Enhancing CO2 Sequestration Through Corn Stalk Biochar-Enhanced Mortar: A Synergistic Approach with Algal Growth for Carbon Capture Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Corn Stalk Biochar
2.2. Mortar Preparation
2.3. Mortar Tests
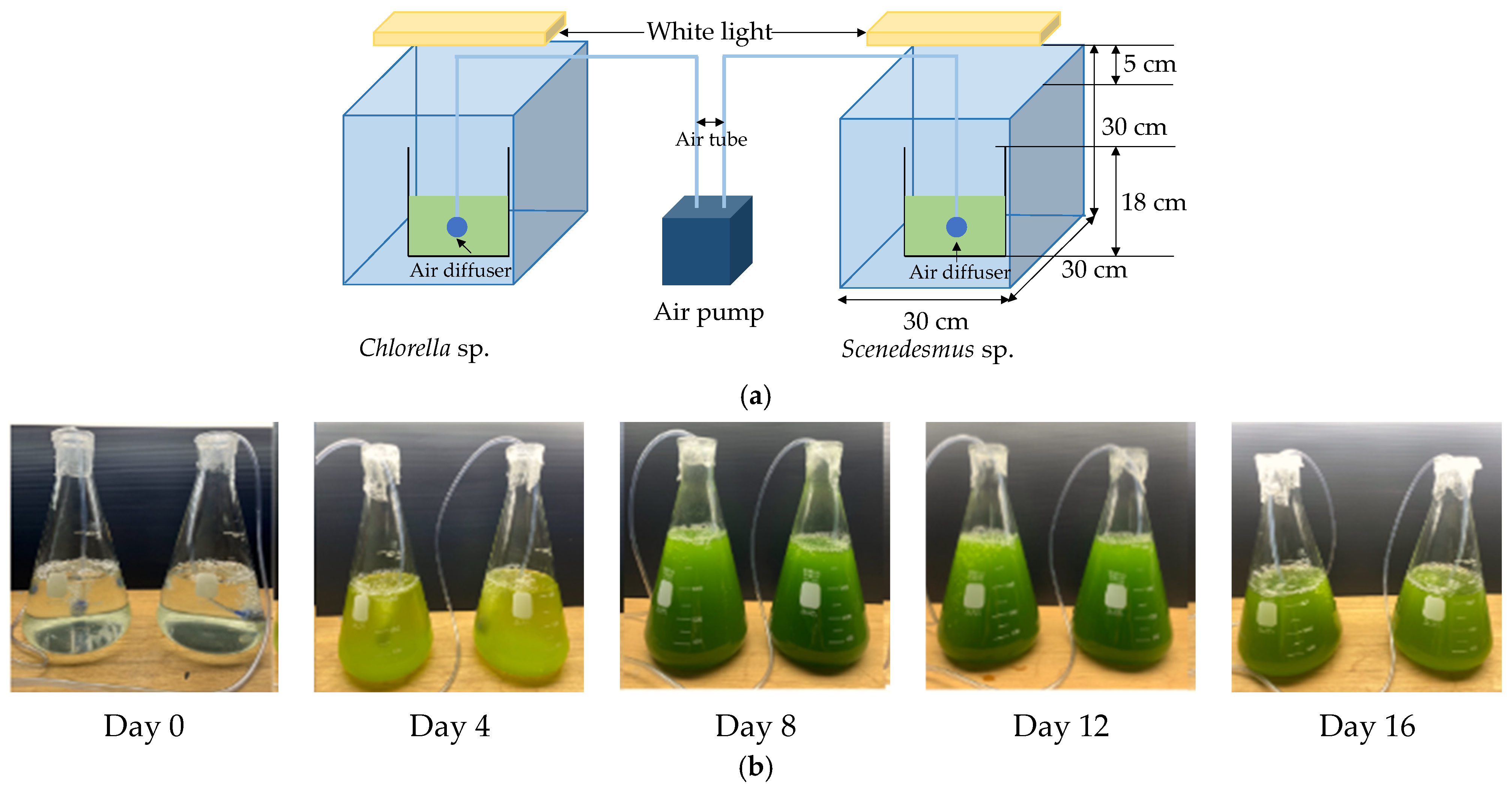
2.4. Microalgae Cultivation on CSB-Enhanced Mortar Specimens
2.4.1. Initial Cultivation of Algae for Biomass Expansion Before Mortar Transfer
2.4.2. Surface Cultivation of Algae on Corn Stalk Biochar-Enhanced Mortar
2.4.3. Assessment of Algal Growth and Their Carbon Dioxide Absorption Capacity
2.5. Data Analysis
3. Results and Discussion
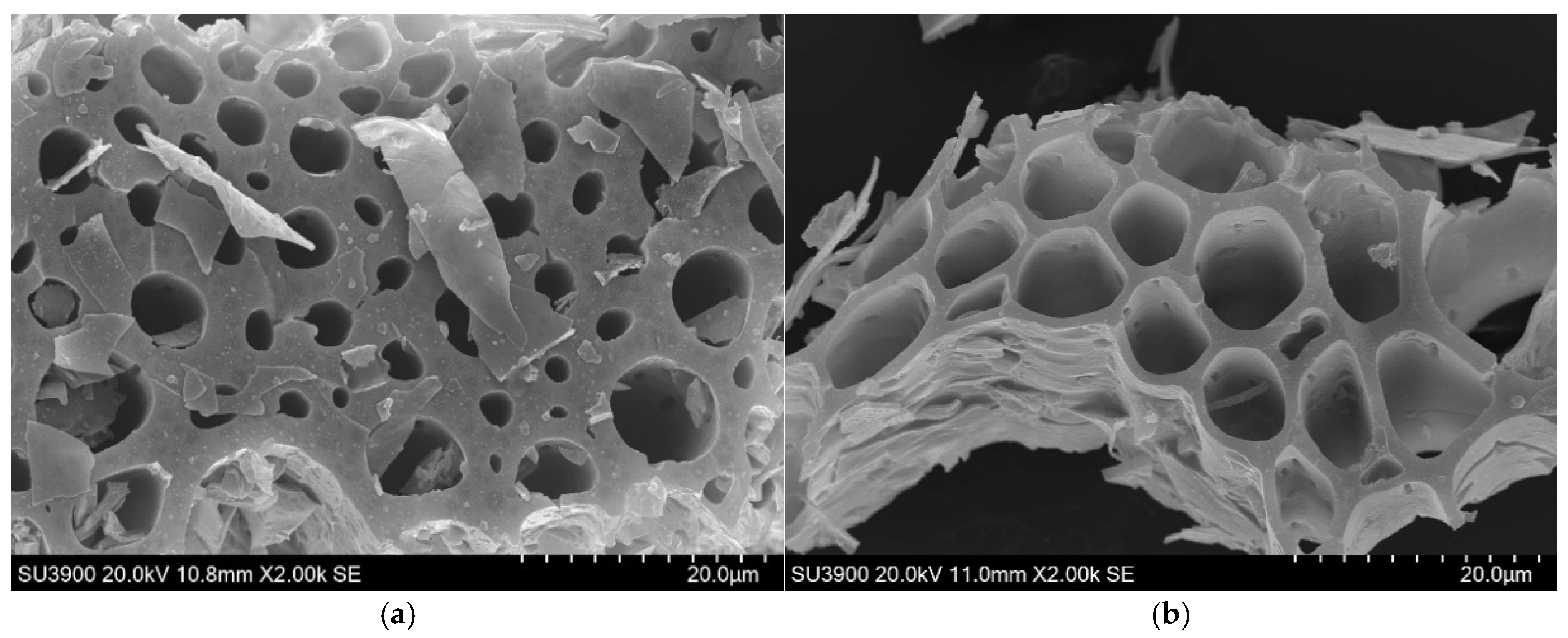
3.1. Characterization of Corn-Stalk Biochar
3.2. Physicochemical Properties of CSB-Enhanced Cement Mortar
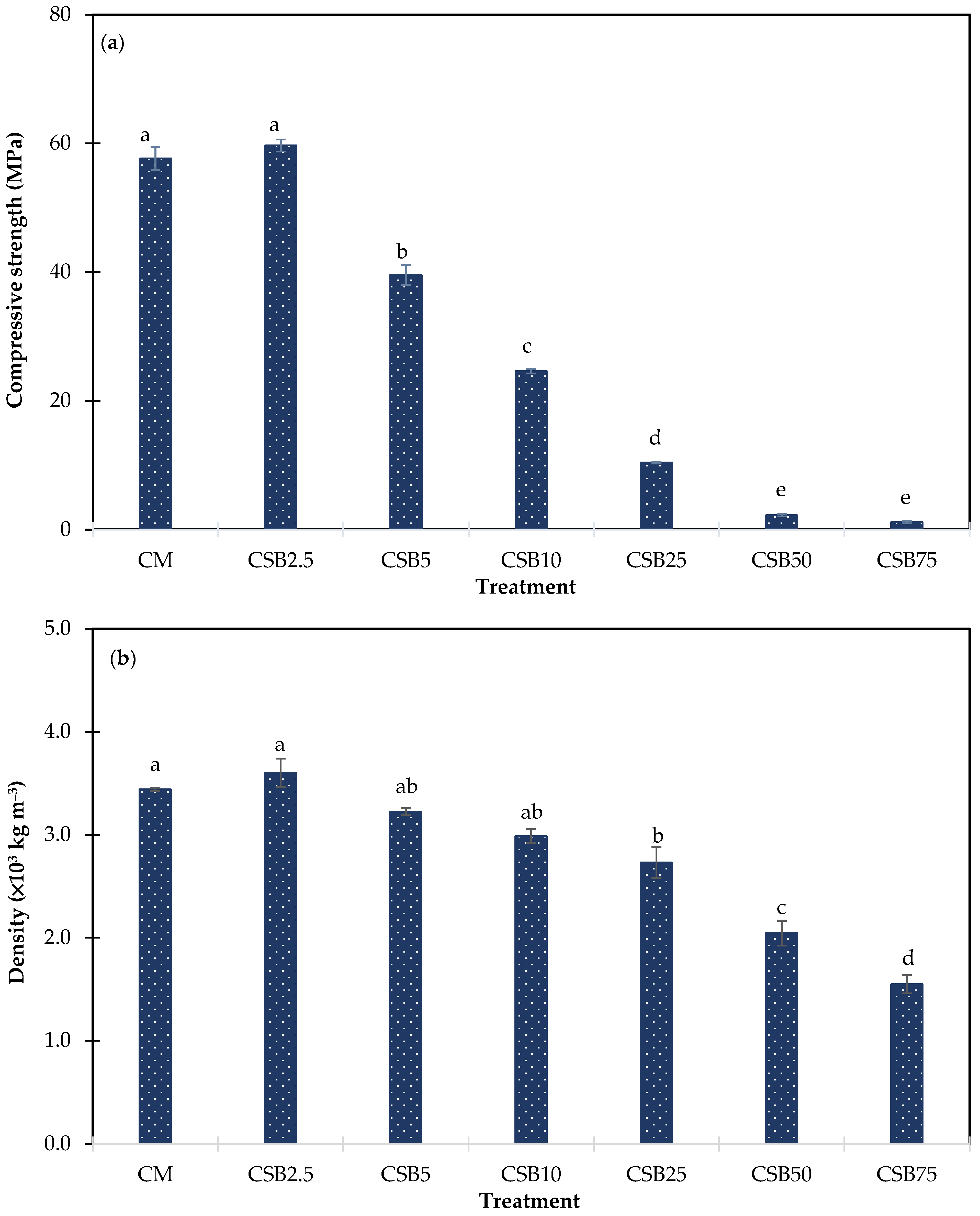
3.2.1. Compressive Strength
3.2.2. Density
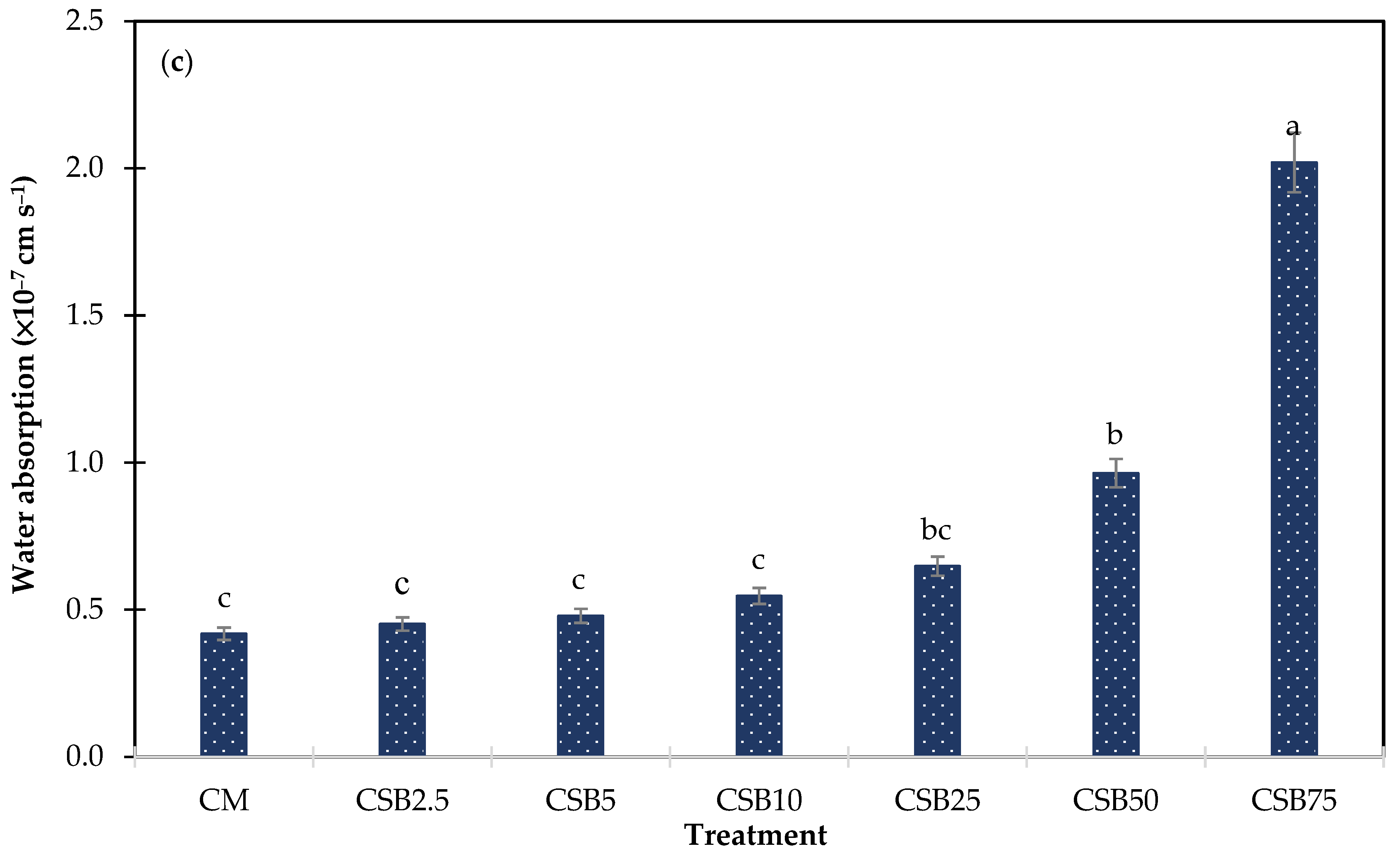
3.2.3. Water Absorption
3.3. Algal Growth Dynamics
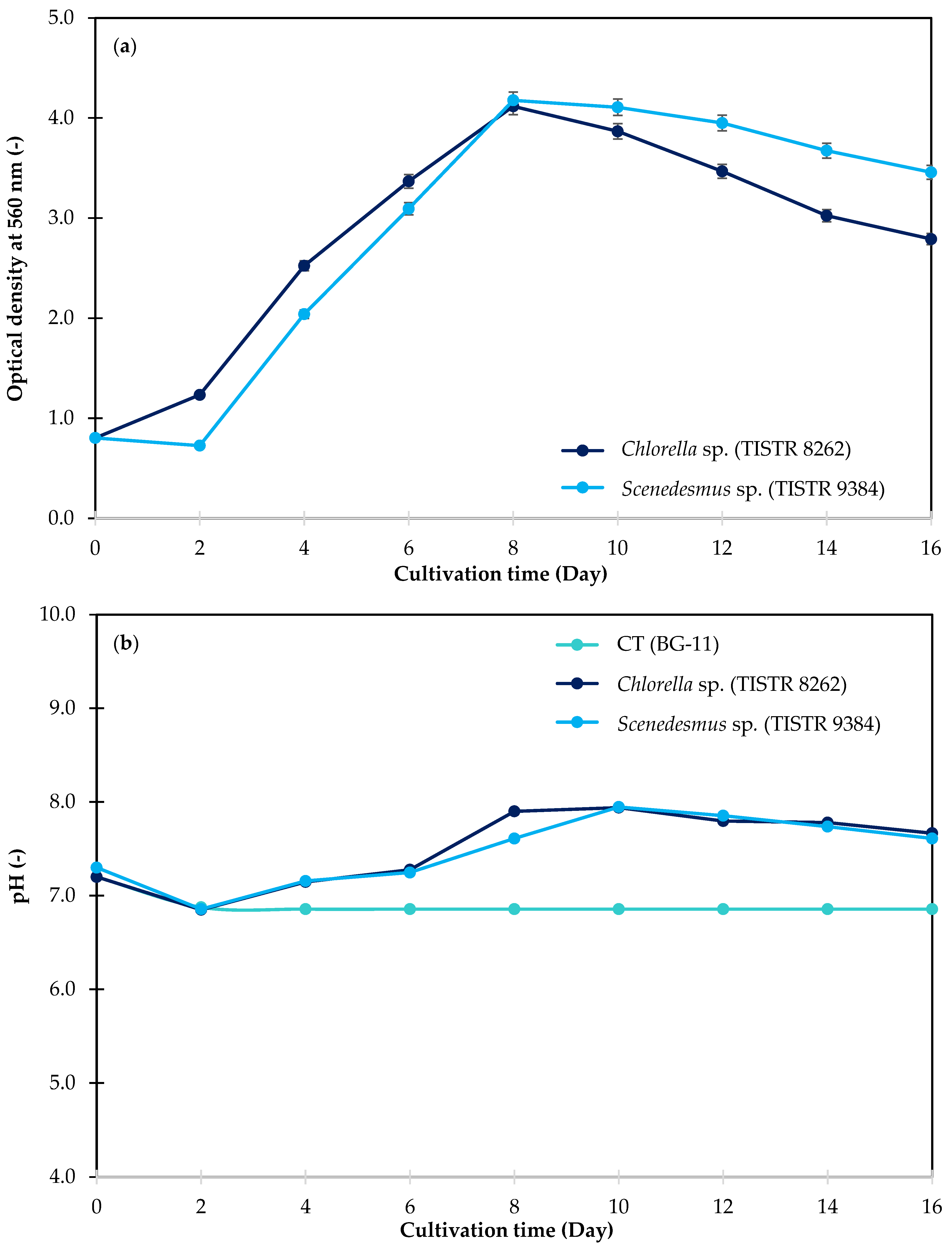
3.3.1. Increment of Optical Density
3.3.2. pH Variations
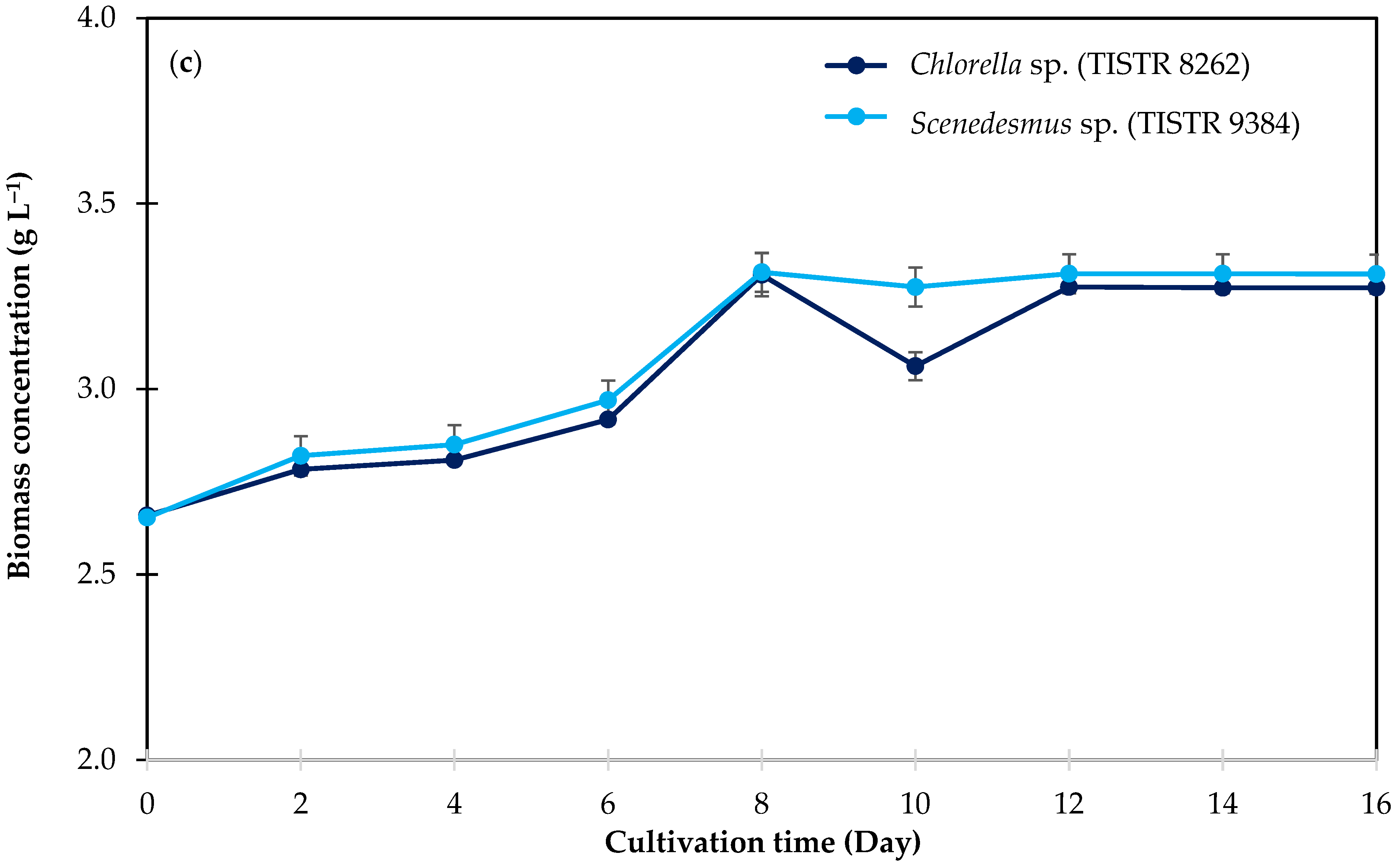
3.3.3. Biomass Accumulation
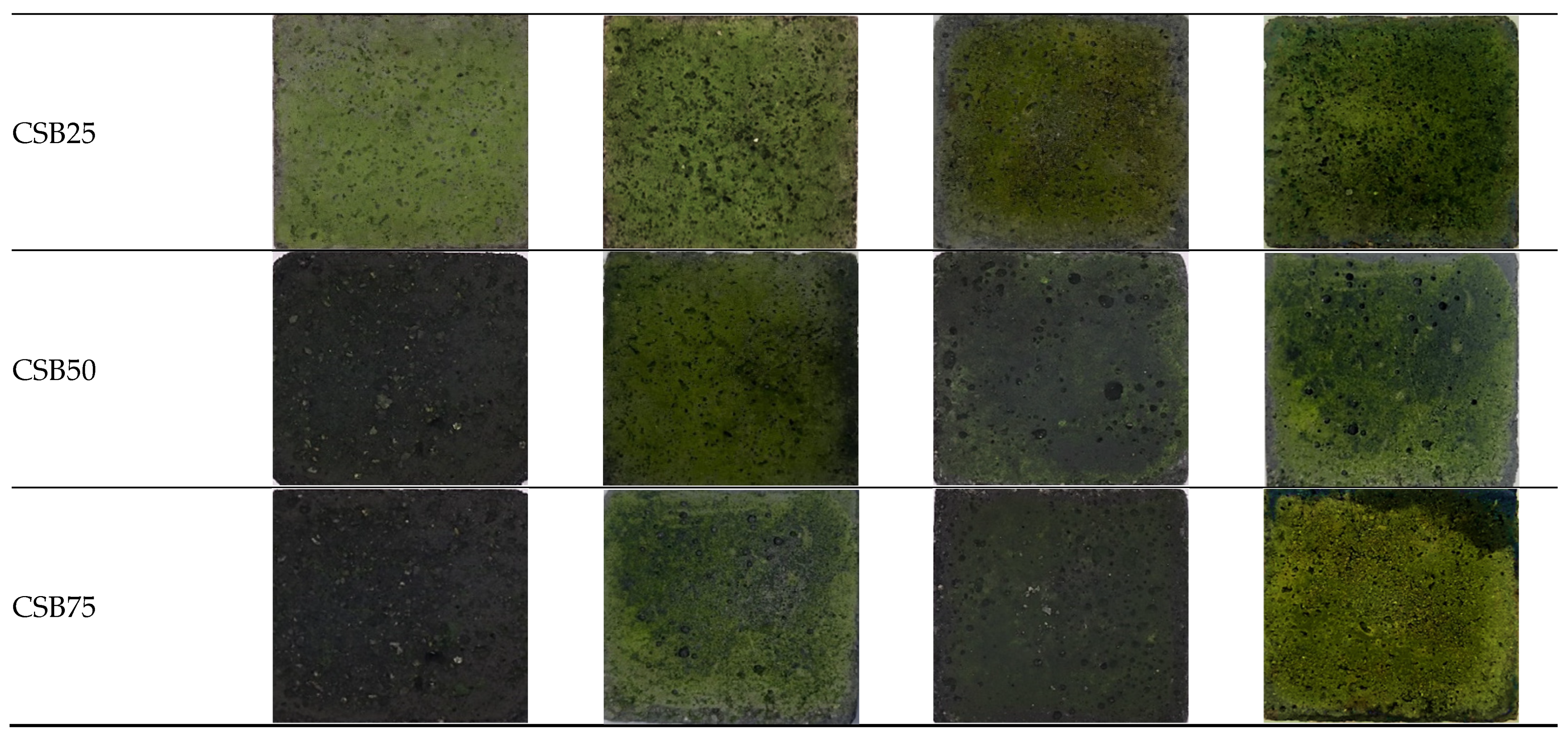
3.4. Algal Growth on Cron Stalk Biochar-Enhanced Mortar and Their Efficiency in CO2 Sequestration
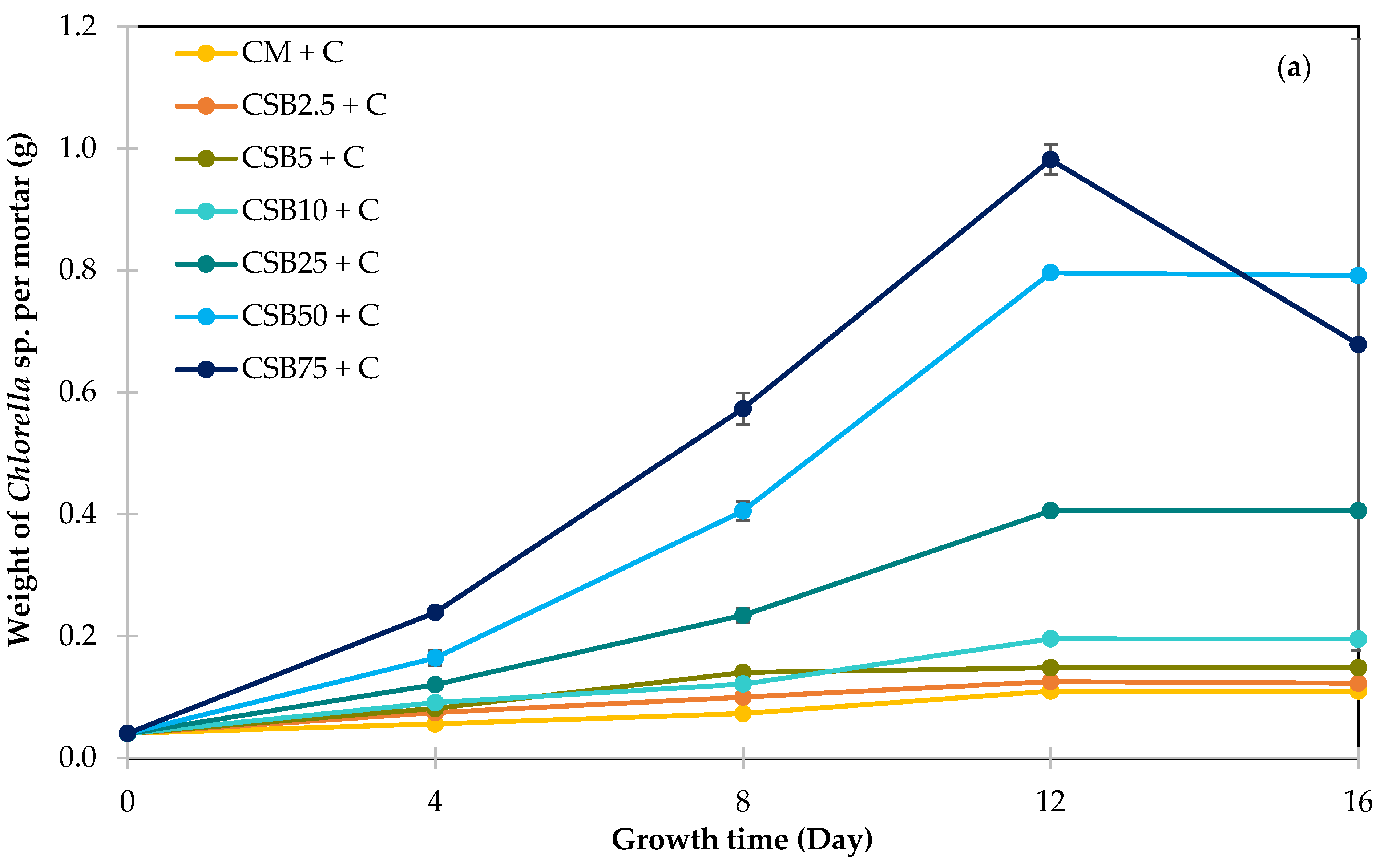
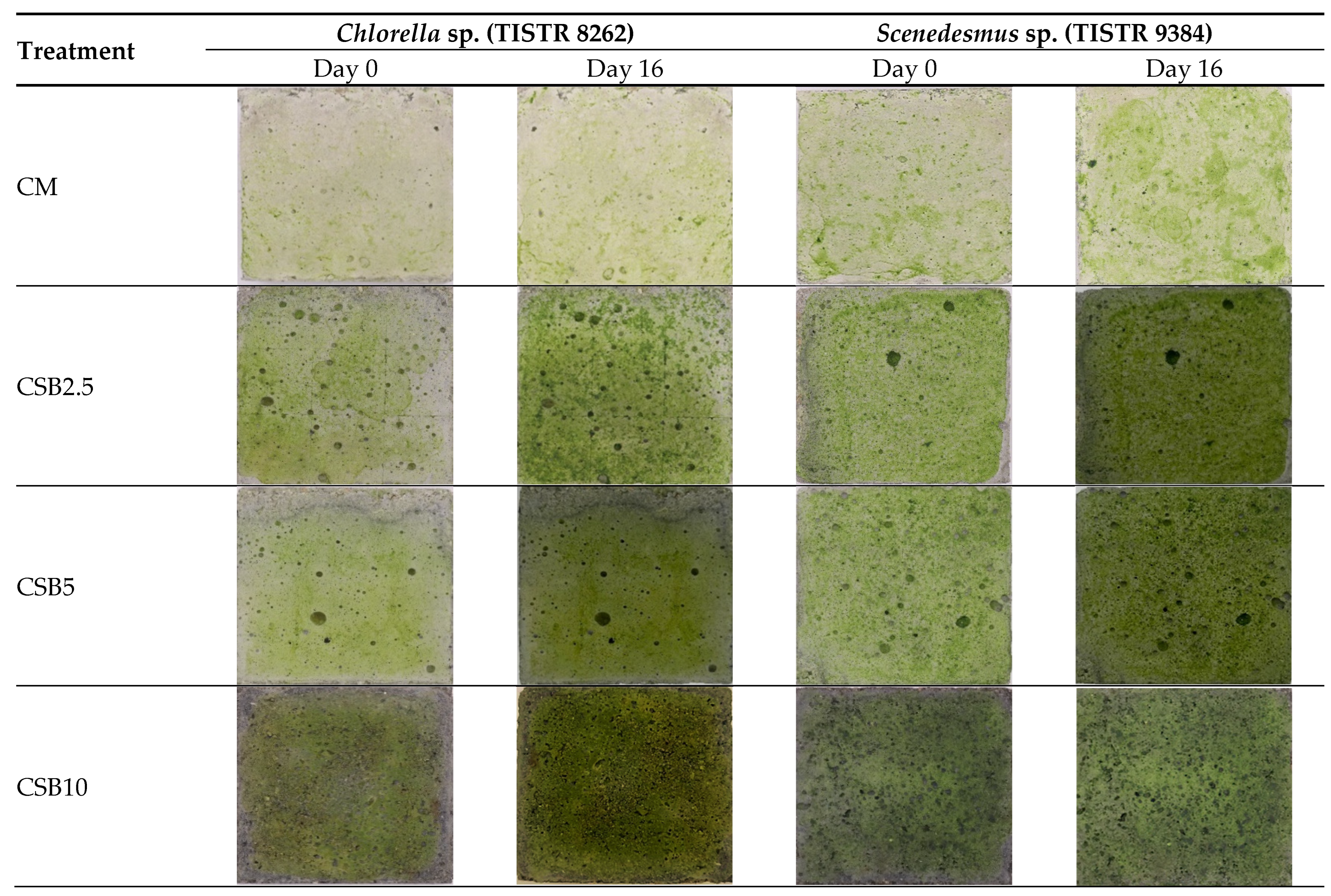
3.4.1. Growth of Chlorella and Scenedesmus Species
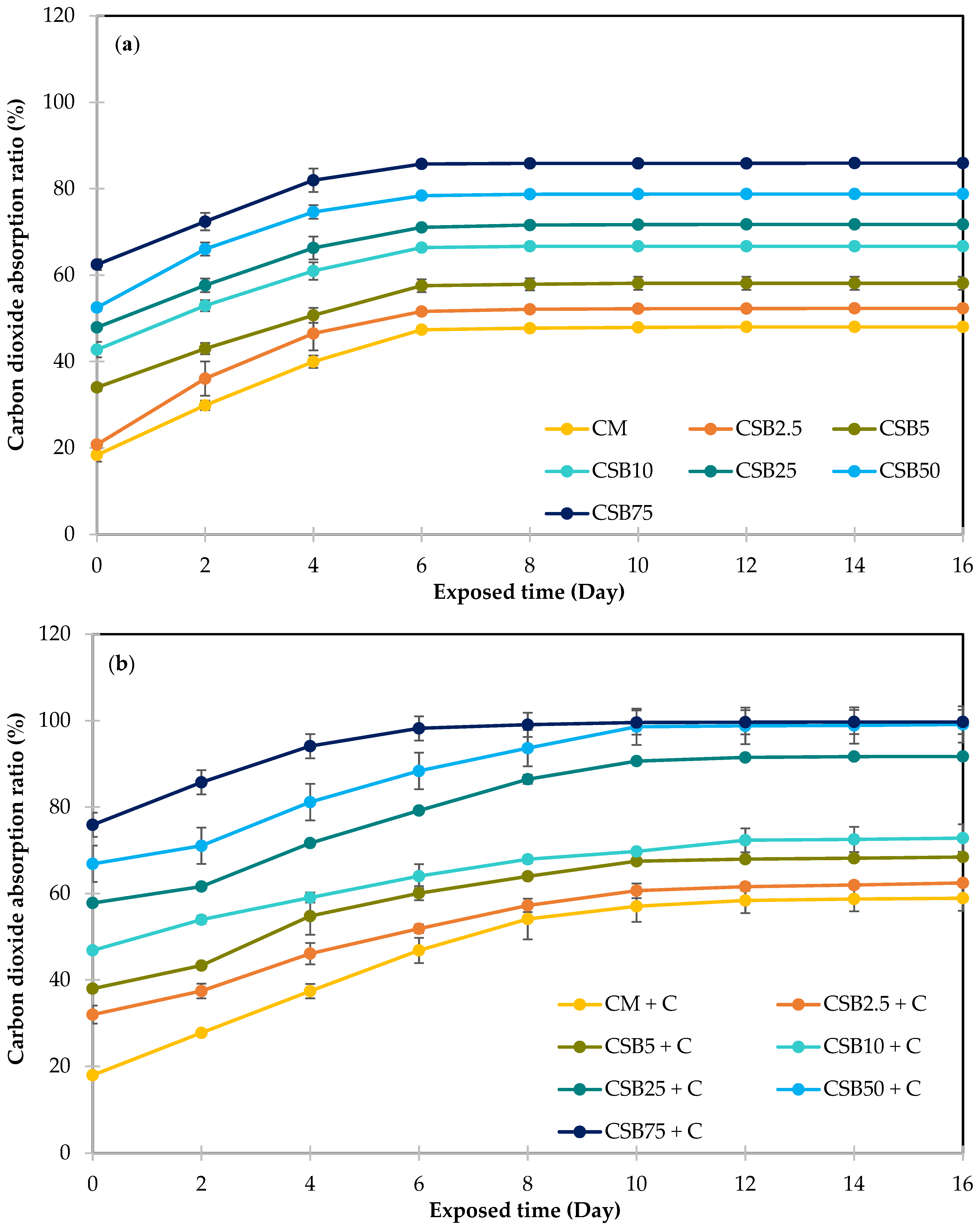
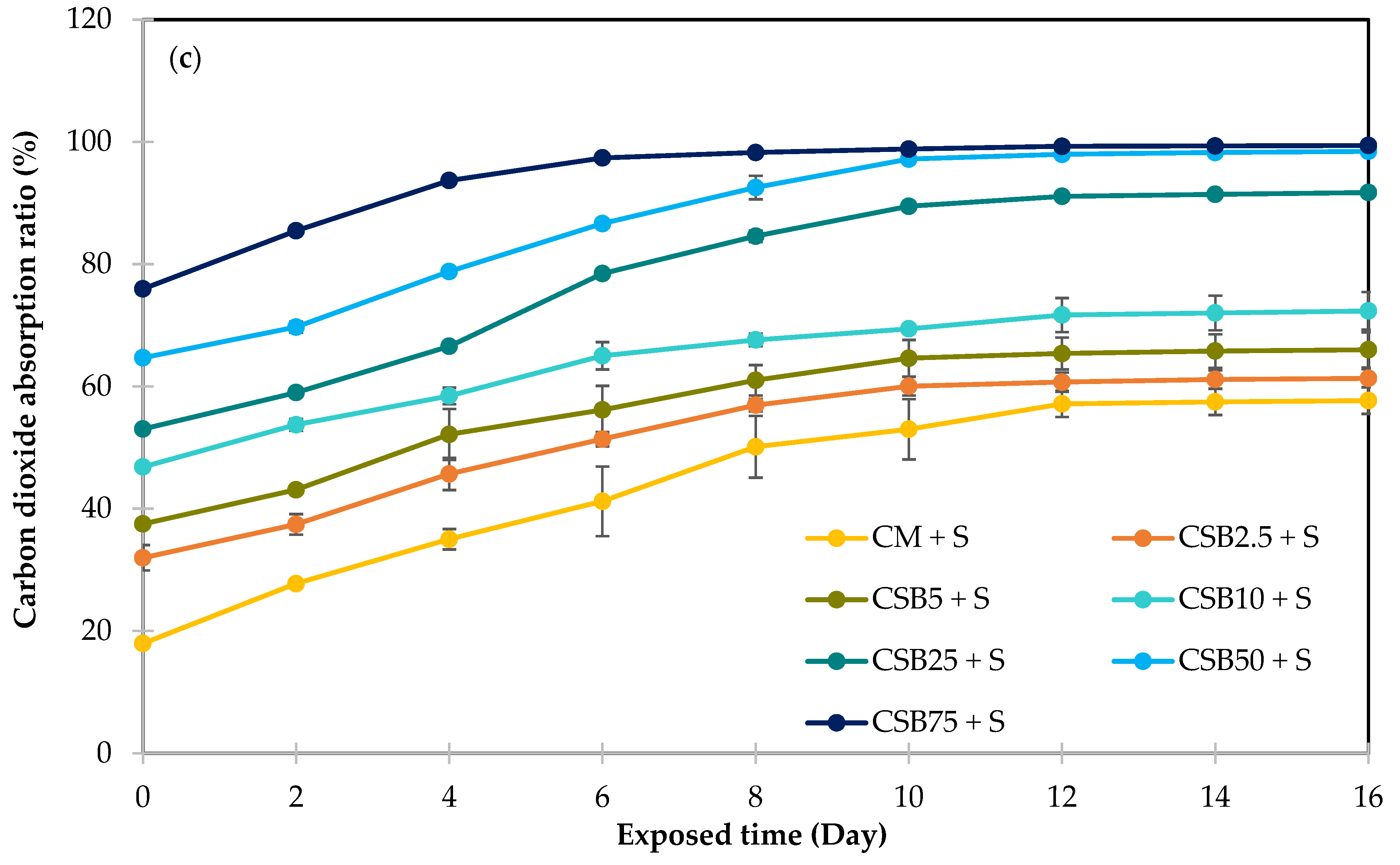
3.4.2. Carbon Dioxide Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marescaux, A.; Thieu, V.; Garnier, J. Carbon dioxide, methane, and nitrous oxide emissions from the human-impacted Seine watershed in France. Sci. Total Environ. 2018, 643, 247–259. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. Available online: https://ipcc.ch/site/assets/uploads/2018/02/WG1AR5_all_final.pdf (accessed on 30 September 2024).
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.W.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, R.; Fu, Q.; Li, T.; Wang, J.; Su, Z.; Shen, W.; Zhou, W.; Wang, Y.; Li, M. Modified biochar reduces the greenhouse gas emission intensity and enhances the net ecosystem economic budget in black soil soybean fields. Soil Tillage Res. 2024, 237, 105978. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, G.; Jalilov, A.S.; Tarkunde, Y.R.; Fei, H.; Tour, J.M. Biochar as a renewable source for high-performance CO2 sorbent. Carbon 2016, 107, 344–351. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Churkina, G.; Organschi, A.; Reyer, C.P.O.; Ruff, A.; Vinke, K.; Liu, Z.; Reck, B.K.; Graedel, T.E.; Schellnhuber, H.J. Buildings as a global carbon sink. Nat. Sustain. 2020, 3, 269–276. [Google Scholar] [CrossRef]
- Praneeth, S.; Saavedra, L.; Zeng, M.; Dubey, B.K.; Sarmah, A.K. Biochar admixtured lightweight, porous and tougher cement mortars: Mechanical, durability and micro computed tomography analysis. Sci. Total Environ. 2021, 750, 142327. [Google Scholar] [CrossRef]
- Suarez-Riera, D.; Restuccia, L.; Ferro, G.A. The use of Biochar to reduce the carbon footprint of cement-based materials. Procedia Struct. Integr. 2020, 26, 199–210. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Liu, G.; Cui, X.; Sun, J. Application of biochar cement-based materials for carbon sequestration. Constr. Build. Mater. 2023, 405, 133373. [Google Scholar] [CrossRef]
- Mrad, R.; Chehab, G. Mechanical and microstructure properties of biochar-based mortar: An internal curing agent for PCC. Sustainability 2019, 11, 2491. [Google Scholar] [CrossRef]
- Aziz, M.A.; Zubair, M.; Saleem, M.; Alharthi, Y.M.; Ashraf, N.; Alotaibi, K.S.; Aga, O.; Al Eid, A.A.A. Mechanical, non-destructive, and thermal characterization of biochar-based mortar composite. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Room, S.; Bahadori-Jahromi, A. Biochar-enhanced carbon-negative and sustainable cement composites: A scientometric review. Sustainability 2024, 16, 10162. [Google Scholar] [CrossRef]
- Rupasinghe, M.; Zhang, Z.; Mendis, P.; Sofi, M. Carbon sequestering biochar incorporated cementitious composites: Evaluation of hygrothermal, mechanical and durability characteristics. Cement Concr. Compos. 2025, 157, 105864. [Google Scholar] [CrossRef]
- Alum, A.; Rashid, A.; Mobasher, B.; Abbaszadegan, M. Cement-based biocide coatings for controlling algal growth in water distribution canals. Cem. Concr. Compos. 2008, 30, 839–847. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E. Algal growth inhibition on cement mortar: Efficiency of water repellent and photocatalytic treatments under UV/VIS illumination. Int. Biodeterior. Biodegrad. 2014, 89, 115–125. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Eyvaz, M.; Al Maskari, T.; Nassani, D.E.; Abu Amr, S.S.; Abujazar, M.S.S.; Bashir, M.J.K. An overview of green bioprocessing of algae-derived biochar and biopolymers: Synthesis, preparation, and potential applications. Energies 2023, 16, 791. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Kong, D.; Lian, L.; Liu, Y. Review on application of algae-based biochars in environmental remediation: Progress, challenge, and perspectives. J. Environ. Chem. Eng. 2023, 11, 111263. [Google Scholar] [CrossRef]
- Behl, K.; Sinha, S.; Sharma, M.; Singh, S.; Joshi, M.; Bhatnagar, A.; Nigam, S. One-time cultivation of Chlorella pyrenoidosa in aqueous dye solution supplemented with biochar for microalgal growth, dye decolorization, and lipid production. Chem. Eng. J. 2019, 364, 552–561. [Google Scholar] [CrossRef]
- Ramos-Ibarra, J.R.; Snell-Castro, R.; Neria-Casillas, J.A.; Choix, F.J. Biotechnological potential of Chlorella sp. and Scenedesmus sp. microalgae to endure high CO2 and methane concentrations from biogas. Bioprocess Biosyst. Eng. 2019, 42, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.; He, M.; Li, D.; Gebreluel, T.; Cheng, B.; Wang, C. Utilization of tannery wastewater for biofuel production: New insights on microalgae growth and biomass production. Sci. Rep. 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Pandian, P.M.; Dharkshith, K.; Muthiah, P. Improvising biodiesel production from Scenedesmus dimorphus via nutrient starvation and optimized pretreatment process. BioEnergy Res. 2024, 17, 2400–2412. [Google Scholar] [CrossRef]
- Gotore, O.; Rameshprabu, R.; Itayama, T. Adsorption performances of corn cob-derived biochar in saturated and semi-saturated vertical-flow constructed wetlands for nutrient removal under erratic oxygen supply. Environ. Chem. Ecotoxicol. 2022, 4, 155–163. [Google Scholar] [CrossRef]
- ASTM C33; Standard Specification for Concrete Aggregates. ASTM International: Philadelphia, PA, USA, 2012.
- ASTM C109/C109M-20b; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens). ASTM International: Philadelphia, PA, USA, 2020.
- ASTM C305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: Philadelphia, PA, USA, 2002.
- ASTM C188-17; Standard Test Method for Density of Hydraulic Cement. ASTM International: Philadelphia, PA, USA, 2023.
- ASTM C1403-15; Standard Test Method for Rate of Water Absorption of Masonry Mortars. ASTM International: Philadelphia, PA, USA, 2022.
- Carneiro, M.; Ranglová, K.; Lakatos, G.E.; Manoel, J.A.C.; Grivalský, T.; Kozhan, D.M.; Toribio, A.; Moreno, G.; Otero, A.; Varela, J.; et al. Growth and bioactivity of two chlorophyte (Chlorella and Scenedesmus) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res. 2021, 56, 102299. [Google Scholar] [CrossRef]
- Wei, X.; Yu, G.; Cao, W.; Feng, M.; Xu, Y.; Jin, M.; Zhang, Y.; Li, T.; Guo, L. Biomass producing and CO2 capturing simultaneously by Chlorella vulgaris: Effect of CO2 concentration and aeration rate. Energy 2024, 306, 132321. [Google Scholar] [CrossRef]
- Yin, D.; Wang, Z.; Wen, X.; Ding, Y.; Hou, X.; Geng, Y.; Li, Y. Effects of carbon concentration, pH, and bubbling depth on carbon dioxide absorption ratio in microalgae medium. Environ. Sci. Pollut. Res. 2019, 26, 32902–32910. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Liu, Z.; Su, Y.; Du, C. Adsorbent biochar derived from corn stalk core for highly efficient removal of bisphenol A. Environ. Sci. Pollut. Res. 2023, 30, 74916–74927. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.P.; Moni, C.; Dieguez-Alonso, A.; de la Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of properties of biochar produced from different types of lignocellulosic biomass by slow pyrolysis at 600 °C. Appl. Energy Combust. Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Yan, S.; Yu, W.; Yang, T.; Li, Q.; Guo, J. The adsorption of corn stalk biochar for Pb and Cd: Preparation, characterization, and batch adsorption study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Mamaghani, Z.G.; Hawboldt, K.A.; MacQuarrie, S. Adsorption of CO2 using biochar—Review of the impact of gas mixtures and water on adsorption. J. Environ. Chem. Eng. 2023, 11, 109643. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Salehi, E.; Askari, M.; Velashjerdi, M.; Arab, B. Phosphoric acid-treated spent tea residue biochar for wastewater decoloring: Batch adsorption study and process intensification using multivariate data-based optimization. Chem. Eng. Process Intensif. 2020, 158, 108170. [Google Scholar] [CrossRef]
- Tang, L.; Yang, G.-D.; Zeng, G.-M.; Cai, Y.; Li, S.-S.; Zhou, Y.; Pang, Y.; Liu, Y.-Y.; Zhang, Y.; Luna, B. Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsh, D.; Hofman, J.; Sedlacek, P.; Bielska, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Li, Q.; Yu, W.; Guo, L.; Wang, Y.; Zhao, S.; Zhou, L.; Jiang, X. Sorption of sulfamethoxazole on inorganic acid solution-etched biochar derived from alfalfa. Materials 2021, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Effect of water entrainment by pre-soaked biochar particles on strength and permeability of cement mortar. Constr. Build. Mater. 2018, 159, 107–125. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Guo, B.; Yang, J.; Shen, Z.; Hou, D.; Ok, Y.S.; Poon, C.S. Biochar as green additives in cement-based composites with carbon dioxide curing. J. Clean. Prod. 2020, 258, 120678. [Google Scholar] [CrossRef]
- Zhao, Z.; El-Naggar, A.; Kau, J.; Olson, C.; Tomlinson, D.; Chang, S.X. Biochar affects compressive strength of Portland cement composites: A meta-analysis. Biochar 2024, 6, 21. [Google Scholar] [CrossRef]
- Liu, W.; Li, K.; Xu, S. Utilizing bamboo biochar in cement mortar as a bio-modifier to improve the compressive strength and crack-resistance fracture ability. Constr. Build. Mater. 2022, 327, 126917. [Google Scholar] [CrossRef]
- Pan, S.Y.; Adhikari, R.; Chen, Y.H.; Li, P.; Chiang, P.C. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- Jhatial, A.A.; Goh, W.I.; Mohamad, N.; Sohu, S.; Lakhiar, M.T. Utilization of palm oil fuel ash and eggshell powder as partial cement replacement-a review. Civ. Eng. J. 2018, 4, 1977–1984. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Novel biochar-concrete composites: Manufacturing, characterization and evaluation of the mechanical properties. Sci. Total Environ. 2018, 616–617, 408–416. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Berardi, U.; Briens, C.; Berruti, F. Biochar from residual biomass as a concrete filler for improved thermal and acoustic properties. Biomass Bioenergy 2019, 120, 77–83. [Google Scholar] [CrossRef]
- Zeidabadi, Z.A.; Bakhtiari, S.; Abbaslou, H.; Ghanizadeh, A.R. Synthesis, characterization and evaluation of biochar from agricultural waste biomass for use in building materials. Constr. Build. Mater. 2018, 181, 301–308. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Adesina, A. Recent advancements in the use of biochar for cementitious applications: A review. J. Build. Eng. 2020, 32, 101705. [Google Scholar] [CrossRef]
- Oukarroum, A. Change in photosystem II photochemistry during algal growth phases of Chlorella vulgaris and Scenedesmus obliquus. Curr. Microbiol. 2016, 72, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Raven, J. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.H.; Li, A.; Fu, L.; Zhou, D. Co-culture of Chlorella and Scenedesmus could enhance total lipid production under bacteria quorum sensing molecule stress. J. Water Process Eng. 2021, 39, 101739. [Google Scholar] [CrossRef]
- Shaima, A.F.; Yasin, N.H.M.; Ibrahim, N.; Takriff, M.S.; Gunasekaran, D.; Ismaeel, M.Y.Y. Unveiling antimicrobial activity of microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J. Biol. Sci. 2022, 29, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.E.; Juarez, A.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; Ríos de Molina, M.d.C. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef]
- Vonshak, A.; Torzillo, G. Chapter 4: Environmental stress physiology. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Wiley-Blackwell: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Gao, K.; Campbell, D.A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: A review. Funct. Plant Biol. 2014, 41, 449–459. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Kuo, C.M.; Lin, C.S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Torzillo, G.; Koblízek, M.; Kopecký, J.; Bernardini, P.; Sacchi, A.; Komenda, J. Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: Changes in chlorophyll fluorescence quenching and the xanthophyll cycle. Planta 1999, 209, 126–135. [Google Scholar] [CrossRef]
- Lacour, T.; Babin, M.; Lavaud, J. Diversity in xanthophyll cycle pigments content and related nonphotochemical quenching (NPQ) among microalgae: Implications for growth strategy and ecology. J. Phycol. 2020, 56, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Giovannacci, D.; Leclaire, C.; Horgnies, M.; Ellmer, M.; Mertz, J.D.; Orial, G.; Chen, J.; Bousta, F. Algal colonization kinetics on roofing and façade tiles: Influence of physical parameters. Constr. Build. Mater. 2013, 48, 670–676. [Google Scholar] [CrossRef]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D.; Deves, O.; Ruot, B. Influence of the intrinsic characteristics of mortars on their biofouling by pigmented organisms: Comparison between laboratory and field-scale experiments. Int. Biodeterior. Biodegrad. 2014, 86, 334–342. [Google Scholar] [CrossRef]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D.; Devès, O.; Ruot, B. Avrami’s law based kinetic modeling of colonization of mortar surface by alga Klebsormidium flaccidum. Int. Biodeterior. Biodegrad. 2013, 79, 73–80. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Hunt, J.; Sanders, E.; Tran, M.; Burk, G.A.; Mlsna, T.E.; Fitzkee, N.C. Effect of biochar on microbial growth: A metabolomics and bacteriological investigation in E. coli. Environ. Sci. Technol. 2019, 53, 2635–2646. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.A.N.; Montero, O.; Maté, A.P.; Debdoubi, A.; Rad, C. The growth of filamentous microalgae is increased on biochar solid supports. Biocatal. Agric. Biotechnol. 2018, 13, 182–185. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. Nitrogen enriched biochar modified by high temperature CO2–ammonia treatment: Characterization and adsorption of CO2. Chem. Eng. J. 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons. Environ. Sci. Technol. 2012, 46, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kan, Y.; Zhao, L.; Cao, X. Chemical transformation of CO2 during its capture by waste biomass derived biochars. Environ. Pollut. 2016, 213, 533–540. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Bian, Z.; Hu, J.; Liu, H. Dynamic hydrophobic hindrance effect of zeolite@zeolitic imidazolate framework composites for CO2 capture in the presence of water. J. Mater. Chem. A 2015, 3, 8091–8097. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Elemento, O.; Giannopoulou, E.G.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Low, C.Y. Use of biochar as carbon sequestering additive in cement mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Chang, J.S.; Miao, X. A two-stage culture strategy for Scenedesmus sp. FSP3 for CO2 fixation and the simultaneous production of lutein under light and salt stress. Molecules 2022, 27, 7497. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.; Li, X.; Yin, Y.; Qin, S.; Ge, B. Enhanced biomass and CO2 sequestration of Chlorella vulgaris using a new mixotrophic cultivation method. Process Biochem. 2020, 90, 168–176. [Google Scholar] [CrossRef]




| Element/Compound | Composition (%) (n = 3) |
|---|---|
| Calcium oxide (CaO) | 63.11 ± 1.20 |
| Silicon dioxide (SiO2) | 16.69 ± 0.66 |
| Aluminum oxide (Al2O3) | 4.40 ± 0.21 |
| Sulfur trioxide (SO3) | 3.51 ± 0.05 |
| Ferric oxide (Fe2O3) | 3.13 ± 0.04 |
| Organic matter (CHNO) | 5.98 ± 0.08 |
| Magnesium oxide (MgO) | 1.60 ± 0.02 |
| Potassium oxide (K2O) | 0.842 ± 0.001 |
| Sodium oxide (Na2O) | 0.121 ± 0.003 |
| Phosphorus pentoxide (P2O5) | 0.120 ± 0.004 |
| Titanium dioxide (TiO2) | 0.292 ± 0.005 |
| Strontium oxide (SrO) | 0.074 ± 0.011 |
| Chloride (Cl−) | 0.032 ± 0.006 |
| Copper oxide (CuO) | 0.030 ± 0.004 |
| Zinc oxide (ZnO) | 0.030 ± 0.005 |
| Manganese oxide (MnO) | 0.024 ± 0.007 |
| Arsenic trioxide (As2O3) | 0.011 ± 0.000 |
| Zirconium dioxide (ZrO2) | 0.013 ± 0.005 |
| Molybdenum trioxide (MoO3) | 0.014 ± 0.004 |
| Rubidium oxide (Rb2O) | 0.004 ± 0.000 |
| Treatment | OPC (g) | Water (ml) | Sand (g) | CSB (g) |
|---|---|---|---|---|
| CM | 250.0 | 175.0 | 687.5 | - |
| CSB2.5 | 243.8 | 175.0 | 687.5 | 6.3 |
| CSB5 | 237.5 | 175.0 | 687.5 | 12.5 |
| CSB10 | 225.0 | 175.0 | 687.5 | 25.0 |
| CSB25 | 187.5 | 175.0 | 687.5 | 62.5 |
| CSB50 | 125.0 | 175.0 | 687.5 | 125.0 |
| CSB75 | 62.5 | 175.0 | 687.5 | 187.5 |
| Parameter | Value (n = 3) |
|---|---|
| Carbon (C, %) | 62.3 ± 0.1 |
| Hydrogen (H, %) | 3.5 ± 0.0 |
| Oxygen (O, %) | 22.3 ± 0.2 |
| Nitrogen (N, %) | 9.3 ± 0.1 |
| Sulfur (S, %) | 3.1 ± 0.0 |
| O/C ratio (-) | 0.4 ± 0.0 |
| H/C ratio (-) | 0.006 ± 0.006 |
| C/H ratio (-) | 18.1 ± 0.1 |
| Surface area (m2 g−1) | 680.3 ± 2.6 |
| Total pore volume (cm3 g−1) | 0.4 ± 0.3 |
| Average pore diameter (nm) | 1.9 ± 0.1 |
| CO2 absorption capacity (mmol g−1) | 2.9 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinyoung, S.; Jeeraro, A.; Udomkun, P.; Kunchariyakun, K.; Graham, M.; Kaewlom, P. Enhancing CO2 Sequestration Through Corn Stalk Biochar-Enhanced Mortar: A Synergistic Approach with Algal Growth for Carbon Capture Applications. Sustainability 2025, 17, 342. https://doi.org/10.3390/su17010342
Sinyoung S, Jeeraro A, Udomkun P, Kunchariyakun K, Graham M, Kaewlom P. Enhancing CO2 Sequestration Through Corn Stalk Biochar-Enhanced Mortar: A Synergistic Approach with Algal Growth for Carbon Capture Applications. Sustainability. 2025; 17(1):342. https://doi.org/10.3390/su17010342
Chicago/Turabian StyleSinyoung, Suthatip, Ananya Jeeraro, Patchimaporn Udomkun, Kittipong Kunchariyakun, Margaret Graham, and Puangrat Kaewlom. 2025. "Enhancing CO2 Sequestration Through Corn Stalk Biochar-Enhanced Mortar: A Synergistic Approach with Algal Growth for Carbon Capture Applications" Sustainability 17, no. 1: 342. https://doi.org/10.3390/su17010342
APA StyleSinyoung, S., Jeeraro, A., Udomkun, P., Kunchariyakun, K., Graham, M., & Kaewlom, P. (2025). Enhancing CO2 Sequestration Through Corn Stalk Biochar-Enhanced Mortar: A Synergistic Approach with Algal Growth for Carbon Capture Applications. Sustainability, 17(1), 342. https://doi.org/10.3390/su17010342






