Abstract
Alkali-activated materials have gained increasing popularity in the field of soil barrier materials due to their high strength and low environmental impact. However, barrier materials made from alkali-activated materials still suffer from long setting times and poor barrier performance in acidic, alkaline, and saline environments, which hinders the sustainable development of green alkali-activated materials. Herein, coconut shell biochar, sodium silicate-based adhesives, and polyether polyol/polypropylene polymers were used for multi-stage material modification. The modified materials were evaluated for barrier performance, rapid formation, and resistance to acidic, alkaline, and saline environments, using metrics such as compressive strength, permeability, mass loss, and VOC diffusion efficiency. The results indicated that adhesive modification reduced the material’s setting time from 72 to 12 h. Polymer modification improved resistance to corrosion by 15–20%. The biochar-containing multi-stage modified materials achieved VOC diffusion barrier efficiency of over 99% in both normal and corrosive conditions. These improvements are attributed to the adhesive accelerating calcium silicate hydration and forming strength-enhancing compounds, the polymer providing corrosion resistance, and biochar enhancing the volatile organic compounds (VOC) barrier properties. The combined modification yielded a highly effective multi-stage green barrier material suitable for rapid barrier formation and corrosion protection. These findings contribute to evaluating multi-level modified barrier materials’ effectiveness and potential benefits in this field and provide new insights for the development of modified, green, and efficient alkali-activated barrier materials, promoting the green and sustainable development of soil pollution control technologies.
1. Introduction
The research on using alkali-activated materials as an alternative to cement-based materials for barrier material applications has been actively conducted in recent years. The silicate cement material (OPC) was once the most popular component of barrier materials due to its low cost and excellent properties, such as Carvalho et al. enhancing Portland cement properties by recycling BOF slag, Zhang et al. investigating Cr(VI) immobilization in Portland cement pastes, and Moon et al. exploring the stabilization of selenium-impacted soils using Portland cement and cement kiln dust, highlighting the role of cement-based materials in environmental remediation and waste containment [1,2,3]. However, the silicate cement production process was particularly energy-intensive and resource-consuming, requiring approximately 5000 MJ of energy and 1.6 tons of calcium- and clay-based raw materials to produce 1 ton of OPC, while the silicate cement-based barrier technology does not possess strong sustainability [4,5]. Furthermore, significant CO2 emissions were generated during the calcination process, with estimates ranging from 0.7 to 1.1 tons of CO2 released per ton of OPC [6]. For alkali-activated materials, partial replacement of cement can significantly reduce CO2 emissions during the material production process and improve its barrier properties [7]. For instance, Fan et al. discovered that using fly ash as a substitute for cement in barrier materials had improved the pollutant barrier performance [8]. Fernández et al. also found that the use of industrial waste-derived precursors and activators in alkali-activated materials significantly contributes to sustainability in material production [9]. Similarly, Li et al. used industrial by-products such as mineral powders as raw materials to synthesize cementitious materials, which improved compressive strength while the negative environmental impact and costs promoted the green development of alkali-activated materials [10]. The widespread use of these green alkali-activated materials can reduce energy consumption and other negative environmental impacts while promoting the sustainable development of soil pollution control technologies.
However, limitations were still encountered, such as prolonged sitting times—generally 7–15 days [11]. In cases of emergency site contamination, the barrier effectiveness might have been insufficient. Additionally, the formation of alkali-activated materials required a specific alkaline environment, which could have affected barrier performance under harsh conditions, such as acids, alkalis, or high salinity. These shortcomings significantly hinder the widespread application of green alkali-activated materials in barrier technologies. At the same time, research aimed at addressing these deficiencies within the field of alkali-activated barrier materials still holds considerable potential. Therefore, it became necessary for faster-setting, more environmentally adaptable barrier materials to be explored, and their performance under highly acidic and alkaline conditions needed to be tested.
To address the limitations of alkali-activated materials (AAMs) and further advance the sustainable development of green alkali-activated barrier technology to optimize cement-based material barrier technology, targeted materials were considered to enhance the barrier performance of these materials. Biochar, recognized for its eco-friendly and porous properties, has been widely used in pollutant adsorption and control. When incorporated into barrier materials, biochar was found to improve their ability to control pollutants further [12]. Previous studies have shown that different biochar types can increase cement-based composites’ compressive strength by approximately 2–13%, while also enhancing the overall sustainability of the material by utilizing waste biomass and reducing the carbon footprint of the production process [13]. Water glass solutions, known for their gelation properties upon water loss and their ability to form silica through chemical reactions, were commonly used as adhesives in concrete materials. Additionally, their production is relatively environmentally friendly. Compared to traditional chemical adhesives, sodium silicate and sodium fluorosilicate adhesives are safer, and the sustainability of materials incorporating them is stronger [14]. Sodium fluorosilicate, as a coagulant additive, was used in conjunction with water glass solutions [15]. To address the challenge of rapidly forming alkali-activated material-based barriers, it was necessary to explore whether water glass solutions could facilitate the formation of these barriers, thus making them suitable for emergency scenarios. Targeting acid, alkali, and salt environments, polyether polyols, as green high-polymer organic materials, were recognized for their excellent environmental adaptability and mechanical properties, particularly in enhancing the resistance of modified barrier materials. Additionally, since these polymers are typically derived from renewable resources and benefit from mature recycling technologies, they possess excellent sustainability. For example, elastomeric polyurethanes made from polyether polyols were found to have a maximum tensile strength of 26.6 MPa, along with good thermal stability and acid-alkali resistance [16]. Due to their favorable environmental adaptability, these materials were often used to prepare anti-corrosion coatings [17]. Polypropylene, known for its good mechanical properties and cost-effectiveness, was widely used in materials science, with existing research indicating its potential application in barrier films [18,19]. The aforementioned modified materials are commonly used and exhibit excellent performance. To impart the advantages of these materials to alkali-activated barrier materials and develop a green, efficient, and sustainable barrier material, it has become essential to utilize these materials as modifiers for the multi-level modification of the base barrier materials.
The research presented here utilized unconfined compressive strength, permeability, chemical compatibility, and VOC diffusion barrier performance as key indicators of the barrier properties of materials. The influence of adhesive incorporation on the rapid barrier performance of these materials was thoroughly examined. In addition, the study investigated the barrier performance of unmodified alkali-activated barrier materials and polymer-modified barrier materials under various environmental conditions, such as acid, alkali, and salt environments, through unconfined compressive strength and permeability tests. Immersion and soil column experiments were also conducted to compare the resistance of unmodified alkali-activated barrier materials and modified barrier materials to acid, alkali, and high-salt corrosion. Furthermore, the actual barrier effectiveness of modified materials against organic pollutants under different environmental conditions was explored. The findings of this research provide valuable insights for the rapid containment and control of soil pollution in environments characterized by acid, alkali, and salt conditions using green and sustainable alkali-activated barrier materials.
2. Materials and Methods
2.1. Raw Materials
The base isolation materials were selected as follows: Ordinary field soil, screened through a 40-mesh sieve and subsequently dried, was used as the base fill material. The optimum moisture content and maximum dry density, determined via compaction testing in accordance with standard highway geotechnical testing procedures, were found to be 19% and 1.63 g/cm3, respectively. Calcium-based bentonite, exhibiting an expansion index of 12.5 mL·2 g−1, was utilized. Industrial by-products incorporated in the study included: (1) ground granulated blast furnace slag (GGBS, S95 grade, Henan Hengyuan New Materials Co., Ltd., Anyang, China), which served as a concrete admixture; (2) Class F low-calcium fly ash (FA, Nanjing Huaneng Power Plant, Nanjing, China); and (3) steel slag (SS, Nanjing Jiuding Trading Co., Ltd., Nanjing, China). The components of various industrial solid wastes are shown in Table 1. Modified materials included: 40-mesh coconut shell biochar (Ningxia Tingyuan Fruitwood Energy Technology Co., Ltd., Ningxia, China); water glass solution, with a modulus of 3.32 and an SiO2 content of approximately 30% (Nantong Mengya New Materials Technology Co., Ltd., Nantong, China); sodium hexafluorosilicate white powder, with an analytical reagent (AR) grade ≥ 99.0% (China National Pharmaceutical Group, Beijing, China); a 2000 molecular weight elastomeric polyether polyol (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China); and 100-mesh polypropylene (PP) powder (Nanjing Weisheng Environmental Protection New Materials Co., Ltd., Nanjing, China).

Table 1.
Main Industrial Solid Waste Components (wt%).
2.2. Preparation and Analysis of Barrier Materials
The evaluation of the barrier material performance typically included common indicators, such as unconfined compressive strength and permeability coefficient. In addition, soil column diffusion experiments were conducted to assess the actual contaminant barrier performance and the practical application potential of the barrier materials. The specific preparation process was as follows: The base alkali-activated materials (such as GGBS and bentonite) were mixed with multi-stage modified materials, including biochar, adhesive, and polymers, in batches using a high-speed mechanical mixer to obtain a slurry. The slurry was then poured into the corresponding molds for unconfined compressive strength (cylindrical specimens with a diameter of 50 mm and height of 50 mm) and permeability (cylindrical specimens with a diameter of 61.8 mm and height of 40 mm) tests, and cured in a standard curing chamber at 23 °C and 70% humidity. Polyether polyols were pre-mixed with the alkali-activated slurry using ultrasonic assistance (40 kHz, 30 min), while polypropylene powder (particle size < 100 μm) was uniformly dispersed through dry ball milling (300 rpm, 2 h). After a fixed curing time, the samples were demolded to obtain the formed barrier material specimens, with the curing time controlled to evaluate the rapid barrier performance of the materials.
Subsequently, the formed barrier material samples were immersed in corresponding acidic, alkaline, and saline solutions (HCl solution with pH = 2, NaOH solution with pH = 12, and 5% CaCl2 solution by mass) to simulate different environmental influences. After a fixed immersion time, the barrier material samples were removed and subjected to unconfined compressive strength and permeability tests. Concurrently, the barrier material samples were placed in soil columns for contaminant barrier experiments. After a fixed diffusion period, the contaminant concentrations in the upper, middle, and lower layers were measured using a meteorological analyzer to assess the diffusion barrier performance of the material. The preparation and analysis flowchart of barrier materials is shown in Figure 1.
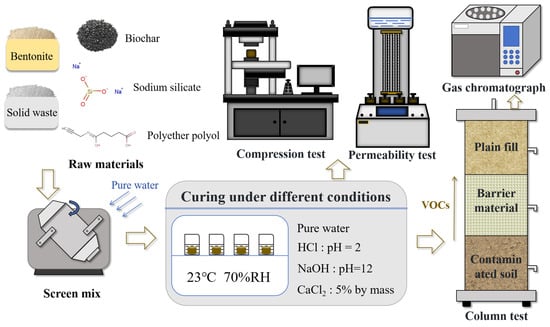
Figure 1.
The preparation and analysis of barrier materials.
2.3. Basic Physical Properties
The aim of this research was to evaluate the fundamental physical properties of various barrier materials. As shown in Table 2, this included unmodified alkali-activated barrier materials (UM), biochar-modified barrier materials (BM-B), sodium silicate-modified barrier materials (BM-S), sodium silicate/fluorosilicate-modified barrier materials (BM-S/F), polyether polyol-modified barrier materials (BM-P), and polyether polyol/polypropylene-modified barrier materials (BM-P/PP).

Table 2.
Sample ID and Composition Ratio (wt%).
The evaluation focused primarily on unconfined compressive strength and permeability coefficient (as per Chinese National Standard, “Highway Geotechnical Test Procedures, JTG E40-2007”) [20] to assess the physical barrier performance of these materials.
2.4. Chemical Compatibility
The chemical compatibility of the barrier material was tested following the Chinese National Standard (Highway Engineering Geotechnical Test Procedures, JTG E40-2007) [21]. Unconfined compressive test specimens were used as soil samples. These soil samples were immersed in corresponding acid, alkali, and salt solutions, specifically a pH = 2 HCl solution, a pH = 12 NaOH solution, and a 5% CaCl2 solution. The erosion of the barrier material after immersion was determined using the immersion method. The mass loss rate of the material, influenced by the acid, alkali, and salt solutions, was calculated using the mass loss rate formula, which is as follows:
where m0 is the initial mass and mt is the mass after erosion.
Additionally, the physical barrier performance of the materials was evaluated after immersion in various environmental solutions, with results compared to a baseline established by materials soaked in pure water. This comparison, along with the mass loss rate, provided insights into the materials’ chemical compatibility and environmental adaptability.
2.5. Evaluation of VOC Barrier Effectiveness
To assess the ability of the material to control the vapor phase migration of organic pollutants, a cylindrical multi-layer soil column with a diameter of 50 mm and a height of 30 cm was employed for the experiment. The column consisted of three layers, each layer of soil or barrier material was filled using a layered compaction method: the lower layer contained contaminated soil with volatile organic compounds (VOCs), the middle layer comprised the green efficient barrier material, and the upper layer consisted of unpolluted soil sourced from the same location as the lower layer. Contaminated soil with varying gradient pollution levels was established, and the barrier effect of the material on the meteorological migration of organic pollutants was evaluated by measuring the time elapsed until pollutants penetrated, specifically, the time at which pollutants appeared in the unpolluted soil of the upper layer. At the same time, the concentration of pollutants in the modified barrier material of the middle layer was measured to comprehensively assess the actual barrier performance of the modified material under different environmental conditions.
2.6. Characterization and Detection Methods
The quantity of specific oxides in the material was determined utilizing X-ray fluorescence (XRF, PANalytical, Almelo, Netherlands). The mineral composition and structure of the samples were investigated through X-ray diffraction (XRD, Smart Lab; Rigaku Corporation, Tokyo, Japan) and Fourier transform infrared spectroscopy (FTIR, Nicolet 5700; Thermo Fisher Scientific, Waltham, USA), respectively. The barrier material’s internal structure and crystal composition were examined using scanning electron microscopy (SEM, Ultra Plus; Carl Zeiss AG, Oberkochen, Germany).
3. Results and Discussion
3.1. Physical Properties of Barrier Materials Prepared from Multiple Solid Wastes
The incorporation of industrial solid waste into barrier materials was found to offer several advantages over traditional cement-based materials, including enhanced mechanical strength and improved environmental friendliness [22,23,24]. In this study, the compressive strength and permeability coefficient of barrier materials, formulated with varying proportions of steel slag (SS), S95-grade ground granulated blast-furnace slag (GGBS), and low-calcium fly ash (FA), were investigated after 24 h of curing.
As illustrated in Figure 2, the compressive strength of the barrier materials increased significantly with a higher proportion of industrial solid waste, particularly with the inclusion of steel slag (SS), which nearly tripled the compressive strength, exceeding the unconfined compressive strength standard (UCSS). This enhancement was attributed to the high hardness of the steel slag [25]. In contrast, regarding the influence of industrial solid waste on the permeability coefficient of the barrier materials, S95-grade GGBS and low-calcium fly ash exhibited a smaller cumulative pore volume and porosity compared to steel slag [26]. Consequently, the changes in the permeability performance of the blended barrier materials were more gradual with varying proportions; all blending ratios, except for the 80% blend, met the permeability coefficient standard (PCS). Additionally, the blending ratio of industrial solid waste was found to significantly affect the barrier performance of the materials [27].
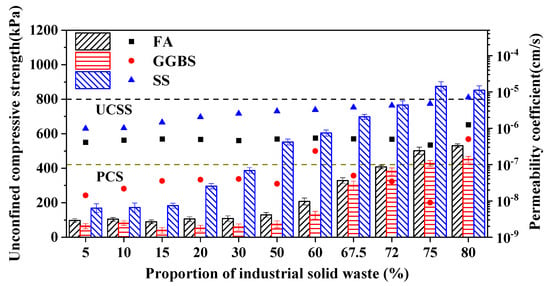
Figure 2.
Selection of industrial solid waste types and blending ratios.
On the one hand, an increase in the solid waste blending ratio resulted in a higher overall porosity of the barrier materials, leading to an increased permeability coefficient [28,29]. This was associated with a reduced proportion of bentonite, which decreased the fine particle content and hindered pore blockage. On the other hand, the compressive strength of the barrier materials was derived from both the inherent hardness of the materials and the alkaline activation effects of industrial solid waste [30]. Variations in solid waste content were found to affect the formation of the gel structure within the materials.
Industrial solid waste was utilized as an alkaline-activated material, while bentonite, a common component in barrier materials, was included. This study explored the relationship between different types and proportions of solid waste and the physical properties of the barrier materials, focusing on identifying the optimal performance achieved through various combinations of industrial solid waste sources and blending ratios. With increasing proportions of solid waste, the compressive strength of the materials improved under the same forming time, peaking at a 75% blending ratio. Due to the significant variation in permeability coefficients with steel slag incorporation and the relatively lower compressive strength of fly ash-blended barrier materials, the mineral powder was ultimately selected as the raw material, demonstrating superior compressive and permeability performance.
3.2. Effects of Biochar on Compressive and Permeability Properties
Biochar, recognized as a green material with a porous structure, was found to serve as an effective agent for controlling pollutants [31]. Among various types of biochar, coconut shell biochar exhibited excellent strength and adsorption properties, and it was demonstrated that its incorporation enhanced the contaminant barrier performance of materials [32]. As illustrated in Figure 3b, the barrier performance parameters of materials incorporating coconut shell biochar were analyzed. It was observed that the modified material’s unconfined compressive strength (UCS) significantly decreased with increasing biochar content, while the permeability coefficient of the material correspondingly increased. This phenomenon was attributed to the relatively low strength of biochar compared to other materials, resulting in a reduction of the barrier material’s overall strength upon incorporating biochar [33]. Furthermore, a dense microstructure usually means fewer voids, which can effectively withstand externally applied pressure, thereby enhancing the material’s compressive strength. The presence of pores, on the other hand, leads to stress concentration, reducing the material’s compressive capacity.

Figure 3.
Selection of biochar blending ratios and mechanisms of modification. (a) Comparison of XRD images of BM-B and UM. (b) Screening of BM-B formulations based on barrier performance. (c,d) Comparison of SEM images of BM-B and UM.
The impact of biochar on material properties, particularly in terms of strength and permeability, is closely related to its microstructure and chemical reactivity. From a microstructural perspective, biochar has a porous structure, and these pores can enhance the material’s permeability. However, this porosity also results in lower strength for biochar itself, as the presence of pores reduces the material’s density and continuity, thus decreasing its mechanical strength. As demonstrated in Figure 3c,d, the incorporation of biochar introduced distinct pore structures into the barrier material, which enhanced the material’s permeability [34], which, in turn, diminished the material’s barrier performance. Furthermore, the presence of coconut shell biochar was evident in the modified barrier materials, as shown in the XRD diffraction patterns in Figure 3a. The biochar played a significant role, particularly due to its unique composition and properties. The carbon-based and mineral components in biochar may react with the matrix material, forming various chemical bonds. These reactions may increase the brittleness of the material, thereby reducing its strength [35].
However, biochar was essential to endow the material with a certain pollutant control capability; it can effectively adsorb and immobilize pollutants through its rich pore structure and surface functional groups. In addition, biochar enhances pollutant control through physical adsorption, chemical reactions, and electrostatic interactions with the pollutants [36]. Therefore, it was concluded that an effective strategy was to wisely integrate an appropriate proportion of biochar into the barrier material to balance satisfactory physical properties and pollutant control capacity. Testing revealed that a biochar incorporation rate of 3% allowed the material to meet physical performance standards while enhancing its ability to control pollutants to some extent.
3.3. Effects of Adhesives on Material Molding Rate
Sodium silicate and sodium fluosilicate, rich in silicate ions, were commonly used as material adhesives. Figure 4a,b illustrates the changes in the forming speed of the modified materials with sodium silicate and sodium fluosilicate. The barrier materials modified with sodium silicate (BM-S) and those co-modified with sodium silicate and sodium fluosilicate (BM-S/F) achieved the required barrier performance within 24 h. Notably, the co-modified barrier materials demonstrated significant progress, reaching basic formability within 12 h—five times faster than the 72 h required for the unmodified materials (BM-B). The reduction in forming time was attributed to the accelerated alkali activation process [37], primarily due to the abundant silicate ions in the modified materials, which expedited the formation of the hardening adhesive—calcium silicate hydrate (C-S-H). The addition of adhesives increases the bonding strength between particles, which in turn enhances the compressive strength. Conversely, if there is weak bonding or slipping between particles, the compressive strength of the material will decrease. As shown in the XRD patterns in Figure 4c, numerous diffraction peaks corresponding to hydrated calcium aluminosilicate confirmed its role as a major contributor to the strength of the barrier materials. Meanwhile, in the Fourier transform infrared spectrum (Figure 4d), the BM-S and BM-S/F modified material samples mainly exhibit strong absorption peaks corresponding to silicon-oxygen bonds.
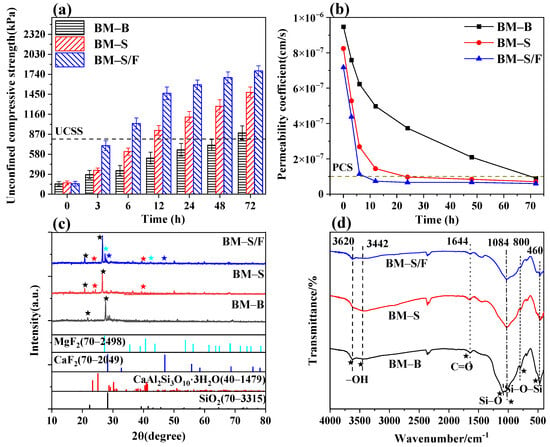
Figure 4.
Effects of adhesives on the formation rate of barrier materials and mechanisms of modification. (a) The effect of adhesive modification on the compressive properties and (b) the permeability properties of materials. (c) XRD images and (d) FTIR images of adhesive-modified samples.
Upon further observation of the BM-S/F modified material, a more prominent absorption peak corresponding to the vibration of the fluorine-silicon bond appears in the lower wavenumber region (such as 800–1000 cm−1). These phenomena further indicate that the molding rate of the barrier material has accelerated after binder modification. Furthermore, the co-modification with sodium fluosilicate further shortened the barrier formation time. XRD and infrared spectroscopy analyses in Figure 4c,d revealed that this phenomenon was due to the additional formation of strengthening compounds—calcium fluoride and magnesium fluoride—resulting from incorporating sodium fluosilicate. This enhancement enabled the materials to achieve higher levels of unconfined compressive strength in a shorter timeframe, thereby facilitating rapid barrier formation.
The above-mentioned factors underscored the effectiveness of modifying barrier materials with the binding properties of sodium silicate and sodium fluosilicate, which enabled the materials to exhibit rapid barrier formation capabilities.
3.4. Effects of Polymers on Material Chemical Compatibility
Polyether polyols (P) and polypropylene (PP) were recognized as environmentally friendly polymers. Polyether polyols were commonly used as anti-corrosion coatings due to their inertness toward acidic and basic reactions [17], while polypropylene was valued for its excellent mechanical properties, high production volume, ease of processing, low cost, and environmental adaptability [38]. As illustrated in Figure 5, the permeability coefficients of the barrier materials were significantly increased following polymer modification, although the trend in compressive strength exhibited slight variation. This discrepancy was attributed to relatively flexible molecular structure of polyether polyols, which contain a higher number of oxygen atoms, resulting in a strength significantly lower than soil materials.
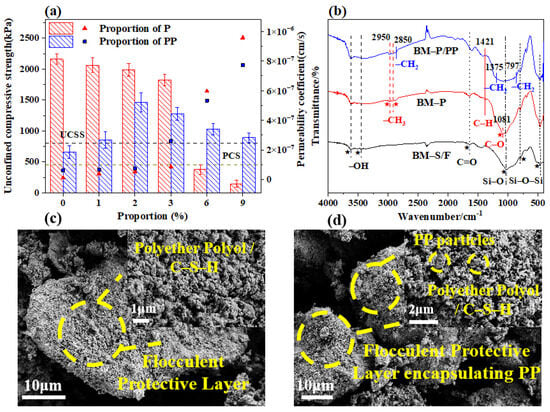
Figure 5.
Mechanical properties and microstructure of polymer-modified barrier materials. (a) Barrier performance and (b) FTIR images of polymer-modified materials. (c,d) SEM images of BM-P and BM-P/PP.
When added, it reduced the overall hardness and rigidity of the material, leading to a decrease in compressive strength. Additionally, polyether polyols could absorb moisture in humid environments, and the presence of moisture affected the material’s mechanical properties, particularly its compressive strength, resulting in a reduction in strength [39]. On the other hand, powdered polypropylene was found to have the capacity to fill the internal pores of the barrier materials effectively at a 2% incorporation ratio. Therefore, it was deemed crucial to employ an appropriate blending ratio to ensure optimal performance of the barrier materials.
Additionally, as illustrated in Figure 5b, in the BM-P group, new absorption peaks corresponding to the methyl and ether bonds introduced by polyether polyols appeared. Additionally, the typical chemical groups in the molecular structure of polyether polyols, such as the oxygen (–O–) and hydroxyl (–OH) groups, made the material potentially affinitive to acid, alkali, and salt solutions [40]. In the BM-P/PP group, a methyl rocking vibration peak appeared due to the incorporation of polypropylene. Polypropylene (PP) is a typical non-polar plastic, composed primarily of carbon-hydrogen units in its molecular structure, and exhibits low chemical reactivity [41]. Furthermore, as shown in Figure 5b, FTIR spectra confirm the characteristic peaks of C–O–C (1100 cm−1) and –CH3 (1375 cm−1) in BM-P/PP, which demonstrate that the polymer interacts with the gel structure through physical adsorption.
This indicated that the blending of the two polymers might have contributed synergistically to providing different roles in resisting environmental degradation. In Figure 5c, it is evident that the addition of polyether polyol promoted the formation of a denser, flocculent protective layer as C–S–H particles adhered to one another. Figure 5d further demonstrates that including polypropylene particles led to their integration within the flocculent matrix, thereby contributing to the overall protective layer. The two polymers, through physical adsorption or chemical crosslinking, formed a protective layer within the composite material that resisted environmental solutions. This layer helped protect key strength components, such as the hydrated calcium silicate gel and the alkaline environment of the material, from degradation by environmental solutions. These findings suggested that the polymer-modified barrier materials possessed significant potential for corrosion resistance. In addition, the alkali activators and the polymers can form a protective film covering the surface of the reactive barrier materials, thereby preventing the reaction between the alkaline compounds in the cement and the reactive silicate minerals.
Further studies were conducted on the resistance of polymer-modified barrier materials to acids, alkalies, and high salinity. Figure 6 illustrates the changes in barrier performance of the modified materials during the molding process in environments with acids, alkalies, and high salinity. Under varying environmental influences, the barrier performance of the materials initially increased and then stabilized. This trend was primarily attributed to the dominant rapid molding performance of the barrier materials within the first 24 h, significantly enhancing their barrier properties. However, as the molding process gradually completed and environmental solutions began eroding the barrier materials, the barrier performance tended to plateau or decline.
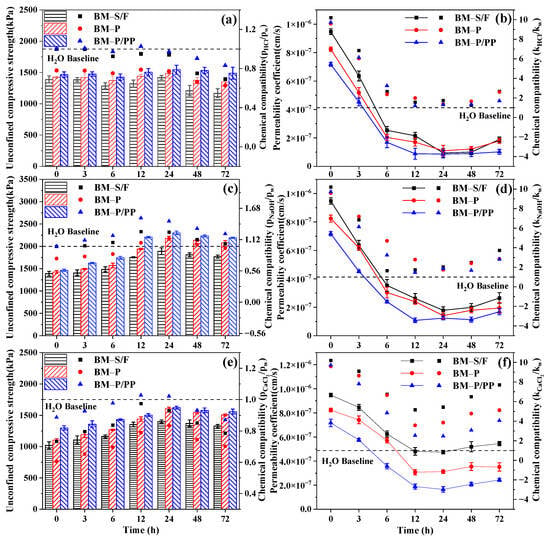
Figure 6.
Barrier properties of polymer-modified materials under the influence of different environmental factors. (a,b) The impact of a strongly acidic environment with pH = 2. (c,d) The impact of a strongly alkaline environment with pH = 12. (e,f) The impact of a high salt environment with a mass fraction of 5%.
Notably, the environmental adaptability of the polyether polyol/polypropylene polymer itself and the adhesive properties of polyether polyol interacted with the gel present in the materials [42], resulting in a denser structure of the barrier materials. Additionally, the hydrophobicity of polypropylene was observed to reduce the erosion from acidic solutions to some extent [43]. The barrier performance parameters and chemical compatibility indices of the polymer-modified materials were significantly superior to those of unmodified materials. Based on this observation, it could be concluded that the polyether polyol/polypropylene modification imparted certain environmental adaptability to the barrier materials.
Moreover, an examination of the chemical compatibility indices in Figure 6b,c revealed that some alkali-treated materials exhibited superior barrier performance. This improvement was due to the hydroxide ions present in the alkaline solution, which enhanced the alkalization of the barrier materials during the molding process [7], allowing for a further enhancement of barrier performance. This indicated that the polymer-modified barrier materials demonstrated excellent alkali resistance and could benefit from exposure to strong alkaline environments. This phenomenon aligned with the fundamental characteristics of alkali-activated materials.
A thorough investigation into the environmental adaptability of polymer-modified barrier materials revealed that exposure to environmental solutions could lead to a decline in material quality. This decline was significant, as material quality is one of the key factors influencing physical barrier properties, such as compressive strength. Therefore, it was essential to evaluate the quality loss of modified materials under the influence of various environmental solutions.
Figure 7a illustrated the quality loss rates of barrier materials with different polymer modifications when exposed to pure water, hydrochloric acid solution with a pH of 2, sodium hydroxide solution with a pH of 12, and calcium chloride solution with a mass fraction of 5%. It was observed that the polymer-modified barrier materials exhibited the greatest stability in pure water. This stability was attributed to the interaction between the polymeric materials and the gel within them, which enhanced the density of the materials [40]. Consequently, the quality loss rate of the modified materials in acidic environments was also lower.
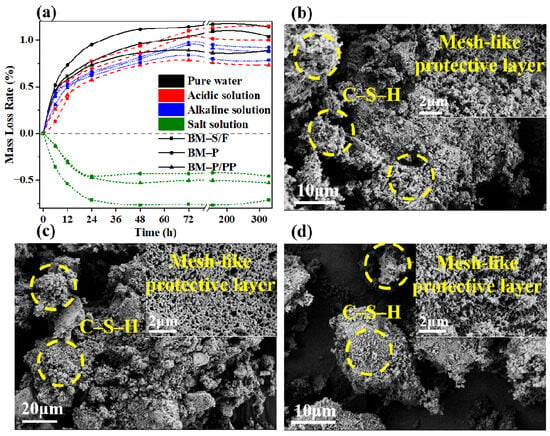
Figure 7.
Analysis of the mechanisms of acid-base resistance in polymer-modified barrier materials. (a) Mass loss of polymer-modified materials under different environmental influences. (b) Polymer-modified materials in acidic environments, (c) in alkaline environments, and (d) in saline environments.
Additionally, under the influence of the alkaline solution, the unmodified barrier materials exhibited a smaller mass loss. The primary contributor to the mass loss in these materials was the reaction between hydroxide ions in the solution and the barrier material, which led to an alkali activation process. This alkali activation was identified as a key mechanism that enhanced the barrier properties of the material. Notably, a higher mass loss rate was indicative of improved material performance.
Moreover, it was noteworthy that the quality of barrier materials increased when exposed to calcium chloride solutions. This increase was attributed to the reaction between calcium ions and sodium silicate, a coagulating agent within the barrier material, which resulted in precipitates adhering to the material’s surface [44,45]. Sodium silicate was found to promote the adhesion of various components within the barrier material, suggesting that the forming process of the barrier material was hindered.
These observations collectively confirmed the environmental adaptability of the modified barrier materials at a macroscopic level. On the other hand, after the polyether polyol/polypropylene acid-base resistant polymer materials are added to the barrier materials, they either exist as free acid-base resistant salt functional groups or are adsorbed onto the gel structure, forming a protective layer that safeguards key strength components in the material, such as hydrated calcium silicate gel, from erosion by environmental solutions under alkaline conditions.
As shown in Figure 7b–d, the polymer modification imparts a mesh-like protective layer to the barrier material that is resistant to acid, base, and salts. This is because the incorporation of the polymer introduces more bonding functional groups and establishes stable connections between the various components of the material, resulting in a more cohesive overall adhesion of the barrier material. Therefore, the erosion process by environmental solutions must overcome the resistance provided by the dense structure of the barrier material and the acid-base-salt-resistant polymers. This was evidenced by increased amounts of crucial strength-giving substances, such as hydrated calcium silicate and hydrated calcium aluminum silicate, in the polymer-modified barrier materials, which also exhibited a more compact distribution of materials. Furthermore, in an alkaline environment conducive to the activation of the barrier material, the network structure formed by the acid-base-resistant polymer and the alkali-activated gel is less prone to erosion compared to acidic and high-salt environments. These phenomena collectively explained, at a microscopic level, the environmental adaptability of the polymer-modified barrier materials.
3.5. VOC Barrier Effectiveness of Modified Materials
In addition to investigating the physical barrier properties of the materials, halogenated hydrocarbons (1,2-dichloroethane), aromatics (toluene), and alcohols (isopropanol) were selected as characteristic pollutants to further examine the organic pollution barrier effectiveness of the modified materials. This investigation aimed to elucidate the practical efficacy of the modified materials on the organic pollution barrier and their feasibility for application.
Testing revealed that the modified materials exhibited significant barrier effects against the three types of VOC pollutants. Under the influence of baseline organic pollution at a concentration of 10,000 ppm, no pollutants were detected in the upper unpolluted soil after 15 days of exposure, and the concentration of pollutants within the barrier material was also found to be negligible compared to the source pollution. This effect was attributed to two factors. First, the modification through biochar endowed the material with a certain degree of adsorption capacity, which allowed some VOCs to be captured by the modified material, thereby achieving a barrier effect [46,47,48]. Second, the swelling characteristics of bentonite [49,50] and the filling effect of polymeric materials [51] greatly enhanced the densification of the barrier material by filling its pores [52], which inhibited the diffusion of VOC pollutants and achieved the desired barrier effect. The compact microstructure in Figure 8 corroborates the role of the bentonite-filled barrier material in sealing the gaps.
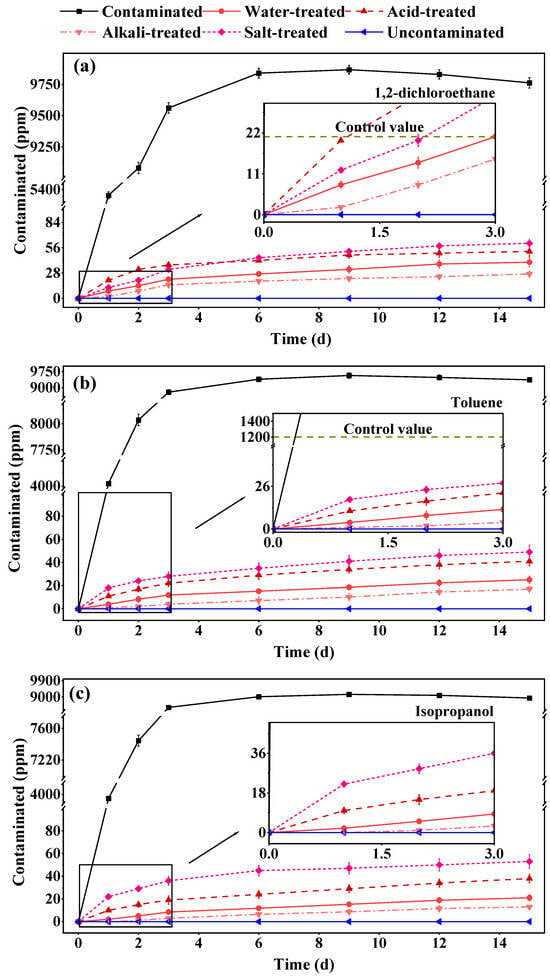
Figure 8.
The different VOC barrier effectiveness of the modified materials. (a) 1,2-dichloroethane. (b) Toluene. (c) Isopropanol.
Moreover, regarding the emergency barrier capability of the modified materials, within three days of incorporation into polluted soil, the concentrations of various VOC pollutants within the barrier materials were maintained below the national regulatory limits. This phenomenon indicated that the modified barrier materials could potentially mitigate short-term or even emergency pollution.
Furthermore, Figure 8 demonstrated that the ability of the modified barrier materials to block organic pollutants remained largely unaffected by acids, alkalies, and high salinity conditions. Although acids and high salinity significantly impacted barrier performance, the materials maintained over 90% efficacy. Notably, the barrier performance was enhanced in alkaline conditions, which aligned with the observed physical property changes of alkaline-activated materials. These findings also suggested that the modified barrier materials exhibited a certain degree of environmental adaptability in their practical barrier performance.
The exceptional barrier performance of multi-level modified materials, achieving over 99% VOC pollutant barrier efficiency while withstanding acidic, alkaline, and saline corrosive environments over a 15-day cycle, is attributed to the modifications at each stage. First, sodium silicate-based adhesive modification promotes rapid hydration of calcium silicate gel and formation of strength-enhancing substances like magnesium and calcium fluorides within 24 h, resulting in low pollutant concentrations in the first 3 days, indicating the material’s suitability for short-term rapid barriers. Second, modification with polyether polyols and polypropylene polymers creates a dense, corrosion-resistant layer, ensuring effective barrier performance in corrosive environments. Finally, biochar-enhanced materials maintain over 99% VOC barrier efficiency even at 10,000 ppm concentrations, highlighting their effectiveness in high-pollutant conditions.
These stages together explain the materials’ excellent barrier properties, including environmental adaptability and rapid performance, while revealing their potential in complex environments and providing solid theoretical support.
4. Conclusions
Modified barrier materials were enhanced by incorporating adhesives, polymers, and biochar, which respectively strengthened the materials, formed protective layers, and improved microstructure. These modifications significantly improved the rapid molding, corrosion resistance, and pollutant adsorption of alkali-activated barrier materials, enabling green alkali-activated barrier materials to sustainably achieve effective performance in complex environments with high pollutant concentrations or corrosive conditions.
Sodium silicate and fluosilicate adhesives reduced molding time from 72 h to 12 h. Polyether polyol and polypropylene polymers preserved over 80% of the materials’ mechanical and chemical properties in acidic, alkaline, and saline environments, compared to 60% in unmodified materials. Biochar-modified materials achieved 99.5 ± 0.3% VOC barrier efficiency at an initial concentration of 10,000 ppm in a sealed system at 25 °C and consistently maintained over 99% VOC barrier performance even under corrosive conditions. These improvements were attributed to faster molding, corrosion resistance, and enhanced pollutant adsorption. Therefore, this green modified alkali-activated barrier material with significant sustainability may be able to replace cement-based barrier materials for rapid pollutant barrier applications in acidic, alkaline, and saline environments.
Future research will further focus on the life cycle assessment and economic evaluation of materials while also exploring a wider range of pollutants beyond VOCs to validate the sustainability and application prospects of multi-stage modified alkali-activated materials as green and efficient barrier materials in various scenarios.
Author Contributions
Y.G.: Writing-original draft, Methodology, Formal analysis, Writing—review and editing. Q.H.: Data curation, Resources, Supervision, Y.H. (Yue Hu): Supervision, Writing—review and editing, X.C. and S.W.: Resources, Writing—review and editing, Z.W. and Y.H. (Yue Hui): Visualization, M.S.: Funding acquisition, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (grant number: 2022YFC3702500).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article material, and further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the National Key Research and Development Program (No. 2022YFC3702500) for their support of this study, and we sincerely thank the editor and all reviewers for their valuable comments and feedback.
Conflicts of Interest
The authors declare no conflict of interest.
Declaration of Generative AI and Al-Assisted Technologies in the Writing Process
During the preparation of this work the authors used ChatGPT3.5 in order to improve language. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
References
- Carvalho, S.-Z.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S.-N. The Recycling Effect of BOF Slag in the Portland Cement Properties. Resour. Conserv. Recycl. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Zhang, M.-T.; Yang, C.-H.; Zhao, M.; Yu, L.-W.; Yang, K.; Zhu, X.-H.; Jiang, X. Immobilization of Cr(VI) by Hydrated Portland Cement Pastes with and without Calcium Sulfate. J. Hazard. Mater. 2018, 342, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-H.; Grubb, D.-G.; Reilly, T.L. Stabilization/Solidification of Selenium-Impacted Soils Using Portland Cement and Cement Kiln Dust. J. Hazard. Mater. 2009, 168, 944–951. [Google Scholar] [CrossRef]
- Wang, C.; Engels, A.; Wang, Z.-H. Overview of Research on China’s Transition to Low-Carbon Development: The Role of Cities, Technologies, Industries, and the Energy System. Renew. Sustain. Energy Rev. 2018, 81 Pt 1, 1350–1364. [Google Scholar] [CrossRef]
- Zou, H.; Cui, S.-T.; Zhao, K.; Cheng, J.-S.; Zheng, Z.-J.; Li, S.-J.; Zhang, Y.-L. Dynamic Mechanical Properties and Microstructure of Ultrafine Slag Powder Cement Paste Utilizing Solid Waste Industrial Tailings Powder and Fly Ash. Process Saf. Environ. Prot. 2024, 191, 813–827. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, D. Transforming Waste into Sustainable Solution: Physicochemical and Geotechnical Evaluation of Cement Stabilized Municipal Solid Waste Incinerator Bottom Ash for Geoenvironmental Applications. Process Saf. Environ. Prot. 2023, 176, 685–695. [Google Scholar] [CrossRef]
- Adesina, A. Synthesis, Characterization, and Efficacy of Alkali-Activated Materials from Mine Tailings: A Review. Waste Manag. 2025, 191, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-C.; Wang, B.-M.; Ai, H.-M.; Qi, Y.; Liu, Z. A Comparative Study on Solidification/Stabilization Characteristics of Coal Fly Ash-Based Geopolymer and Portland Cement on Heavy Metals in MSWI Fly Ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, Á. Sustainable Alkali Activated Materials: Precursor and Activator Derived from Industrial Wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Sadegh, F.; Sadegh, N.; Wongniramaikul, W.; Apiratikul, R.; Choodum, A. Adsorption of Volatile Organic Compounds on Biochar: A Review. Process Saf. Environ. Prot. 2024, 182, 559–578. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Effect of Silica Fume on Efflorescence Formation and Alkali Leaching of Alkali-Activated Slag. J. Clean. Prod. 2021, 315, 128210. [Google Scholar] [CrossRef]
- Tang, W.; Ye, C.-W.; Zhang, Q.; Li, J.; Ao, F.; Zheng, B.; Luo, Y. Study on the Carbon Sequestration Performance and Barrier Mechanism of Biochar Cement-Based Vertical Cutoff Walls for Phenol Pollution in Groundwater. J. Environ. Chem. Eng. 2024, 12, 114560. [Google Scholar] [CrossRef]
- Zhao, Z.; El-Naggar, A.; Kau, J.; Olson, C.; Tomlinson, D.; Chang, S.X. Biochar Affects Compressive Strength of Portland Cement Composites: A Meta-Analysis. Biochar 2024, 6, 21. [Google Scholar] [CrossRef]
- Matinfar, M.; Nychka, J.-A. A Review of Sodium Silicate Solutions: Structure, Gelation, and Syneresis. Adv. Colloid Interface Sci. 2023, 322, 103036. [Google Scholar] [CrossRef]
- Li, B.; Trueman, B.F.; Munoz, S.; Locsin, J.M.; Gagnon, G.A. Impact of Sodium Silicate on Lead Release and Colloid Size Distributions in Drinking Water. Water Res. 2021, 190, 116709. [Google Scholar] [CrossRef]
- Sun, N.; Di, M.; Liu, Y. Lignin-Containing Polyurethane Elastomers with Enhanced Mechanical Properties via Hydrogen Bond Interactions. Int. J. Biol. Macromol. 2021, 184, 1–8. [Google Scholar] [CrossRef]
- Fang, C.-Q.; Pan, S.-F.; Wang, Z.; Zhou, X.; Lei, W.-Q.; Cheng, Y.-L. Synthesis of Waterborne Polyurethane Using Snow as Dispersant: Structures and Properties Controlled by Polyols Utilization. J. Mater. Sci. Technol. 2019, 35, 1491–1498. [Google Scholar] [CrossRef]
- Feng, X.-M.; Wang, B.; Wang, X.; Wen, P.-Y.; Cai, W.; Hu, Y.; Liew, K.M. Molybdenum Disulfide Nanosheets as Barrier Enhancing Nanofillers in Thermal Decomposition of Polypropylene Composites. Chem. Eng. J. 2016, 295, 278–287. [Google Scholar] [CrossRef]
- Zielińska, D.; Siwińska-Ciesielczyk, K.; Bula, K.; Peplińska, B.; Jesionowski, T.; Borysiak, S. Advanced Nanocellulose Hybrid Fillers for Sustainable Polypropylene Biomaterials with Enhanced Oxygen Barrier Properties. Appl. Mater. Today 2023, 34, 101897. [Google Scholar] [CrossRef]
- JTG E40-2007; Highway Geotechnical Test Procedures. China Communications Press: Beijing, China, 2007.
- JTG E40-2007; Highway Engineering Geotechnical Test Procedures. China Communications Press: Beijing, China, 2007.
- Hu, Y.; Huan, Q.; Lai, J.-H.; Yao, X.-Y.; Song, C.-Y.; Song, M. Modification of Multi-Source Industrial Solid Waste with Porous Materials to Produce Highly Polymerized Silica Gel: Microstructure Optimization and CO2 Mineralization Enhancement Mechanism. Sep. Purif. Technol. 2024, 336, 126225. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Song, B.; Huan, Q.; Hu, Y.; Song, M. Ambient pressure carbonation curing of cold-bonded fly ash lightweight aggregate using coal-fired flue gas for properties enhancement and CO2 sequestration. J. Environ. Chem. Eng. 2024, 12, 114208. [Google Scholar] [CrossRef]
- Wei, H.; Song, B.; Huan, Q.; Song, C.-Y.; Wang, S.-F.; Song, M. Preparation of iron tailings-based porous ceramsite and its application to lead adsorption: Characteristic and mechanism. Sep. Purif. Technol. 2024, 342, 126839. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Wang, T.; Xia, J.-L.; Lu, M.-X.; Xue, Y.-J. Kill two birds with one stone: Contribution of steel slag on enhancing the performance of coal gangue-based cementitious materials and simultaneous sulfur fixation. J. Environ. Chem. Eng. 2023, 11, 111169. [Google Scholar] [CrossRef]
- Palankar, N.; Ravi Shankar, A.-U.; Mithun, B.-M. Durability studies on eco-friendly concrete mixes incorporating steel slag as coarse aggregates. J. Clean. Prod. 2016, 129, 437–448. [Google Scholar] [CrossRef]
- Yang, P.; Liu, L.; Suo, Y.-L.; Zhu, M.-B.; Xie, G.; Deng, S.-C. Mechanical properties, pore characteristics and microstructure of modified magnesium slag cemented coal-based solid waste backfill materials: Affected by fly ash addition and curing temperature. Process Saf. Environ. Prot. 2023, 176, 1007–1020. [Google Scholar] [CrossRef]
- Vieira, C.-S.; Pereira, P.-M.; Lopes, M.d.L. Recycled construction and demolition wastes as filling material for geosynthetic reinforced structures: Interface properties. J. Clean. Prod. 2016, 124, 299–311. [Google Scholar] [CrossRef]
- Poinot, T.; Laracy, M.E.; Aponte, C.; Jennings, H.M.; Ochsendorf, J.A.; Olivetti, E.A. Beneficial use of boiler ash in alkali-activated bricks. Resour. Conserv. Recycl. 2018, 128, 1–10. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.-R.; Xu, W.-R.; Yuan, Q.; Niu, D. Hydration characteristics and microstructure of alkali-activated slag concrete: A review. Engineering 2023, 20, 162–179. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-Clay, Biochar-Microorganism, and Biochar-Enzyme Composites for Environmental Remediation: A Review. Environ. Chem. Lett. 2023, 21, 1837–1862. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Park, J.-W.; Choung, J.-H.; Choi, D.-K. Adsorption characteristics of SO2 on activated carbon prepared from coconut shell with potassium hydroxide activation. Environ. Sci. Technol. 2002, 36, 1086–1092. [Google Scholar] [CrossRef]
- Xu, W.-J.; Zhang, Y.-Y.; Li, M.; Qu, F.; Poon, C.-S.; Zhu, X.-H.; Tsang, D.C.W. Durability and micromechanical properties of biochar in biochar-cement composites under marine environment. J. Clean. Prod. 2024, 450, 141842. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, J.-S.; Wang, Y.; Guo, H. Gas permeability and emission in unsaturated vegetated landfill cover with biochar addition. Biochar 2023, 5, 47. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.-Y. Hydration, strength, durability, and workability of biochar-cement binary blends. J. Build. Eng. 2021, 42, 103064. [Google Scholar] [CrossRef]
- Su, Y.; Wen, Y.; Yang, W.; Zhang, X.; Xia, M.; Zhou, N.; Xiong, Y.; Zhou, Z. The mechanism transformation of ramie biochar’s cadmium adsorption by aging. Bioresour. Technol. 2021, 330, 124947. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Luo, J.; Chang, H.; Wang, P.-H.; Moon, R.J.; Kumar, S. Cellulose nanocrystals effect on the stabilization of polyacrylonitrile composite films. Carbon 2018, 134, 92–102. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Li, X.; Cai, T.; Chung, T.-S. Anti-fouling behavior of hyperbranched polyglycerol-grafted poly(ether sulfone) hollow fiber membranes for osmotic power generation. Environ. Sci. Technol. 2014, 48, 9898–9907. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Cheng, Y.; Song, F.; Ding, Y.; Shao, M. Self-reinforced composites based on polypropylene fiber and graphene nano-platelets/polypropylene film. Carbon 2022, 189, 586–595. [Google Scholar] [CrossRef]
- Sunder, A.; Mülhaupt, R.; Haag, R.; Frey, H. Hyperbranched Polyether Polyols: A Modular Approach to Complex Polymer Architectures. Adv. Mater. 2000, 12, 235–239. [Google Scholar] [CrossRef]
- Wang, X.-T.; Liang, Y.-F.; Pu, Z.-C.; He, J.; Yang, S.-Q. Transforming waste to treasure: Superhydrophobic coatings from recycled polypropylene for high-value application. Prog. Org. Coat. 2024, 188, 108248. [Google Scholar] [CrossRef]
- Xie, G.; Liu, L.; Suo, Y.-L.; Yang, P.; Zhang, C.-X.; Qu, H.-S.; Lv, Y. Hydration mechanism of calcium chloride modified coal gasification slag-based backfill materials. Process Saf. Environ. Prot. 2024, 182, 127–138. [Google Scholar] [CrossRef]
- Qu, R.-J.; Wang, Y.; Li, D.; Wang, L.-J. Rheological behavior of nanocellulose gels at various calcium chloride concentrations. Carbohydr. Polym. 2021, 274, 118660. [Google Scholar] [CrossRef] [PubMed]
- Huan, Q.; Wibowo, H.; Yan, M.; Song, M. A review of CO2 mineral storage: Current processes, typical applications, and life cycle assessment. J. Environ. Chem. Eng. 2024, 12, 114785. [Google Scholar] [CrossRef]
- Xing, J.; Dong, W.; Liang, N.; Huang, Y.; Wu, M.; Zhang, L.-J.; Chen, Q. Sorption of organic contaminants by biochars with multiple porous structures: Experiments and molecular dynamics simulations mediated by three-dimensional models. J. Hazard. Mater. 2023, 458, 131953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Xia, B.; Liu, Z.-H.; Tan, C.-Q.; Song, C.-Y.; Fujii, M.; Ma, L.; Song, M. Defect engineering boosted peroxydisulfate activation of dual-vacancy Cu–Fe spinel oxides for soil organics decontamination. ACS ES&T Eng. 2024, 4, 2025–2035. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, X.-F.; Wang, Z.; Peng, K.-M.; Lu, L.-J.; Liu, J. Composite-polymer modified bentonite enhances anti-seepage and barrier performance under high-concentration heavy-metal solution. J. Clean. Prod. 2022, 376, 134253. [Google Scholar] [CrossRef]
- Souza, R.-F.C.; Pejon, O.-J. Pore size distribution and swelling behavior of compacted bentonite/claystone and bentonite/sand mixtures. Eng. Geol. 2020, 275, 105738. [Google Scholar] [CrossRef]
- Bawn, C. High Polymers. Nature 1957, 180, 672–673. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Luo, X.-F.; Song, Z.-J.; Lu, K.; Zhu, S.-W.; Yang, Y.-S.; Zhang, Y.-T.; Fang, W.-X.; Jin, J. Microporous polymer adsorptive membranes with high processing capacity for molecular separation. Nat. Commun. 2022, 13, 4169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).