Conservation Value to Bats: Assessing Multiple Functional Habitats in a Nature Preserve at the Urban-Agricultural Interface via Temporal Ecology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Recording Sites, and Detector Setup
2.2. Acoustic Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, K.R.; Anderson, R.C.; Schwartz, M.W. The Tallgrass Prairie Mosaic. In Conservation in Highly Fragmented Landscapes; Schwartz, M.W., Ed.; Springer: New York, NY, USA, 1997; pp. 55–87. ISBN 978-1-4757-0656-7. [Google Scholar]
- Albrecht, H.; Haider, S. Species Diversity and Life History Traits in Calcareous Grasslands Vary along an Urbanization Gradient. Biodivers. Conserv. 2013, 22, 2243–2267. [Google Scholar] [CrossRef]
- Boutin, C.; Jobin, B.; Bélanger, L.; Choinière, L. Plant Diversity in Three Types of Hedgerows Adjacent to Cropfields. Biodivers. Conserv. 2002, 11, 1–25. [Google Scholar] [CrossRef]
- van der Ree, R.; McCarthy, M.A. Inferring Persistence of Indigenous Mammals in Response to Urbanisation. Anim. Conserv. 2005, 8, 309–319. [Google Scholar] [CrossRef]
- Van Der Walt, L.; Cilliers, S.S.; Kellner, K.; Du Toit, M.J.; Tongway, D. To What Extent Does Urbanisation Affect Fragmented Grassland Functioning? J. Environ. Manag. 2015, 151, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, R.; Bengtsson, J.; Berg, Å.; Cousins, S.A.O.; Eriksson, O.; Gustafsson, T.; Hasund, K.P.; Lenoir, L.; Pihlgren, A.; Sjödin, E.; et al. A Landscape Perspective on Conservation of Semi-Natural Grasslands. Agric. Ecosyst. Environ. 2008, 125, 213–222. [Google Scholar] [CrossRef]
- Shafer, C.L. Terrestrial Nature Reserve Design at the Urban/Rural Interface. In Conservation in Highly Fragmented Landscapes; Schwartz, M.W., Ed.; Springer: New York, NY, USA, 1997; pp. 345–378. ISBN 978-1-4757-0656-7. [Google Scholar]
- Volenec, Z.M.; Dobson, A.P. Conservation Value of Small Reserves. Conserv. Biol. 2020, 34, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Steffan-Dewenter, I.; Kruess, A.; Thies, C. Contribution of Small Habitat Fragments to Conservation of Insect Communities of Grassland–Cropland Landscapes. Ecol. Appl. 2002, 12, 354–363. [Google Scholar] [CrossRef]
- Kendal, D.; Zeeman, B.J.; Ikin, K.; Lunt, I.D.; McDonnell, M.J.; Farrar, A.; Pearce, L.M.; Morgan, J.W. The Importance of Small Urban Reserves for Plant Conservation. Biol. Conserv. 2017, 213, 146–153. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Janousek, W.M.; McCarty, J.P.; Wolfenbarger, L.L. Effects of urbanization on site occupancy and density of grassland birds in tallgrass prairie fragments. J. Field Ornithol. 2014, 85, 258–273. [Google Scholar] [CrossRef]
- Schwartz, M.W.; van Mantgem, P.J. The Value of Small Preserves in Chronically Fragmented Landscapes. In Conservation in Highly Fragmented Landscapes; Schwartz, M.W., Ed.; Springer: New York, NY, USA, 1997; pp. 379–394. ISBN 978-1-4757-0656-7. [Google Scholar]
- Divoll, T.J.; Aldrich, S.P.; Haulton, G.S.; O’Keefe, J.M. Endangered Myotis Bats Forage in Regeneration Openings in a Managed Forest. For. Ecol. Manag. 2022, 503, 119757. [Google Scholar] [CrossRef]
- Best, T.L.; Geluso, K.N. Summer Foraging Range of Mexican Free-Tailed Bats (Tadarida brasiliensis Mexicana) from Carlsbad Cavern, New Mexico. Swna 2003, 48, 590–596. [Google Scholar] [CrossRef]
- Hall, E.M.; Bennett, V.J. Seasonal Variation in Home Range Size of Evening Bats (Nycticeius humeralis) in an Urban Environment. J. Mammal. 2021, 102, 1497–1506. [Google Scholar] [CrossRef]
- Turner, M.; Gardner, R.; O’Neill, R. Landscape Ecology in Theory and Practice: Pattern and Process; Springer: New York, NY, USA, 2001; ISBN 978-0-387-95123-2. [Google Scholar]
- Schwartz, M.W. Choosing the Appropriate Scale of Reserves for Conservation. Annu. Rev. Ecol. Syst. 1999, 30, 83–108. [Google Scholar] [CrossRef]
- Hofmann, J.E. A Limited Survey of the Bats of the Middle Fork River Forest Preserve; Illinois Natural History Survey: Champaign County, IL, USA, 2003. [Google Scholar]
- Barton, A.; Miller, E.; Bergeson, S. Bat Diversity Survey of a Nature Preserve Complex near, Fort Wayne, Indiana. Proc. Indiana Acad. Sci. 2020, 129, 115–123. [Google Scholar]
- Coleman, J.L.; Barclay, R.M.R. Prey Availability and Foraging Activity of Grassland Bats in Relation to Urbanization. J. Mammal. 2013, 94, 1111–1122. [Google Scholar] [CrossRef]
- Vanreusel, W.; Van Dyck, H. When Functional Habitat Does Not Match Vegetation Types: A Resource-Based Approach to Map Butterfly Habitat. Biol. Conserv. 2007, 135, 202–211. [Google Scholar] [CrossRef]
- Krausman, P.R.; Morrison, M.L. Another Plea for Standard Terminology. J. Wildl. Manag. 2016, 80, 1143–1144. [Google Scholar] [CrossRef]
- Kunz, T.H.; Fenton, M.B. Bat Ecology; University of Chicago Press: Chicago, IL, USA, 2003; ISBN 978-0-226-46206-6. [Google Scholar]
- Kunz, T.H. Resource Utilization: Temporal and Spatial Components of Bat Activity in Central Iowa. J. Mammal. 1973, 54, 14–32. [Google Scholar] [CrossRef]
- Mohan, R.; Chhaya, V.; Krishnan, A. Seasonality and Interspecific Temporal Partitioning in a Semiarid Grassland Bat Assemblage of Northwestern India. J. Arid. Environ. 2022, 205, 104818. [Google Scholar] [CrossRef]
- Mariton, L.; Kerbiriou, C.; Bas, Y.; Zanda, B.; Le Viol, I. Even Low Light Pollution Levels Affect the Spatial Distribution and Timing of Activity of a “Light Tolerant” Bat Species. Environ. Pollut. 2022, 305, 119267. [Google Scholar] [CrossRef]
- Li, H.; Crihfield, C.; Feng, Y.; Gaje, G.; Guzman, E.; Heckman, T.; Mellis, A.; Moore, L.; Romo Bechara, N.; Sanchez, S.; et al. The Weekend Effect on Urban Bat Activity Suggests Fine Scale Human-Induced Bat Movements. Animals 2020, 10, 1636. [Google Scholar] [CrossRef]
- Rivero, J.; Mena Alvarez, J.L. Hourly Activity Patterns of the Insectivorous Bat Assemblage in the Urban-Rural Landscape of Lima, Peru. J. Mammal. 2023, 104, 1–13. [Google Scholar] [CrossRef]
- Sedlock, J.L.; Stuart, A.M.; Horgan, F.G.; Hadi, B.; Como Jacobson, A.; Alviola, P.A.; Alvarez, J.D.V. Local-Scale Bat Guild Activity Differs with Rice Growth Stage at Ground Level in the Philippines. Diversity 2019, 11, 148. [Google Scholar] [CrossRef]
- Beilke, E.A.; Blakey, R.V.; O’Keefe, J.M. Bats Partition Activity in Space and Time in a Large, Heterogeneous Landscape. Ecol. Evol. 2021, 11, 6513–6526. [Google Scholar] [CrossRef]
- Parker, K.A., Jr.; Li, H.; Kalcounis-Rueppell, M.C. Species-Specific Environmental Conditions for Winter Bat Acoustic Activity in North Carolina, United States. J. Mammal. 2020, 101, 1502–1512. [Google Scholar] [CrossRef]
- Gorman, K.M.; Barr, E.L.; Ries, L.; Nocera, T.; Ford, W.M. Bat Activity Patterns Relative to Temporal and Weather Effects in a Temperate Coastal Environment. Glob. Ecol. Conserv. 2021, 30, e01769. [Google Scholar] [CrossRef]
- Deeley, S.; Ford, W.M.; Kalen, N.J.; Freeze, S.R.; St. Germain, M.; Muthersbaugh, M.; Barr, E.; Kniowski, A.; Silvis, A.; De La Cruz, J. Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity 2022, 14, 319. [Google Scholar] [CrossRef]
- Schaik, J.v.; Janssen, R.; Bosch, T.; Haarsma, A.-J.; Dekker, J.J.A.; Kranstauber, B. Bats Swarm Where They Hibernate: Compositional Similarity between Autumn Swarming and Winter Hibernation Assemblages at Five Underground Sites. PLoS ONE 2015, 10, e0130850. [Google Scholar] [CrossRef]
- Li, H.; Wilkins, K.T. Predator-Prey Relationship between Urban Bats and Insects Impacted by Both Artificial Light at Night and Spatial Clutter. Biology 2022, 11, 829. [Google Scholar] [CrossRef]
- Kerbiriou, C.; Barré, K.; Mariton, L.; Pauwels, J.; Zissis, G.; Robert, A.; Le Viol, I. Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity 2020, 12, 165. [Google Scholar] [CrossRef]
- Frick, W.F.; Kingston, T.; Flanders, J. A Review of the Major Threats and Challenges to Global Bat Conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Frick, W.F.; Puechmaille, S.J.; Hoyt, J.R.; Nickel, B.A.; Langwig, K.E.; Foster, J.T.; Barlow, K.E.; Bartonička, T.; Feller, D.; Haarsma, A.-J.; et al. Disease Alters Macroecological Patterns of North American Bats. Glob. Ecol. Biogeogr. 2015, 24, 741–749. [Google Scholar] [CrossRef]
- Russo, D.; Coleman, J.L.; Ancillotto, L.; Korine, C. Ecosystem Services by Bats in Urban Areas. In Urban Bats; Springer: Cham, Switzerland, 2022; pp. 167–180. ISBN 978-3-031-13173-8. [Google Scholar]
- Tuneu-Corral, C.; Puig-Montserrat, X.; Riba-Bertolín, D.; Russo, D.; Rebelo, H.; Cabeza, M.; López-Baucells, A. Pest Suppression by Bats and Management Strategies to Favour It: A Global Review. Biol. Rev. 2023, 98, 1564–1582. [Google Scholar] [CrossRef]
- Park, K.J. Mitigating the Impacts of Agriculture on Biodiversity: Bats and Their Potential Role as Bioindicators. Mamm. Biol. 2015, 80, 191–204. [Google Scholar] [CrossRef]
- Moretto, L.; Ancillotto, L.; Li, H.; Threlfall, C.G.; Jung, K.; Avila-Flores, R. City Trees, Parks, and Ponds: Green and Blue Spaces as Life Supports to Urban Bats. In Urban Bats: Biology, Ecology, and Human Dimensions; Moretto, L., Coleman, J.L., Davy, C.M., Fenton, M.B., Korine, C., Patriquin, K.J., Eds.; Fascinating Life Sciences; Springer International Publishing: Cham, Switzerland, 2022; pp. 107–121. ISBN 978-3-031-13173-8. [Google Scholar]
- Dickson, T.L.; Hayes, B.A.; Bragg, T.B. Effects of 34 Years of Experimentally Manipulated Burn Seasons and Frequencies on Prairie Plant Composition. Rangel. Ecol. Manag. 2019, 72, 82–91. [Google Scholar] [CrossRef]
- Dere, A.L.; Miller, A.W.; Hemje, A.M.; Parcher, S.K.; Capalli, C.A.; Bettis, E.A. Solute Fluxes through Restored Prairie and Intensively Managed Critical Zones in Nebraska and Iowa. Front. Earth Sci. 2019, 7, 24. [Google Scholar] [CrossRef]
- Manning, D.W.P.; Dere, A.L.; Miller, A.W.; Coleman, T.J. Evidence for Pulse-Shunt Carbon Exports from a Mixed Land-Use, Restored Prairie Watershed. Freshw. Sci. 2022, 41, 284–298. [Google Scholar] [CrossRef]
- White, J.A.; Lemen, C.A.; Freeman, P.W. Acoustic Detection Reveals Fine-Scale Distributions of Myotis lucifugus, Myotis septentrionalis, and Perimyotis subflavus in Eastern Nebraska. Wnan 2016, 76, 27–35. [Google Scholar] [CrossRef]
- Benedict, R.A. Reproductive Activity and Distribution of Bats In Nebraska. West. N. Am. Nat. 2004, 64, 231–248. [Google Scholar]
- Blakey, R.V.; Webb, E.B.; Kesler, D.C.; Siegel, R.B.; Corcoran, D.; Johnson, M. Bats in a Changing Landscape: Linking Occupancy and Traits of a Diverse Montane Bat Community to Fire Regime. Ecol. Evol. 2019, 9, 5324–5337. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Dere, A.; Coleman, T. High-Frequency Data Reveal Differential Dissolved and Suspended Solids Behavior from a Mixed Restored Prairie and Agricultural Catchment. Sci. Total Environ. 2021, 753, 141731. [Google Scholar] [CrossRef]
- Li, H.; Kalcounis-Rueppell, M. Separating the Effects of Water Quality and Urbanization on Temperate Insectivorous Bats at the Landscape Scale. Ecol. Evol. 2018, 8, 667–678. [Google Scholar] [CrossRef]
- Thieurmel, B.; Elmarhraoui, A. Suncalc: Compute Sun Position, Sunlight Phases, Moon Position and Lunar Phase. 2019. Available online: https://cran.r-project.org/web/packages/suncalc/index.html (accessed on 2 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 2 March 2024).
- Wickham, H.; Chang, W.; Ggplot2: An Implementation of the Grammar of Graphics. R Package Version 0.7. 2008. Available online: http://CRAN.R-project.org/package=ggplot2 (accessed on 2 March 2024).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 1-4419-2764-6. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. JABES 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Fill, C.; Allen, C.; Twidwell, D.; Benson, J. Spatial Distribution of Bat Activity in Agricultural Fields: Implications for Ecosystem Service Estimates. Ecol. Soc. 2022, 27, 11. [Google Scholar] [CrossRef]
- Trubitt, R.T.; Hovick, T.J.; Gillam, E.H.; McGranahan, D.A. Habitat Associations of Bats in a Working Rangeland Landscape. Ecol. Evol. 2019, 9, 598–608. [Google Scholar] [CrossRef]
- Geluso, K.; Benedict, R.; Kock, F. Seasonal Activity and Reproduction in Bats of East-Central Nebraska. Trans. Neb. Acad. Sci. Affil. Soc. 2004, 29, 33–44. [Google Scholar]
- Münzer, O.; Li, H.; Schaetz, B.; Kurta, A. Selection of Maternity Roosts by Evening Bats (Nycticeius humeralis) in a Riparian Forest at the Northern Edge of Their Range. Acta Chiropterologica 2023, 25, 101–111. [Google Scholar] [CrossRef]
- Kaarakka, H.M.; White, J.P.; Redell, J.A.; Luukkonen, R.A. Notes on Capture and Roost Characteristics of Three Female Evening Bats (Nycticeius Humeralis) in Southern Wisconsin: An Expanding Species? Am. Midl. Nat. 2018, 180, 168. [Google Scholar] [CrossRef]
- Genoways, H.; Freeman, P.; Grell, C. Extralimital Records of the Mexican Free-Tailed Bat (Tadarida brasiliensis Mexicana) in the Central United States and Their Biological Significance. Trans. Neb. Acad. Sci. Affil. Soc. 2000, 26, 85–96. [Google Scholar]
- McCracken, G.F.; Bernard, R.F.; Gamba-Rios, M.; Wolfe, R.; Krauel, J.J.; Jones, D.N.; Russell, A.L.; Brown, V.A. Rapid Range Expansion of the Brazilian Free-Tailed Bat in the Southeastern United States, 2008–2016. J. Mammal. 2018, 99, 312–320. [Google Scholar] [CrossRef]
- Ommundsen, P.; Lausen, C.; Matthias, L. First Acoustic Records of the Brazilian Free-Tailed Bat (Tadarida brasiliensis) in British Columbia. Northwestern Nat. 2017, 98, 132–136. [Google Scholar] [CrossRef]
- Larsen, R.J.; Boegler, K.A.; Genoways, H.H.; Masefield, W.P.; Kirsch, R.A.; Pedersen, S.C. Mist Netting Bias, Species Accumulation Curves, and the Rediscovery of Two Bats on Montserrat (Lesser Antilles). Acta Chiropterologica 2007, 9, 423–435. [Google Scholar] [CrossRef][Green Version]
- Flaquer, C.; Torre, I.; Arrizabalaga, A. Comparison of Sampling Methods for Inventory of Bat Communities. J. Mammal. 2007, 88, 526–533. [Google Scholar] [CrossRef]
- Allen, M.C.; Kwait, R.; Vastano, A.; Kisurin, A.; Zoccolo, I.; Jaffe, B.D.; Angle, J.C.; Maslo, B.; Lockwood, J.L. Sampling Environmental DNA from Trees and Soil to Detect Cryptic Arboreal Mammals. Sci. Rep. 2023, 13, 180. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service Endangered Species Status for Northern Long-Eared Bat. 2022. Available online: https://www.federalregister.gov/documents/2022/11/30/2022-25998/endangered-and-threatened-wildlife-and-plants-endangered-species-status-for-northern-long-eared-bat (accessed on 9 December 2023).
- White, J.A.; Freeman, P.W.; Shehan, M.I.; Lemen, C.A. Decline of the Northern Long-Eared Myotis (Myotis septentrionalis) in the Eastern Great Plains after the Arrival of White-Nose Syndrome. Wnan 2022, 82, 86–93. [Google Scholar] [CrossRef]
- Holloway, G.L.; Barclay, R.M. Importance of Prairie Riparian Zones to Bats in Southeastern Alberta. Écoscience 2000, 7, 115–122. [Google Scholar] [CrossRef]
- Paquette, S.R.; Garant, D.; Pelletier, F.; Bélisle, M. Seasonal Patterns in Tree Swallow Prey (Diptera) Abundance Are Affected by Agricultural Intensification. Ecol. Appl. 2013, 23, 122–133. [Google Scholar] [CrossRef]
- Grüebler, M.U.; Morand, M.; Naef-Daenzer, B. A Predictive Model of the Density of Airborne Insects in Agricultural Environments. Agric. Ecosyst. Environ. 2008, 123, 75–80. [Google Scholar] [CrossRef]
- Vonhof, M.J.; Betts, B.J. Nocturnal Activity Patterns of Lactating Silver-Haired Bats (Lasionycteris Noctivagans): The Influence of Roost-Switching Behavior. Acta Chiropterologica 2010, 12, 283–291. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M. Roost Switching, Roost Sharing and Social Cohesion: Forest-Dwelling Big Brown Bats, Eptesicus Fuscus, Conform to the Fission–Fusion Model. Anim. Behav. 2004, 68, 495–505. [Google Scholar] [CrossRef]
- Roemer, C.; Coulon, A.; Disca, T.; Bas, Y. Bat Sonar and Wing Morphology Predict Species Vertical Niche. J. Acoust. Soc. Am. 2019, 145, 3242–3251. [Google Scholar] [CrossRef]
- Peurach, S.C. High-Altitude Collision between an Airplane and a Hoary Bat, Lasiurus cinereus. Bat Res. News 2003, 44, 2–3. [Google Scholar]
- Lacki, M.J.; Hayes, J.P.; Kurta, A. Bats in Forests: Conservation and Management; JHU Press: Baltimore, MD, USA, 2007; ISBN 978-0-8018-8499-3. [Google Scholar]
- Ormsbee, P.C.; Kiser, J.D.; Perlmeter, S.I. Importance of Night Roosts to the Ecology of Bats. In Bats in Forests: Conservation and Management; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; JHU Press: Baltimore, MD, USA, 2007; ISBN 978-0-8018-8499-3. [Google Scholar]
- Benedict, R.A.; Benedict, S.K.; Howell, D.L. Use of Buildings by Indiana Bats (Myotis sodalis) and Other Bats in South-Central Iowa. Amid 2017, 178, 29–35. [Google Scholar] [CrossRef]
- Detweiler, L.W.; Bernard, R.F. Wildlife Use of Anthropogenic Structures: A Comprehensive Review of Bridge Use by Bats. Acta Chiropterologica 2023, 25, 135–157. [Google Scholar] [CrossRef]
- Geluso, K.; Keele, E.C.; Pauley, N.M.; Gomez, I.R.; Tye, S.P. Night-Roosting Behaviors for the Northern Long-Eared Myotis (Myotis septentrionalis) Under a Bridge Revealed by Time-Lapse Photography. Amid 2018, 179, 287–293. [Google Scholar] [CrossRef]
- Downs, N.C.; Cresswell, W.J.; Reason, P.; Sutton, G.; Wells, D.; Wray, S. Sex-Specific Habitat Preferences of Foraging and Commuting Lesser Horseshoe Bats Rhinolophus hipposideros (Borkhausen, 1797) in Lowland England. Acta Chiropterologica 2016, 18, 451–465. [Google Scholar] [CrossRef]
- Bondo, K.J.; Willis, C.K.R.; Metheny, J.D.; Kilgour, R.J.; Gillam, E.H.; Kalcounis-Rueppell, M.C.; Brigham, R.M. Bats Relocate Maternity Colony after the Natural Loss of Roost Trees. J. Wildl. Manag. 2019, 83, 1753–1761. [Google Scholar] [CrossRef]
- Schorr, R.A.; Siemers, J.L. Population Dynamics of Little Brown Bats (Myotis lucifugus) at Summer Roosts: Apparent Survival, Fidelity, Abundance, and the Influence of Winter Conditions. Ecol. Evol. 2021, 11, 7427–7438. [Google Scholar] [CrossRef]
- Nelson, J.J.; Gillam, E.H. Influences of Landscape Features on Bat Activity in North Dakota. J. Wildl. Manag. 2020, 84, 382–389. [Google Scholar] [CrossRef]
- Schimpp, S.A.; Li, H.; Kalcounis-Rueppell, M.C. Determining Species Specific Nightly Bat Activity in Sites with Varying Urban Intensity. Urban Ecosyst. 2018, 21, 541–550. [Google Scholar] [CrossRef]
- Farrow, L.J.; Broders, H.G. Loss of Forest Cover Impacts the Distribution of the Forest-Dwelling Tri-Colored Bat (Perimyotis subflavus). Mamm. Biol. 2011, 76, 172–179. [Google Scholar] [CrossRef]
- Newman, B.A.; Loeb, S.C.; Jachowski, D.S. Winter Roosting Ecology of Tricolored Bats (Perimyotis subflavus) in Trees and Bridges. J. Mammal. 2021, 102, 1331–1341. [Google Scholar] [CrossRef]
- Shute, K.E.; Loeb, S.C.; Jachowski, D.S. Summer Roosting Ecology of the Northern Yellow Bat and Tri-Colored Bat in Coastal South Carolina. Sena 2021, 20, 459–476. [Google Scholar] [CrossRef]
- Braun de Torrez, E.C.; Ober, H.K.; McCleery, R.A. Critically Imperiled Forest Fragment Supports Bat Diversity and Activity within a Subtropical Grassland. J. Mammal. 2018, 99, 273–282. [Google Scholar] [CrossRef]
- Frey-Ehrenbold, A.; Bontadina, F.; Arlettaz, R.; Obrist, M.K. Landscape Connectivity, Habitat Structure and Activity of Bat Guilds in Farmland-Dominated Matrices. J. Appl. Ecol. 2013, 50, 252–261. [Google Scholar] [CrossRef]
- Kalda, O.; Kalda, R.; Liira, J. Multi-Scale Ecology of Insectivorous Bats in Agricultural Landscapes. Agric. Ecosyst. Environ. 2015, 199, 105–113. [Google Scholar] [CrossRef]
- Straka, T.M.; Wolf, M.; Gras, P.; Buchholz, S.; Voigt, C.C. Tree Cover Mediates the Effect of Artificial Light on Urban Bats. Front. Ecol. Evol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Shafer, C.L. Values and Shortcomings of Small Reserves: Dealing with the Smallest Habitat Fragments When Some of Them Are All That Is Left. BioScience 1995, 45, 80–88. [Google Scholar] [CrossRef]
- Bellon, A.M. Does Animal Charisma Influence Conservation Funding for Vertebrate Species under the US Endangered Species Act? Environ. Econ. Policy Stud. 2019, 21, 399–411. [Google Scholar] [CrossRef]
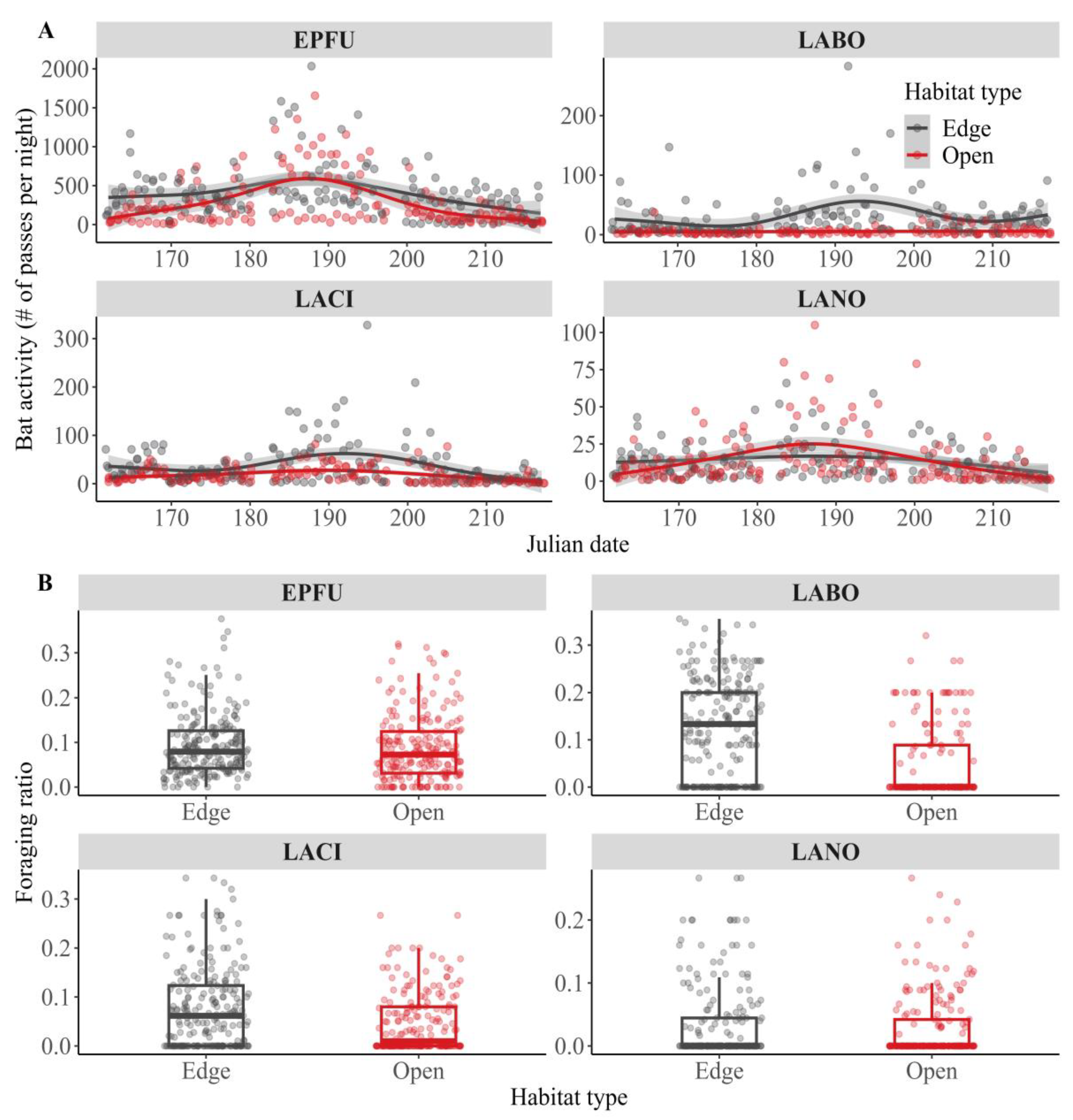

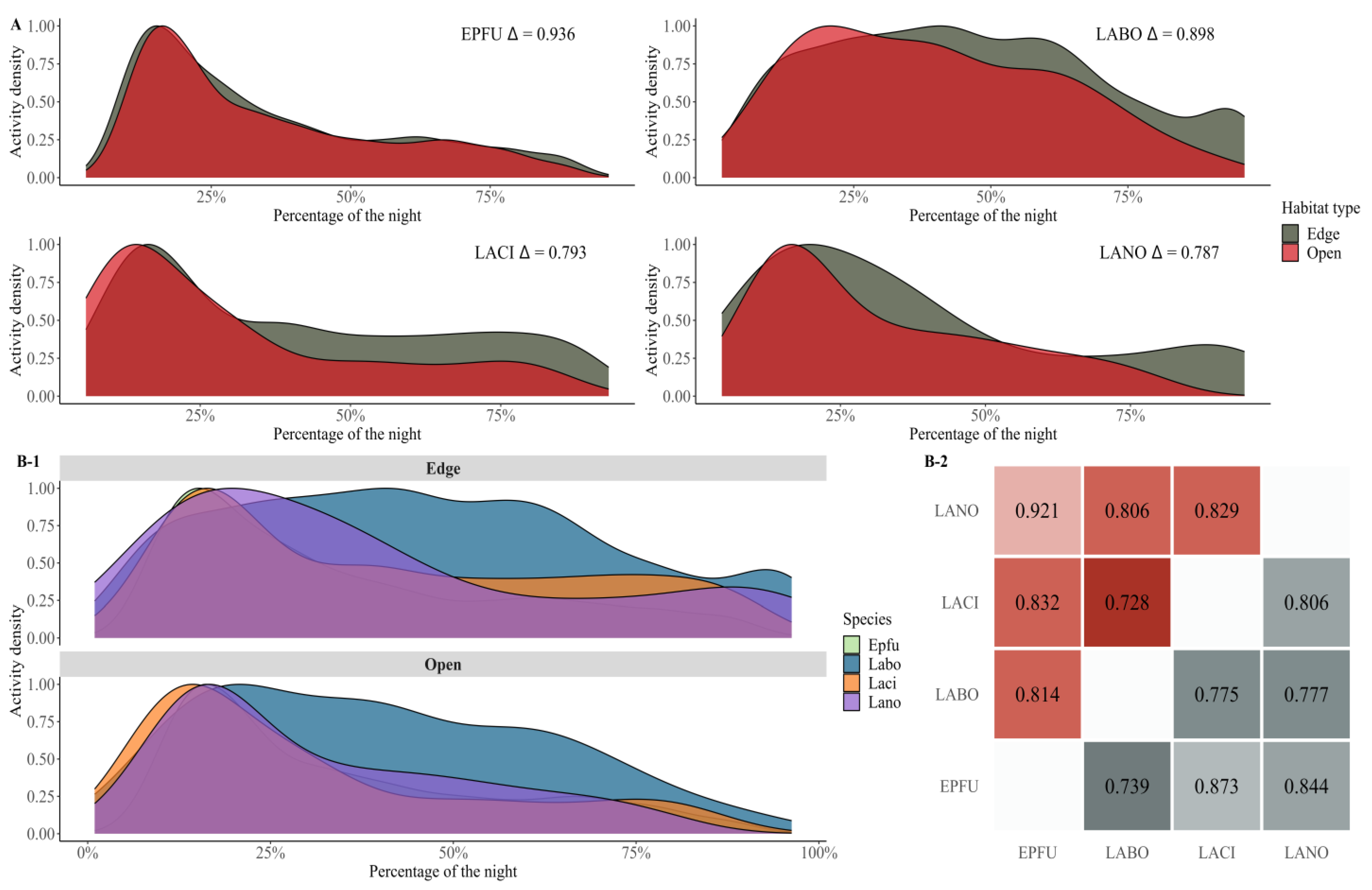
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; White, J.A. Conservation Value to Bats: Assessing Multiple Functional Habitats in a Nature Preserve at the Urban-Agricultural Interface via Temporal Ecology. Sustainability 2024, 16, 2858. https://doi.org/10.3390/su16072858
Li H, White JA. Conservation Value to Bats: Assessing Multiple Functional Habitats in a Nature Preserve at the Urban-Agricultural Interface via Temporal Ecology. Sustainability. 2024; 16(7):2858. https://doi.org/10.3390/su16072858
Chicago/Turabian StyleLi, Han, and Jeremy A. White. 2024. "Conservation Value to Bats: Assessing Multiple Functional Habitats in a Nature Preserve at the Urban-Agricultural Interface via Temporal Ecology" Sustainability 16, no. 7: 2858. https://doi.org/10.3390/su16072858
APA StyleLi, H., & White, J. A. (2024). Conservation Value to Bats: Assessing Multiple Functional Habitats in a Nature Preserve at the Urban-Agricultural Interface via Temporal Ecology. Sustainability, 16(7), 2858. https://doi.org/10.3390/su16072858





