A Review of Nature-Based Solutions for Valorizing Aromatic Plants’ Lignocellulosic Waste Through Oyster Mushroom Cultivation
Abstract
:1. Introduction
2. Methodology
3. Overview of Aromatic Plant Industries
3.1. Kinds of Products, Market Trends, and Legislation
3.2. Lignocellulosic Waste Management
4. Oyster Mushroom Potentiality in Biodegradation of Organic Waste: A Nature-Based Solution
5. Critical Aspects of Oyster Mushroom Cultivation on Lignocellulosic Aromatic Plants’ Waste
5.1. Isolation
5.2. Substrate Preparation, Inoculation, and Incubation
5.2.1. Medicinal Plant-Based Substrates: Pre-Treatment Protocols
5.2.2. Inoculation and Incubation
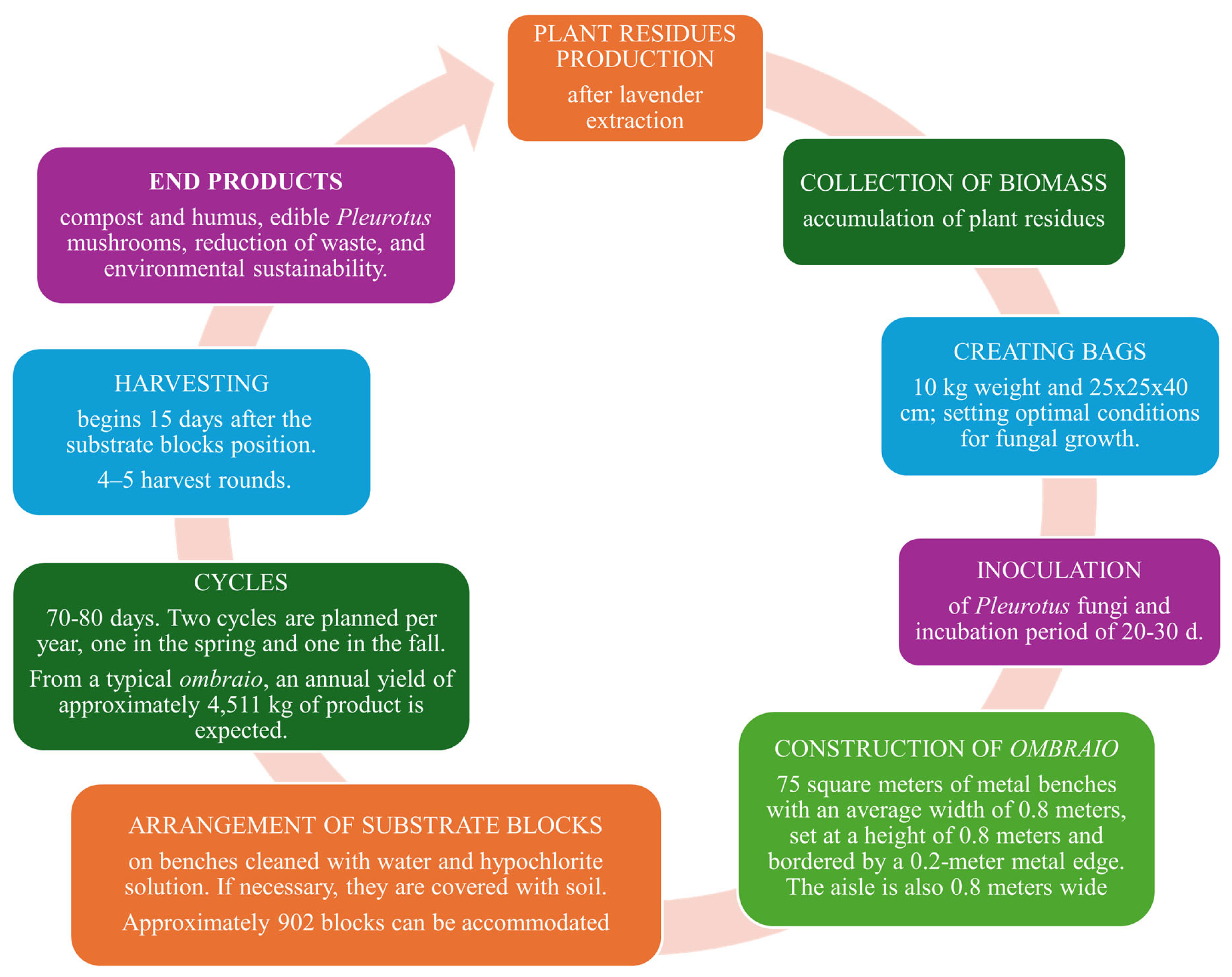
5.3. Cultivation Systems
6. Oyster Mushroom vs. Aromatic Plant Lignocellulosic Waste: Feasibility and Economic Advantages
Feasibility and Economic Benefits of the Process
7. Oyster Mushrooms with High Nutritional Value
8. Composition and Potential Applications of Spent Substrates
9. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Marcelino, S.; Gaspar, P.D.; Paço, A. Sustainable Waste Management in the Production of Medicinal and Aromatic Plants—A Systematic Review. Sustainability 2023, 15, 13333. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Product. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Towards the Circular Economy: Economic and Business Rationale for an Accelerated Transition. 2013. Available online: https://www.greenpolicyplatform.org/research/towards-circular-economy-economic-and-business-rationale-accelerated-transition (accessed on 15 January 2025).
- Moustakas, K.; Loizidou, M.; Malamis, D. A review on the current status and future challenges of sustainable management of agricultural biomass waste. Renew. Sustain. Energy Rev. 2020, 119, 109558. [Google Scholar]
- Sánchez, C. Cultivation of Pleurotus spp. on agro-industrial residues: A review. Bioresour. Technol. 2010, 101, 695–696. [Google Scholar]
- Levin, L.; Herrmann, C.; Papinutti, V. Optimization of lignocellulolytic enzyme production by Pleurotus species. Int. Biodeterior. Biodegrad. 2008, 62, 439–446. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Saha, A.; Basak, B.B. Scope of Value Addition and Utilization of Residual Biomass from Medicinal and Aromatic Plants. Ind. Crop. Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Semenova, E.; Kurakov, A.V.; Nazarov, V.; Presnyakova, V.; Markelova, N.; Karaseva, E.; Kurdyukov, E.E.; Tsokalo, I.; Minkina, T.; Rajput, V.D. Biotransformation of Wastes of Essential Oil Industry by Strains Agaricus bisporus (J.E. Lange) Imbach, Lentinula edodes (Berk.) Pegler, and Pleurotus ostreatus (Jacq.) P. Kumm. Horticulturae 2023, 9, 450. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of Medicinal and Aromatic Plants for Specialty Industrial Materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Stefanova, P.; Blazheva, D.; Vladev, V.; Petkova, N.; Slavov, A.; Denev, P.; Karashanova, D.; Zaharieva, R.; et al. Waste Rose Flower and Lavender Straw Biomass—An Innovative Lignocellulose Feedstock for Mycelium Bio-Materials Development Using Newly Isolated Ganoderma resinaceum Ga1m. J. Fungi 2021, 7, 866. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Benvenuti, M.; Di Piazza, S.; Salis, A.; Cecchi, G.; Zotti, M.; Scarfì, S.; Damonte, G. A Novel Method for the Extraction and Characterization of Metabolites from Basidiomycota: Pleurotus Ostreatus (Jacq.) P. Kumm., 1871 as a Case Study. Sep. Sci. Plus 2023, 6, 2300116. [Google Scholar] [CrossRef]
- Regulation (EU) No 1307/2013 of the European Parliament and of the Council of 17 December 2013 Establishing Rules for Direct Payments to Farmers Under Support Schemes Within the Framework of the Common Agricultural Policy and Repealing Council Regulation. 2024, pp. 1–91. Available online: https://eur-lex.europa.eu/eli/reg/2013/1307/oj/eng (accessed on 15 January 2025).
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. 2024, pp. 1–24. Available online: https://eur-lex.europa.eu/eli/reg/2002/178/oj/eng (accessed on 15 January 2025).
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides (Text with EEA Relevance) This Document Has Been Published in a Special Edition. 2024, pp. 1–25. Available online: https://eur-lex.europa.eu/eli/dir/2009/128/oj/eng (accessed on 15 January 2025).
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products Text with EEA Relevance This Document Has Been Published in a Special Edition (s). 2024, pp. 1–186. Available online: https://eur-lex.europa.eu/eli/reg/2012/528/oj/eng (accessed on 15 January 2025).
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration This Document Has Been Published in a Special Edition (s) (BG, RO, HR) in Force: This. 2024, pp. 1–18. Available online: https://eur-lex.europa.eu/eli/dir/2006/118/oj/eng (accessed on 15 January 2025).
- Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species in Force: This Act Has Been Changed. Current Consolidated Version. 2024, Volume 192, pp. 1–30. Available online: https://eur-lex.europa.eu/eli/reg/2014/1143/oj/eng (accessed on 15 January 2025).
- DECRETO LEGISLATIVO 21 Maggio 2018. n. 75. 2024, pp. 1–9. Available online: https://www.gazzettaufficiale.it/eli/id/2018/06/06/18G00086/SG (accessed on 15 January 2025).
- Giuliani, C.; Maleci Bini, L.; Flamini, G. Essential oil composition and morphological investigation of a population of Lavandula angustifolia cultivated in Tuscany (Italy). Nat. Prod. Commun. 2014, 9, 391–394. [Google Scholar]
- Verma, R.S.; Rahman, L.; Verma, R.K. Aromatic plants: Traditional knowledge and future perspectives. J. Essential Oil Res. 2020, 32, 97–109. [Google Scholar]
- Baratta, M.T.; Dorman, H.J.D.; Deans, S.G.; Figueiredo, A.C.; Barroso, J.G.; Ruberto, G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998, 13, 235–244. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Composition of the essential oil of Rosmarinus officinalis L. from Italy and comparison with other countries. J. Essential Oil Res. 2007, 19, 526–531. [Google Scholar]
- European Commission. Rural Development in the EU: Statistical and Economic Information Report; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- ISMEA (Istituto di Servizi per il Mercato Agricolo Alimentare). Rapporto Sull’agricoltura Biologica in Italia; ISMEA: Rome, Italy, 2022. [Google Scholar]
- Euromonitor International. Essential Oils in Western Europe—Market Report; Euromonitor International: London, UK, 2021. [Google Scholar]
- ICE Agenzia (Italian Trade Agency). Il Mercato Degli Oli Essenziali Nel Mondo; ICE Agenzia: Rome, Italy, 2020. [Google Scholar]
- Yarin, T.; Banga Krishi Vishwavidyalaya, U.; Behar, C.; Bengal, W.; Babli Dutta, I.; Kumar Murmu, D.; Shrilekha Das, I. Valorization of Medicinal and Aromatic Plants Waste: Review Article. Pharma Innov. 2022, 11, 532–537. [Google Scholar]
- Silva, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach. Foods 2024, 13, 1464. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Universal Recovery Strategy. In Food Waste Recovery Processing Technologies and Industrial Techniques; Academic Press: Cambridge, MA, USA, 2015; Chapter 3; pp. 59–81. [Google Scholar] [CrossRef]
- Olofsson, J.; Börjesson, P. Residual Biomass as Resource—Life-Cycle Environmental Impact of Wastes in Circular Resource Systems. J. Clean. Prod. 2018, 196, 997–1006. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Rajasekharan, R. PROCESS FOR CHEMICAL CONVERSION U.S. Cl. OF CELLULOSE SOLATED FROM CPC C07D 307/46 (2013.01) AROMATIC SPENT BOMASS TO HYDROXY USPC 549/479 METHYL FURFURAL. U.S. Patent 2014/0350271 A1, 27 November 2014. [Google Scholar]
- Wang, Q.; Rehman, M.; Peng, D.; Liu, L. Antioxidant Capacity and α-Glucosidase Inhibitory Activity of Leaf Extracts from Ten Ramie Cultivars. Ind. Crop. Prod. 2018, 122, 430–437. [Google Scholar] [CrossRef]
- Gómez, L.D.; Amalfitano, C.; Andolfi, A.; Simister, R.; Somma, S.; Ercolano, M.R.; Borrelli, C.; McQueen-Mason, S.J.; Frusciante, L.; Cuciniello, A.; et al. Valorising Faba Bean Residual Biomass: Effect of Farming System and Planting Time on the Potential for Biofuel Production. Biomass Bioenergy 2017, 107, 227–232. [Google Scholar] [CrossRef]
- Mahato, N.; Agarwal, P.; Mohapatra, D.; Sinha, M.; Dhyani, A.; Pathak, B.; Tripathi, M.K.; Angaiah, S. Processes Biotransformation of Citrus Waste-II: Bio-Sorbent Materials for Removal of Dyes, Heavy Metals and Toxic Chemicals from Polluted Water. Processes 2021, 9, 1544. [Google Scholar] [CrossRef]
- Riaz, B.; Ansari, T.M.; Hanif, M.A.; Riaz, S.; Khan, M.A.; Riaz, M.; Jilani, M.I. Utilization of Extensively Available Environmental Waste Mentha Spicata for Uptake of Pb(II) from Aqueous Solutions. Asian J. Chem. 2013, 25, 4551–4555. [Google Scholar] [CrossRef]
- Zein, R.; Satrio Purnomo, J.; Ramadhani, P.; Safni; Alif, M.F.; Putri, C.N. Enhancing Sorption Capacity of Methylene Blue Dye Using Solid Waste of Lemongrass Biosorbent by Modification Method. Arab. J. Chem. 2023, 16, 104480. [Google Scholar] [CrossRef]
- Deshmukh, Y.; Yadav, V.; Nigam, N.; Yadav, A.; Khare, P. Quality of Bio-Oil by Pyrolysis of Distilled Spent of Cymbopogon flexuosus. J. Anal. Appl. Pyrolysis 2015, 115, 43–50. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential Oils and Distilled Straws of Lavender and Lavandin: A Review of Current Use and Potential Application in White Biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Sarkar, S.; Skalicky, M.; Hossain, A.; Brestic, M.; Saha, S.; Garai, S.; Ray, K.; Brahmachari, K.; Krishi Vigyan Kendra, N.; Chandra Krishi Viswavidyalaya, B.; et al. Sustainability Management of Crop Residues for Improving Input Use Efficiency and Agricultural Sustainability. Sustainability 2010, 12, 9808. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Stewart, N.; Joyce, B.; Baxter, H.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E.A.; Poovaiah, C.R. Dual Utilization of Medicinal and Aromatic Crops as Bioenergy Feedstocks. J. Agric. Food Chem. 2018, 66, 8744–8752. [Google Scholar] [CrossRef]
- Okuda, Y. Sustainability Perspectives for Future Continuity of Mushroom Production: The Bright and Dark Sides. Front. Sustain. Food Syst. 2022, 6, 1026508. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Arzú, R.F.; Yu, F.Q. Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 9783030373788. [Google Scholar]
- K, O.J.; Adegoke Adebayo, E. Oyster Mushroom (Pleurotus Species); A Natural Functional Food. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 254. [Google Scholar] [CrossRef]
- Lim, M.; Shu, Y. The Future is Fungi: How Fungi Can Feed Us, Heal Us, Free Us and Save Our World; Thames & Hudson Australia: Cremorne, Australia, 2022. [Google Scholar]
- Gregori, A.; Svagelj, M.; Pohleven, J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol. Biotechnol. 2007, 45, 238–249. [Google Scholar]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of oyster mushroom (Pleurotus ostreatus Kumm. P.) on different substrates. AMB Express 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Sharma, N.; Shafeeq, H.; Ganjoo, A.; Singh, D.; Gairola, S.; Babu, V. Valorization of Distillation Wastes of Aromatic Crops for the Cultivation of Biofortified Pleurotus florida. Waste Biomass Valorization 2023, 14, 1649–1656. [Google Scholar] [CrossRef]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y.B. Green Potential of Pleurotus spp. in Biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef]
- Bhagarathi, L.K.; Subramanian, G.; DaSilva, P.N.B. A Review of Mushroom Cultivation and Production, Benefits and Therapeutic Potentials. World J. Biol. Pharm. Health Sci. 2023, 15, 01–056. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Molecules Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative Study on the Growth and Yield of Pleurotus ostreatus Mushroom on Different Lignocellulosic By-Products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef]
- Mandeel, Q.A.; Al-Laith, A.A.; Mohamed, S.A. Cultivation of Oyster Mushrooms (Pleurotus spp.) on Various Lignocellulosic Wastes. World J. Microbiol. Biotechnol. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Jin, Z.; Hou, Q.; Niu, T. Effect of Cultivating Pleurotus ostreatus on Substrates Supplemented with Herb Residues on Yield Characteristics, Substrates Degradation, and Fruiting Bodies’ Properties. J. Sci. Food Agric. 2020, 100, 4901–4910. [Google Scholar] [CrossRef]
- Di Piazza, S.; Cecchi, G.; Rosa, E.; Zotti, M. The Cultivation of Macrofungi. In Encyclopedia of Mycology; Elsevier: Amsterdam, Netherlands, 2021; pp. 396–404. [Google Scholar] [CrossRef]
- Homolka, L. Preservation of Live Cultures of Basidiomycetes—Recent Methods. Fungal Biol. 2014, 118, 107–125. [Google Scholar] [CrossRef]
- Voyron, S.; Roussel, S.; Munaut, F.; Varese, G.C.; Ginepro, M.; Declerck, S.; Filipello Marchisio, V. Vitality and Genetic Fidelity of White-Rot Fungi Mycelia Following Different Methods of Preservation. Mycol. Res. 2009, 113, 1027–1038. [Google Scholar] [CrossRef]
- Piattoni, F.; Leonardi, P.; Siham, B.; Iotti, M.; Zambonelli, A. Viability and infectivity of Tuber borchii after cryopreservation. Cryo Lett. 2017, 38, 58–64. [Google Scholar]
- Leonardi, P.; Puliga, F.; Iotti, M.; Piattoni, F.; Zambonelli, A. Ultra-low freezing to preserve the lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2018, 20. [Google Scholar] [CrossRef]
- Duggar, B.M. The Principles of Mushroom Growing and Mushroom Spawn Making (No. 85); US Government Printing Office: Washington, DC, USA, 1905. [Google Scholar]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.J.; Athanassiou, A. Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties OPEN. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing Cultivation of Cordyceps militaris for Fast Growth and Cordycepin Overproduction Using Rational Design of Synthetic Media. Comput. Struct. Biotechnol. J. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; F Guiné, R.P.; João Lima, M.; Kumar, N.; Kaushik, R.; Ahmed, N.; Nath Yadav, A.; Kumar, H.; Houhoula, D.; et al. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; TörOs, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Sustainability Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Lyu, X.; Chen, T.; Chen, J.; Chen, X.; Guo, S.; Olech, M.; Barh, A.; Rocio Rodriguez Arcos, I. Utilization of Functional Agro-Waste Residues for Oyster Mushroom Production: Nutritions and Active Ingredients in Healthcare. Front. Plant Sci. 2023, 13, 1085022. [Google Scholar] [CrossRef] [PubMed]
- Dal Molim, G.; de Souza Braga, M.; Satiko Kikuchi, I.; R Nemţanu, M.; Dua, K.; de Jesus Andreoli Pinto, T. The microbial quality aspects and decontamination approaches for the herbal medicinal plants and products: An in-depth review. Curr. Pharma Des. 2016, 22, 4264–4287. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Citation: Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Ogbu, C.C.; Okey, S.N. Agro-industrial waste management: The circular and bioeconomic perspective. In Agricultural Waste-New Insights; Books on Demand: Pasig City, Philippines, 2023. [Google Scholar]
- Bejenaru, L.E.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Manda, C.-V.; Mogo¸sanu, G.D.; Bejenaru, C.; Bejenaru, L.E.; Radu, A.; Segneanu, A.-E.; et al. Innovative Strategies for Upcycling Agricultural Residues and Their Various Pharmaceutical Applications. Plants 2024, 13, 2133. [Google Scholar] [CrossRef]
- Sangeeta; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom Processing: Innovative Techniques and Technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [CrossRef]
- Matidza, T.L. Investigations Of Various Agro-Wastes As Substrates For Cultivation Of Oyster Mushrooms (Pleurotus ostreatus (Jacq.:Fr) P. Kumm and Pleurotus pulmonarius (Fr.) Quèl). Master Thesis, University of Venda, Thohoyandou, South Africa, 2022. [Google Scholar]
- Donnini, D.; Gargano, M.L.; Perini, C.; Savino, E.; Murat, C.; Di Piazza, S.; Altobelli, E.; Salerni, E.; Rubini, A.; Rana, G.L.; et al. Wild and Cultivated Mushrooms as a Model of Sustainable Development. Plant Biosyst. 2013, 147, 226–236. [Google Scholar] [CrossRef]
- Ferri, F.; Zjalic, S.; Reverberi, M.; Fabbri, A.A.; Fanelli, C. I Funghi—Coltivazione e Proprietà Medicinali; Edagricole: Bologna, Italy, 2007; p. 271. [Google Scholar]
- Jasinska, A. Sustainability of Mushroom Cultivation Systems. Sustainability of Mushroom Cultivation Systems. Horticulturae 2023, 9, 1191. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Salami, A.O.; Bankole, F.A.; Olawole, O.I. Effect of Different Substrates on the Growth and Protein Content of Oyster Mushroom (Pleurotus florida). Int. J. Biol. Chem. Sci. 2016, 10, 475. [Google Scholar] [CrossRef]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase. Carbon. Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Atila, F.; Cetin, M. Bioconversion of Lavender Oil Extraction Wastes through Cultivation of Pleurotus eryngii Var. ferulae: Its Effects on Yield, Nutritional Content and Antioxidant Capacity of the Mushroom. Biocatal. Agric. Biotechnol. 2024, 58, 103138. [Google Scholar] [CrossRef]
- Yang, D.; Liang, J.; Wang, Y.; Sun, F.; Tao, H.; Xu, Q.; Zhang, L.; Zhang, Z.; Ho, C.T.; Wan, X. Tea Waste: An Effective and Economic Substrate for Oyster Mushroom Cultivation. J. Sci. Food Agric. 2016, 96, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Ka-logeropoulos, N.; Zervakis, G.I. Enhancing the Nutritional and Functional Properties of Pleurotus citrinopileatus Mushrooms through the Exploitation of Winery and Olive Mill Wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef]
- Atila, F. A Useful Way to Dispose of Phenolic-Rich Agro-Industrial Wastes: Mushroom Cultivation. Turkey EJENS 2019, 3, 32–41. [Google Scholar]
- Omarini, A.; Nepote, V.; Grosso, N.R.; Zygadlo, J.A.; Albertó, E. Sensory Analysis and Fruiting Bodies Characterisation of the Edible Mushrooms Pleurotus ostreatus and Polyporus tenuiculus Obtained on Leaf Waste from the Essential Oil Production Industry. Int. J. Food Sci. Technol. 2010, 45, 466–474. [Google Scholar] [CrossRef]
- Di Piazza, S.; Benvenuti, M.; Damonte, G.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Fungi and Circular Economy: Pleurotus ostreatus Grown on a Substrate with Agricultural Waste of Lavender, and Its Promising Biochemical Profile. Recycling 2021, 6, 40. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Kumar, K. Nutraceutical Potential and Processing Aspects of Oyster Mushrooms (Pleurotus Species). Curr. Nutr. Food Sci. 2018, 16, 3–14. [Google Scholar] [CrossRef]
- Agnihotri, C.; Agnihotri, S.; Kamal, S.; Singh, B.P. Mushroom Bioactives: Traditional Resources with Nutraceutical Importance. In Traditional Resources and Tools for Modern Drug Discovery; Das Talukdar, A., Patra, J.K., Das, G., Nath, D., Eds.; Interdisciplinary Biotechnological Advances; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Drzewiecka, B.; Wessely-Szponder, J.; Świeca, M.; Espinal, P.; Fusté, E.; Fernández-De La Cruz, E. Bioactive Peptides and Other Immunomodulators of Mushroom Origin. Biomedicines 2024, 12, 1483. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant Compounds from Edible Mushrooms as Potential Candidates for Treating Age-Related Neurodegenerative Diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Kumar, U.; Singh, P.K.; Singh, R.P.; Madheshiya, P.; Kharwar, S. Utilizing Residual Biomass from Medicinal and Aromatic Plants: Scope for Value Enhancement. In Sustainable Landscape Planning and Natural Resources Management; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D., Tsay, H.S., Shyur, L.F., Wu, Y.C., Wang, S.Y., Eds.; Medicinal and Aromatic Plants of the World; Springer: Singapore, 2017; Volume 4. [Google Scholar] [CrossRef]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Straetkvern, O.; Straetkvern, K.O. Spent Substrate from Mushroom Cultivation: Exploitation Potential toward Various Applications and Value-Added Products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent Advances and Future Directions on the Valorization of Spent Mushroom Substrate (SMS): A Review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.; Almeida, M.; Paié-Ribeiro, J.; Barros, A.N.; Rodrigues, M. Unlocking the Potential of Spent Mushroom Substrate (SMS) for Enhanced Agricultural Sustainability: From Environmental Benefits to Poultry Nutrition. Life 2023, 13, 1948. [Google Scholar] [CrossRef]
- Mayans, B.; Antón-Herrero, R.; García-Delgado, C.; Delgado-Moreno, L.; Guirado, M.; Pérez-Esteban, J.; Eymar, E. Bioremediation of petroleum hydrocarbons polluted soil by spent mushroom substrates: Microbiological structure and functionality. J. Hazard. Mat. 2024, 473, 134650. [Google Scholar] [CrossRef]
- Gupta, G.; Maurya, S.; Jha, P.N.; Chauhan, P.S. Valorization of Mushroom Spent Mushroom Substrate: A Sustainable Approach to Remediation of Xenobiotic Compounds-A Comprehensive Review. Groundw. Sustain. Dev. 2024, 26, 101290. [Google Scholar] [CrossRef]
| Costs | Unit of Measurement | Amount | Unit Cost | Tot | Variable Costs | Express Costs |
|---|---|---|---|---|---|---|
| Cost of plastic bags and inoculum | Number | 1.805 | 0.15 | 270.78 | X | x |
| Purchases of various means, boxes, and minute tools | Estimation | 1 | 200.00 | 200.00 | X | x |
| Insurances | Estimate—breakdown from total company | 1 | 50.00 | 50.00 | X | x |
| Tax consulting | Estimate—breakdown from total company | 1 | 50.00 | 50.00 | X | x |
| Membership costs | Estimate—breakdown from total company | 1 | 50.00 | 50.00 | x | |
| Waste disposal costs (plastic wrap) | Estimate—breakdown from total company | 1 | 50.00 | 50.00 | X | x |
| Fuels for the marketing of the product | Estimation | 1 | 100.00 | 100.00 | X | x |
| Electricity for a packaging machine, fans, and refrigerator | Estimation | 1 | 300.00 | 300.00 | X | x |
| Plastic maintenance and renewal | Estimation | 1 | 200.00 | 200.00 | X | x |
| Waged labor | Hours | 120 | 14.00 | 1680.00 | X | x |
| Entrepreneur labor and family | Hours | 218 | 14.00 | 3052.00 | X | x |
| Taxes and contributions | Estimate—breakdown from total company | 1 | 50.00 | 50.00 | x | |
| Land capital depreciation (wood shade) | Calculated quotas | 1 | 75.00 | 75.00 | x | |
| Processing room depreciation | Calculated quotas | 1 | 300.00 | 300.00 | x | |
| Depreciation of electrical and similar equipment | Calculated quotas | 1 | 375.00 | 375.00 | x | |
| Depreciation of pallets and structures for processing/conditioning | Calculated quotas | 1 | 180.00 | 180.00 | x | |
| Transport van depreciation | Calculated quotas | 1 | 320.00 | 320.00 | x | |
| Bag wrapping machine depreciation | Calculated quotas | 1 | 625.00 | 625.00 | x | |
| Cold storage depreciation | Calculated quotas | 1 | 375.00 | 375.00 | x | |
| Directional work of the entrepreneur | Hours | 8 | 18.00 | 144.00 | x | |
| Interests | Computation | 73.77 | X | x | ||
| Land capital cost | Estimation | 1 | 25.00 | 25.00 | ||
| Total Costs | 8545.44 | |||||
| Aggregation of Cost Items | Tot | |||||
| Total attrition factors | 470.68 | |||||
| Other costs | 800.00 | |||||
| Partial attrition factors | 2250.00 | |||||
| Waged labor | 1680.00 | |||||
| Labor entrepreneur and family | 3052.00 | |||||
| Directional work of the entrepreneur | 144.00 | |||||
| Taxes | 50.00 | |||||
| Interests | 73.77 | |||||
| Land capital cost | 25.00 | |||||
| TOTAL COSTS | 8545.44 | |||||
| Variable costs | 6026.44 | |||||
| Express costs | 5004.44 | |||||
| Revenue by Product Type | Unit of Measure | Total Product in kg (2 Cycles) | Price in Euro/KG | Total | ||
| Fungi, 2 cycles | kg | 4511.28 | 2.00 | 9022.56 | ||
| Total Income or Plv. | 9022.56 | |||||
| Results | Total | |||||
| Gross income = revenue—variable costs | 2996.11 | |||||
| Net income = revenue—explicit costs | 4018.11 | |||||
| Profit = revenue—total costs | 477.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, M.; Cecchi, G.; Canonica, L.; Di Piazza, S. A Review of Nature-Based Solutions for Valorizing Aromatic Plants’ Lignocellulosic Waste Through Oyster Mushroom Cultivation. Sustainability 2025, 17, 4410. https://doi.org/10.3390/su17104410
Zotti M, Cecchi G, Canonica L, Di Piazza S. A Review of Nature-Based Solutions for Valorizing Aromatic Plants’ Lignocellulosic Waste Through Oyster Mushroom Cultivation. Sustainability. 2025; 17(10):4410. https://doi.org/10.3390/su17104410
Chicago/Turabian StyleZotti, Mirca, Grazia Cecchi, Laura Canonica, and Simone Di Piazza. 2025. "A Review of Nature-Based Solutions for Valorizing Aromatic Plants’ Lignocellulosic Waste Through Oyster Mushroom Cultivation" Sustainability 17, no. 10: 4410. https://doi.org/10.3390/su17104410
APA StyleZotti, M., Cecchi, G., Canonica, L., & Di Piazza, S. (2025). A Review of Nature-Based Solutions for Valorizing Aromatic Plants’ Lignocellulosic Waste Through Oyster Mushroom Cultivation. Sustainability, 17(10), 4410. https://doi.org/10.3390/su17104410








