Abstract
Addressing the complex physicochemical properties of coal gangue from typical mining areas in Inner Mongolia, this study focuses on this area’s abundant reserves coupled with the low utilization rate and significant strength variability of ecological slope protection materials. Notably, research on the alkalization–carbonization of coal gangue remains scarce. To bridge this gap, we propose a method leveraging the moisture migration behavior of coal gangue porous media. By utilizing continuous displacement high-temperature steam carbon sequestration enhancement technology, internal moisture is gradually and precisely controlled to induce the formation of high-temperature carbonic acid gas. This process facilitates internal carbon sequestration and effectively locks in the sequestration effect. This approach enables effective loading of sulfurized CO2 composite gases in a reversible manner, achieving passive carbon sequestration driven by moisture migration. Consequently, it enhances the negative carbon content within the aggregates while bolstering their mechanical properties. After alkalization pretreatment with various concentrations and three hours of carbon sequestration, the microhardness of the aggregate surface and transition zone were observed to have increased by 24.3% and 36.4%, respectively. Additionally, the compressive and splitting tensile strengths of coal gangue concrete rose by 4.8 MPa and 0.4 MPa, respectively, while porosity decreased by up to 3.6%, and the proportion of harmful pores dropped from 11.22% to 6.54%. A strong correlation between the proportion of harmless/low-harm pores and strength development was observed. Overall, the high-temperature carbonic acid steam displacement method with sulfurized CO2 composite gases effectively improves the physicochemical properties of coal gangue aggregates and enhances surface activity and hydration in the interface transition zone, meeting the engineering standards for in situ ecological remediation in Inner Mongolia’s mining areas.
1. Introduction
Inner Mongolia’s coal reserves are substantial, and its annual output of coal gangue is high. The raw coal output reached 1.21 billion tons in 2023, and coal gangue accounted for approximately 10% to 25% of this output [1,2]. In light of the pressing demand for engineering materials in slope construction for typical landfills, the delicate ecological conditions found in typical mining areas [3,4,5], and the inadequacy of natural sand and stone resources in terms of meeting engineering standards, it is not economically viable to transport these materials from abroad [6]. Therefore, utilizing coal gangue as a raw material for engineering purposes holds urgent practical significance.
There exist significant differences in the physicochemical properties of natural coal gangue and natural crushed-stone aggregates. Specifically, natural coal gangue has lower bulk density and apparent density, higher water absorption capacity [7], and a greater crushing value [8]. Furthermore, the surfaces of gangue aggregates tend to liquefy when exposed to water, resulting in poor structural adhesion and larger pores within the interfacial transition zone of coal gangue concrete [9]. GAO et al. have indicated that the direct use of natural coal gangue as coarse aggregates can lead to a reduction in the mechanical properties of concrete by 19.4% to 49.5% [10].
Currently, the primary methods for improving the performance of unconventional aggregates, which have suboptimal properties, include surface coating, high-temperature heat treatment, and chemical activation. These techniques primarily act on the surface or interior of the aggregates through different mechanisms, thereby improving their microstructures and enhancing their physical and mechanical properties, durability, and interfacial bonding performance with respect to cement matrices [11,12,13]. However, they are relatively cumbersome, and from a cost–benefit analysis perspective, their advantages are not significant [14]. For instance, Pang et al. optimized the interfacial transition zone of coal gangue aggregates by coating them with cement fly ash slurry mixed with corn fiber, effectively enhancing the 28-day strength of the coal gangue [15]. Carolina et al. observed that mussel shells heat-treated at 135 °C for 30 min can be used as aggregates in plain concrete. The suitable substitution rate of mussel aggregates (whether sand or gravel) for natural aggregates could reach up to 25%, and when sand and gravel were considered together, the substitution rate could reach up to 12.5%. These percentages are considered appropriate for both structural and non-structural applications of plain concrete [16]. Furthermore, Li et al.’s research shows that interface modification mediated by silane coupling agents can enhance the interfacial compatibility of high-filled coal gangue/polyethylene composites. At an optimal concentration of a 2% relative mass fraction of silane coupling agent KH550, the density and hardness of the surface-treated composite were significantly improved [17].
Among the methods for enhancing the strength of solid-waste aggregates, CO2 gas has emerged as a significant improvement method due to its unique chemical properties and environmental friendliness. Moreover, the carbonation of aggregates is simple and easy to perform, with no other by-products generated during the process, aligning with the concepts of sustainability and green development [18,19,20]. Through carbonation treatment, CO2 reacts with active components in solid-waste aggregates to form stable compounds, thereby enhancing the strength and durability of the aggregates [21,22,23]. For instance, Shuo Yang et al. demonstrated that the strength of industrial waste residues such as steel slag was significantly improved after carbonation treatment, with notable reductions in the crushing value and water absorption rate. This, in turn, improved the overall performance of carbonated steel slag concrete, including its strength and volume stability [24]. Renjie Mi et al. pre-infiltrated coarse coral aggregates with Ca(OH)2 slurry and successfully sequestered CO2 in the form of CaCO3. The proposed CO2 sequestration method did not compromise the strength development of the concrete or the pH value of the concrete pore solution [25].
Currently, the enhancement methods for coal gangue aggregate are relatively limited, and the procedures involved are cumbersome and not easy to perform. This paper introduces a method that involves pre-alkalizing gangue aggregates and subsequently utilizing waste gas from thermal power plants (primarily CO2) as the experimental gas source. Based on the moisture migration behavior of porous media materials derived from coal gangue, steam is used to carry CO2 waste gas to form high-temperature reversible carbonic acid gas, which is then allowed to permeate the interior of the aggregates. As the internal liquid of the aggregates condenses, a carbon fixation reaction begins. High-speed centrifugation is employed to remove pore water from the aggregates, enabling the mixed gas to passively enter the pores. This establishes a continuous carbon sequestration system designed to meet the material requirements for slope construction in typical mining areas (C25 and above). To evaluate the impact of this method, the mechanical properties of coal gangue concrete were tested, and pore data were examined using nuclear magnetic resonance (NMR). Microhardness testing was conducted to observe changes in mechanical properties within the interfacial transition zone. Additionally, X-ray diffraction (XRD), energy-dispersive spectroscopy (EDS), and scanning electron microscopy (SEM) were utilized to observe the morphology of the hydration products. These assessments were conducted to evaluate the influence of alkali–CO2 mineralization under continuous carbon sequestration conditions on the strength and microstructure of coal gangue concrete. With a pretreatment concentration of 0.05 mol/L, the properties of the gangue aggregates meet the requirements for aggregate materials used in slope construction in general mining areas. This method comprehensively enhances the mechanical properties of coal gangue concrete, reduces harmful pores within the concrete, and improves the hydration effect in the interfacial transition zone. Overall, this research provides technical insights for promoting the engineering application of gangue aggregates in typical coal mines as well as achieving green ecological construction and sustainable development in mining areas.
2. Materials and Methods
2.1. Materials
The coal gangue used in this experiment was sourced from the Huangbai secondary mining area in Wuhai City, Inner Mongolia, China. Its primary chemical composition is presented in Table 1. The coarse gangue aggregate had a maximum particle size of 19.0 mm, with the grading curve detailed in Figure 1b. The compressive strength in the cylinder test was 3.08 MPa, the crushing value was 24%, and the water absorption rate stood at 4.04%. Sulfurized CO2 composite gas was obtained from the waste gas of the Qifeng Mining Thermal Power Plant in Wuhai City. For this experiment, P.C 42.5 composite Portland cement was selected (Table 2), with natural river sand serving as the fine aggregate (with the corresponding grading curve shown in Figure 1a). Tap water from Wuhai City was utilized for the experimental procedures.

Table 1.
Coal gangue composition.
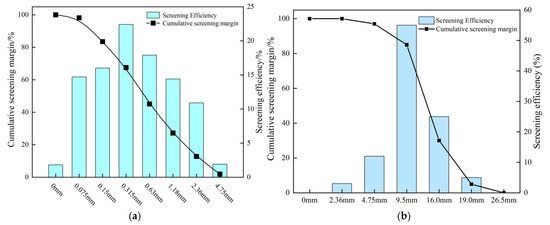
Figure 1.
Aggregate grading: (a) natural sand grading; (b) coal gangue grading.

Table 2.
P-O 42.5 cement components.
2.2. Alkalization and Carbon Fixation of Coal Gangue Aggregate and Its Preparation
In this study, gangue aggregates were soaked in various concentrations of calcium hydroxide alkalizing solutions, which were grouped as follows: CG-0 with 0 mol/L, CG-0.5 with 0.01 mol/L, CG-1 with 0.02 mol/L, CG-2 with 0.05 mol/L, CG-4 with 0.10 mol/L, and CG-8 with 0.19 mol/L. Subsequently, the gangue aggregates were placed in a high-temperature steam-based carbon mineralization enhancement device for 3 h. The effective component in the exhaust gas was kept at a CO2 concentration of 15%, with an SO2 content of approximately 96 mg/m3. The operational process, which featured a mixer speed of 240 r/min, was conducted under normal pressure throughout. Changes in humidity and temperature served as the primary driving forces for the inward migration of sulfurized CO2. The operational process is illustrated in Figure 2. The concrete mix proportions were as follows: cement/sand/gangue aggregate at a mass ratio of 1:1.34:2.65, with a water-to-binder ratio of 0.45. The aggregates were prepared in a saturated surface-dry state to produce 100 mm specimens, which were then subjected to standard curing for 28 days.
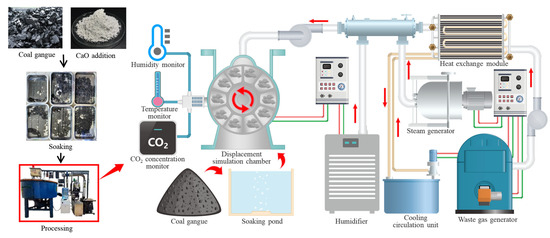
Figure 2.
Aggregate treatment.
The high-temperature steam carbon mineralization enhancement device utilizes steam to carry thermoelectric waste gases such as CO2 and SO2, forming high-temperature dibasic acid reversible gases. These gases are then infused into the interior of the aggregates, where the internal liquid condenses and initiates a carbon mineralization reaction. By employing high-speed centrifugation, the pore water within the aggregates is removed, allowing the mixed gases to passively enter the pores, thereby establishing a continuous carbon mineralization system. Through this continuous carbon mineralization process, the resulting material undergoes a carbon mineralization effect from the inside out, locking in the carbon mineralization products. The sulfurized CO2 composite gases are effectively loaded in the form of reversible gases, enabling passive carbon mineralization of the aggregates dominated by material moisture migration. This approach establishes a CG aggregate enhancement route with coal gangue serving as the skeleton and diverse calcium salts serving as the strength supplement.
In this study, the sulfurized CO2 composite gas, consisting of SO2 and CO2, exhibited the general properties of acidic oxides. Under the influence of high-temperature steam, these oxides form unstable dibasic acids, namely, H2SO3 and H2CO3. Both SO2 and CO2 have densities greater than that of air. At elevated temperatures, their molecular kinetic energy increases, leading to significant jumps in their originally layered distribution and the formation of a spatial network morphology with alternating fusion of SO2 and CO2, demonstrating certain “sulfurization” characteristics. Furthermore, as the high-temperature steam carries the sulfurized CO2 composite gas into the interior of the aggregates, SO2 and CO2 react with Ca(OH)2 solution, generating highly water-soluble Ca(HCO3)2 and Ca(HSO3)2 at high concentrations. This further facilitates the steam-guided migration of Ca(HCO3)2 and Ca(HSO3)2 into the interior of the aggregates. As they migrate near the interior of the aggregates, the increased migration resistance of the steam and decreased migration rate prompt some SO2 to rapidly capture O2, forming SO3, which reacts with the previously formed Ca(HSO3)2 to produce CaSO4. This substance mainly assists in forming the side channels of the pore passages. With prolonged carbon mineralization, CaSO4 reacts with hydrated tricalcium aluminate in the concrete to form high-sulfur and low-sulfur hydrated calcium sulfoaluminate with expansive properties. The expansiveness of these substances blocks the lateral secondary pores, resulting in a regular morphology of the main pore channels. Subsequently, with the support of Ca(HSO3)2, carbonic acid steam accumulates in the medium-sized pore channels, enhancing the carbon mineralization effect and achieving multi-tiered filling and solidification within the pores, significantly improving the physicochemical properties of the aggregates and eliminating stability issues (Figure 3).
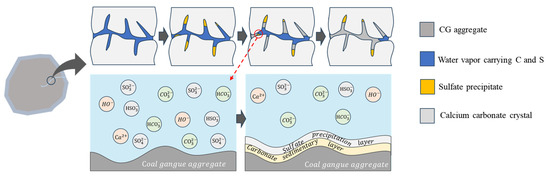
Figure 3.
The principle of aggregate treatment.
2.3. Test Method
2.3.1. Measurement of Mechanical Properties of Coal Gangue Aggregate and Concrete
The samples’ mechanical properties were evaluated through several tests; the water absorption and cylinder compressive strength of the aggregates were measured according to GB/T 17431.2-2010 [26]. Additionally, the compressive strength and splitting tensile strength of 100 mm cubic specimens of gangue concrete were determined based on GB/T 50081-2019 [27]. The compressive strength tests were conducted using a universal testing machine with a loading rate of 2.4 KN/s, and the specimens were tested at ages of 1d, 3d, 7d, and 28d. The final compressive strength value was taken as the average of three tests. The splitting tensile strength tests were also performed using a universal testing machine but at a loading rate of 0.05 MPa/s; additionally, the specimens were tested at an age of 28d, and the final strength value was the average of three tests.
2.3.2. NMR Pore Structure Determination
Nuclear magnetic resonance (NMR) spectroscopy was employed to measure the pore distribution of coal gangue concrete. Cores with a diameter of 40 mm and a height of 48 mm were drilled from the specimens using a core-drilling machine. The samples were then vacuumed for 24 h and subsequently saturated with water. A MesoMR-60 NMR imaging analyzer (Suzhou, China) was used to conduct CPMG pulse sequence testing on the fully water-saturated specimens, offering high precision, non-destructiveness, and continuity in pore size measurement [28].
2.3.3. Microhardness Test and EDS Test
After cutting, mounting, and polishing the coal gangue concrete slice samples, energy-dispersive spectroscopy (EDS) analysis was conducted using a Czech TESCAN MIRA LMS (Brno, Czech Republic). Microhardness testing was performed using a Dutch INNOVATEST FALCON507 Vickers hardness tester (Maastricht, The Netherlands). The arrangement of test points is shown in Figure 4 below.
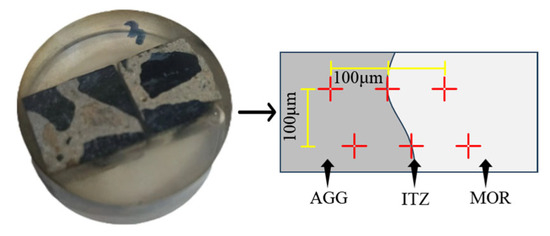
Figure 4.
Distribution of microhardness measurement points.
2.3.4. XRD Test
The chemical compositions of the coal gangue concrete samples were determined using X-ray diffraction (XRD). Coal gangue concrete fragments were selected, crushed, and ground to prepare the XRD samples (with a particle size of approximately 80 mesh). Data collection was performed using a Rigaku SmartLab SE X-ray diffractometer (Dolní Břežany, Czech Republic) with a scanning rate of 5°/min.
2.3.5. SEM Micromorphology Analysis
The micro-morphology of the samples was observed using a field-emission scanning electron microscope (FE-SEM). Fragments of the paste and aggregate bond from the coal gangue concrete were selected as SEM samples (with a maximum length of <10 mm and a thickness of <5 mm). The microstructure of the samples was observed using a field-emission scanning electron microscope (TESCAN MIRA LMS, Czech Republic).
3. Results and Discussion
3.1. Mechanical Properties of Coal Gangue Aggregate and Concrete
The compressive strength of the gangue aggregates increased after carbon sequestration, with the CH-0.5 group showing an increase of 19.3% (Figure 5). The water absorption rate decreased, with the CH-4 group exhibiting a water absorption rate of 3.1%, a decrease of 22.5%. The compressive strength first increased and then decreased as the alkalization concentration increased, while the water absorption rate showed an opposite trend, first decreasing and then increasing. The enhancement in compressive strength and reduction in water absorption rate of the aggregates reveal the blocking effect of the sulfurized CO2 composite gas on the pore channels of the gangue.
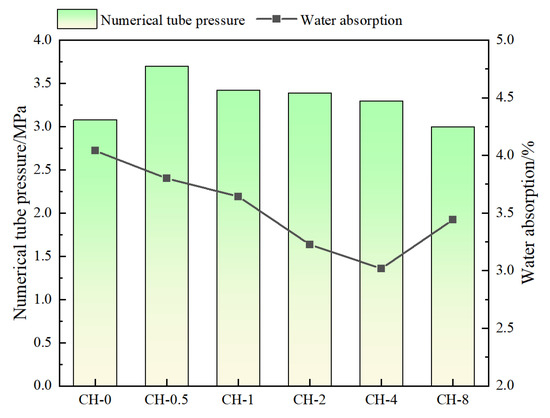
Figure 5.
Aggregate barrel compressive strength and water absorption rate.
The compressive strength and splitting tensile strength of the gangue concrete at various ages increased to varying degrees. Compared to the baseline group, the CH-0.5 group exhibited a 28-day compressive strength of 27.4 MPa, an increase of 4.8 MPa, and a splitting tensile strength of 2.40 MPa, an increase of 0.42 MPa (Figure 6 and Figure 7).
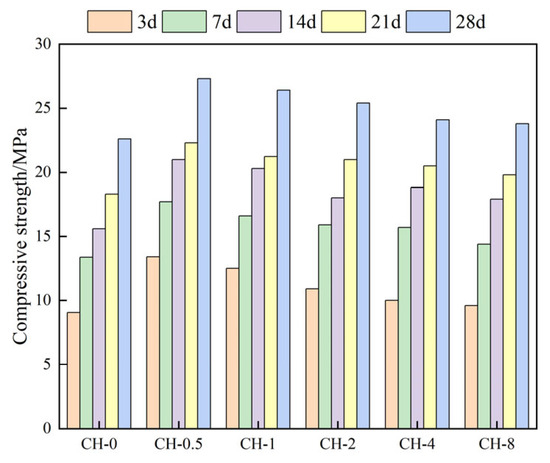
Figure 6.
Compressive strength of standard specimens.
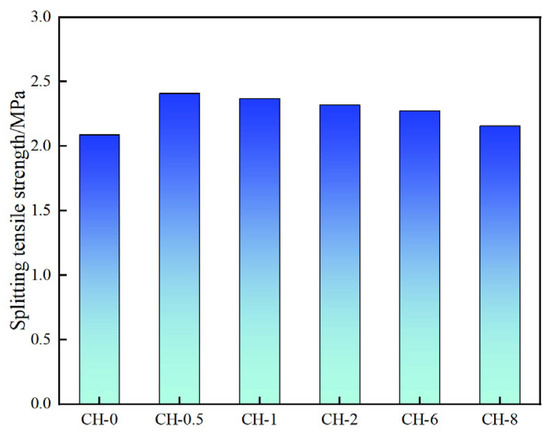
Figure 7.
Splitting compressive strength of standard specimens.
Due to natural bedding defects and their relatively low inherent strength, the mechanical strength of coal gangue aggregates in coal gangue concrete is lower than that of cement paste, resulting in, primarily, aggregate failure and aggregate-paste debonding during splitting. After pre-alkalization and high-temperature steam carbonation treatment, the mechanical properties and surface activity of the gangue aggregates enhanced, improving the interfacial bonding and internal stress tolerance of the coal gangue concrete. However, excessively high pre-alkalization concentrations led to excessively high CaO content at the interface, disrupting the formation of C-S-H gel at the ITZ (Interface Transition Zone), weakening cement-based-aggregate interfacial bonding and resulting in weakened strength development in the CH-1 group over time.
3.2. Analysis of the Pore Characteristics of Gangue Concrete (via NMR)
Under high-temperature steam carbonation enhancement treatment, there is a strong positive correlation between porosity and fluid saturation in coal gangue concrete with varying degrees of pre-alkalization (Figure 8). Compared to the baseline group (CH-0), the porosity of CH-0.5 decreased by 0.15%, a reduction of 3.6%. However, as the pretreatment concentration increased, the porosity of CH-8 increased by 0.83% compared to CH-0.5. Compared to the baseline group, the free-fluid saturation of CH-0.5 decreased by 0.3%, while the free-fluid saturation of CH-8 increased by 0.34%. The proportion of connected pores in CH-0.5 decreased by 11.2% compared to the baseline group, while the proportion of connected pores in CH-8 increased by 14.0% compared to the baseline group. By combining the changes in the nuclear magnetic resonance relaxation time distribution (as shown in Figure 9) [29,30], the pore radii were classified into four ranges: <0.02 μm (non-harmful pores), 0.02–0.05 μm (lightly harmful pores), 0.05–0.2 μm (harmful pores), and >0.2 μm (highly harmful pores). Each group of concrete was classified according to these pore radius ranges (see Figure 10 for details) [31].
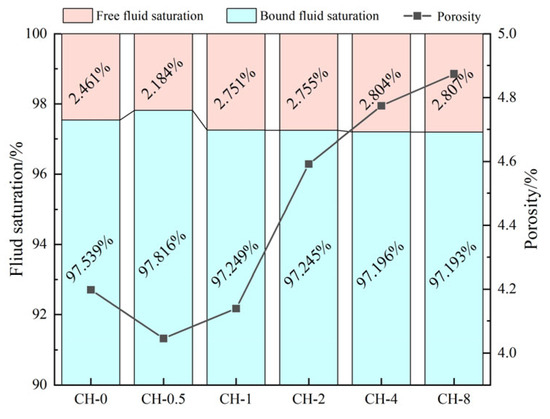
Figure 8.
Fluid saturation and porosity obtained through NMR analysis.
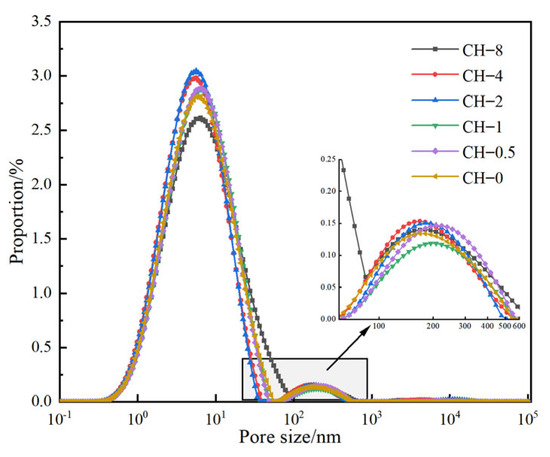
Figure 9.
T2 spectra following NMR pore inversion.
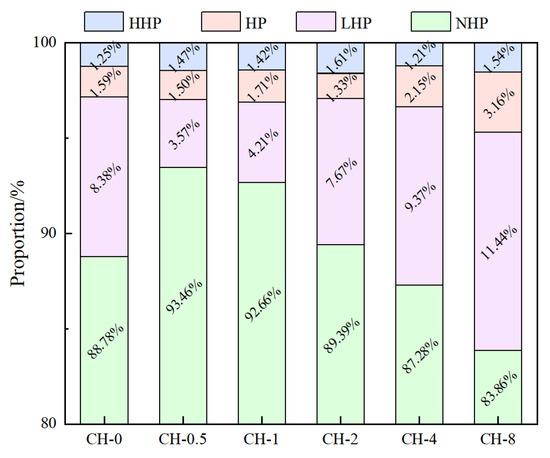
Figure 10.
Aperture classification following NMR pore inversion.
The most probable pore diameters of all groups are basically around 6 nm, with similar pore distribution characteristics. Among them, the proportions of highly harmful pores and harmful pores remain basically unchanged. Compared to the baseline group (CH-0), the proportion of non-harmful pores in CH-0.5 increased from 88.78% to 93.46%, an increase of 5.1%, while the proportion of lightly harmful pores decreased from 8.38% to 3.57%, a decrease of 57.4%. When the alkalization concentration (CH concentration) reached 0.10 mol/L or higher, the proportion of harmful pores increased from 1.50% to 2.15% and 3.61%, the proportion of lightly harmful pores increased from 3.57% to 11.44%, and the proportion of non-harmful pores decreased significantly from 93.46% to 83.86%.
Alkalization at an appropriate concentration can reduce the proportion of harmful pores. As the degree of alkalization increases, the proportion of pores that are harmful increases. The increase in alkalization concentration on the surface of aggregates directly affects porosity and the proportion of smaller pore diameters (<0.05 μm), promoting the conversion trend of non-harmful pores into lightly harmful pores.
Furthermore, based on the Pearson correlation analysis model [32], we established the correlation between porosity (Por), free-fluid saturation (FFS), and the proportions of non-harmful pores (NHP), lightly harmful pores (LHP), harmful pores (HP), and highly harmful pores (HHP) and the alkalization concentration (the concentrations of CaO and C). By considering the CaO distribution characteristics associated with moisture migration in porous medium materials and fully accounting for the impact of sulfurized CO2 composite gas solidification on the pore distribution and development of coal gangue concrete, a relationship based on statistical analysis was established between pore structure parameters and the strength of coal gangue under continuous displacement high-temperature steam carbonation enhancement (Figure 11).
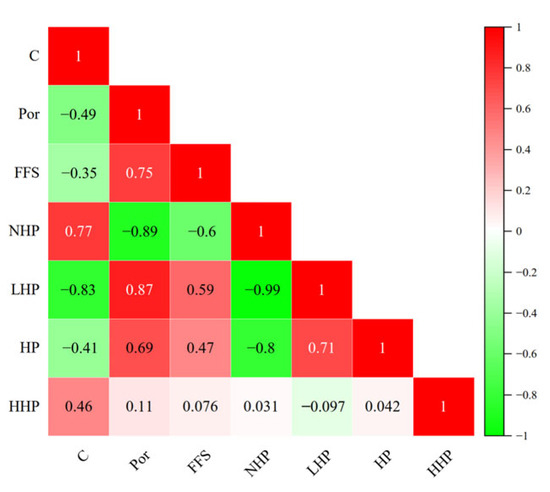
Figure 11.
Correlation heat map including porosity parameters.
After analysis, the Pearson correlation coefficient between the alkalization concentration and the proportion of non-harmful pores was determined to be −0.97, and the Pearson correlation coefficient between the alkalization concentration and the proportion of lightly harmful pores was 0.93. Additionally, there was a strong negative correlation between non-harmful pores and lightly harmful pores, which is consistent with the experimental results. To demonstrate the correlation between the alkalization concentration of coal gangue concrete and the proportion of non-harmful pores within it under high-temperature steam carbonation enhancement treatment, the following model was established:
In the equation above, A represents the pore characteristic matrix, while ω and φ are pore characteristic coefficients. Specifically, ,
The results demonstrate that there is a clear numerical relationship between the alkalization concentration under continuous displacement high-temperature steam carbonation treatment and the proportion of non-harmful pores in coal gangue concrete. As the alkalization concentration increases, the proportion of non-harmful pores exhibits a decreasing trend, and the magnitude of this change gradually diminishes.
At the microscopic level, SO2 and CO2 in the sulfurized CO2 composite gas react with CaO remaining in the tiny pores of coal gangue aggregates to form calcium salts with low solubility, such as CaCO3 and Ca(HSO3)2, filling smaller pores, increasing the proportion of non-harmful pores, and reducing the water absorption rate of the aggregates. When subjected to varying alkalization concentrations, which influence the CaO concentration at the pore openings, under conditions of high CaO concentrations in the environment, SO2 and CO2 are rapidly adsorbed at the pore entrances. Consequently, CaCO3, Ca(HSO3)2, and CaSO4 precipitate outside the pore structure, hindering the infiltration of SO2 and CO2 into the interior of the pore structure and thereby reducing the sealing effectiveness on finer pores. The deposited CaSO4 can react with hydrated tricalcium aluminate in the concrete to produce expansive high-sulfur and low-sulfur hydrated calcium sulfoaluminate, exacerbating the debonding between the deposited layer and the aggregate surface and promoting the development of pores in the interfacial transition zone of coal gangue concrete. Meanwhile, the sealing of pores can effectively reduce the overall water-carrying capacity of the aggregates [33], and the reduced water absorption rate of the aggregates can lower the water/cement ratio in the interfacial transition zone [34], decrease the accumulation of free water on the aggregate surface, optimize the loose packing of cement particles around the aggregates, improve pore distribution, reduce harmful pores, and positively affect the interfacial bonding [35,36]. Therefore, within the scope of this study, an alkalization concentration of 0.05 mol/L is the most beneficial range for pore development in coal gangue concrete under high-temperature steam carbonation enhancement.
3.3. Microhardness of Coal Gangue Concrete
Through a comparative analysis of the micromechanics of the gangue aggregate surface, the interfacial hardness of the ITZ (interfacial transition zone), and the microhardness of the paste near the ITZ, positive enhancement characteristics of carbon sequestration treatment on gangue concrete were observed (as shown in Figure 12). The maximum microhardness of the CH-0.5 aggregate surface reaches 121.5 kg/mm2, representing a 24.3% increase compared to the baseline group. The highest microhardness within the interfacial transition zone of the CH-0.5 aggregates is 76.3 kg/mm2, which is a 36.4% increase compared to the baseline group. Additionally, the maximum microhardness of CH-0.5 paste is 99.1 kg/mm2, indicating a 23.2% improvement over the CH-0 baseline group.
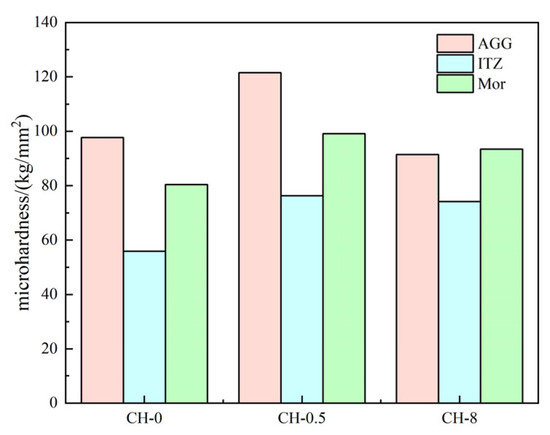
Figure 12.
Microhardness values of ITZ.
The reaction of SO2 and CO2 in a sulfurized CO2 composite gas with CaO produces calcium salts with low solubility, such as CaCO3 and Ca(HSO3)2, which fill small pores and enhance the pore structure of coal gangue, thereby increasing the microhardness of the aggregates. However, in the case of high CaO concentrations, the deposition layer formed by the carbon sequestration products reduces the filling of small pores within the coal gangue aggregates, leading to the significantly higher microhardness in CH-0.5 aggregates compared to the baseline group and CH-8 group. Additionally, both pore filling and deposition layers can prevent the migration of moisture into the interior of the aggregates, reducing the water-carrying capacity of the aggregates and preventing an increase in pores at the ITZ interface due to internal moisture evaporation during the later stages of concrete hydration for aggregates with a high water-carrying capacity [37,38]. Macroscopically, the microhardness in the interfacial transition zone of the CH-0.5 group is similar to that of the CH-8 group but higher than that of the baseline group. Overall, the carbon sequestration reaction can seal the pores on the aggregate surface, reduce the overall water-carrying capacity of the gangue aggregate surface, and improve the hydration effect at the ITZ. The microhardness values of the aggregate surface and its surrounding area of influence correlate with the degree of pre-alkalization, but excessive alkalization has a significant negative impact on the enhancement of the microstructural strength of gangue concrete.
3.4. Analysis of Hydration Characteristics of Coal Gangue Concrete
Samples from the baseline group (CH-0), CH-0.5, and CH-8 specimens were collected and subjected to XRD analysis. Figure 13 presents an XRD comparison between the baseline, CH-0.5, and CH-8 groups. As illustrated in Figure 13, the transition zone of coal gangue concrete under continuous displacement and high-temperature steam-based carbon mineralization enhancement primarily consists of calcium carbonate, sulfur dioxide, calcium hydroxide, and silicon dioxide, among other substances. The baseline group, CH-0.5, and CH-8 exhibited typical quartz peaks at 2θ values of 20.8°, 26.6°, and 50.1°, respectively. Notably, all three groups—the baseline, CH-0.5, and CH-8—displayed calcium carbonate (CaCO3) reflection peaks at approximately 29.4°, 39.4°, and 47.1° in 2θ, indicating the presence of a certain amount of calcium carbonate. In particular, the reflection peaks of calcium hydroxide Ca(OH)2, at 2θ values of approximately 18.1°, 34.1°, and 50.8°, exhibited a clear correlation with the alkaline treatment concentration. Specifically, the peak intensity of Ca(OH)2 in the CH-0.5 group was significantly lower than that in the baseline and CH-8 groups.
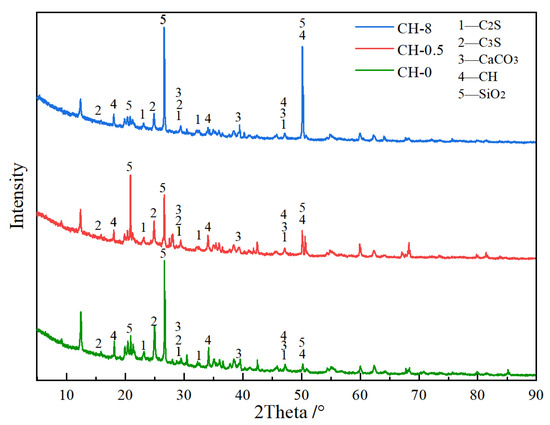
Figure 13.
XRD spectrum containing ITZ.
The main components within the transition zone of coal gangue concrete under enhanced carbon sequestration with continuous displacement of high-temperature steam are calcium carbonate, sulfur dioxide, calcium hydroxide, and silica phases, among others. Compared to the baseline group, the calcium hydroxide peak in CH-0.5 is significantly lower due to the reaction between carbon in the exhaust gas and CH, resulting in the formation of insoluble crystalline substances and thus decreasing the relative content of calcium hydroxide. The resurgence in CH peak height in CH-8 indicates that CO2 treatment for a limited time (3 h) cannot fully consume high concentrations of CH. Furthermore, the rapid reaction between high concentrations of CH and sulfurized CO2 composite gas produces products such as CaCO3 and CaSO4, which accumulate at the openings of pore structures and on the surface, inhibiting the reaction process and affecting the overall carbon sequestration effect.
As shown in Figure 14, there is a clear positional relationship between the distribution of Ca and C and the paste–ITZ–gangue aggregate. Within the paste region and the gangue aggregate, the content of C and Ca may fluctuate with distance but generally remains within a relatively stable range. In the ITZ region, the content of C and Ca varies with distance, and the distance between the last Ca peak and the first C peak can be considered the ITZ region.
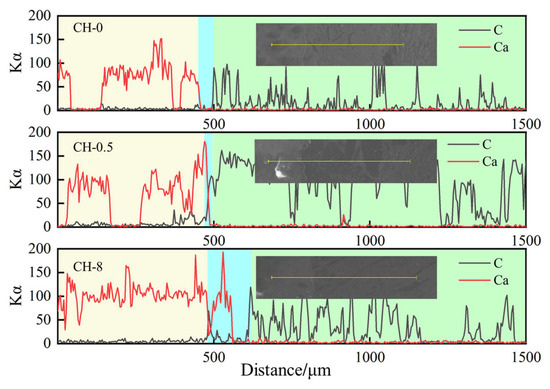
Figure 14.
EDS spectrum containing ITZ.
The results indicate that CH-0.5 has a thinner transition zone, making it superior to both the baseline group and the CH-8 group in this respect. The sulfurized CO2 composite gas under the continuous carbon sequestration system can effectively react with CaO within the aggregates, and the formed deposition products block the pore-opening structures. This reduces the water/cement ratio in the interfacial transition zone and also prevents the migration of cement components and moisture into the aggregates, resulting in a thinner C-Si boundary zone.
At the pore openings, a “trapping effect” occurs due to the high concentration of CaO, which causes the sulfurized CO2 composite gas to combine with Ca2+ to form a relatively thick deposition layer. As high-temperature steam carries the sulfurized CO2 composite gas into the interior of the aggregates, SO2 and CO2 react with the Ca(OH)2 solution, generating Ca(HCO3)2 and Ca(HSO3)2 in a high-concentration state readily soluble in water. This further facilitates the steam-induced migration of Ca(HCO3)2 and Ca(HSO3)2 towards the interior of the aggregates. When these substances migrate to the trapping zone at the pore openings, resistance increases, and the migration rate decreases, leading to deposition outside the pore structure. The reducing property of some SO2 components prompts them to rapidly capture O2, forming SO3, which in turn converts the originally formed Ca(HSO3)2 into CaSO4. The reaction between CaSO4 in the deposition layer and trihydrate aluminum calcium sulfate in the concrete produces expansive ettringite (both high-sulfur and low-sulfur types), exacerbating the debonding between the deposition layer and the aggregate surface. Consequently, a thicker C-Si interface zone is formed in the CH-8 group.
3.5. Observation of Microscopic Morphology of Gangue Concrete
To further investigate the distribution characteristics of hydration products and the influence of the carbon sequestration system on the hydration of coal gangue concrete, SEM was employed to compare the micromorphological features of hydration products between the baseline group (CH-0), CH-0.5, and CH-8 (as shown in Figure 15). By comparing the micro morphologies, it was observed that the surfaces of the aggregates in the baseline group exhibited no significant protrusions, with a distinct interfacial transition zone. The surface morphologies of the left and right sections were markedly different, with only a small amount of hydration products interwoven and no cubic carbonation product particles present. A few platelet-like calcium hydroxide (CH) particles were observed. In CH-0.5, notable filamentous calcium silicate hydrate (C-S-H) gels were observed to be interwoven and cemented, with cubic products (calcite) encapsulated within. The surface structure of the interfacial transition zone was rich, with only a few platelets and lamellae interspersed. In CH-8, filamentous C-S-H existed but were not tightly interwoven. Additionally, a large number of amorphous hydration products adhered to the surface of the aggregates. Obvious cracks were present, but the hydration products filling the cracks were not tightly packed, and no significant growth of cubic calcite (CC) was observed.
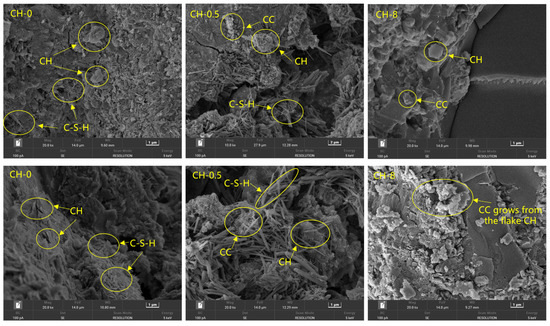
Figure 15.
SEM image containing hydration products.
During the initial stages of hydration, hydration product crystals precipitate from saturated salt solutions. However, the crystallization process requires overcoming potential energy barriers, and crystal nuclei can drive liquid-phase molecules to overcome these energy barriers, thereby further promoting crystal growth [39,40,41,42]. The primary reason for the well-developed hydration interface in CH-0.5 is that the carbonation products form micro-particles adhering to the surfaces of the aggregates, serving as crystal nuclei, which reduce the energy barriers for the crystallization of hydration products and induce the growth of hydration product crystals. However, with high CH concentrations (CH-8), the deep carbonation reaction is suppressed, with less calcium carbonate adhering to the surfaces of the aggregates, resulting in poor hydration development at the interfacial transition zone (ITZ).
Based on the aforementioned aspects, the continuous displacement of high-temperature steam for enhanced carbon sequestration treatment significantly improves the performance of coal gangue concrete. The sulfurized CO2 composite gas forms granular deposits within the pores and microcracks of the gangue aggregates. These deposits not only strengthen the internal structure of the aggregates but also significantly enhance their chemical activity. The attachment of carbon sequestration products on the surface of the aggregates alters their micro-morphology, forming granular protrusions that serve as nucleation sites for hydration product crystals. These protrusions reduce the energy barriers for the crystallization of hydration products, thereby better inducing the growth of C-S-H (calcium silicate hydrate) crystals [43,44].
Furthermore, the carbon sequestration treatment effectively reduces the water-cement ratio at the ITZ (interfacial transition zone) and decreases the thickness of the ITZ. By sealing the microcracks on the surface of the aggregates, the carbon sequestration products reduce the overall water content in the aggregates and increase the density packing of cement particles. This contributes to the formation of a more stable aggregate–cement cementitious matrix, improving the overall performance of the material and reducing the formation of harmful pores. The effective sealing of gangue aggregates under the continuous displacement of high-temperature steam for carbon sequestration treatment enhances the properties of the aggregates and improves gangue–mortar interfacial transition.
4. Conclusions
This study aimed to optimize coal gangue performance through sulfurized CO2 composite gas–steam carbon sequestration, enhancing coal gangue concrete properties and elucidating underlying mechanisms. Variations in exhaust gas composition, type, and treatment conditions influenced outcomes. Based on experimental results, the following conclusions were drawn:
- (1)
- Pre-alkalized coal gangue treated with sulfurized CO2 composite gas served as an effective carbon sequestration carrier. At a pretreatment concentration of 0.5 mol/L, the gangue aggregate’s water absorption rate was 3.1%, and its compressive strength increased by 0.6 MPa. Consequently, coal gangue concrete’s compressive strength improved by a maximum of 20.7%.
- (2)
- Continuous displacement of high-temperature steam during carbon sequestration significantly affected pore distribution. At 0.5 mol/L, pore development improved, with a porosity reduction rate of 3.6% and a harmful pore reduction rate of 5.3%. Pores smaller than 0.02 μm positively correlated with pre-alkalization concentration, while pores between 0.02 μm and 0.05 μm in size negatively correlated with smaller pores.
- (3)
- Pre-alkalized coal gangue aggregates treated with sulfurized CO2 composite gas exhibited improved interfacial transition properties in concrete. Treatment with 0.5 mol/L of calcium hydroxide (CH) strengthened the aggregates, forming CaCO3 at the interfacial transition zone (ITZ), reducing its thickness. The surface hardness of aggregates increased by 24.3%, and ITZ hardness increased by 36.4%.
- (4)
- At alkalization concentrations of 0.19 mol/L and above, carbon sequestration products like CaCO3 were generated, but the carbonation effect was poor. The ITZ of coal gangue concrete thickened, weakening strength enhancement to only 5.3% compared to the baseline. Harmful pore proportion increased by 43.8%, and residual CH had significant negative effects.
Future research will focus on the long-term applicability of these findings in the coal-mining region of Inner Mongolia Autonomous Region, China, addressing the urgent need for engineering materials in landfill slope construction. Our study aims to provide technical references for green ecological governance in coal mining areas, promoting sustainable development through advanced engineering applications of gangue aggregates.
Author Contributions
Conceptualization, H.S. and H.W.; methodology, W.Z.; software, Q.L.; validation, H.W., Q.L. and W.Z.; formal analysis, H.S.; investigation, H.S.; resources, H.S. and Q.L.; data curation, H.S.; writing—original draft preparation, H.S.; writing—review and editing, H.S.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Numbers: 52469024; 52069024) and the Major Science and Technology Special Project of Inner Mongolia Autonomous Region (Grant Number: 2021ZD0007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, models, and code generated or used during the study appear in the submitted article.
Acknowledgments
The authors would like to express their gratitude to all those who have contributed to this research. Special thanks go to the funding agencies, including the National Natural Science Foundation of China (Grant Numbers: 52469024; 52069024) and the Major Science and Technology Special Project of Inner Mongolia Autonomous Region (Grant Number: 2021ZD0007), for their financial support that partially enabled this research.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving Strength of Calcinated Coal Gangue Geopolymer Mortars Via Increasing Calcium Content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Song, W.; Hou, W. High-Capacity Utilization of Coal Gangue as Supplementary Cementitious Material, Geopolymer, and Aggregate: A Review. Constr. Build. Mater. 2024, 435, 136857. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Pei, Y.; Qiao, W.; Wu, Y. Classification of the Type of Eco-Geological Environment of a Coal Mine District: A Case Study of an Ecologically Fragile Region in Western China. J. Clean. Prod. 2018, 174, 1513–1526. [Google Scholar] [CrossRef]
- Li, L.; Fan, Z.; Xiong, K.; Shen, H.; Guo, Q.; Dan, W.; Li, R. Current Situation and Prospects of the Studies of Ecological Industries and Ecological Products in Eco-Fragile Areas. Environ. Res. 2021, 201, 111613. [Google Scholar] [CrossRef]
- Xiao, W.; Lv, X.; Zhao, Y.; Sun, H.; Li, J. Ecological Resilience Assessment of an Arid Coal Mining Area Using Index of Entropy and Linear Weighted Analysis: A Case Study of Shendong Coalfield, China. Ecol. Indic. 2020, 109, 105843. [Google Scholar] [CrossRef]
- Ayenagbo, K.; Ngui, J.; James Gondwe, K.; Rongcheng, W. The Transportation and Marketing Implications of Sand and Gravel and Its Environmental Impact in Lome-Togo. J. Econ. Int. Financ. 2021, 3, 125–138. [Google Scholar]
- Zhou, M.; Dou, Y.; Zhang, Y.; Zhang, Y.; Zhang, B. Effects of the Variety and Content of Coal Gangue Coarse Aggregate on the Mechanical Properties of Concrete. Constr. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Lei, B.; Yang, S.; Liu, Y.; Zhang, Z.; Wang, Y.; Wang, Y. Coal Mine Solid Waste Backfill Process in China: Current Status and Challenges. Sustainability 2023, 15, 13489. [Google Scholar] [CrossRef]
- Wen, P.; Wang, C.; Song, L.; Niu, L.; Chen, H. Durability and Sustainability of Cement-Stabilized Materials Based on Utilization of Waste Materials: A Literature Review. Sustainability 2021, 13, 11610. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.; Guo, L. Application of Coal Gangue as a Coarse Aggregate in Green Concrete Production: A Review. Materials 2021, 14, 6803. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-Friendly Geopolymer Prepared from Solid Wastes: A Critical Review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, G.; Wu, C.; Li, J.; Wu, S.; Jiang, W.; Wang, X.; Wang, W.; Feng, M. Investigation of Hierarchical Porous Cold Bonded Lightweight Aggregates Produced from Red Mud and Solid-Waste-Based Cementitious Material. Constr. Build. Mater. 2021, 308, 124990. [Google Scholar] [CrossRef]
- Choi, H.; Choi, H.; Lim, M.; Inoue, M.; Kitagaki, R.; Noguchi, T. Evaluation on the Mechanical Performance of Low-Quality Recycled Aggregate through Interface Enhancement between Cement Matrix and Coarse Aggregate by Surface Modification Technology. Int. J. Concr. Struct. Mater. 2016, 10, 87–97. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, J.; Jia-Wei, L.; Jizhi, Z.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, Ç.; Pan, B.; Li, X. A Review of China’s Municipal Solid Waste (Msw) and Comparison with International Regions: Management and Technologies in Treatment and Resource Utilization. J. Clean. Prod. 2021, 293, 126144. [Google Scholar] [CrossRef]
- Pang, S.; Li, J.; Xie, F.; Wang, G.; Fan, H.; Zhu, K. Research on Improving the Flexural Performance of Alkali-Activated Geopolymer Cemented Coal Gangue through Layered Addition of Fibers. J. Build. Eng. 2024, 96, 110549. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Martínez-Abella, F.; Carro-López, D. Performance of Mussel Shell as Aggregate in Plain Concrete. Constr. Build. Mater. 2017, 139, 570–583. [Google Scholar] [CrossRef]
- Li, C.; Liao, H.; Gao, H.; Cheng, F. Enhancing Interface Compatibility in High-Filled Coal Gangue/Polyethylene Composites through Silane Coupling Agent-Mediated Interface Modification. Compos. Sci. Technol. 2024, 251, 110546. [Google Scholar] [CrossRef]
- Last, G.V.; Schmick, M.T. A Review of Major Non-Power-Related Carbon Dioxide Stream Compositions. Environ. Earth Sci. 2015, 74, 1189–1198. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C. Potential of CO2 Sequestration through Construction and Demolition (C&D) Waste—An Overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar]
- Tam, V.W.; Butera, A.; Le, K.N.; Li, W. Utilising CO2 Technologies for Recycled Aggregate Concrete: A Critical Review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Qi, Z.; Feng, P.; Shen, X.; Lu, J.; Ye, S.; Wang, H.; Ling, T.; Ran, Q. Utilization of Solid Wastes to Sequestrate Carbon Dioxide in Cement-Based Materials and Methods to Improve Carbonation Degree: A Review. J. CO2 Util. 2023, 72, 102502. [Google Scholar]
- Feng, C.; Cui, B.; Huang, Y.; Guo, H.; Zhang, W.; Zhu, J. Enhancement Technologies of Recycled Aggregate–Enhancement Mechanism, Influencing Factors, Improvement Effects, Technical Difficulties, Life Cycle Assessment. Constr. Build. Mater. 2022, 317, 126168. [Google Scholar] [CrossRef]
- Sakir, S.; Raman, S.N.; Safiuddin, M.; Amrul Kaish, A.B.M.; Mutalib, A.A. Utilization of by-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction. Sustainability 2020, 12, 3888. [Google Scholar] [CrossRef]
- Yang, S.; Mo, L.; Deng, M. Effects of Ethylenediamine Tetra-Acetic Acid (Edta) on the Accelerated Carbonation and Properties of Artificial Steel Slag Aggregates. Cem. Concr. Compos. 2021, 118, 103948. [Google Scholar] [CrossRef]
- Mi, R.; Yu, T.; Poon, C.S. Feasibility of Utilising Porous Aggregates for Carbon Sequestration in Concrete. Environ. Res. 2023, 228, 115924. [Google Scholar] [CrossRef]
- Gb/T 17431.2-2010; Light Aggregates and Their Test Methods—Part 2: Test Methods for Light Aggregates. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China & Standardization Administration of China: Beijing, China, 2010.
- Gb-T 50081-2019; Standard for Inspection and Evaluation of Concrete Strength. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2019.
- de J Cano-Barrita, P.F.; Castellanos, F.; Ramírez-Arellanes, S.; Cosmes-López, M.F.; Reyes-Estevez, L.R.; Hernández-Arrazola, S.E.; Ramírez-Ortíz, A.E. Monitoring Compressive Strength of Concrete by Nuclear Magnetic Resonance, Ultrasound, and Rebound Hammer. ACI Mater. J. 2015, 112, 147. [Google Scholar] [CrossRef]
- Grunewald, E.; Knight, R. A Laboratory Study of Nmr Relaxation Times and Pore Coupling in Heterogeneous Media. Geophysics 2009, 74, E215–E221. [Google Scholar] [CrossRef]
- An, C.; Yan, B.; Alfi, M.; Mi, L.; Killough, J.E.; Heidari, Z. Estimating Spatial Distribution of Natural Fractures by Changing Nmr T2 Relaxation with Magnetic Nanoparticles. J. Pet. Sci. Eng. 2017, 157, 273–287. [Google Scholar] [CrossRef]
- Wu, Z.W. Discussion on the Recent Development Direction of Concrete Science and Technology. J. Chin. Ceram. Soc. 1979, 3, 262–270. [Google Scholar]
- Dutilleul, P.; Stockwell, J.D.; Frigon, D.; Legendre, P. The Mantel Test Versus Pearson’s Correlation Analysis: Assessment of the Differences for Biological and Environmental Studies. J. Agric. Biol. Environ. Stat. 2000, 5, 131–150. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of Calcium Sulfoaluminate Formation from Tricalcium Aluminate, Calcium Sulfate and Calcium Oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Vargas, P.; Restrepo-Baena, O.; Tobón, J.I. Microstructural Analysis of Interfacial Transition Zone (Itz) and Its Impact on the Compressive Strength of Lightweight Concretes. Constr. Build. Mater. 2017, 137, 381–389. [Google Scholar] [CrossRef]
- Yang, B.; Kamali-Bernard, S.; Bernard, F. Microstructure, Tensile Strength and Shear Strength of Aggregate-Mortar Interface: Effect of Aggregate Mineralogy. Constr. Build. Mater. 2023, 388, 131721. [Google Scholar] [CrossRef]
- Chinchillas-Chinchillas, M.J.; Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Gómez-Soberón, J.M.; Corral-Higuera, R. Sem Image Analysis in Permeable Recycled Concretes with Silica Fume. A Quantitative Comparison of Porosity and the Itz. Materials 2019, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, B.; Dai, Q.; Yu, X. Investigation of Internal Curing Effects on Microstructure and Permeability of Interface Transition Zones in Cement Mortar with Sem Imaging, Transport Simulation and Hydration Modeling Techniques. Constr. Build. Mater. 2015, 76, 366–379. [Google Scholar] [CrossRef]
- Wong, H.S.; Zobel, M.; Buenfeld, A.R.; Zimmerman, R.W. Influence of the Interfacial Transition Zone and Microcracking on the Diffusivity, Permeability and Sorptivity of Cement-Based Materials after Drying. Mag. Concr. Res. 2009, 61, 571–589. [Google Scholar] [CrossRef]
- Espinosa-Marzal, R.M.; Scherer, G.W. Advances in Understanding Damage by Salt Crystallization. Acc. Chem. Res. 2010, 43, 897–905. [Google Scholar] [CrossRef]
- Lutsko, J.F. How Crystals Form: A Theory of Nucleation Pathways. Sci. Adv. 2019, 5, eaav7399. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Jean-Paul, C. Modeling Capillary Flows by Conservation of Acceleration and Surface Energy. Int. J. Multiph. Flow 2024, 171, 104672. [Google Scholar]
- Shen, P.; Gu, Z.; Lu, J.; Zhang, Y.; Jiang, Y.; Xuan, D.; Zhang, S.; Poon, C.S. Preparation of Reactive Urchin-Like Recycled Concrete Aggregate by Wet Carbonation: Towards Improving the Bonding Capability of the Interfacial Transition Zone in Recycled Aggregate Concrete. Cem. Concr. Compos. 2023, 143, 105235. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, L.; Liu, J.; Ma, Y.; Liu, Y.; Xiao, Z.; Shi, C. Accelerators for Normal Concrete: A Critical Review on Hydration, Microstructure and Properties of Cement-Based Materials. Cem. Concr. Compos. 2022, 134, 104762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).