Litter and Pruning Biomass in Mango Orchards: Quantification and Nutrient Analysis
Abstract
1. Introduction
2. Materials and Methods
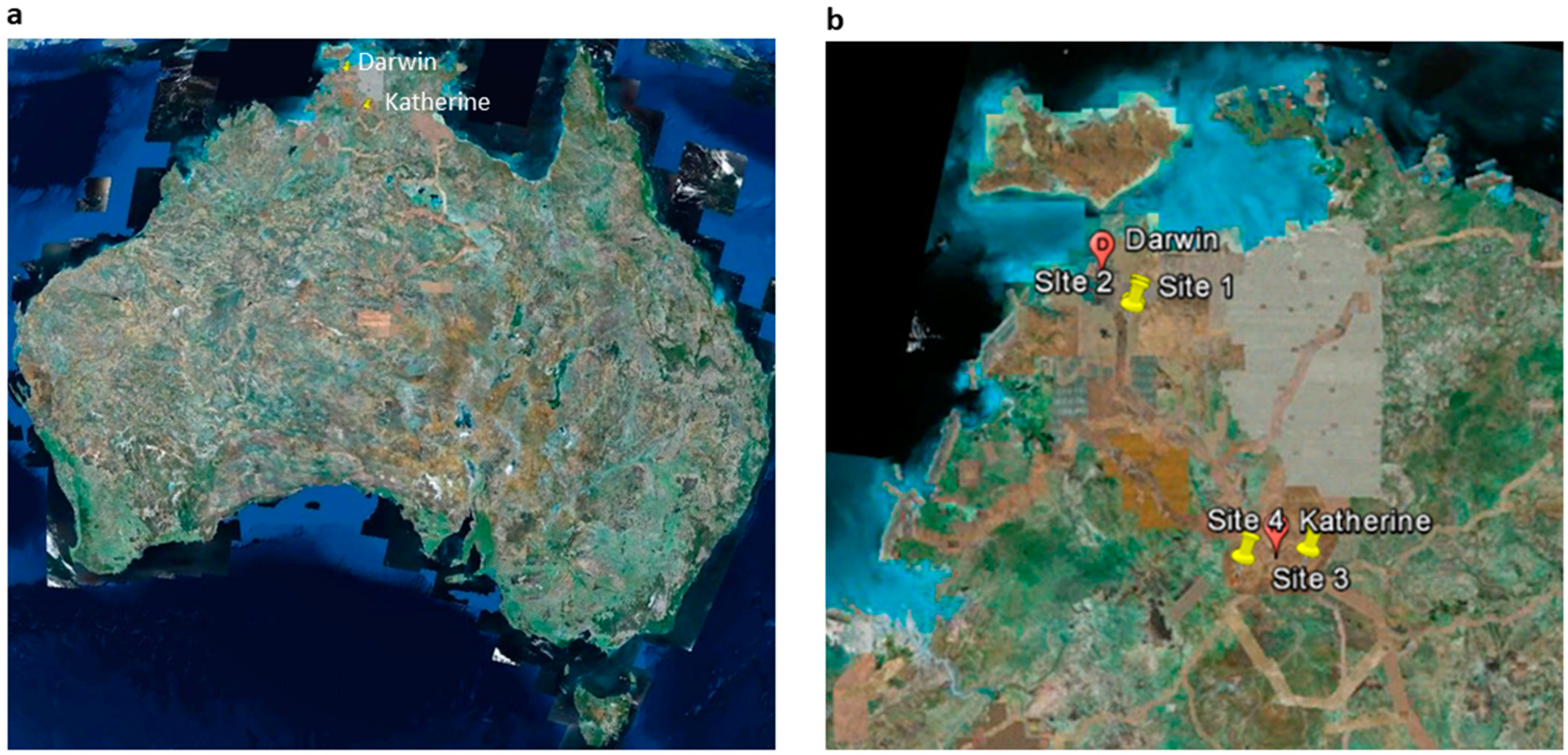
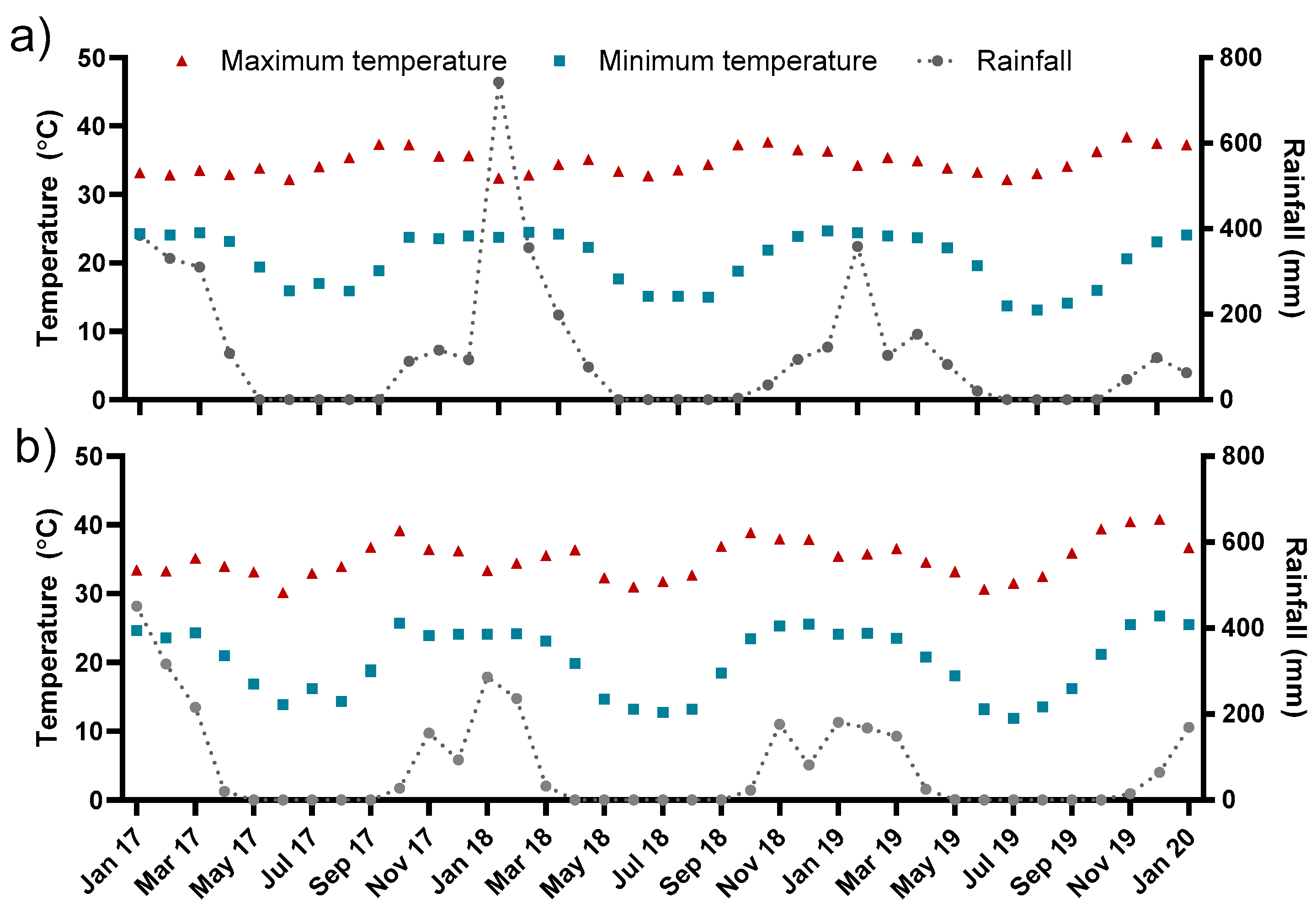
2.1. Experimental Sites
2.2. Litter and Pruning Biomass Collection
2.3. Sample Processing and Nutrient Analysis
2.4. Statistical Analysis
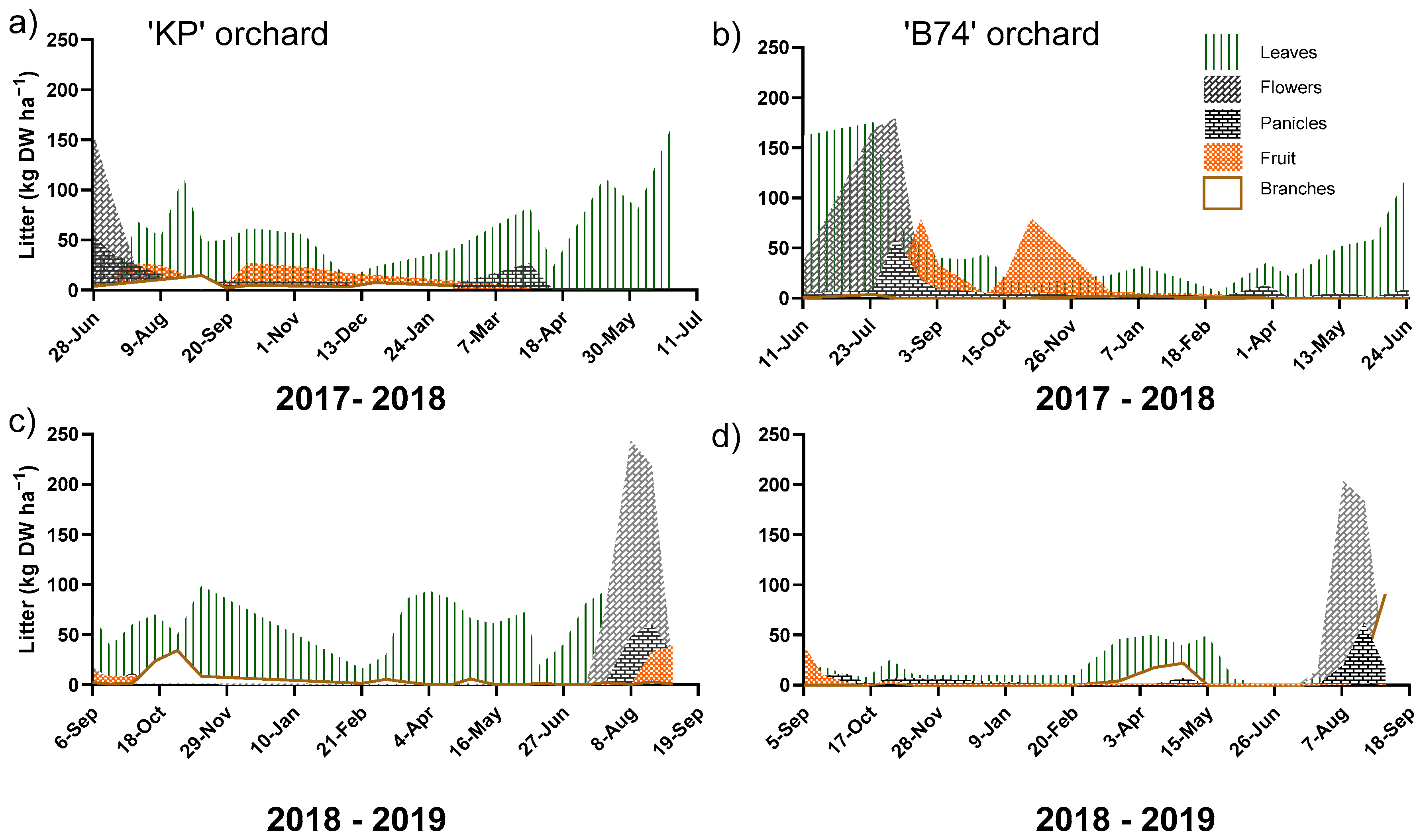
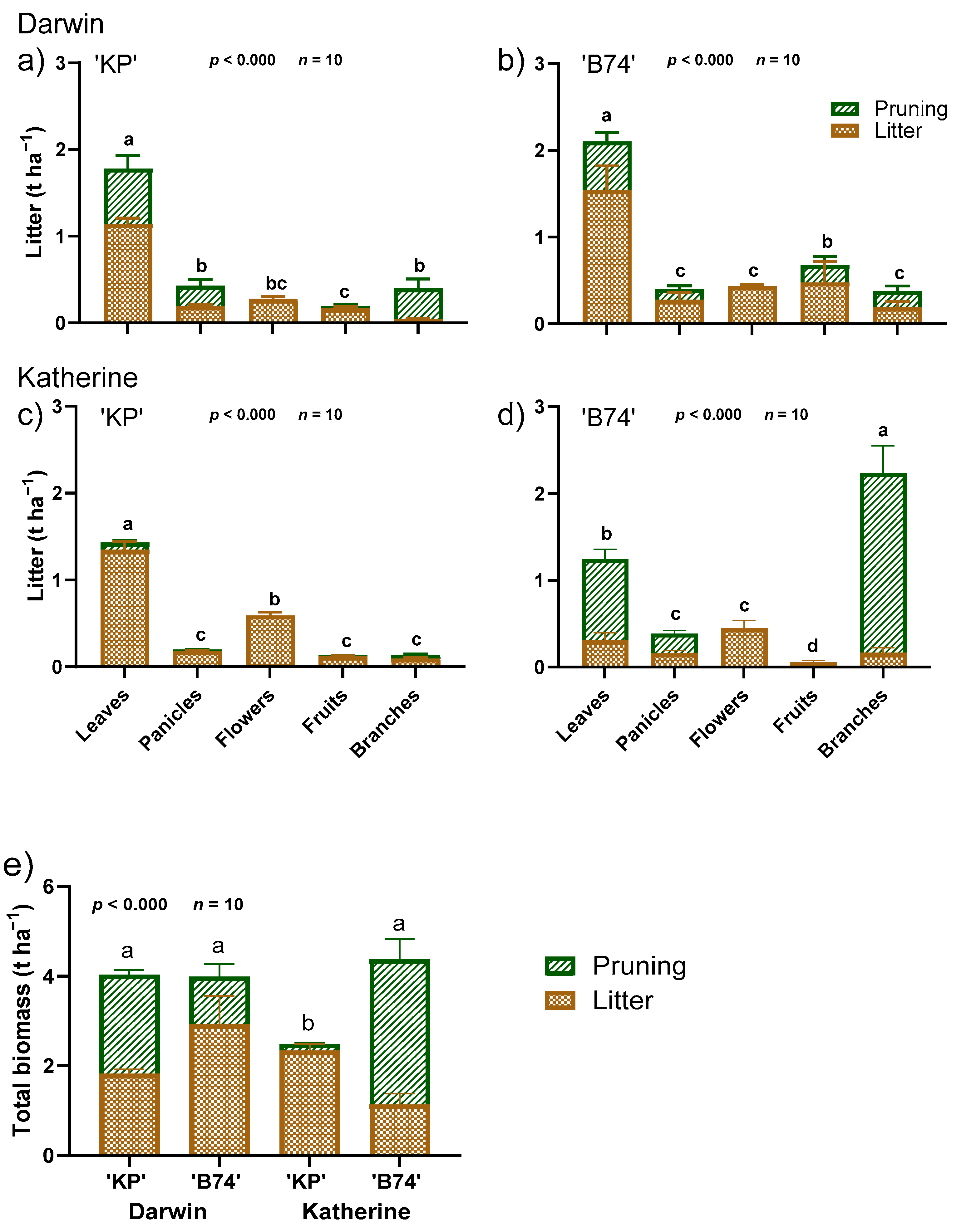
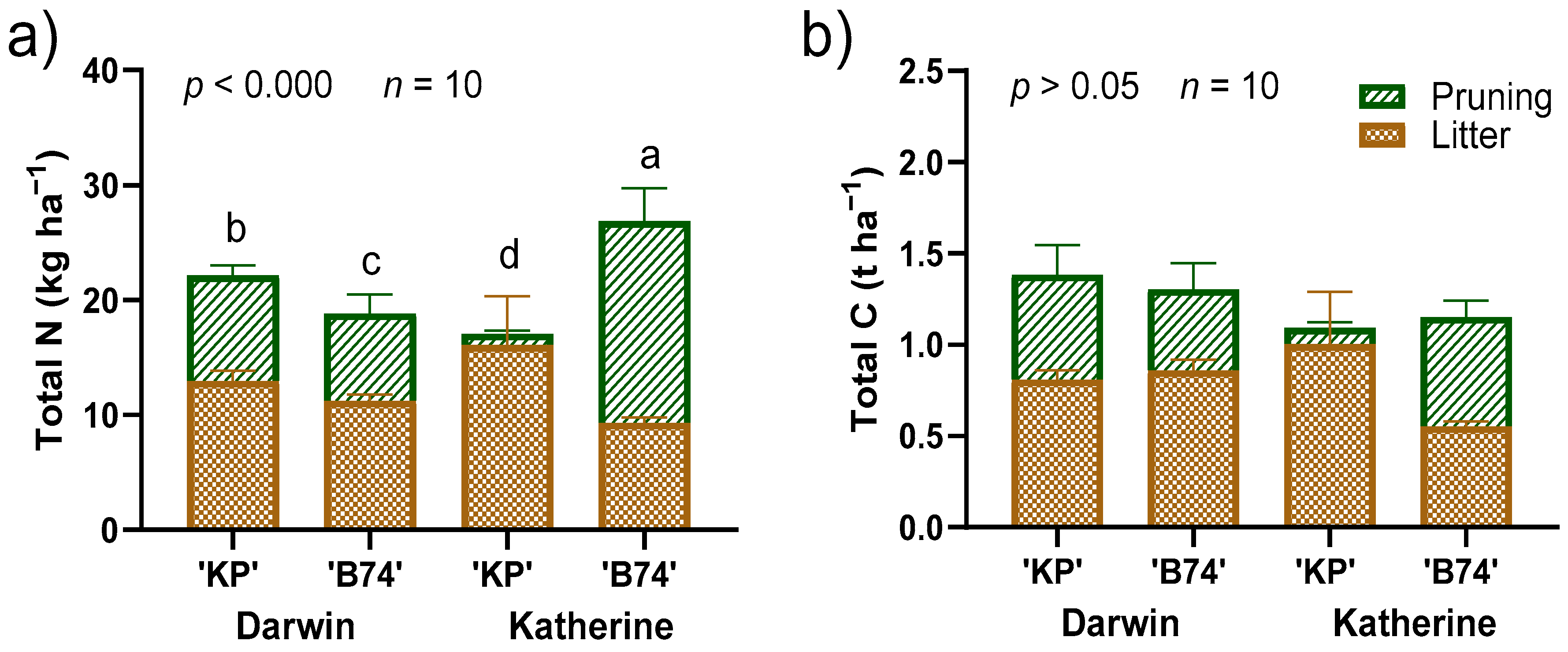
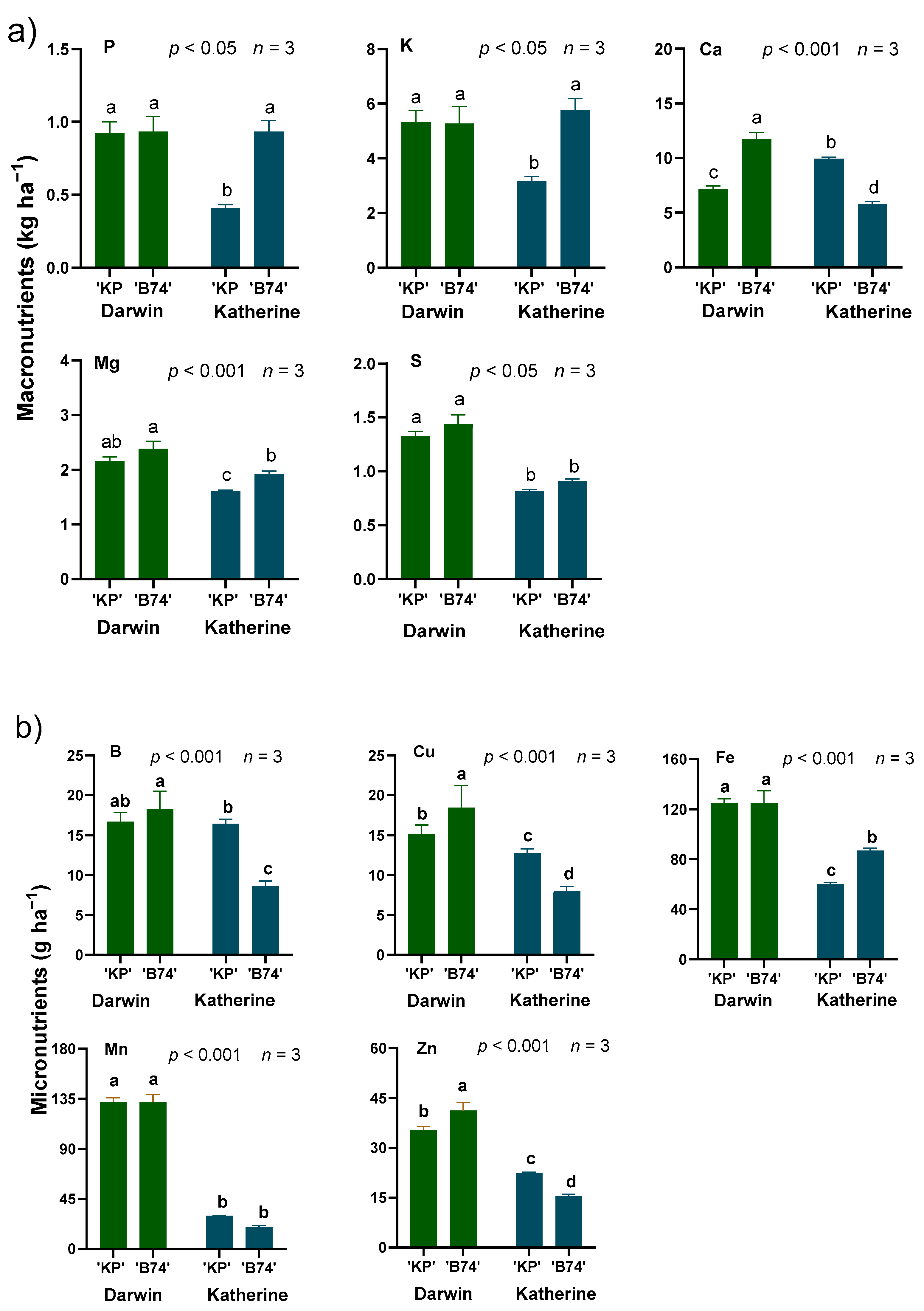
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| AUD | Australian Dollar |
| BBCH | Biologische Bundesanstalt, Bundessortenamt und CHemical Industry |
| BOM | Bureau of Meteorology |
| ICP-MS | Inductively Coupled Plasma–Mass Spectroscopy |
| IRRI | International Rice Research Institute |
| NUE | Nitrogen-Use Efficiency |
| RAAF | Royal Australian Air Force |
| STAR | Statistical Tool for Agricultural Research |
References
- Amruddin, A.; Mahmood, M.R.; Supardjo, D.; Safruddin, S.; Susilatun, H.R. Analysis of climate change impacts on agricultural production and adaptation strategies for farmers: Agricultural policy perspectives. Glob. Int. J. Innov. Res. 2024, 2, 374–383. [Google Scholar] [CrossRef]
- Pratap, D.; Tamuly, G.; Ganavi, N.R.; Anbarasan, S.; Pandey, A.K.; Singh, A.; Priya, P.; Debnath, A.; Iberaheem, M. Climate change and global agriculture: Addressing challenges and adaptation strategies. J. Exp. Agric. Int. 2024, 46, 799. [Google Scholar] [CrossRef]
- Webb, N.P.; Marshall, N.; Stringer, L.C.; Reed, M.S.; Chappell, A.; Herrick, J. Land degradation and climate change: Building climate resilience in agriculture. Front. Ecol. Environ. 2017, 15, 450–459. [Google Scholar] [CrossRef]
- Saikanth, D.R.K.; Kumar, S.; Rani, M.; Sharma, A.; Srivastava, S.; Vyas, D.; Singh, G.A.; Kumar, S. A Comprehensive review on climate change adaptation strategies and challenges in agriculture. Int. J. Environ. Clim. Change 2023, 13, 10–19. [Google Scholar] [CrossRef]
- Çakmakç, R.; Salık, M.A.; Çakmakç, S. Assessment and principles of environmentally sustainable food and agriculture systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, W.; Fernie, A.R.; Wen, W. Combining novel technologies with interdisciplinary basic research to enhance horticultural crops. Plant J. 2022, 109, 35–46. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Lakra, J.; Sharma, U. Appraisal of carbon capture, storage and utilization through fruit crops. Front. Environ. Sci. 2021, 9, 258. [Google Scholar] [CrossRef]
- Bally, I.S.E.; Dillon, N.L. Mango (Mangifera indica L.) breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2018; Volume 3, pp. 811–896. [Google Scholar] [CrossRef]
- Singh, S.P.; Nandi, A.K. A financial viability and relative profitability of mango orcharding in Lucknow District of Uttar Pradesh. Econ. Affairs 2020, 65, 77–83. [Google Scholar] [CrossRef]
- Sarker, F.I.M.G.W.; Biswas, J.C.; Maniruzzaman, M. Climate change adaptation and economic profitability: Crop land shifting to mango orchard in Rajshahi region. Bangladesh Rice J. 2014, 18, 8–17. [Google Scholar] [CrossRef]
- Arnoldus, M.; Clausen, B. Production Cost and Profitability of Mango Farming in Ghana: A Benchmark Study Between 4 Countries; Sense GIZ: Cape Town, South Africa, 2019; pp. 1–51. [Google Scholar]
- Ayala-Garay, A.V.; Almaguer-Vargas, G.; De la Trinidad-Pérez, N.K.; Caamal-Cauich, I.; Rendón, R. Competitividad de la producción de mango (Mangifera indica L.) en Michoacán. Rev. Chapingo Serie Hortic. 2009, 15, 133–140. [Google Scholar] [CrossRef]
- Blare, T.; Ballen, F.H.; Singh, A.; Haley, N.; Crane, J.H. Profitability and cost estimates for producing Mango (Mangifera indica L.) in south Florida. EDIS 2022, 2022. [Google Scholar] [CrossRef]
- Testa, R.; Tudisca, S.; Schifani, G.; Di Trapani, A.M.; Migliore, G. Tropical fruits as an opportunity for sustainable development in rural areas: The case of mango in small-sized Sicilian farms. Sustainability 2018, 10, 1436. [Google Scholar] [CrossRef]
- Bennett, D.M.; Dickinson, G.R. Economic Case Study of Intensive Mango Systems: A Comparison of the Profitability of Conventional (Low, Medium and High Density) and Trellis (High Density) Mango Canopy Systems in North Queensland Based on Early Trial Results; CRCNA: Queensland, Autralia, 2021; pp. 1–28. [Google Scholar]
- Kiloes, A.M.; Joyce, D.C.; Abdul Aziz, A. Exploring the challenges and opportunities of mango export from Indonesia: Insights from stakeholder interviews. Qual. Rep. 2024, 29, 811–830. [Google Scholar] [CrossRef]
- Ronie, M.E.; Aziz, A.H.A.; Kobun, R.; Pindi, W.; Roslan, J.; Putra, N.R.; Mamat, H. Unveiling the potential applications of plant by-products in food—A review. Waste Manag. Bull. 2024, 2, 183–203. [Google Scholar] [CrossRef]
- Johnson, G.I. Introduction of the mango to Australia. Proc. R. Soc. Qld. 2000, 109, 83–90. [Google Scholar]
- Hort Innovation. Australian Horticulture Statistics Handbook; Hort Innovation: Sydney, NSW, Australia, 2024; pp. 100–103. Available online: https://www.horticulture.com.au/contentassets/a36fdfa2427d4ad284c426663b06f15c/hort-innovation-ahsh-2023-24-fruit-r2.pdf (accessed on 7 May 2025).
- NT Mangoes, Northern Territory, Australia. Available online: https://ntfarmers.org.au/commodities/mangoes/ (accessed on 28 June 2024).
- Pandeya, H.R.; Friedl, J.; De Rosa, D.; Asis, C.T.; Tilbrook, J.; Scheer, C.; Bristow, M.; Grace, P.R.; Rowlings, D.W. Combined effect of nitrogen fertiliser and leaf litter carbon drive nitrous oxide emissions in tropical soils. Nutr. Cycl. Agroecosyst. 2020, 118, 207–222. [Google Scholar] [CrossRef]
- Smith, S.; Hill, J. Supporting Sustainable Development–Risks and Impacts of Plant Industries on Soil Condition, Technology Bulletin 340; Northern Territory Government: Darwin, NT, Australia, 2011; pp. 1–27. Available online: https://industry.nt.gov.au/__data/assets/pdf_file/0005/233258/tb340.pdf (accessed on 20 January 2025).
- Derpsch, R.; Kassam, A.; Reicosky, D.; Friedrich, T.; Calegari, A.; Basch, G.; Gonzalez-Sanchez, E.; dos Santos, D.R. Nature’s laws of declining soil productivity and Conservation Agriculture. Soil Secur. 2024, 14, 100127. [Google Scholar] [CrossRef]
- Schmidt, M.; Corre, M.D.; Kim, B.; Morley, J.; Göbel, L.; Sharma, A.S.I.; Setriuc, S.; Veldkamp, E. Nutrient saturation of crop monocultures and agroforestry indicated by nutrient response efficiency. Nutr. Cycl. Agroecosyst. 2021, 119, 69–82. [Google Scholar] [CrossRef]
- Mosier, S.; Córdova, S.C.; Robertson, G.P. Restoring soil fertility on degraded lands to meet food, fuel, and climate security needs via perennialization. Front. Sustain. Food Syst. 2021, 5, 706142. [Google Scholar] [CrossRef]
- Khalsa, S.D.S.; Smart, D.R.; Muhammad, S.; Armstrong, C.M.; Sanden, B.L.; Houlton, B.Z.; Brown, P.H. Intensive fertilizer use increases orchard N cycling and lowers net global warming potential. Sci. Total Environ. 2020, 722, 137889. [Google Scholar] [CrossRef]
- Chave, J.; Navarrete, D.; Almeida, S.; Álvarez, E.; Aragão, L.E.O.C.; Bonal, D.; Châtelet, P.; Silva-Espejo, J.E.; Goret, J.Y.; von Hildebrand, P.; et al. Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 2010, 7, 43–55. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.; Wang, H.; He, Y. Research progress on the relationship between leaf senescence and quality, yield and stress resistance in horticultural plants. Front. Plant Sci. 2022, 13, 1044500. [Google Scholar] [CrossRef] [PubMed]
- Estiarte, M.; Campioli, M.; Mayol, M.; Penuelas, J. Variability and limits of nitrogen and phosphorus resorption during foliar senescence. Plant Commun. 2023, 4, 100503. [Google Scholar] [CrossRef]
- Asis, C.A.; Niscioli, A. Impact of twig-tip dieback on leaf nutrient status and resorption efficiency of mango (Mangifera indica L.) Trees. Horticulturae 2024, 10, 678. [Google Scholar] [CrossRef]
- Mitra, S. Mango cultivation practices in the tropics: Good agricultural practices to maximize sustainable yields. In Achieving Sustainable Cultivation of Mangoes; Sauco, V.G., Lu, P., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 149–163. [Google Scholar]
- Sayer, E.J.; Tanner, E.V. Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J. Ecol. 2010, 98, 1052–1062. [Google Scholar] [CrossRef]
- Google EarthTM. Available online: https://earth.google.com/web/ (accessed on 25 April 2025).
- Ahammad, R.; Sangha, K. A Literature Review of the Horticulture Sector in Northern Australia; The Cooperative Research Centre for Developing Northern Australia: Darwin, Northern Territory, Australia, 2022; pp. 1–29. [Google Scholar]
- Asis, C.A.; Tilbrook, J.; Anson, D.; Niscioli, A.; Bristow, M.; Friedl, J.; Rowlings, D. Estimating nitrogen uptake efficiency of mango varieties from foliar KNO3 application using a 15N tracer technique. Nitrogen 2024, 5, 1124–1134. [Google Scholar] [CrossRef]
- Isbell, R.F. ; National Committee on Soil and Terrain. The Australian Soil Classification, 3rd ed.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 67–73. [Google Scholar]
- Climate Data Online, Bureau of Meteorology, Australia. Available online: http://www.bom.gov.au/climate/data/index.shtml (accessed on 3 January 2025).
- Junaedi, A.; Hardiwinoto, S.; Supriyo, H.; Mindawati, N. Litter productivity and leaf litter nutrient return of three native tree species in drained tropical peatland, Riau-Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 533, 012007. [Google Scholar] [CrossRef]
- Morffi-Mestre, H.; Ángeles-Pérez, G.; Powers, J.S.; Andrade, J.L.; Huechacona Ruiz, A.H.; May-Pat, F.; Chi-May, F.; Dupuy, J.M. Multiple factors influence seasonal and interannual litterfall production in a tropical dry forest in Mexico. Forests 2020, 11, 1241. [Google Scholar] [CrossRef]
- Yao, M.K.; Koné, A.W.; Otinga, A.N.; Kassin, E.K.; Tano, Y. Carbon and nutrient cycling in tree plantations vs. natural forests: Implication for an efficient cocoa agroforestry system in West Africa. Reg. Environ. Change 2021, 21, 44. [Google Scholar] [CrossRef]
- Finotti, R.; Freitas, S.R.; Cerqueira, R.; Vieira, M.V. A method to determine the minimum number of litter traps in litterfall studies. Biotropica 2003, 35, 419–421. [Google Scholar] [CrossRef]
- Scott: Automation That Transforms. Available online: https://scottautomation.com/en/ (accessed on 23 November 2024).
- Berauer, B.J.; Wilfahrt, P.A.; Schuchardt, M.A.; Schlingmann, M.; Schucknecht, A.; Jentsch, A. High land-use intensity diminishes stability of forage provision of mountain pastures under future climate variability. Agronomy 2021, 11, 910. [Google Scholar] [CrossRef]
- Hernández-Delgado, P.M.; Aranguren, M.; Reig, C.; Fernández-Galván, D.; Mesejo, C.; Martínez-Fuentes, A.; Galán-Saúco, V.; Agustí, M. Phenological growth stages of mango (Mangifera indica L.) according to the BBCH scale. Sci. Hortic. 2011, 130, 536–540. [Google Scholar] [CrossRef]
- LECO Corporation 928 Series: Macro Determination Through Combustion. Available online: https://www.leco.com/products/928-series/ (accessed on 23 January 2025).
- Agilent: Gas Chromatography/Mass Spectrometry (GC/MS). Available online: https://www.agilent.com/en/product/gas-chromatography-mass-spectrometry-gc-ms (accessed on 23 January 2025).
- Htwe, N.M.; Phyu, S.L.; Thu, C.N. Assessment of genetic variability and character association of Myanmar local rice (Oryza sativa L.) germplasm. J. Exp. Agric. Int. 2019, 40, 1–10. [Google Scholar] [CrossRef]
- GraphPad: Comprehensive Analysis and Powerful Statistics, Simplified. Available online: https://www.graphpad.com/scientific-software/prism/ (accessed on 23 November 2024).
- Savaci, G.; Sariyildiz, T. Effects of stand age on litter quality, decomposition rate and nutrient release of Kazdagi fir (Abies nordmanniana subsp. equi-trojani). iForest Biogeosci. For. 2020, 13, 396. [Google Scholar] [CrossRef]
- Morffi-Mestre, H.; Ángeles-Pérez, G.; Powers, J.S.; Andrade, J.L.; Feldman, R.E.; May-Pat, F.; Chi-May, F.; Dupuy-Rada, J. Leaf litter decomposition rates: Influence of successional age, topography and microenvironment on six dominant tree species in a tropical dry forest. Front. For. Glob. Change 2023, 6, 1082233. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Miranda, I.S.; de Sousa, A.M.L. Can mango orchards rehabilitate degraded areas by nutrient cycling? J. Environ. Manag. 2019, 231, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Navnage, N.P.; Pharande, A.L.; Kadlag, A.D.; Bainade, S.P. Litter dynamics of mango trees grown in different shrink-swell soil series of Maharashtra. Intl. J. Adv. Chem. Res. 2021, 3, 38–42. [Google Scholar] [CrossRef]
- Murovhi, N.R.; Materechera, S.A.; Mulugeta, S.D. Seasonal changes in litter fall and its quality from three sub-tropical fruit tree species at Nelspruit, South Africa. Agrofor. Syst. 2012, 86, 61–71. [Google Scholar] [CrossRef]
- Pereira, D.G.C.; Portugal, A.F.; Giustolin, T.A.; Maia, V.M.; Megda, M.X.V.; Kondo, M.K. Litter decomposition and nutrient release in different land use systems in the Brazilian semi-arid region. Catena 2023, 231, 107345. [Google Scholar] [CrossRef]
- Patrício, M.D.S.; Nunes, L.F.; Pereira, E.L. Litterfall and litter decomposition in chestnut high forest stands in northern Portugal. For. Syst. 2012, 21, 259–271. [Google Scholar] [CrossRef]
- Wu, Z.D.; Wang, Y.X.; Cai, Z.J.; You, Z.M.; Zhang, W.J.; Weng, B.Q. Amount and decomposition characteristics of litters in citrus orchard in Fuzhou, China. J. Ecol. Rural Environ. 2010, 26, 231–234. [Google Scholar]
- Pérez Méndez, V.; Herrero Romero, M.; Hormaza Urroz, J.I. Different factors involved in the low fruit set of mango (Mangifera indica). Acta Hortic. 2019, 1231, 43–48. [Google Scholar] [CrossRef]
- Tilbrook, J.; Niscioli, A.; Rowlings, D.; Asis, C. Optimising Nutrient Management for Improved Productivity and Fruit Quality in Mango (RRDP1720), Final Report Submitted to the Cotton Research and Development Corporation—More Profit from Nitrogen Program; CRDC: Narrabri, NSW, Australia, 2021. [Google Scholar]
- Musvoto, C.; Campbell, B.M.; Kirchmann, H. Decomposition and nutrient release from mango and miombo woodland litter in Zimbabwe. Soil Biol. Biochem. 2000, 32, 1111–1119. [Google Scholar] [CrossRef]
- de Almeida, C.X.; Pita Junior, J.L.; Rozane, D.E.; de Souza, H.A.; Hernandes, A.; Natale, W.; Farraudo, A. Nutrient cycling in mango trees. Ciênc. Agrár. 2014, 35, 259–266. [Google Scholar] [CrossRef]
- Naik, S.K.; Maurya, S.; Mukherjee, D.; Singh, A.K.; Bhatt, B.P. Rates of decomposition and nutrient mineralization of leaf litter from different orchards under hot and dry sub-humid climate. Arch. Agron. Soil Sci. 2018, 64, 560–573. [Google Scholar] [CrossRef]
- García-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Parsons, S.A.; Congdon, R.A.; Lawler, I.R. Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol. 2014, 203, 873–882. [Google Scholar] [CrossRef]
- Peguero, G.; Sardans, J.; Asensio, D.; Fernández-Martínez, M.; Gargallo-Garriga, A.; Grau, O.; Llusià, J.; Margalef, O.; Márquez, L.; Ogaya, R.; et al. Nutrient scarcity strengthens soil fauna control over leaf litter decomposition in tropical rainforests. Proc. R. Soc. B 2019, 286, 20191300. [Google Scholar] [CrossRef]
- Anaya, C.A.; Jaramillo, V.J.; Martínez-Yrízar, A.; García-Oliva, F. Large rainfall pulses control litter decomposition in a tropical dry forest: Evidence from an 8-year study. Ecosystems 2012, 15, 652–663. [Google Scholar] [CrossRef]
- Vickery, B.S. The Limit to Soil Organic Carbon Sequestration in Tropical Soils. Bachelor’s Thesis, Queensland University of Technology, Brisbane, Queensland, Australia, 2019. [Google Scholar]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Sione, S.M.J.; Ledesma, S.; Aceñolaza, P.G.; Wilson, M.G. Biomass Carbon and Nitrogen allocation in different tree species: Do tree compartments and size affect C: N relationship? Silva Finnica 2022, 56, 10755. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef]
- Gentile, R.M.; Boldingh, H.L.; Campbell, R.E.; Gee, M.; Gould, N.; Lo, P.; McNally, S.; Park, K.C.; Richardson, A.C.; Stringer, L.D.; et al. System nutrient dynamics in orchards: A research roadmap for nutrient management in apple and kiwifruit. A review. Agron. Sustain. Dev. 2022, 42, 64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niscioli, A.; Asis, C.A.; Tilbrook, J.; Anson, D.; Guinto, D.; Bristow, M.; Rowlings, D. Litter and Pruning Biomass in Mango Orchards: Quantification and Nutrient Analysis. Sustainability 2025, 17, 4452. https://doi.org/10.3390/su17104452
Niscioli A, Asis CA, Tilbrook J, Anson D, Guinto D, Bristow M, Rowlings D. Litter and Pruning Biomass in Mango Orchards: Quantification and Nutrient Analysis. Sustainability. 2025; 17(10):4452. https://doi.org/10.3390/su17104452
Chicago/Turabian StyleNiscioli, Alan, Constancio A. Asis, Joanne Tilbrook, Dallas Anson, Danilo Guinto, Mila Bristow, and David Rowlings. 2025. "Litter and Pruning Biomass in Mango Orchards: Quantification and Nutrient Analysis" Sustainability 17, no. 10: 4452. https://doi.org/10.3390/su17104452
APA StyleNiscioli, A., Asis, C. A., Tilbrook, J., Anson, D., Guinto, D., Bristow, M., & Rowlings, D. (2025). Litter and Pruning Biomass in Mango Orchards: Quantification and Nutrient Analysis. Sustainability, 17(10), 4452. https://doi.org/10.3390/su17104452






