Comparison of Fuel Properties of Alternative Fuels from Insect Lipids and Their Blending with Diesel Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Black Soldier Fly Larvae (BSFL) Oil
2.2. Preparation of the Blends
2.3. Analytical Methods
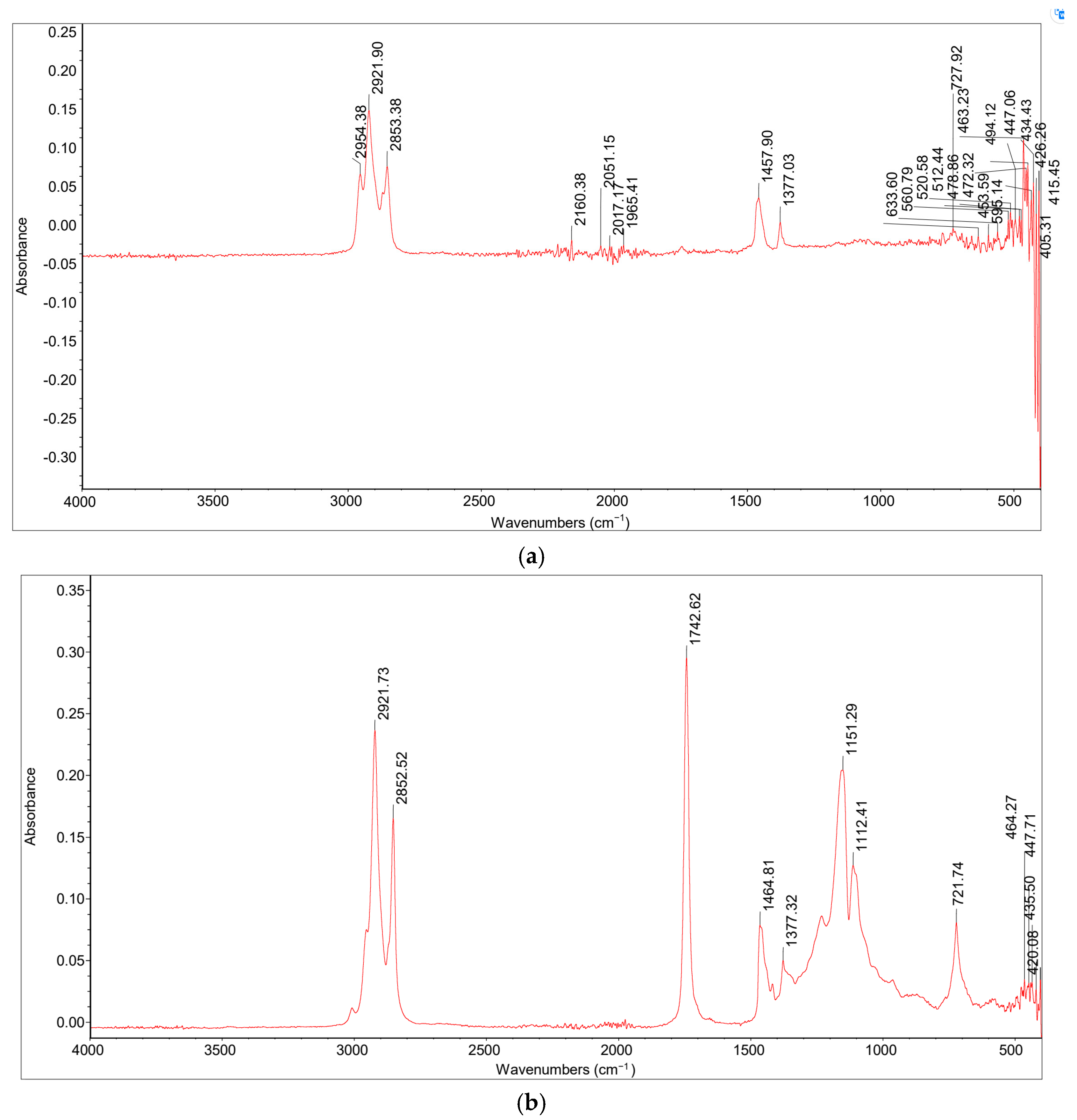
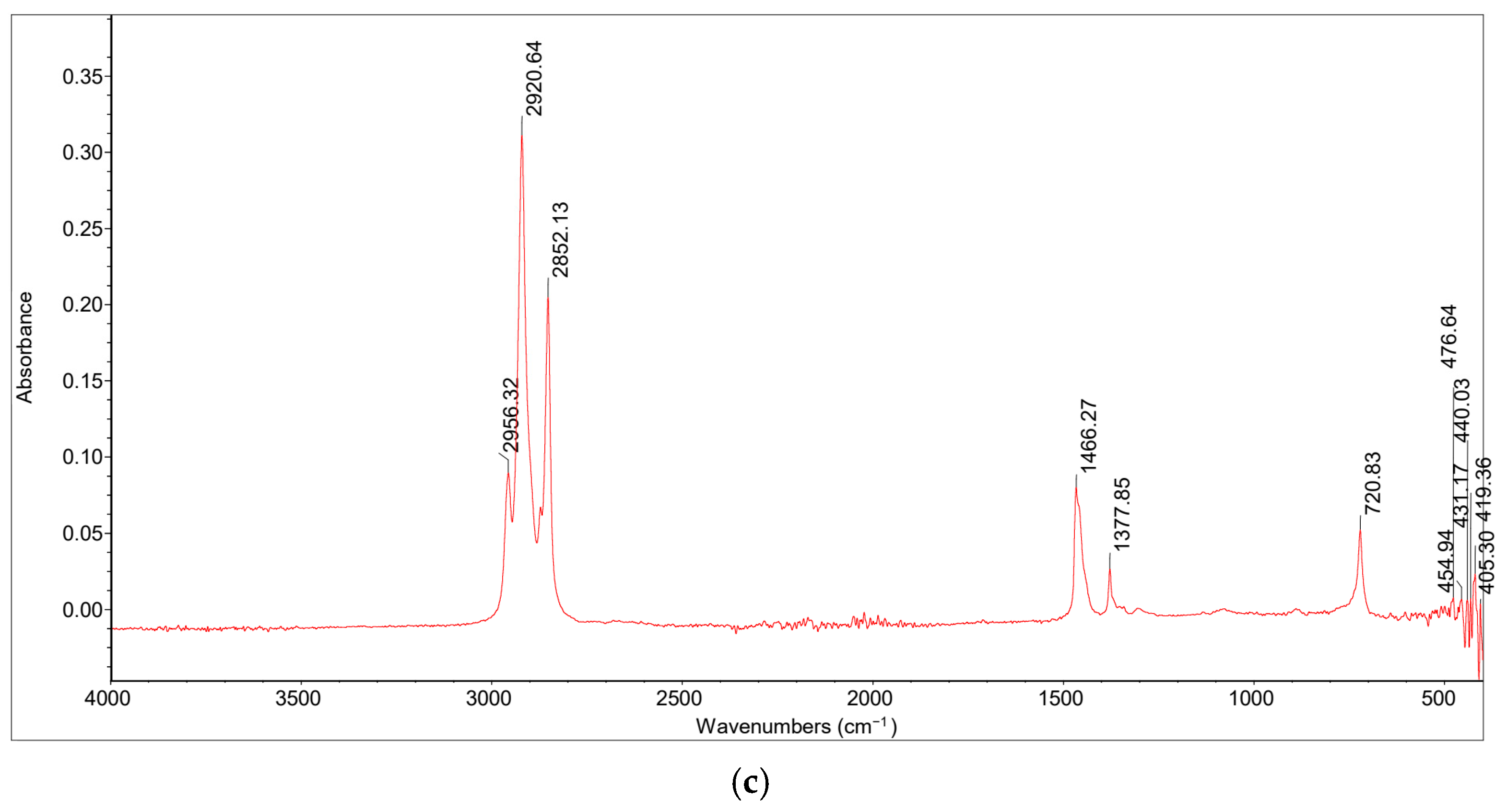
2.3.1. Fourier Transform-Infrared (FT-IR) Spectroscopy
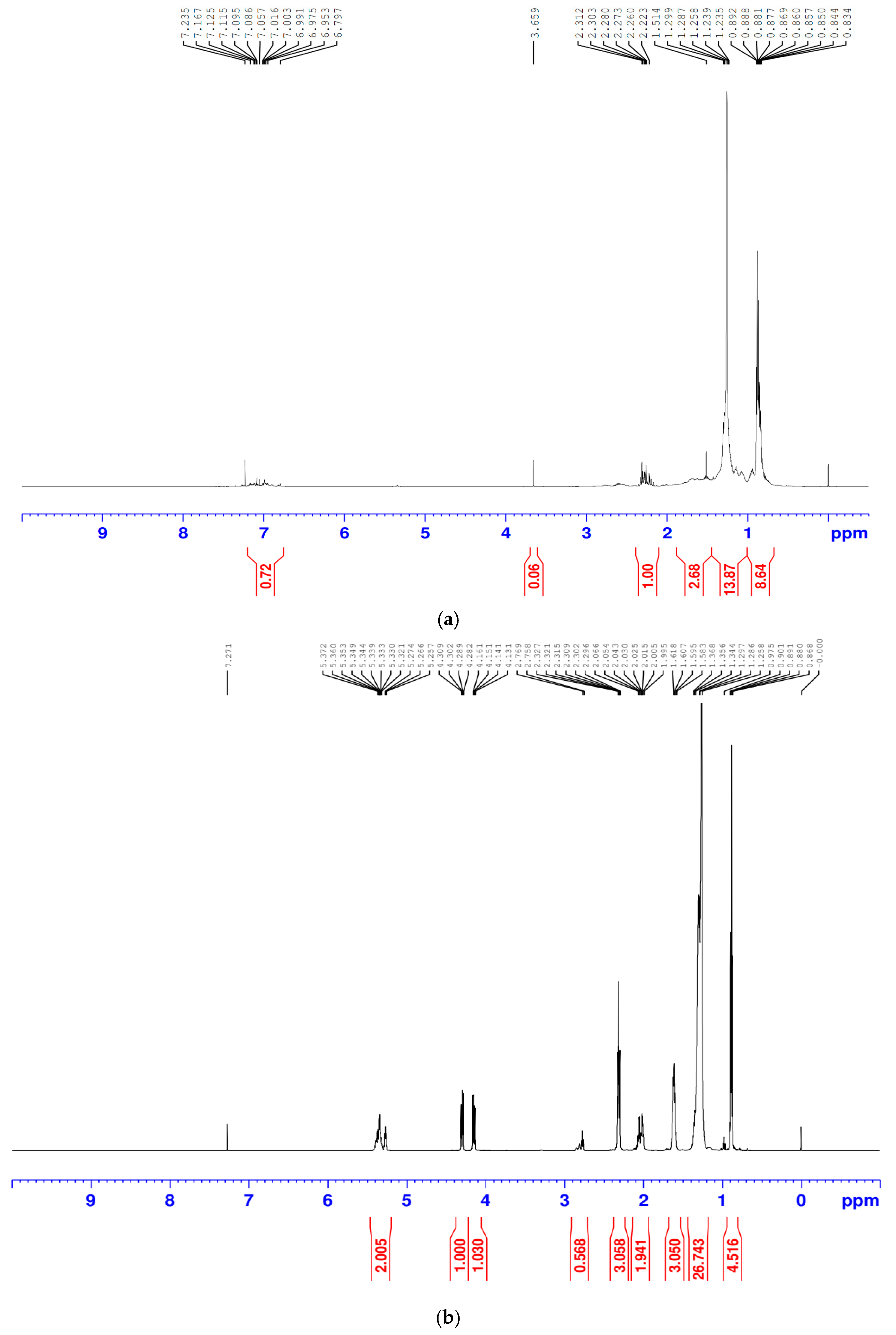
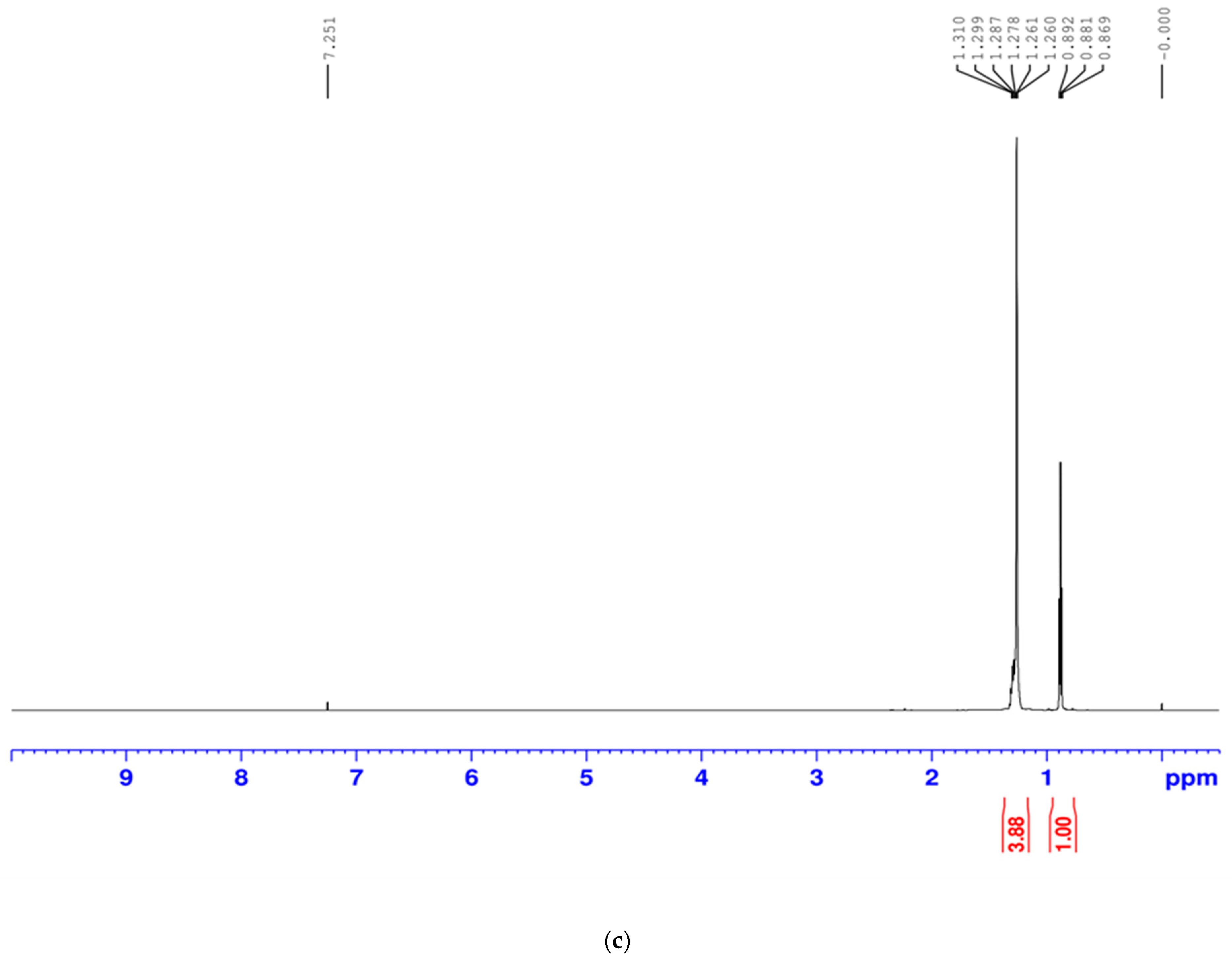
2.3.2. Proton Nuclear Magnetic Resonance (1H NMR)
2.3.3. 13C Nuclear Magnetic Resonance (13C NMR)
2.3.4. GC Analysis
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Physicochemical Fuel Properties
3. Results and Discussion
3.1. Effect of Feeding Mixtures on BSFL Growth
3.2. Fuel Properties Analysis
3.2.1. Properties of Commercial Diesel, IO, and HIO
FT-IR
1H-NMR
13C-NMR
3.2.2. Properties of Blends
IO Blends
HIO Blends
3.2.3. Comparison of Fuel Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirjola, L.; Kuuluvainen, H.; Timonen, H.; Saarikoski, S.; Teinilä, K.; Salo, L.; Datta, A.; Simonen, P.; Karjalainen, P.; Kulmala, K.; et al. Potential of Renewable Fuel to Reduce Diesel Exhaust Particle Emissions. Appl. Energy 2019, 254, 113636. [Google Scholar] [CrossRef]
- Arvidsson, R.; Persson, S.; Fröling, M.; Svanström, M. Life Cycle Assessment of Hydrotreated Vegetable Oil from Rape, Oil Palm and Jatropha. J. Clean. Prod. 2011, 19, 129–137. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” Fuel Production from Biomass: Critical Review on Techno-Economic Feasibility and Sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Palankoev, T.A.; Dementiev, K.I.; Khadzhiev, S.N. Promising Processes for Producing Drop-in Biofuels and Petrochemicals from Renewable Feedstock (Review). Pet. Chem. 2019, 59, 438–446. [Google Scholar] [CrossRef]
- Bullermann, J.; Meyer, N.C.; Krafft, A.; Wirz, F. Comparison of Fuel Properties of Alternative Drop-in Fuels with Standard Marine Diesel and the Effects of Their Blends. Fuel 2024, 357, 129937. [Google Scholar] [CrossRef]
- Bortel, I.; Vávra, J.; Takáts, M. Effect of HVO Fuel Mixtures on Emissions and Performance of a Passenger Car Size Diesel Engine. Renew. Energy 2019, 140, 680–691. [Google Scholar] [CrossRef]
- Roy, P.; Jahromi, H.; Rahman, T.; Adhikari, S.; Feyzbar-Khalkhali-Nejad, F.; Barbary Hassan, E.; Oh, T.S. Understanding the Effects of Feedstock Blending and Catalyst Support on Hydrotreatment of Algae HTL Biocrude with Non-Edible Vegetable Oil. Energy Convers. Manag. 2022, 268, 115998. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel. Catalysts 2019, 9, 293. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia Illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of Dairy Manure by Black Soldier Fly (Diptera: Stratiomyidae) for Biodiesel and Sugar Production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the Potential of Lipids from Black Soldier Fly: New Paradigm for Biodiesel Production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric Acid from the Black Soldier Fly (Hermetia Illucens) and Its Potential Applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Park, J.Y.; Jung, S.; Na, Y.G.; Jeon, C.H.; Cheon, H.Y.; Yun, E.Y.; Lee, S.H.; Kwon, E.E.; Kim, J.K. Biodiesel Production from the Black Soldier Fly Larvae Grown on Food Waste and Its Fuel Property Characterization as a Potential Transportation Fuel. Environ. Eng. Res. 2022, 27, 200704. [Google Scholar] [CrossRef]
- Lee, J.E.; Jang, H.S.; Yun, Y.J.; Han, G.B.; Park, Y.K.; Yang, Y.C.; Jang, J.H. Application of the Hydrodeoxygenation of Black Soldier Fly Larvae Lipids in Green Diesel Production. Sustainability 2024, 16, 584. [Google Scholar] [CrossRef]
- KS M 2610:2023; Diesel Fuel Oil. Korean Agency for Technology and Standards: Eumseong, Republic of Korea, 2023.
- Han, G.B.; Jang, J.H.; Ahn, M.H.; Suh, Y.W.; Choi, M.; Park, N.K.; Lee, M.E.; Kim, J.K.; Jeong, B. Operation of Bio-Aviation Fuel Manufacturing Facility via Hydroprocessed Esters and Fatty Acids Process and Optimization of Fuel Property for Turbine Engine Test. Korean J. Chem. Eng. 2021, 38, 1205–1223. [Google Scholar] [CrossRef]
- Moser, B.R.; Evangelista, R.L.; Jham, G. Fuel Properties of Brassica Juncea Oil Methyl Esters Blended with Ultra-Low Sulfur Diesel Fuel. Renew. Energy 2015, 78, 82–88. [Google Scholar] [CrossRef]
- Alves, A.A.A.; Alves, R.S.; de Medeiros, P.Y.G.; Maia, L.C.; Feitosa, F.X.; de Sant’Ana, H.B. Distillation Analysis of Diesel-Biodiesel Mixtures: A Comparative Study with ASTM Norms, Experimental Data, and Novel Correlations. Fuel 2025, 383, 133864. [Google Scholar] [CrossRef]
- ASTM D4052-22; Standard Test Method for Density and Relative Density of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D5453-19a; Standard Test Method for Determination of Total Nitrogen in Fuel Oils and Lubricants by High Temperature Catalytic Conversion and Chemiluminescence Detection. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D4737-21; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Reduced Pressure. ASTM International: West Conshohocken, PA, USA, 2021.
- KS M ISO 3104:2020; Standard Test Method for Transparent and Opaque Liquids—Determination of Kinematic Viscosity. Korean Agency for Technology and Standards: Eumseong, Republic of Korea, 2020.
- KS M ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. Korean Standards Association: Seoul, Republic of Korea, 2016.
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Widya Yuwono, N.; Nisa’, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black Soldier Fly Larvae (BSFL) and Their Affinity for Organic Waste Processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Kandasamy, S.; Surendran, R.P.; Kumar, R.; Rajan, D.K.; Rajarajeswaran, J. Food Waste-Derived Black Soldier Fly (Hermetia Illucens) Larval Resource Recovery: A Circular Bioeconomy Approach. Process Saf. Environ. Prot. 2024, 184, 170–189. [Google Scholar] [CrossRef]
- Coronado, M.A.; Montero, G.; García, C.; Valdez, B.; Ayala, R.; Pérez, A. Quality Assessment of Biodiesel Blends Proposed by the New Mexican Policy Framework. Energies 2017, 10, 631. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Production and Characterization of Biodiesel from Algae. Fuel Process. Technol. 2014, 120, 79–88. [Google Scholar] [CrossRef]
- Sarker, M.; Rashid, M.M.; Molla, M. Waste Plastic Conversion into Hydrocarbon Fuel like Low Sulfur Diesel. J. Environ. Sci. Eng. 2011, 5, 446–452. [Google Scholar]
- Abbas, O.; Rebufa, C.; Dupuy, N.; Permanyer, A.; Kister, J. Assessing Petroleum Oils Biodegradation by Chemometric Analysis of Spectroscopic Data. Talanta 2008, 75, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Windarsih, A.; Jatmiko, T.H.; Anggraeni, A.S.; Rahmawati, L. Machine Learning-Assisted FT-IR Spectroscopy for Identification of Pork Oil Adulteration in Tuna Fish Oil. Vib. Spectrosc. 2024, 134, 103715. [Google Scholar] [CrossRef]
- Kothiyal, N.C.; Kumar, V.; Kumar, N.; Saruchi; Pandey, S.; Ansar, S. Reduction of Carcinogenic PAHs from Petrodiesel Engine Exhaust by Blending of Green Diesel (A New Development in Renewable Fuels) with Different Biodiesel Fuels. Environ. Technol. Innov. 2022, 25, 102089. [Google Scholar] [CrossRef]
- Sihlovec, F.; Vrtiška, D.; Šimáček, P. The Use of Multivariate Statistics and Mathematically Modeled IR Spectra for Determination of HVO Content in Diesel Blends. Fuel 2025, 379, 132963. [Google Scholar] [CrossRef]
- Vuković, J.P.; Srića, V.; Novak, P. Fast Determination of Diesel Fuel Oxidation Stability by 1H NMR Spectroscopy. Acta Chim. Slov. 2015, 62, 233–236. [Google Scholar]
- Raj, M.R.G.; Rani, M.P.; Rameshkumar, K.B. Detection of Coconut Oil Adulteration with Palm Oil through NMR Spectroscopic Method. Grasas Y Aceites 2024, 75, 2012. [Google Scholar]
- Edwards, J.C. A Review of Applications of NMR Spectroscopy in the Petroleum Industry. In Spectroscopic Analysis of Petroleum Products and Lubricants; Chapter 16; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Krivdin, L.B. Recent Advances in 1D and 2D Liquid-Phase and Solid-State NMR Studies of Biofuel. Renew. Energy 2025, 243, 122592. [Google Scholar] [CrossRef]
- Renna, M.; Gasco, L.; Livorsi, L.; Mele, M.; Conte, G.; Meneguz, M.; Lussiana, C. Growth Performance, Proximate Composition and Fatty Acid Profile of Black Soldier Fly Larvae Reared on Two Grape Pomace Varieties. Animal 2024, 18, 101240. [Google Scholar] [CrossRef] [PubMed]
- Noriega, A.K.; Tirado, A.; Méndez, C.; Marroquín, G.; Ancheyta, J. Hydrodeoxygenation of Vegetable Oil in Batch Reactor: Experimental Considerations. Chin. J. Chem. Eng. 2020, 28, 1670–1683. [Google Scholar] [CrossRef]
- Rabaev, M.; Landau, M.V.; Vidruk-Nehemya, R.; Koukouliev, V.; Zarchin, R.; Herskowitz, M. Conversion of Vegetable Oils on Pt/Al2O3/SAPO-11 to Diesel and Jet Fuels Containing Aromatics. Fuel 2015, 161, 287–294. [Google Scholar] [CrossRef]
- Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of Palm Oil Renewable Diesel through Hydroisomerization and Formulation of an Optimal Blend. Fuel 2017, 209, 442–448. [Google Scholar] [CrossRef]
- Magalhães, A.M.S.; Pereira, E.; Meirelles, A.J.A.; Sampaio, K.A.; Maximo, G.J. Proposing Blends for Improving the Cold Flow Properties of Ethylic Biodiesel. Fuel 2019, 253, 50–59. [Google Scholar] [CrossRef]
- Appavu, P.; Ramanan, M.V.; Venu, H. Quaternary Blends of Diesel/Biodiesel/Vegetable Oil/Pentanol as a Potential Alternative Feedstock for Existing Unmodified Diesel Engine: Performance, Combustion and Emission Characteristics. Energy 2019, 186, 115856. [Google Scholar] [CrossRef]
- Alagu, K.; Venu, H.; Jayaraman, J.; Raju, V.D.; Subramani, L.; Appavu, P. Novel Water Hyacinth Biodiesel as a Potential Alternative Fuel for Existing Unmodified Diesel Engine: Performance, Combustion and Emission Characteristics. Energy 2019, 179, 295–305. [Google Scholar] [CrossRef]
- ASTM D975-24a; Standard Specification for Diesel Fuel. ASTM International: West Conshohocken, PA, USA, 2024.
- Bezergianni, S.; Dimitriadis, A. Comparison between Different Types of Renewable Diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]



| Feeding Mixtures | Meat Byproduct (wt%) | Wet Food Waste (wt%) | Dried Food Waste (wt%) | Processed Food Waste (wt%) |
|---|---|---|---|---|
| M1 | 0 | 100 | 0 | 0 |
| M2 | 20 | 80 | 0 | 0 |
| M3 | 0 | 80 | 20 | 0 |
| M4 | 0 | 50 | 50 | 0 |
| M5 | 30 | 40 | 30 | 0 |
| M6 | 0 | 0 | 0 | 100 |
| M7 | 0 | 20 | 0 | 80 |
| M8 | 0 | 50 | 0 | 50 |
| Crude Protein (% w/w) | Crude Lipid (% w/w) | Crude Ash (% w/w) | Crude Fiber (% w/w) | Moisture (% w/w) | Salt Content (% w/w) | |
|---|---|---|---|---|---|---|
| Meat byproduct | 28.3 | 3.4 | 2.5 | 0.2 | 65.0 | trace |
| Wet food waste | 3.0 | 1.2 | 3.0 | 3.0 | 87.0 | 1.5 |
| Dried food waste | 23.1 | 9.4 | 16.6 | 16.2 | 3.8 | 1.2 |
| Processed food waste | 4.5 | 4.6 | 2.6 | 2.7 | 80.7 | 0.7 |
| Feeding Mixture | Mean ± SE | ||
|---|---|---|---|
| Moisture (%) | Lipid Yield (%) | Feeding Mixture Price (USD/kg) | |
| M1 | 6.65 ± 0.2 | 30.21 ± 3.3 | 0.014 |
| M2 | 5.71 ± 0.5 | 25.27 ± 1.0 | 0.014 |
| M3 | 6.33 ± 0.1 | 31.57 ± 2.6 | 0.008 |
| M4 | 4.71 ± 0.2 | 33.2 ± 1.6 | 0.001 |
| M5 | 3.89 ± 0.1 | 26.08 ± 0.5 | 0.006 |
| M6 | 4.68 ± 0.5 | 49.54 ± 1.1 | 0.210 |
| M7 | 3.63 ± 0.4 | 48.71 ± 1.3 | 0.171 |
| M8 | 4.02 ± 0.2 | 45.21 ± 0.5 | 0.112 |
| Experimental Values | Standard Values | ||||
|---|---|---|---|---|---|
| Property | Unit | Commercial Diesel | HIO | HIO 5 | Diesel Standard No. 1-D S15 (ASTM D975) |
| Density at 15 °C | kg/m3 | 823.1 | 770.2 | 820.0 | - |
| Kinematic viscosity at 40 °C | mm2/s | 2.205 | 2.196 | 2.182 | 1.3–2.4 |
| Sulfur | mg/kg | 6.7 | Max 1.0 | 5.6 | Max 15 |
| Flash point | °C | 43 | 72.0 | 43 | Min 38 |
| Cetane index | NA | 49.4 | 84.8 | 51.7 | Min 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Jang, H.S.; Yun, Y.J.; Yang, Y.C.; Jang, J.H. Comparison of Fuel Properties of Alternative Fuels from Insect Lipids and Their Blending with Diesel Fuel. Sustainability 2025, 17, 4295. https://doi.org/10.3390/su17104295
Lee JE, Jang HS, Yun YJ, Yang YC, Jang JH. Comparison of Fuel Properties of Alternative Fuels from Insect Lipids and Their Blending with Diesel Fuel. Sustainability. 2025; 17(10):4295. https://doi.org/10.3390/su17104295
Chicago/Turabian StyleLee, Ji Eun, Hyun Sung Jang, Yeo Jin Yun, Young Cheol Yang, and Jung Hee Jang. 2025. "Comparison of Fuel Properties of Alternative Fuels from Insect Lipids and Their Blending with Diesel Fuel" Sustainability 17, no. 10: 4295. https://doi.org/10.3390/su17104295
APA StyleLee, J. E., Jang, H. S., Yun, Y. J., Yang, Y. C., & Jang, J. H. (2025). Comparison of Fuel Properties of Alternative Fuels from Insect Lipids and Their Blending with Diesel Fuel. Sustainability, 17(10), 4295. https://doi.org/10.3390/su17104295






