Toxic Effects of Liquors Generated During Kraft Pulp Production Process on Aerobic Biomass and Growth of Selenastrum capricornutum
Abstract
1. Introduction
2. Materials and Methods
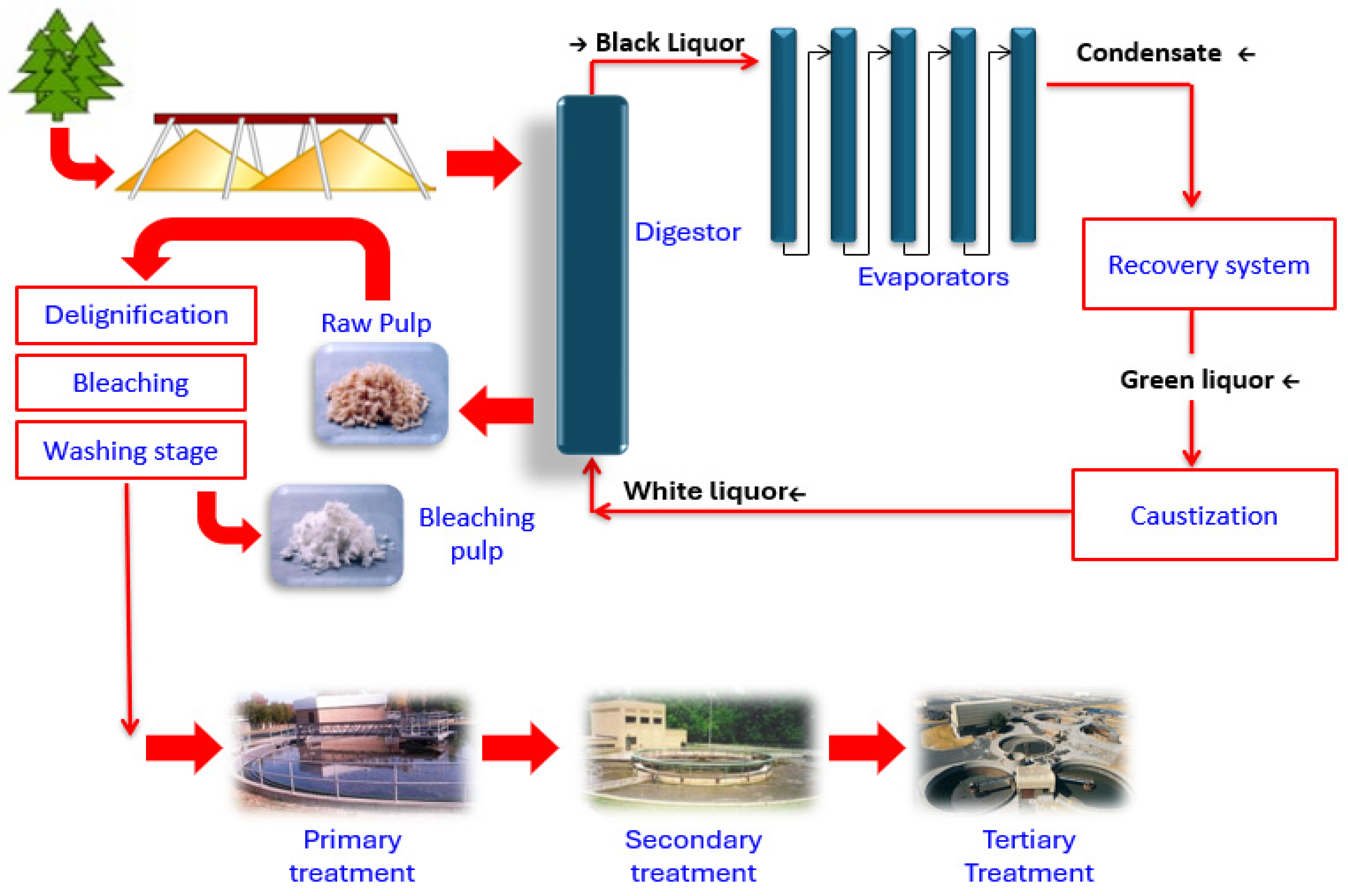
2.1. Collection of Kraft Pulp Effluent and Accidental Toxic Spill Flows
2.2. Physicochemical Characterization of a Kraft Pulp Effluent and Liquors Formed as By-Products of the Kraft Pulp Process
2.3. Measurement via Respirometry of Aerobic Biomass Activity of an Activated Sludge System Exposed to Different COD Concentrations of White, Black, and Green Liquors and Condensate
2.4. Determination of Selenastrum capricornutum Growth and Inhibition Rates upon Exposure to Different COD Concentrations of White, Black, and Green Liquors and Condensate
3. Results and Discussion
3.1. Physicochemical Characterization of a Kraft Pulp Process Effluent Used as Feed for a Laboratory-Scale Activated Sludge System and White, Black, and Green Liquors and Condensate
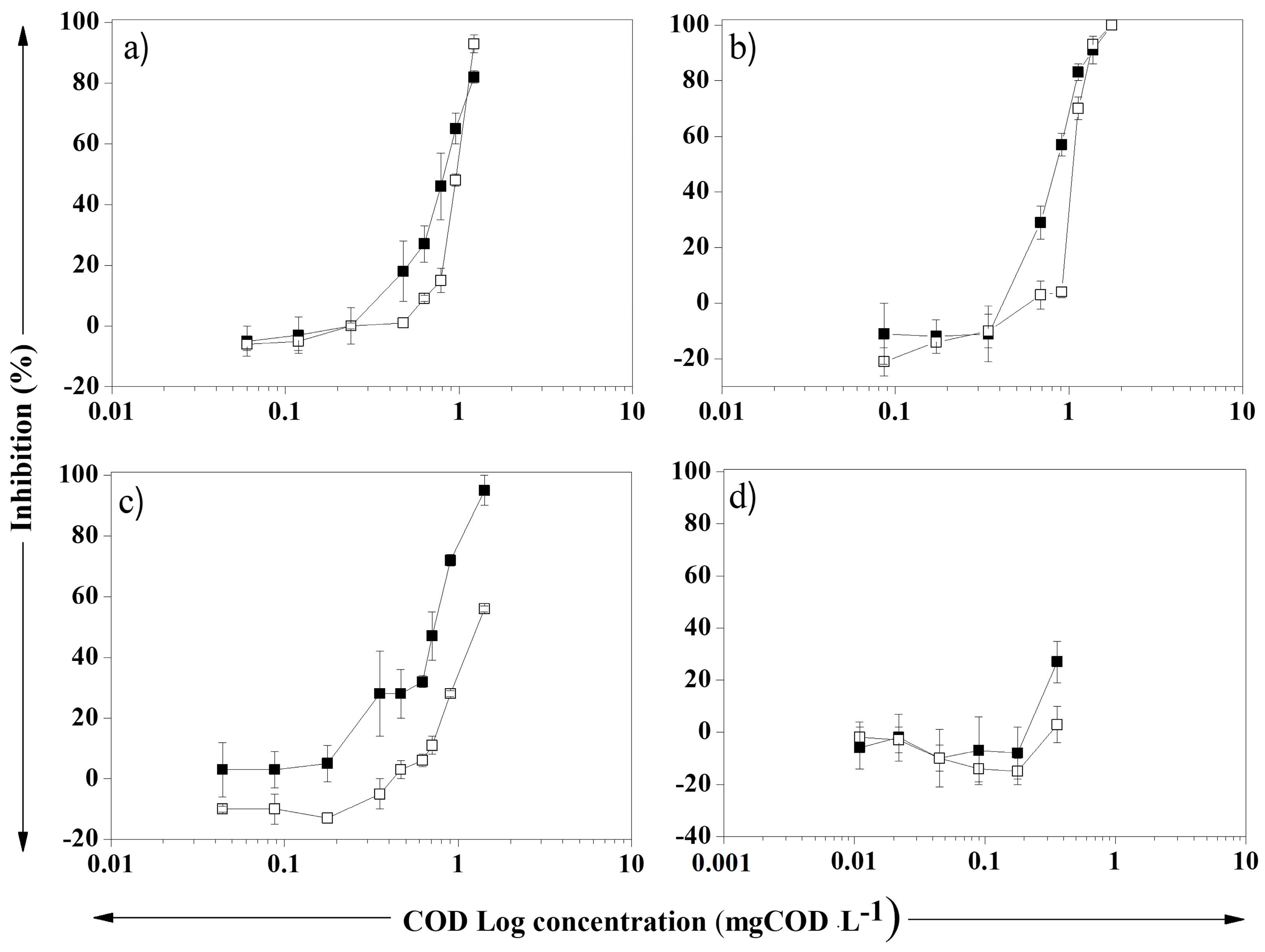
3.2. Assessment of the Toxicity of Liquors Generated During the Kraft Pulp Production Process to the Aerobic Biomass of an Activated Sludge Treatment System
3.3. Evaluation of the Toxicity of Liquors Produced in the Kraft Pulp Process to Selenastrum capricornutum, a Bioindicator of Acute Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidal, G.; González, Y.; Piña, B.; Jarpa, M.; Gómez, G. Minimization of environmental impact Kraft pulp mill effluents: Current practices and future perspectives towards sustainability. Sustainability 2021, 13, 9288. [Google Scholar] [CrossRef]
- Pola, L.; Collado, S.; Oulego, P.; Díaz, M. Kraft black liquor as renewable source of value-added chemicals. Chem. Eng. J 2022, 448, 137728. [Google Scholar] [CrossRef]
- Singh, A.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. toxicol 2019, 211, 202–216. [Google Scholar] [CrossRef]
- Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelic, S.; Vogel, F.; Konttinen, J.; Joronen, T. Sub-and supercritical water liquefaction of kraft lignin and black liquor derived lignin. Energy 2020, 13, 3309. [Google Scholar] [CrossRef]
- Coimbra, E.-C.; Mounteer, A.; Vital do Carmo, A.; Michielsen, M.; Tótola, L.; Guerino, J.; Goncalves, J.; Da silva, P. Electrocoagulation of kraft pulp bleaching filtrates to improve biotreatability. Process Saf. Environ. Prot. 2021, 147, 346–355. [Google Scholar] [CrossRef]
- Jardim, J.; Hart, P.; Lucia, L.; Jameel, H.; Chang, H. The effect of the Kraft pulping process, wood species, and pH on lignin recovery from black liquor. Fibers 2022, 10, 16. [Google Scholar] [CrossRef]
- Morales, G.; Pesante, S.; Vidal, G. Effects of black liquor shocks on activated sludge treatment of bleached kraft pulp mill wastewater. J. Environ. Sci. Health Part A-Toxic/Hazard Subst. Environ. Eng. 2015, 50, 639–645. [Google Scholar]
- Sousa, A.M.; Pinto, I.S.S.; Machado, L.; Gando-Ferreira, L.; Quina, M.J. Sustainability of Kraft pulp mills: Bleaching technologies and sequences with reduced water use. J. Ind. Eng. Chem. 2023, 125, 58–70. [Google Scholar] [CrossRef]
- Chamorro, S.; Hernández, L.; Sáez, K.; Gómez, G.; Vidal, G. Effects of black liquor shocks on the stability of activated sludge treatment of kraft pulp mill effluent: Morphological alteration in Daphnia magna and mutagenicity and genotoxicity response in Salmonella typhimurium. Sustainability 2022, 14, 3869. [Google Scholar] [CrossRef]
- Chamorro, S.; Hernandez, V.; Matamoros, V.; Dominguez, C.; Becerra, J.; Vidal, G.; Piña, B.; Bayona, J.M. Chemical characterization of organic microcontaminant sources and biological effects in riverine sediments impacted by urban sewage and pulp mill discharges. Chemosphere 2013, 90, 611–619. [Google Scholar] [CrossRef]
- Diaz, A.I.; Laca, A.; Lima, N.; Diaz, M. Treatment of Kraft black liquor using basidiomycete and ascomycete fungi. Process Saf. Environ. Protect 2022, 168, 67–76. [Google Scholar] [CrossRef]
- Goycoechea, N.; López, I.; Borzacconi, L. Optimization of anaerobic digestion and solubilization of biosludges from the Kraft cellulose industry using thermal hydrolysis as pretreatment. J. Environ. Manag. 2023, 344, 118504. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Del-Río, P.; Rubira, A.; Pérez, M.; Garrote, G. Co-valorization of discarded wood pinchips and sludge from pulp and paper industry for production of advanced biofuels. Ind. Crop. Prod. 2024, 209, 117992. [Google Scholar] [CrossRef]
- Sandberg, M. Mill case, simulation, and laboratory plant study of black liquor spill effects on a multiple stage biological treatment plant. Can. J. Civ. Eng. 2009, 36, 839–849. [Google Scholar] [CrossRef]
- Kelly, C.R.; Hargreaves, T.L.; Golden, R.; Holm, S.E.; Deardorff, T.L.; Festa, J.L. Toxicity investigations associated with Daphnia magna and Pimephales promelas exposed to spent pulping liquor from an elemental chlorine free kraft mill. In Pulp and Paper Mill Effluent Environmental Fate and Effects, 1st ed.; Borton, D.L., Thomas, J.F., Fisher, R.P., Hall, T.J., Eds.; DEStech Publications: Lancaster, PA, USA, 2004; pp. 304–309. [Google Scholar]
- Nielsen, P.; Keiding, K. Disintegration of activated sludge flocs in presence of sulfide. Water Res. 1998, 32, 313–320. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Küster, E.; Dorusch, F.; Altenburger, R. Effects of hydrogen sulfide to Vibrio fischeri, Scenedesmus vacuolatus, and Daphnia magna. Environ. Toxicol. Chem. 2005, 24, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development (OECD). Test No. 209: Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Environmental Protection Agency (EPA). Method 1003.0: Green Alga, Selenastrum capricornutum, Growth Test; Chronic Toxicity, 2002, 4th ed. EPA-821-R-02-013. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/method_1003_2002.pdf (accessed on 10 May 2025).
- Villamar, C.A.; Jarpa, M.; Decap, J.; Vidal, G. Aerobic moving bed biorreactor performance: A comparative study of removal efficiencies of Kraft mill effluents from Pinus radiata and Eucalyptus globulus as raw material. Water Sci. Technol. 2009, 59, 507–514. [Google Scholar] [CrossRef][Green Version]
- Henricson, K. Chemical Recovery Cycle. In Educational Course Material and Only for Internal and Personal Use During the Course: “An Introduction to Chemical Pulping Technology”; Lappeenranta University of Technology: Lappeenranta, Finland, 2005; Volume 33. [Google Scholar]
- Kumar, V.; Verma, P. Microbial valorization of kraft black liquor for production of platform chemicals, biofuels, and value-added products: A critical review. J. Environ. Manag. 2024, 366, 121631. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, Y.; Mahar, R.; Wang, Z.; Nie, Y. Effects and model of alkaline waste activated sludge treatment. Bioresour. Technol. 2008, 99, 5140–5144. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis: Changing view of the dose-response, a personal account of the history and current status. Mutant. Res.–Rev. Mutat. Res. 2002, 511, 181–189. [Google Scholar] [CrossRef]
- Morya, R.; Kumar, M.; Tyagi, I.; Pandey, A.; Park, J.; Raj, T.; Sirohi, R.; Kumar, V.; Kim, S. Recent advances in black liquor valorization. Bioresour. Technol. 2022, 350, 126916. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Chen, Q.; Duan, S. Transcriptional Analysis of Chlorella pyrenoidosa Exposed to Bisphenol A. Int. J. Environ. Res. Public Health 2019, 16, 1374. [Google Scholar] [CrossRef] [PubMed]
- Rasooly-Garmaroody, E.; Esmaeli-Jafarzadeh, A.; Kermanian, H.; Ramezani, O. Spent black liquor as an alternative carbon source for the synthesis of bacterial cellulose. Cell Chem. Technol. 2022, 56, 749–756. [Google Scholar] [CrossRef]
- Brown, D.; Grunden, A.; Pawlak, J. Statistical optimization of black liquor-containing media for growth and lactic acid production by Paenibacillus glucanolyticus SLM1. Bioresour. Technol. Rep. 2021, 13, 100629. [Google Scholar] [CrossRef]
- Chauhan, V.; Singh, G.; Ramamurthy, V. Eucalyptus kraft black liquor enhances growth and productivity of spirulina in outdoor cultures. Biotechnol. Prog. 1995, 11, 457–460. [Google Scholar] [CrossRef]


| Parameter | Unit | Range | Mean ± SD * |
|---|---|---|---|
| pH | - | 6.62–6.67 | 6.65 ± 0.04 |
| EC * | mS/cm | 2.80–2.81 | 2.81 ± 0.01 |
| COD | mg/L | 611.0–638.5 | 624.8 ± 19.5 |
| BOD5 | mg/L | 324.0–360.0 | 342.0 ± 25.5 |
| TSS | mg/L | 0.03–0.06 | 0.05 ± 0.02 |
| VSS | g/L | 0.02–0.05 | 0.03 ± 0.02 |
| Color | Abs | 0.09–0.10 | 0.10 ± 0.00 |
| Lignosulfonic acids | Abs | 0.05–0.05 | 0.05 ± 0.00 |
| Lignin | Abs | 2.39–2.41 | 2.40 ± 0.01 |
| Aromatic compounds | Abs | 0.49–0.53 | 0.52 ± 0.03 |
| Total phenols | mg/L | 159.7–161.4 | 160.6 ± 1.2 |
| TP | mg/L | 2.7–2.7 | 2.7 ± 0.0 |
| TN | mg/L | 0.5–0.8 | 0.7 ± 0.2 |
| NO3− | mg/L | <0.1 | – |
| NO2− | mg/L | 0.03–0.05 | 0.04 ± 0.01 |
| Parameter | Unit | Liquor | |||
|---|---|---|---|---|---|
| White | Black | Green | Condensate | ||
| pH | - | 13.0 ± 0.1 | 13.0 ± 0.1 | 13.0 ± 0.1 | 7.6 ± 0.1 |
| EC * | mS/cm | 32.95 | 89.90 | 37.13 | 0.03 |
| COD * | mg/L | 1911 ± 209 | 141,350 ± 2275 | 2755 ± 95 | 358 ± 7 |
| TOC * | mg/L | 1341.0 ± 2.5 | 3072.0 ± 3.1 | 1948.0 ± 3.5 | 71.7 ± 0.5 |
| Color | Abs | - | 5.500 | - | 0.014 |
| Lignosulfonic acids | Abs | 0.500 | 21.500 | - | 0.058 |
| Lignin | Abs | 2.0 | 51.5 | 2.0 | 0.258 |
| Aromatic compounds | Abs | 3.4 | 62.5 | 6.0 | 0.6 |
| Total phenols | mg/L | 203 | 4056 | 406 | 37 |
| Liquor | EC20 * | EC50 * |
|---|---|---|
| (mgCOD/L) | ||
| White | 117.8 | 168.9 |
| Black | 1400.0 | 2854.5 |
| Green | 129.9 | 300.3 |
| Condensate | 167.1 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidd, C.; Morales, G.; Monsalves, N.; Vidal, G. Toxic Effects of Liquors Generated During Kraft Pulp Production Process on Aerobic Biomass and Growth of Selenastrum capricornutum. Sustainability 2025, 17, 4494. https://doi.org/10.3390/su17104494
Hidd C, Morales G, Monsalves N, Vidal G. Toxic Effects of Liquors Generated During Kraft Pulp Production Process on Aerobic Biomass and Growth of Selenastrum capricornutum. Sustainability. 2025; 17(10):4494. https://doi.org/10.3390/su17104494
Chicago/Turabian StyleHidd, Constanza, Gabriela Morales, Naomi Monsalves, and Gladys Vidal. 2025. "Toxic Effects of Liquors Generated During Kraft Pulp Production Process on Aerobic Biomass and Growth of Selenastrum capricornutum" Sustainability 17, no. 10: 4494. https://doi.org/10.3390/su17104494
APA StyleHidd, C., Morales, G., Monsalves, N., & Vidal, G. (2025). Toxic Effects of Liquors Generated During Kraft Pulp Production Process on Aerobic Biomass and Growth of Selenastrum capricornutum. Sustainability, 17(10), 4494. https://doi.org/10.3390/su17104494









