Study on Non-Metal-Induced EPFRs in PM2.5 Generated from Flue Gas of Cellulose Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
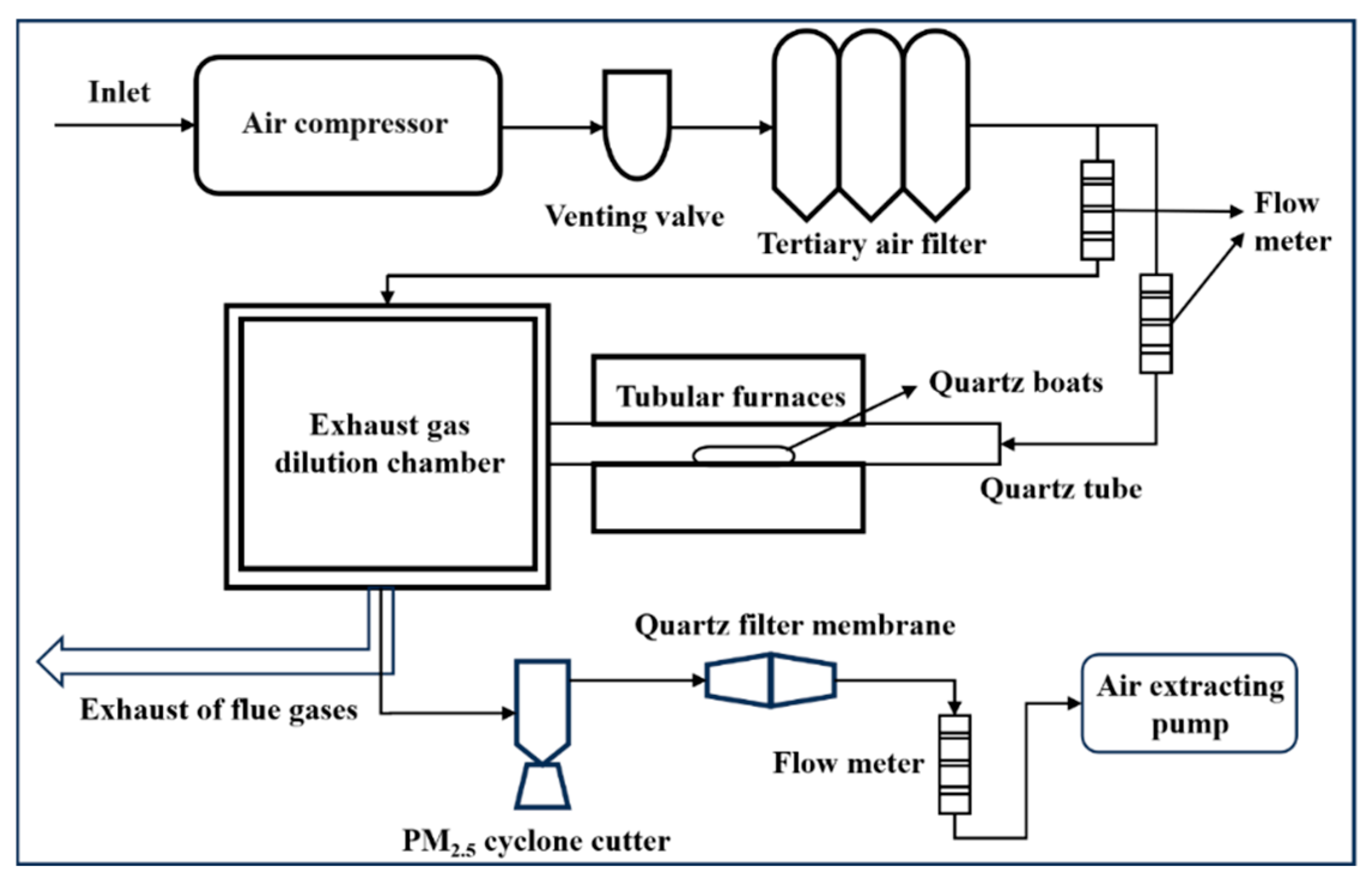
2.2. Sample Preparation and Collection Methods
2.3. Sample Solvent Extraction Treatment Method
2.4. OC/EC Analysis
2.5. Environmental Persistent Free Radical Detection
2.6. Evaluation of the Decay Period of Environmentally Persistent Free Radicals
3. Results
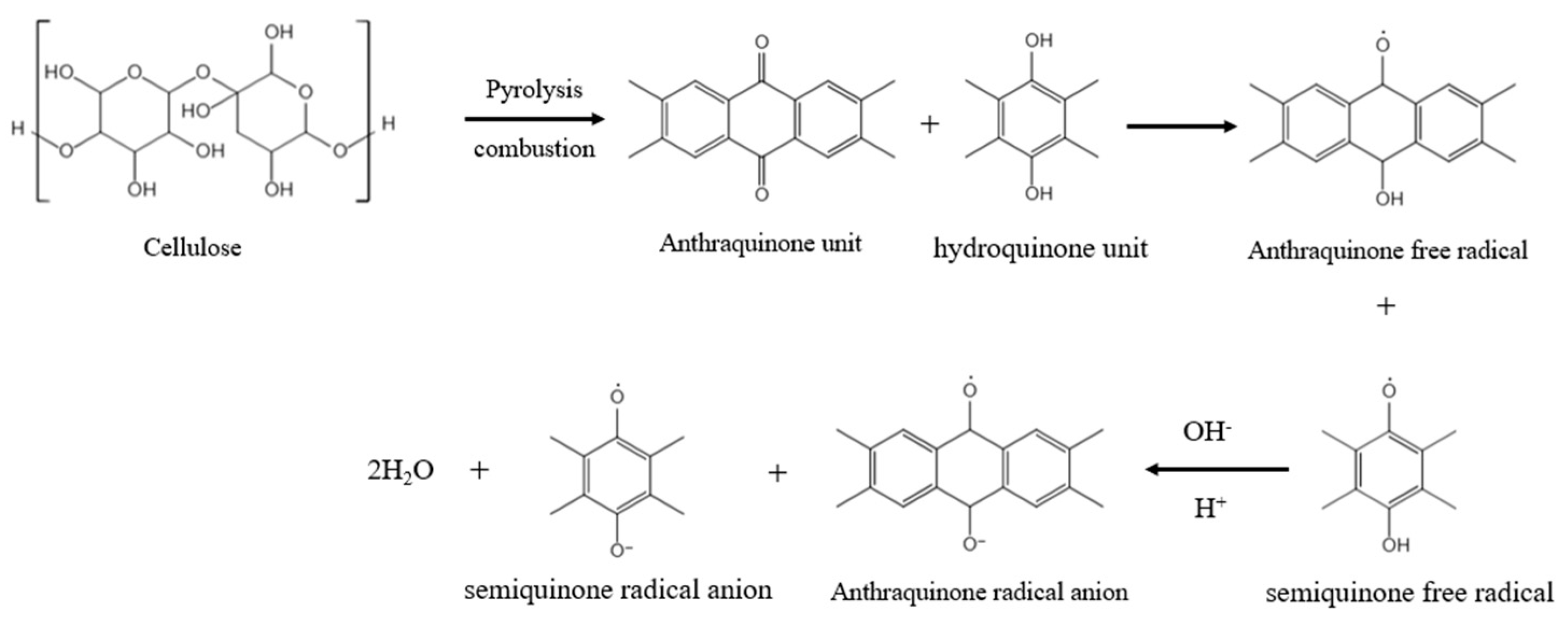
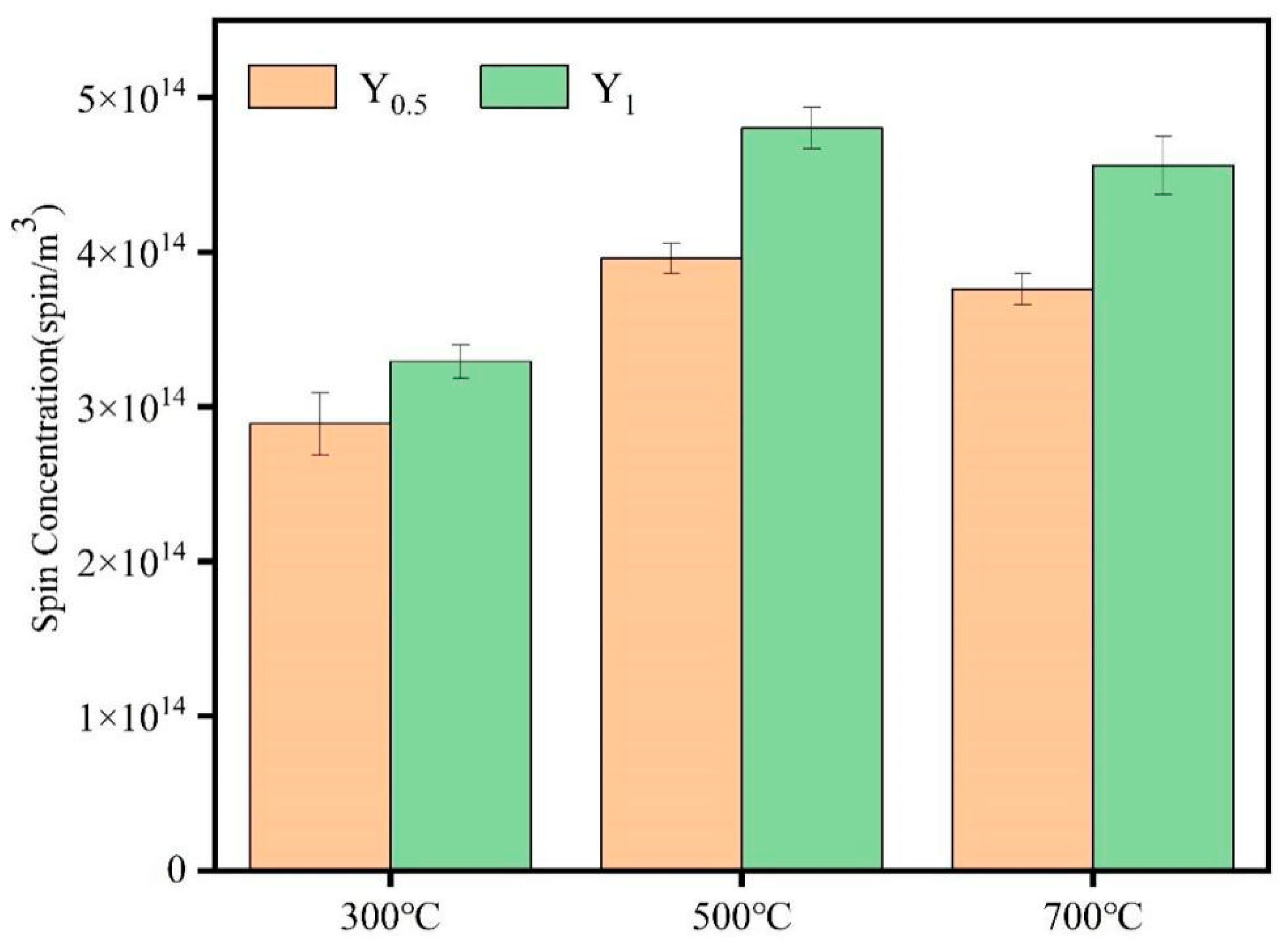
3.1. Emission Characteristics of Non-Metal-Induced EPFRs in PM2.5 Generated from Cellulose Combustion
3.2. Influencing Factors of Non-Metal-Induced EPFRs in PM2.5 Produced by Cellulose Combustion
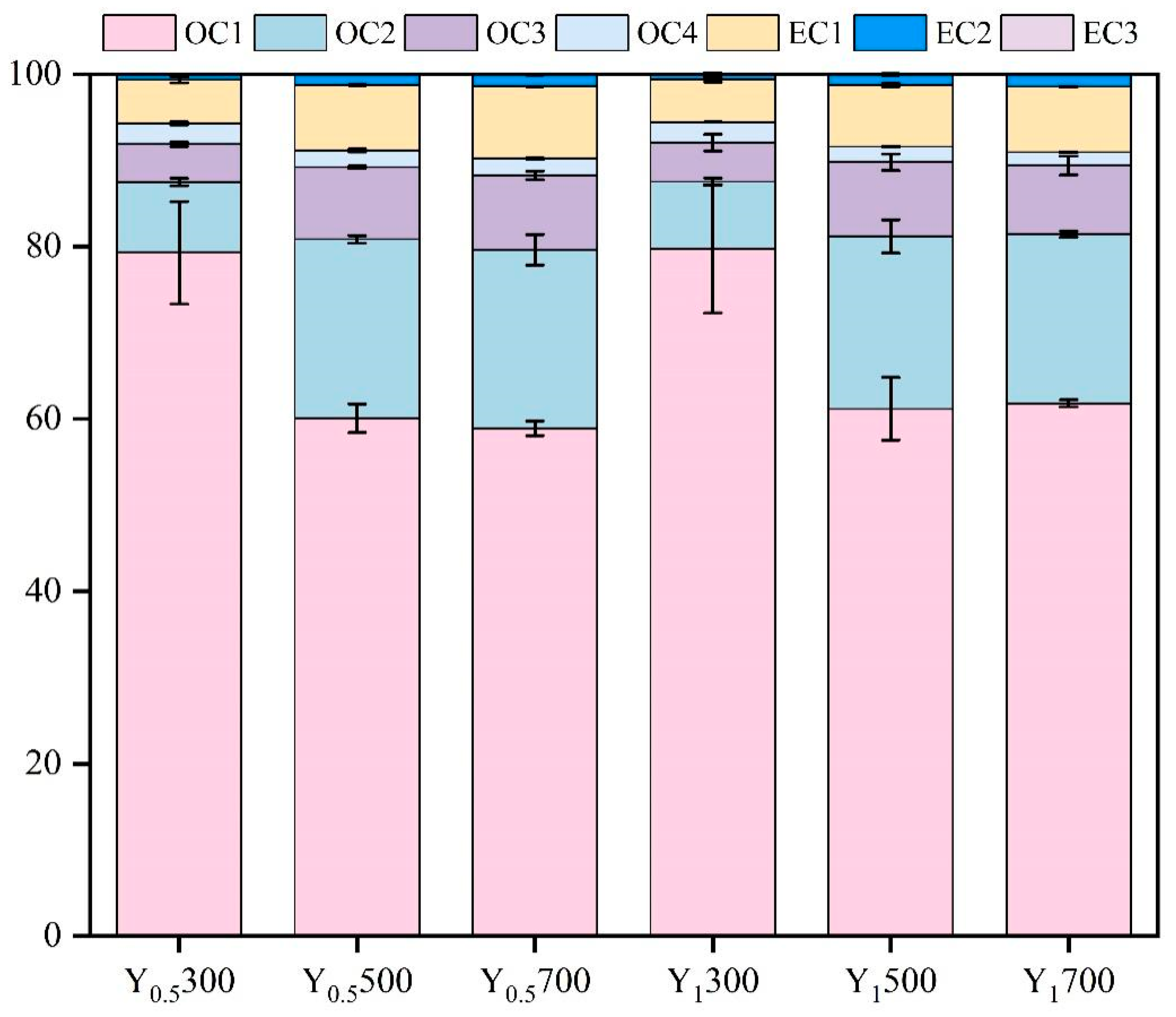
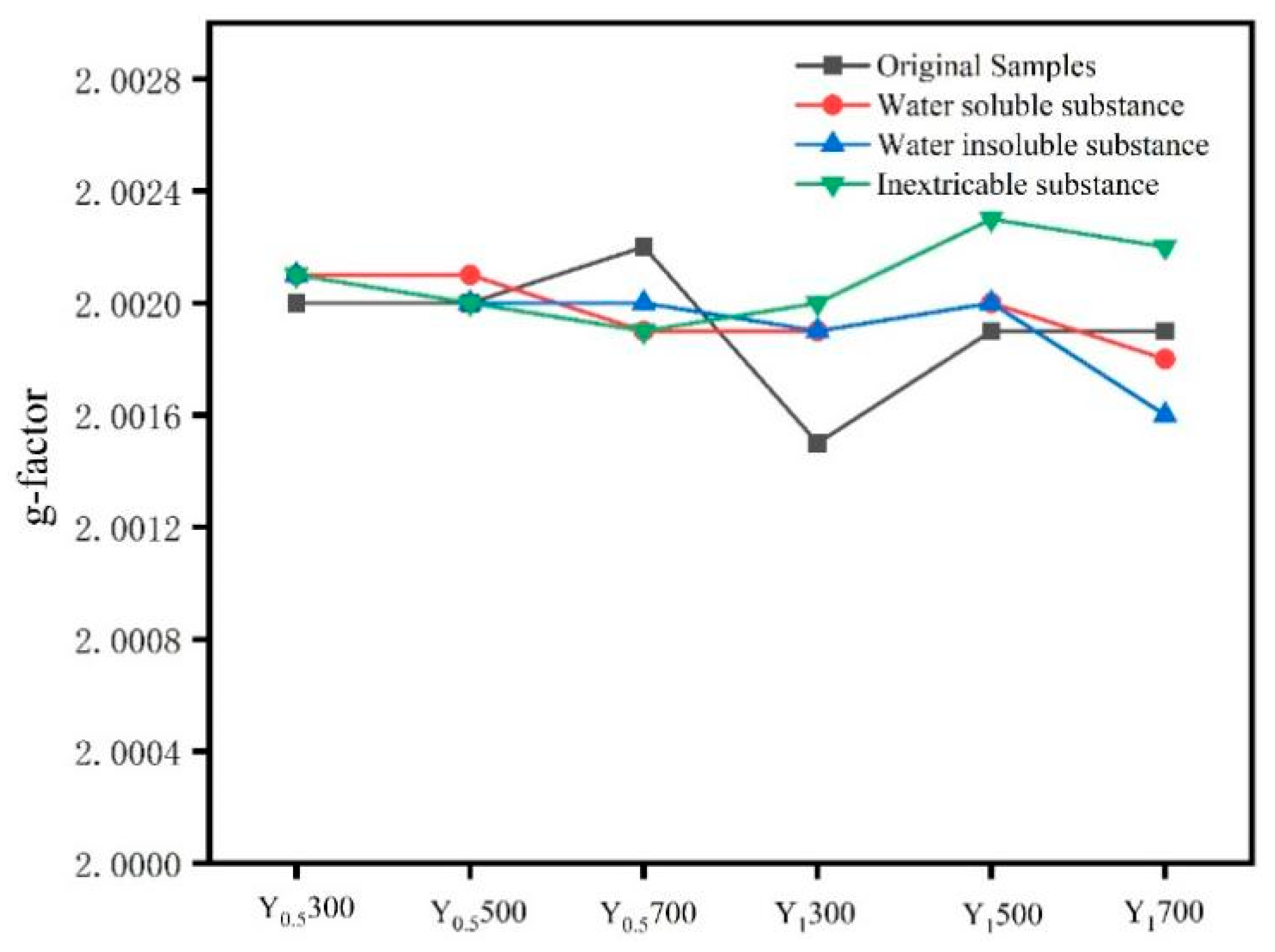
3.3. Distribution Characteristics of Non-Metal-Induced EPFRs in Different Extracted Fractions of PM2.5 Samples
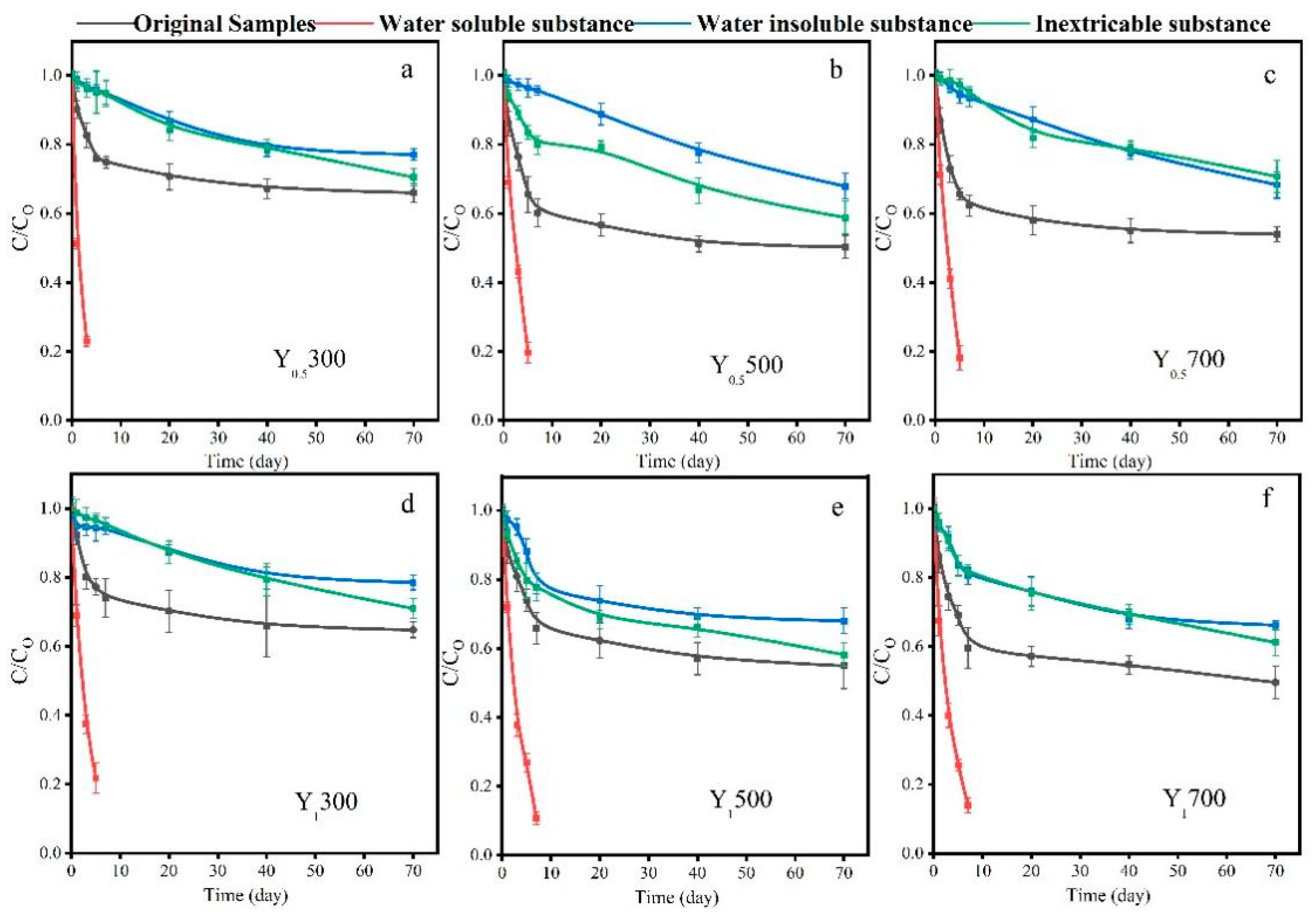
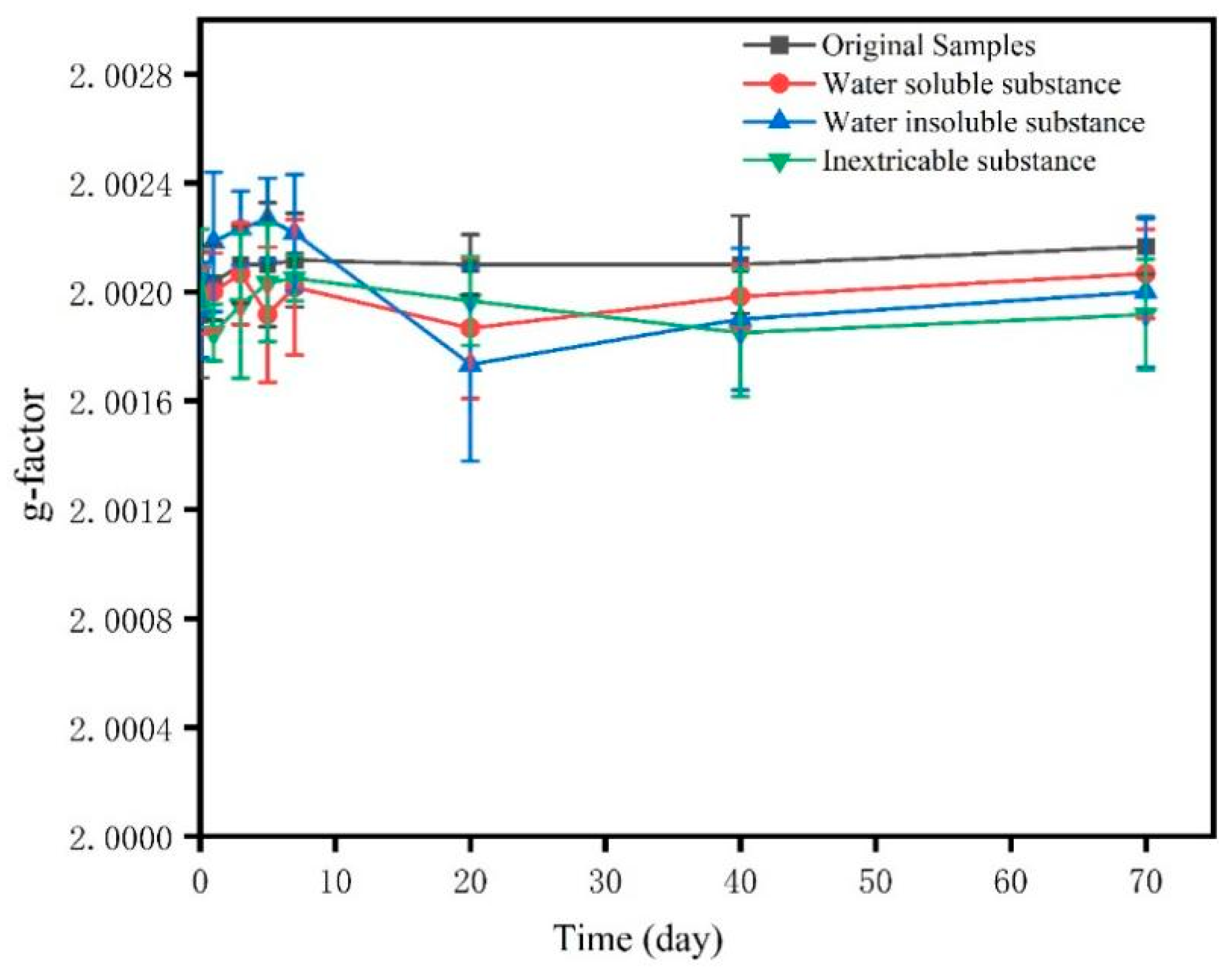
3.4. Decay Characteristics of Non-Metal-Induced EPFRs Generated from Cellulose Combustion
4. Conclusions
- In this study, under current experimental conditions, temperature and the air ratio have a certain influence on the concentration of non-metal-induced EPFRs in PM2.5 generated from the combustion of cellulose. Among them, the concentration of non-metal-induced EPFRs in the sample is the highest at 500 °C and when there is an air ratio of 1. In addition, temperature and the air ratio have no significant effect on the types of non-metal-induced EPFRs, which are all carbon-centered radicals.
- A large quantity of non-metal-induced EPFRs are generated in PM2.5 emissions from cellulose combustion, with concentrations at the level of 1014 spins/m3, similar to the EPFRs concentration in PM2.5 from the atmosphere of Xi’an during the period from 2018 to 2021. The non-metal-induced EPFRs in this study are important contributors to EPFRs in the actual atmosphere. In addition, extraction of PM2.5 samples generated from cellulose combustion reveals that non-metal-induced EPFRs are mainly concentrated in inextricable substances. Their g-factors are in the range of 2.0015~2.0023, which differs significantly from the g-factor range from 2.0028 to 2.0033 for EPFRs in actual atmospheric particles before and after extraction. It is speculated that non-metal-induced EPFRs are not primary contributors to actual atmospheric particulate matters.
- Unlike the half-life of metal-induced EPFRs ranging from hours to months, most non-metal-induced EPFRs in PM2.5 samples from cellulose combustion have long lifetimes, with half-lives of several years or longer, and their types remain relatively stable during the decay process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Sun, Z. Research progress on a new class of environmentally harmful substances, environmentally persistent free radicals (EPFRs). Bull. Mineral. Petrol. Geochem. 2012, 31, 287–290. [Google Scholar]
- Saravia, J.; Lee, G.I.; Lomnicki, S.; Dellinger, B.; Cormier, S.A. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: A review. J. Biochem. Mol. Toxicol. 2013, 27, 56–68. [Google Scholar] [CrossRef]
- Dugas, T.R.; Lomnicki, S.; Cormier, S.A.; Dellinger, B.; Reams, M. Addressing emerging risks: Scientific and regulatory challenges associated with environmentally persistent free radicals. Int. J. Environ. Res. Public. Health 2016, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhu, Q.; Zhao, Y.; Wu, X.; Xu, Y. Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere. Environ. Sci. Technol. 2017, 51, 7936–7944. [Google Scholar] [CrossRef]
- Wang, P.; Pan, B.; Li, H.; Huang, Y.; Dong, X.; Ai, F.; Liu, L.; Wu, M.; Xing, B. The overlooked occurrence of environmentally persistent free radicals in an area with low-rank coal burning, Xuanwei, China. Environ. Sci. Technol. 2018, 52, 1054–1061. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, M.; Xu, Y. Pollution Characteristics and Generation Mechanism of Environmental Persistent Free Radicals. Sci. China: Chem. 2018, 48, 1226–1235. [Google Scholar]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhao, S.; Nulaji, G.; Tao, K.; Wang, F.; Sharma, V.K.; Wang, C. Environmentally persistent free radicals in soils of past coking sites: Distribution and stabilization. Environ. Sci. Technol. 2017, 51, 6000–6008. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Fan, X.; Wang, T. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: Roles of soil characteristics and ambient conditions. J. Hazard. Mater. 2019, 362, 214–223. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Qu, B.; Tian, Y. Photoformation of environmentally persistent free radicals on particulate organic matter in aqueous solution: Role of anthracene and formation mechanism. Chemosphere 2022, 291, 132815. [Google Scholar] [CrossRef]
- Han, L.; Chen, B. Mechanism of environmental persistent free radical production and environmental chemical behavior. Prog. Chem. 2017, 29, 1008–1020. [Google Scholar]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panayiotidis, M.I. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem.-Biol. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef]
- Burn, B.R.; Varner, K.J. Environmentally persistent free radicals (EPFRs) compromise left ventricular function during ischemia/reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H998–H1006. [Google Scholar] [CrossRef]
- Balakrishna, S.; Lomnicki, S.; McAvey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, S.A. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part. Fibre Toxicol. 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting Free Radicals in Biochars and Determining Their Ability to Inhibit the Germination and Growth of Corn, Wheat and Rice Seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar] [CrossRef]
- Singer, L.S. Free radicals in coals and synthetic fuels: L. Petrakis and D. W. Grandy Elsevier, Amsterdam, 1983. Fuel 1985, 64, 1334–1335. [Google Scholar] [CrossRef]
- Valavanidis, A.; Iliopoulos, N.; Gotsis, G.; Fiotakis, K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard. Mater. 2008, 156, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Chen, T.; Zhan, M.X.; Guo, Y.; Xiao-Dong, L.I. Correlation of Persistent Free Radicals, PCDD/Fs and Metals in Waste Incineration Fly Ash. Environ. Sci. 2016, 37, 1163–1170. [Google Scholar]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. Int. 2007, 31, 521–528. [Google Scholar] [CrossRef]
- Nwosu, U.G.; Khachatryan, L.; Youm, S.G.; Roy, A.; Aln, D.C.; Nesterov, E.E.; Dellinger, B.; Cook, R.L. Model System Study of Environmentally Persistent Free Radicals Formation in a Semiconducting Polymer Modified Copper Clay System at Ambient Temperature. RSC Adv. 2016, 6, 43453–43462. [Google Scholar] [CrossRef]
- Kiruri, L.W.; Lavrent, K.; Barry, D.; Slawo, L. Effect of copper oxide concentration on the formation and persistency of environmentally persistent free radicals (EPFRs) in particulates. Environ. Sci. Technol. 2014, 48, 2212–2217. [Google Scholar] [CrossRef]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Formation and stabilization of combustion-generated environmentally persistent free radicals on an Fe(III)2O3/silica surface. Environ. Sci. Technol. 2011, 45, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, H.; Wang, M.; Mu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Zhang, L.; Zhang, Z. Dominant fraction of EPFRs from nonsolvent-extractable organic matter in fine particulates over Xi’an, China. Environ. Sci. Technol. 2018, 52, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Q.; Mou, Z.; Wang, Y.; Shen, Z.; Zhang, L.; Li, Y. Properties and sources of Environmental persistent free radicals (EPFRs) in PM2.5 in Xi ’an. J. Environ. Sci. 2019, 39, 197–203. [Google Scholar] [CrossRef]
- Ding, J.; Chen, Q.; Zhang, Z.; Han, Y. Impact of the “Three-year Action Plan” on the Pollution Characteristics and Sources of Atmospheric Persistent Free Radicals. China Environ. Sci. 2023, 43, 5041–5051. [Google Scholar]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhao, Y.; Wu, X.; Xu, Y. Pivotal roles of metal oxides in the formation of environmentally persistent free radicals. Environ. Sci. Technol. 2017, 51, 12329–12336. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Lifetime of combustion-generated environmentally persistent free radicals on Zn (II) O and other transition metal oxides. J. Environ. Monit. 2012, 14, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.F.; Erdman, J.; Saraceno, A.J. Investigation of the Nature of Free Radicals in Petroleum Asphaltenes and Related Substances by Electron Spin Resonance. Anal. Chem. 1962, 34, 694–700. [Google Scholar] [CrossRef]
- Senesi, N. Application of electron spin resonance (ESR) spectroscopy in soil chemistry. In Advances in Soil Science; Springer: New York, NY, USA, 1990; Volume 14, pp. 77–130. [Google Scholar]
- Watanabe, A.; McPhail, D.B.; Maie, N.; Kawasaki, S.; Anderson, H.A.; Cheshire, M.V. Electron spin resonance characteristics of humic acids from a wide range of soil types. Org. Geochem. 2005, 36, 981–990. [Google Scholar] [CrossRef]
- Yang, J.; Pan, B.; Li, H.; Liao, S.; Zhang, D.; Wu, M.; Xing, B. Degradation of p-nitrophenol on biochars: Role of persistent free radicals. Environ. Sci. Technol. 2016, 50, 694–700. [Google Scholar] [CrossRef]
- Guo, C.; Hasan, F.; Lay, D.; Dela Cruz, A.L.N.; Ghimire, A.; Lomnicki, S.M. Phytosampling—A supplementary tool for particulate matter (PM) speciation characterization. Environ. Sci. Pollut. Res. 2021, 28, 39310–39321. [Google Scholar] [CrossRef]
- Maskos, Z.; Khachatryan, L.; Dellinger, B. Precursors of radicals in tobacco smoke and the role of particulate matter in forming and stabilizing radicals. Energy Fuels 2005, 19, 2466–2473. [Google Scholar] [CrossRef]
- Apicella, B.; Carpentieri, A.; Alfè, M.; Barbella, R.; Tregrossi, A.; Pucci, P.; Ciajolo, A. Mass spectrometric analysis of large PAH in a fuel-rich ethylene flame. Proc. Combust. Inst. 2007, 31, 547–553. [Google Scholar] [CrossRef]
- Rüger, C.P.; Sklorz, M.; Schwemer, T.; Zimmermann, R. Characterisation of ship diesel primary particulate matter at the molecular level by means of ultra-high-resolution mass spectrometry coupled to laser desorption ionisation—Comparison of feed fuel, filter extracts and direct particle measurements. Anal. Bioanal. Chem. 2015, 407, 5923–5937. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Zhang, S.; Peng, C.; Zhang, L.; Yang, F.; Tian, M.; Huang, R.; Wang, Q.; Chen, Q.; Yao, X. Characteristics and potential exposure risks of environmentally persistent free radicals in PM2.5 in the three gorges reservoir area, Southwestern China. Chemosphere 2020, 252, 126425. [Google Scholar] [CrossRef] [PubMed]


| Category | Name | Details |
|---|---|---|
| Reagents | Methanol | chromatographic grade, Macklin, Van Alstyne, TX, USA, ≥99.5% |
| Reagents | Dichloromethane | chromatographic grade, Chengdu Cologne Chemical Co., Ltd., Chengdu, China ≥99.5% |
| Reagents | n-Hexane | chromatographic grade, Chengdu Cologne Chemical Co., Ltd., Chengdu, China ≥99.5% |
| Reagents | Ethanol | analytical grade, Tianjin KeMiOu Chemical Reagent Co., Ltd., Tianjin, China ≥99.5% |
| Materials | Quartz filter membrane | TISSUQUARTZ2500QAT-UP, Pall Life Science, Pensacola, FL, USA |
| Materials | Cellulose | D50, 180–280 um, Shanghai Macroscopic Biochemical Technology Co., Ltd., Shanghai, China |
| Instruments | Tube furnace | OTF-1200X, Hefei Kejing Material Technology Co., Ltd., Hefei, China |
| Instruments | OC/EC analyzer | DRI 2001A, Atmoslytic, Calabasas, CA, USA |
| Instruments | Electron paramagnetic resonance spectrometer | EMX Plus, Bruker, Leipzig, Germany |
| Sample Number | Sample Weight (g) | Combustion Temperature (°C) | Gas Supply Flow Rate (L/min) | Gas Dilution Flow Rate (m3/h) | Sampling Flow Rate (L/min) |
|---|---|---|---|---|---|
| Y1300 | 5.00 | 300 | Air 0.65 | 2 | 16.7 |
| Y1500 | 5.00 | 500 | Air 0.65 | 2 | 16.7 |
| Y1700 | 5.00 | 700 | Air 0.65 | 2 | 16.7 |
| Y0.5300 | 5.00 | 300 | Air 0.325 Nitrogen 0.325 | 2 | 16.7 |
| Y0.5500 | 5.00 | 500 | Air 0.325 Nitrogen 0.325 | 2 | 16.7 |
| Y0.5700 | 5.00 | 700 | Air 0.325 Nitrogen 0.325 | 2 | 16.7 |
| Sample Number | The Concentration of EPFRs (spins/m3) | g-Factor | ΔHP-P |
|---|---|---|---|
| Y1300 | 3.29 × 1014 ± 1.102 × 1013 | 2.0015 | 4.7948 |
| Y1500 | 4.80 × 1014 ± 1.343 × 1013 | 2.0019 | 4.9094 |
| Y1700 | 4.56 × 1014 ± 1.868 × 1013 | 2.0019 | 5.2605 |
| Y0.5300 | 2.89 × 1014 ± 2.104 × 1013 | 2.0020 | 4.8367 |
| Y0.5500 | 3.96 × 1014 ± 1.202 × 1013 | 2.0020 | 4.9961 |
| Y0.5700 | 3.76 × 1014 ± 1.059 × 1013 | 2.0022 | 4.7163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; An, B.; Chen, J.; Zhang, Y.; Yu, G. Study on Non-Metal-Induced EPFRs in PM2.5 Generated from Flue Gas of Cellulose Combustion. Sustainability 2025, 17, 301. https://doi.org/10.3390/su17010301
Zhang L, An B, Chen J, Zhang Y, Yu G. Study on Non-Metal-Induced EPFRs in PM2.5 Generated from Flue Gas of Cellulose Combustion. Sustainability. 2025; 17(1):301. https://doi.org/10.3390/su17010301
Chicago/Turabian StyleZhang, Lixin, Boru An, Jingmin Chen, Yuwei Zhang, and Guojiao Yu. 2025. "Study on Non-Metal-Induced EPFRs in PM2.5 Generated from Flue Gas of Cellulose Combustion" Sustainability 17, no. 1: 301. https://doi.org/10.3390/su17010301
APA StyleZhang, L., An, B., Chen, J., Zhang, Y., & Yu, G. (2025). Study on Non-Metal-Induced EPFRs in PM2.5 Generated from Flue Gas of Cellulose Combustion. Sustainability, 17(1), 301. https://doi.org/10.3390/su17010301






