meng, X.; Liu, S.; Zou, J.; Osborne, B. The effect of substituting inorganic fertilizer with manure on soil N2O and CH4 emissions and crop yields: A global meta-analysis. Field Crops Res. 2025, 326, 109831. [Google Scholar] [CrossRef]
- Fan, L.; Dippold, M.A.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; Dorodnikov, M. Anaerobic oxidation of methane in paddy soil: Role of electron acceptors and fertilization in mitigating CH4 fluxes. Soil Biol. Biochem. 2020, 141, 107685. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, L.; Zhao, X.; Agathokleous, E.; Wang, S.; Ren, B.; Yang, W.; Liu, J.; Jin, J.; Huang, H.; et al. Long-term fertilization enhances the activity of anaerobic oxidation of methane coupled to nitrate reduction and associated microbial abundance in paddy soils. Soil Biol. Biochem. 2023, 185, 109130. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Lüke, C.; Jetten, M.S.M. Enrichment of anaerobic nitrate-dependent methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Ma, L.; Chadwick, D.; Chen, X. Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from China’s vegetable systems: A meta-analysis. Environ. Pollut. 2021, 269, 116143. [Google Scholar] [CrossRef]
- Li, Q.; Andom, O.; Li, Y.; Cheng, C.; Deng, H.; Sun, L.; Li, Z. Responses of grape yield and quality, soil physicochemical and microbial properties to different planting years. Eur. J. Soil Biol. 2024, 120, 103587. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.; Li, D.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Minkina, T.; Singh, R.K.; Singh, P.; Li, Y. Interactive role of silicon and plant–rhizobacteria mitigating abiotic stresses: A new approach for sustainable agriculture and climate change. Plants 2020, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2021, 25, 100173. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Liu, Y.; Zhang, R.; Li, M. Non-negligible roles of archaea in coastal carbon biogeochemical cycling. Trends Microbiol. 2023, 31, 586–600. [Google Scholar] [CrossRef]
- Jin, L.; Jin, N.; Wang, S.; Li, J.; Meng, X.; Xie, Y.; Wu, Y.; Luo, S.; Lyu, J.; Yu, J.; et al. Changes in the microbial structure of the root soil and the yield of Chinese baby cabbage by chemical fertilizer reduction with bio-organic fertilizer application. Microbiol. Spectr. 2022, 10, 1215–1222. [Google Scholar] [CrossRef]
- He, C.; Wang, R.; Ding, W.; Li, Y. Effects of cultivation soils and ages on microbiome in the rhizosphere soil of Panax ginseng. Appl. Soil Ecol. 2022, 174, 104397. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Niu, Y.; Jiang, W.; Wang, Y.; Liu, S.; Wei, W. 44-Years of Fertilization Altered Soil Microbial Community Structure by Changing Soil Physical, Chemical Properties and Enzyme Activity. J. Soil Sci. Plant Nutr. 2024, 24, 3150–3161. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Zhou, Y.; Zhang, B.; Peng, Y.; Zhuo, Y.; Ai, W.; Gao, C.; Wu, B.; Liu, D.; et al. Straw and straw biochar differently affect fractions of soil organic carbon and microorganisms in farmland soil under different water regimes. Environ. Technol. Innov. 2023, 32, 103412. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Ghimire, R.; Sainju, U.M.; Jia, Y.; Zhao, F. Responses of soil bacterial community and enzyme activity to organic matter components under long-term fertilization on the Loess Plateau of China. Appl. Soil Ecol. 2021, 166, 103992. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillusspecies in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2019, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Anand, G.; Pandey, R.; Bar, M.; Yadav, D. Employing Bacillus and Pseudomonas for phytonematode management in agricultural crops. World J. Microbiol. Biotechnol. 2024, 40, 331. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. npj Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yang, Z.; Yuan, X.; Lin, W.; Xiong, D.; Yang, Y. Effects of precipitation exclusion and warming on soil soluble carbon and nitrogen in a young Cunninghamia lanceolata plantation. J. Appl. Econ. 2018, 29, 2217–2223. [Google Scholar] [CrossRef]
- Xiong, R.; Qian, D.; Qiu, Z.; Hou, Y.; Li, Q.; Shen, W. Land-use intensification exerts a greater influence on soil microbial communities than seasonal variations in the Taihu Lake region, China. Sci. Total Environ. 2024, 943, 173630. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Zhu, G.; Xu, A.; Zhang, R.; Bryant, R.; Drohan, P.J.; Long, H.; Willemsen, V. Stable soil moisture promotes shoot performance and shapes the root-rhizosphere microbiome. Agric. Water Manag. 2025, 310, 109354. [Google Scholar] [CrossRef]


| Planting Year | Treatment | Chao1 Index 1 | Shannon Index 2 | Simpson Index 3 | |||
|---|---|---|---|---|---|---|---|
| Bacteria | Archaea | Bacteria | Archaea | Bacteria | Archaea | ||
| 1 | N | 1680.041 | 21.000 | 8.241 | 2.606 | 0.986 | 0.772 |
| T25 | 1472.243 | 19.000 | 7.730 | 2.157 | 0.967 | 0.707 | |
| T50 | 1437.960 | 12.000 | 7.864 | 2.616 | 0.977 | 0.806 | |
| T75 | 1496.493 | 12.000 | 8.415 | 3.044 | 0.987 | 0.838 | |
| O | 1624.528 | 9.333 | 8.181 | 2.506 | 0.987 | 0.783 | |
| 3 | N | 2116.373 | 30.000 | 9.580 | 4.161 | 0.996 | 0.917 |
| T25 | 2185.900 | 37.200 | 9.683 | 4.092 | 0.997 | 0.922 | |
| T50 | 1840.515 | 12.000 | 8.547 | 2.855 | 0.987 | 0.827 | |
| T75 | 1499.000 | 15.000 | 8.690 | 2.982 | 0.989 | 0.813 | |
| O | 1578.085 | 29.250 | 8.101 | 3.169 | 0.971 | 0.820 | |
| 6 | N | 1807.015 | 18.000 | 8.905 | 2.647 | 0.994 | 0.756 |
| T25 | 1745.147 | 13.000 | 8.996 | 2.629 | 0.994 | 0.798 | |
| T50 | 1918.951 | 21.600 | 8.576 | 2.753 | 0.981 | 0.717 | |
| T75 | 1660.791 | 12.000 | 7.991 | 2.688 | 0.983 | 0.781 | |
| O | 1635.243 | 6.000 | 8.546 | 2.218 | 0.991 | 0.750 | |
| 9 | N | 1358.882 | 19.000 | 8.473 | 1.789 | 0.991 | 0.480 |
| T25 | 1343.459 | 17.000 | 8.671 | 2.257 | 0.993 | 0.642 | |
| T50 | 1413.673 | 14.500 | 8.160 | 2.871 | 0.989 | 0.795 | |
| T75 | 1520.940 | 12.500 | 8.078 | 2.791 | 0.979 | 0.814 | |
| O | 1441.507 | 14.500 | 8.554 | 3.088 | 0.993 | 0.850 | |
| Phylum | Genus | ||
|---|---|---|---|
| Eigenvalues | Axis1 | 0.2136 | 0.2029 |
| Axis2 | 0.1391 | 0.1197 | |
| Axis3 | 0.0894 | 0.0779 | |
| Axis4 | 0.0413 | 0.0536 | |
| P | Catalase | 0.002 | 0.312 |
| NH4+-N | 0.002 | 0.258 | |
| NO2−-N | 0.048 | 0.016 | |
| Urease | 0.234 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhao, C.; Liu, X.; Dong, Y.; Liu, W.; Chen, Q.; Ding, S.; Zhang, J.; Guo, B.; Gao, X. The Impact of Organic Fertilizer Substitution on Microbial Community Structure, Greenhouse Gas Emissions, and Enzyme Activity in Soils with Different Cultivation Durations. Sustainability 2025, 17, 4541. https://doi.org/10.3390/su17104541
Guo Y, Zhao C, Liu X, Dong Y, Liu W, Chen Q, Ding S, Zhang J, Guo B, Gao X. The Impact of Organic Fertilizer Substitution on Microbial Community Structure, Greenhouse Gas Emissions, and Enzyme Activity in Soils with Different Cultivation Durations. Sustainability. 2025; 17(10):4541. https://doi.org/10.3390/su17104541
Chicago/Turabian StyleGuo, Yanke, Changsheng Zhao, Xuzhen Liu, Yanan Dong, Wei Liu, Qingfeng Chen, Shigang Ding, Jing Zhang, Beibei Guo, and Xinguo Gao. 2025. "The Impact of Organic Fertilizer Substitution on Microbial Community Structure, Greenhouse Gas Emissions, and Enzyme Activity in Soils with Different Cultivation Durations" Sustainability 17, no. 10: 4541. https://doi.org/10.3390/su17104541
APA StyleGuo, Y., Zhao, C., Liu, X., Dong, Y., Liu, W., Chen, Q., Ding, S., Zhang, J., Guo, B., & Gao, X. (2025). The Impact of Organic Fertilizer Substitution on Microbial Community Structure, Greenhouse Gas Emissions, and Enzyme Activity in Soils with Different Cultivation Durations. Sustainability, 17(10), 4541. https://doi.org/10.3390/su17104541







