Soil Quality Evaluation in Mono and Mixed Eucalypt Plantation
Abstract
1. Introduction
2. Material and Methods
2.1. Location Description and Soil Sampling Strategy
2.2. Soil Quality Indicators and Assessment (SMAF Application)
2.3. Soil Characterization
2.4. SMAF Application
2.5. Statistical Analyses
3. Results
3.1. Soil Quality Indicators in Pure and Intercropped Eucalyptus Plantations
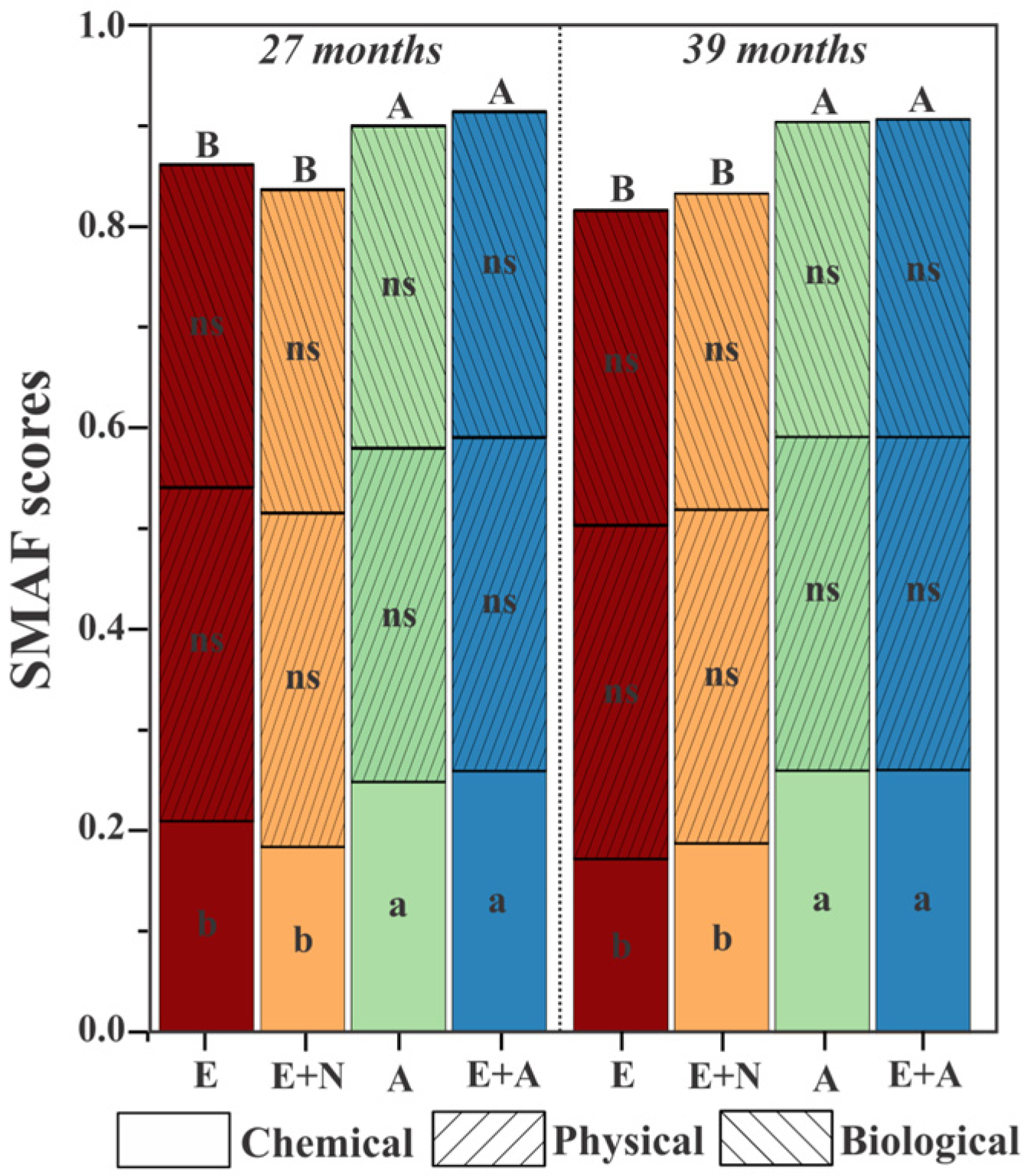
3.2. Soil Quality Indicators Scores
3.3. Soil Quality Indicators Indexes
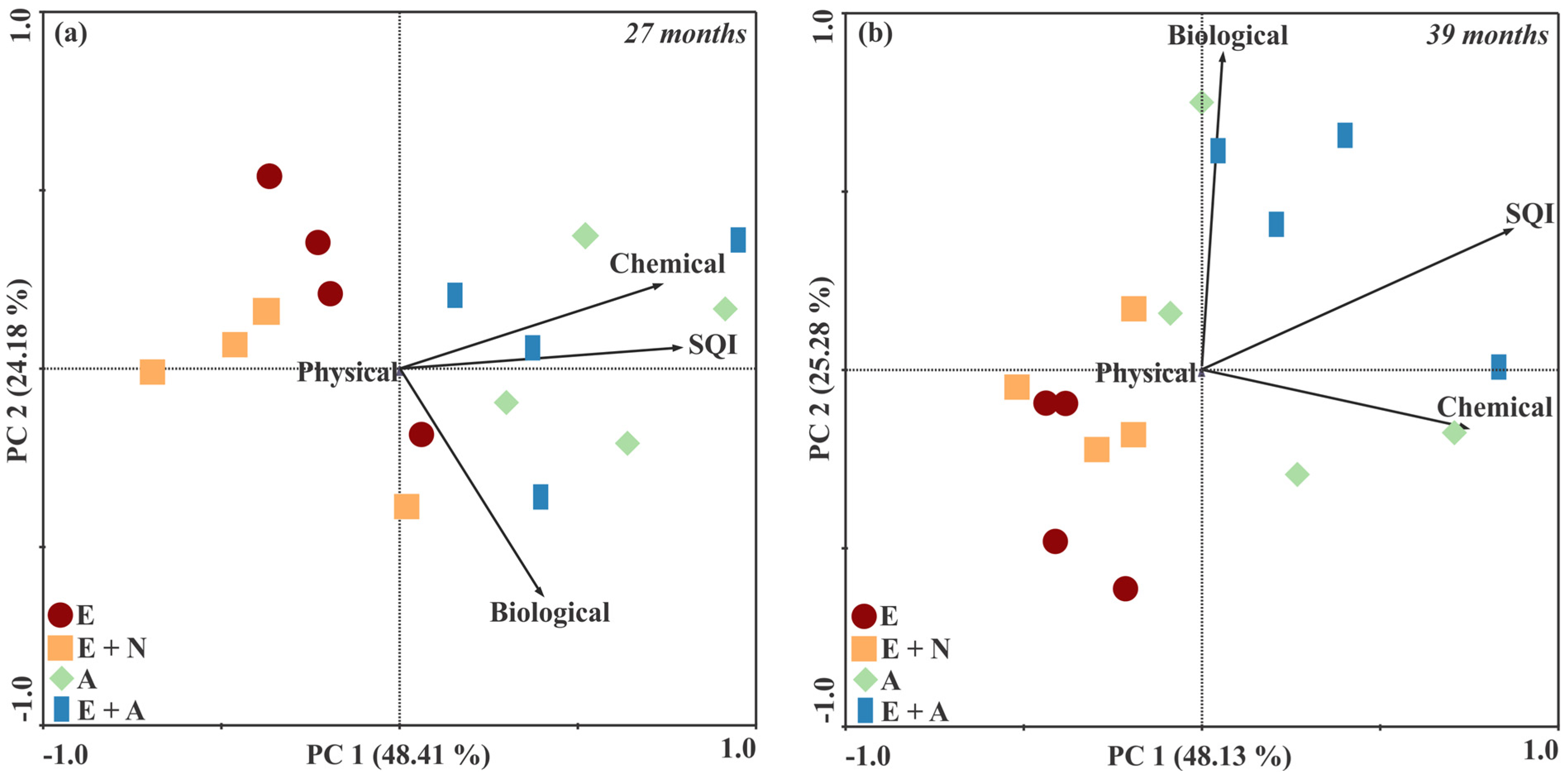
3.4. Principal Component Analysis and Spearman Ranking Correlation Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ABRAF. Anuário Estatístico: Associação Brasileira de Produtores de Florestas Plantadas. Anuário Estatístico ABRAF 2019, 146, 10. [Google Scholar]
- Araújo, A.S.F.; Silva, E.F.L.; Nunes, L.A.P.L.; Carneiro, R.F.V. The Effect of Converting Tropical Native Savanna to Eucalyptus grandis Forest on Soil Microbial Biomass. Land Degrad. Dev. 2010, 21, 540–545. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.B.; Lima, W.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Paula, R.R.; Bouillet, J.P.; José, J.L.; Trivelin, P.C.O.; de C. Balieiro, F.; Nouvellon, Y.; de C. Oliveira, J.; de Deus Júnior, J.C.; Bordron, B.; Laclau, J.P. Nitrogen Fixation Rate of Acacia mangium Wild at Mid Rotation in Brazil Is Higher in Mixed Plantations with Eucalyptus grandis Hill Ex Maiden than in Monocultures. Ann. Sci. 2018, 75, 14. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Santana, M.C.; Zagatto, M.R.G.; Brandani, C.B.; Wang, J.T.; Verma, J.P.; Singh, B.K.; Cardoso, E.J.B.N. Nitrogen-Fixing Trees in Mixed Forest Systems Regulate the Ecology of Fungal Community and Phosphorus Cycling. Sci. Total Environ. 2021, 758, 143711. [Google Scholar] [CrossRef] [PubMed]
- Koutika, L.S.; Mareschal, L. Acacia and Eucalypt Change P, N and C Concentrations in POM of Arenosols in the Congolese Coastal Plains. Geoderma Reg. 2017, 11, 37–43. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Karlen, D.L.; Veum, K.S.; Sudduth, K.A.; Obrycki, J.F.; Nunes, M.R. Soil Health Assessment: Past Accomplishments, Current Activities, and Future Opportunities. Soil Tillage Res. 2019, 195, 104365. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Janzen, H.H.; Janzen, D.W.; Gregorich, E.G. The ‘Soil Health’ Metaphor: Illuminating or Illusory? Soil Biol. Biochem. 2021, 159, 108167. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Gura, I.; Mnkeni, P.N.S. Crop Rotation and Residue Management Effects under No till on the Soil Quality of a Haplic Cambisol in Alice, Eastern Cape, South Africa. Geoderma 2019, 337, 927–934. [Google Scholar] [CrossRef]
- Çelik, İ.; Günal, H.; Acir, N.; Barut, Z.B.; Budak, M. Soil Quality Assessment to Compare Tillage Systems in Cukurova Plain, Turkey. Soil Tillage Res. 2021, 208, 104892. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Cerri, C.E.P.; Tormena, C.A.; Cerri, C.C. A Soil Management Assessment Framework (SMAF) Evaluation of Brazilian Sugarcane Expansion on Soil Quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226. [Google Scholar] [CrossRef]
- Laclau, J.P.; Bouillet, J.P.; Gonçalves, J.L.M.; Silva, E.V.; Jourdan, C.; Cunha, M.C.S.; Moreira, M.R.; Saint-André, L.; Maquère, V.; Nouvellon, Y.; et al. Mixed-Species Plantations of Acacia mangium and Eucalyptus grandis in Brazil. 1. Growth Dynamics and Aboveground Net Primary Production. Ecol. Manag. 2008, 255, 3905–3917. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Zagatto, M.R.G.; Brandani, C.B.; Mescolotti, D.d.L.; Cotta, S.R.; Gonçalves, J.L.M.; Cardoso, E.J.B.N. Acacia Changes Microbial Indicators and Increases C and N in Soil Organic Fractions in Intercropped Eucalyptus Plantations. Front. Microbiol. 2018, 9, 337107. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Honda, H. Description of Cellular Patterns by Diricblet Domains: The Two-Dimensional Case. J. Theor. Biol. 1978, 72, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Saint-André, L.; M’Bou, A.T.; Mabiala, A.; Mouvondy, W.; Jourdan, C.; Roupsard, O.; Deleporte, P.; Hamel, O.; Nouvellon, Y. Age-Related Equations for above- and below-Ground Biomass of a Eucalyptus Hybrid in Congo. Ecol. Manag. 2005, 205, 199–214. [Google Scholar] [CrossRef]
- Wienhold, B.J.; Karlen, D.L.; Andrews, S.S.; Stott, D.E. Protocol for Indicator Scoring in the Soil Management Assessment Framework (SMAF). Renew. Agric. Food Syst. 2009, 24, 260–266. [Google Scholar] [CrossRef]
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2010, 74, 107–119. [Google Scholar] [CrossRef]
- Mendes, I.C.; Sousa, D.M.G.; Dantas, O.D.; Lopes, A.A.L.; Reis Junior, F.B.; Oliveira, M.I.; Chaer, G.M. Soil Quality and Grain Yield: A Win–Win Combination in Clayey Tropical Oxisols. Geoderma 2021, 388, 114880. [Google Scholar] [CrossRef]
- Jiménez, P.; Ortiz, O.; Tarrasón, D.; Ginovart, M.; Bonmatí, M. Effect of Differently Post-Treated Dewatered Sewage Sludge on β-Glucosidase Activity, Microbial Biomass Carbon, Basal Respiration and Carbohydrates Contents of Soils from Limestone Quarries. Biol. Fertil. Soils 2007, 44, 393–398. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.; Alves, P.R.L.; de Paula, A.M.; Nakatani, A.S.; Pereira, J.M.; Nogueira, M.A. Soil quality: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil quality? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarela, H.; Quaggio, J.A. Análise Química para a Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; 285p. Available online: http://lab.iac.sp.gov.br/Publicacao/Raij_et_al_2001_Metod_Anal_IAC.pdf/ (accessed on 20 March 2023).
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brggke, P.C.; Jenkinon, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 775–833. [Google Scholar] [CrossRef]
- Simonet, P.; Grosjean, M.C.; Misra, A.K.; Nazaret, S.; Cournoyer, B.; Normand, P. Frankia Genus-Specific Characterization by Polymerase Chain Reaction. Appl. Environ. Microbiol. 1991, 57, 3278–3286. [Google Scholar] [CrossRef]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP Procedure for Studying the Diversity of NifH Genes in Communities of Nitrogen Fixers in Soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High—Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.M.; Benedetti, V. Nutrição e Fertilizacão Florestal; IPEF: Piracicaba, Brazil, 2000; 427p, Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&id=297693&biblioteca=vazio&busca=autoria:%22GON%C3%87ALVES,%20J.%20L.%22&qFacets=autoria:%22GON%C3%87AVES,%20J.%20L.%22&sort=&paginacao=t&paginaAtual=1/ (accessed on 17 April 2023).
- Bazani, J.H.; Gonçalves, J.L.M.; Rocha, J.H.T.; Melo, E.S.A.C.; Prieto, M. Nutrição fosfatada em plantações de eucalipto. Informações Agronômicas 2014, 148, 11. Available online: http://www.ipni.net/publication/ia-brasil.nsf/0/642B9B97BDFBC80C83257DB600698A31/$FILE/Page1-11-148.pdf/ (accessed on 23 April 2023).
- Lorenzi, J.O.; Monteiro, P.A.; Miranda Filho, H.S.; Van Raij, B. Raízes e tubérculos. In Recomendações de Adubação e Calagem para o Estado de São Paulo; IAC. Boletim Técnico, 100; Van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 1997; pp. 221–229. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 13 March 2020).
- Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research, R Package, version 1.3-5; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 13 March 2020).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data, R Package, version 2.4; R Foundation for Statistical Computing: Vienna, Austria, 2009; Available online: https://cran.r-project.org/web/packages/gplots/index.html/ (accessed on 11 April 2023).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R Package, version 1.1-2; R Foundation for Statistical Computing: Vienna, Austria, 2014. Available online: https://CRAN.R-project.org/package=RColorBrewer/ (accessed on 19 March 2023).
- Tng, D.Y.P.; Janos, D.P.; Jordan, G.J.; Weber, E.; Bowman, D.M.J.S. Phosphorus Limits Eucalyptus grandis Seedling Growth in an Unburnt Rain Forest Soil. Front. Plant Sci. 2014, 5, 98324. [Google Scholar] [CrossRef]
- Daniel, B.; Cardoso, E.J.B.N. Intercropping Acacia mangium stimulates AMF colonization and soil phosphatase activity in Eucalyptus grandis. Sci. Agric. 2018, 75, 102–110. [Google Scholar] [CrossRef]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-Functionality of Organic Matter in Soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Gomes de Sousa, D.M.; Chaer, G.M.; Bueno dos Reis Junior, F.; Goedert, W.J.; de Carvalho Mendes, I. Interpretation of Microbial Soil Indicators as a Function of Crop Yield and Organic Carbon. Soil Sci. Soc. Am. J. 2013, 77, 461–472. [Google Scholar] [CrossRef]
- Stege, P.W.; Messina, G.A.; Bianchi, G.; Olsina, R.A.; Raba, J. Determination of β-Glucosidase Activity in Soils with a Bioanalytical Sensor Modified with Multiwalled Carbon Nanotubes. Anal. Bioanal. Chem. 2010, 397, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.A.; Araujo, A.S.F.; Santana, M.C.; Lima, A.Y.V.; de Araujo, V.L.V.P.; Verma, J.P.; Cardoso, E.J.B.N. Enzymatic Stoichiometry in Tropical Soil under Pure and Mixed Plantations of Eucalyptus with N2-Fixing Trees. Sci. Agric. 2022, 80, e20210283. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Greacen, E.L.; Sands, R. Compaction of forest soils. A review. Soil Res. 1980, 18, 163–189. [Google Scholar] [CrossRef]
- Labelle, E.R.; Hansson, L.; Högbom, L.; Jourgholami, M.; Laschi, A. Strategies to Mitigate the Effects of Soil Physical Disturbances Caused by Forest Machinery: A Comprehensive Review. Curr. For. Rep. 2022, 8, 20–37. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Gonçalves, J.L.M.; Moreira, M.Z.; Trivelin, P.C.O.; Jourdan, C.; Silva, E.V.; Piccolo, M.C.; Tsai, S.M.; Galiana, A. Mixed-Species Plantations of Acacia mangium and Eucalyptus grandis in Brazil. 2: Nitrogen Accumulation in the Stands and Biological N2 Fixation. Ecol. Manag. 2008, 255, 3918–3930. [Google Scholar] [CrossRef]
- Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of Nitrogen Fixation and Nitrogenase-like Sequences amongst Microbial Genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Durrer, A.; Gumiere, T.; Gonçalves, J.L.M.; Robin, A.; Bouillet, J.P.; Wang, J.; Verma, J.P.; Singh, B.K.; Cardoso, E.J.B.N. Mixed Eucalyptus Plantations Induce Changes in Microbial Communities and Increase Biological Functions in the Soil and Litter Layers. Ecol. Manag. 2019, 433, 332–342. [Google Scholar] [CrossRef]
- Cabugao, K.G.; Timm, C.M.; Carrell, A.A.; Childs, J.; Lu, T.Y.S.; Pelletier, D.A.; Weston, D.J.; Norby, R.J. Root and Rhizosphere Bacterial Phosphatase Activity Varies with Tree Species and Soil Phosphorus Availability in Puerto Rico Tropical Forest. Front. Plant Sci. 2017, 8, 293593. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global Patterns of Phosphatase Activity in Natural Soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Moraes, J.L.G.; Bouillet, J.P.; Guillemot, J.; Brancalion, P.H.S.; Teixeira, J.C.M.; Vicente, A.F.; Behling, M.; Laclau, J.P. Establishing mixed-species planted forests for restoration and production in Brazil. Unasylva 2023, 74, 55–59. [Google Scholar] [CrossRef]



| Treatments 1 | Sampling Period | Soil Quality Indicators (Mean Values) 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| SOC | MBC | pH | P | BD | BG | K | ||
| E | 27 months | 4.17 Aa* | 478 Ba | 4.0 ns | 3.44 Bb | 1.21 Aa | 81.93 Aa | 195 Bb |
| E + N | 3.57 Ba | 562 Ba | 3.6 ns | 3.40 Bb | 1.21 Aa | 83.73 Aa | 185 Bb | |
| A | 4.46 Aa | 815 Aa | 4.1 ns | 5.70 Ab | 1.16 Ba | 87.31 Aa | 185 Bb | |
| E + A | 4.38 Aa | 839 Aa | 4.3 ns | 5.90 Ab | 1.05 Ba | 92.66 Aa | 205 Ab | |
| E | 39 months | 2.75 Bb | 475 Aa | 3.8 ns | 2.40 Bb | 1.21 Aa | 74.44 Aa | 312 Ba |
| E + N | 2.84 Bb | 477 Aa | 3.7 ns | 3.30 Bb | 1.21 Aa | 76.74 Aa | 351 Aa | |
| A | 2.93 Bb | 617 Aa | 4.2 ns | 5.80 Aa | 1.16 Ba | 74.24 Ab | 351 Aa | |
| E + A | 4.45 Aa | 667 Aa | 4.3 ns | 6.30 Aa | 1.04 Ba | 81.41 Aa | 351 Aa | |
| Treatments 1 | Sampling Period | SMAF Scores (Mean Values) 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| SOC | MBC | pH | P | BD | BG | K | ||
| E | 27 months | 1 ns* | 1 ns | 0.34 Ba | 0.55 Ba | 0.99 ns | 0.89 Ba | 1 ns |
| E + N | 1 ns | 1 ns | 0.17 Ca | 0.49 Ba | 0.99 ns | 0.90 Ba | 1 ns | |
| A | 1 ns | 1 ns | 0.39 Bb | 0.85 Aa | 0.99 ns | 0.92 Aa | 1 ns | |
| E + A | 1 ns | 1 ns | 0.49 Aa | 0.84 Aa | 0.99 ns | 0.93 Aa | 1 ns | |
| E | 39 months | 1 ns | 1 ns | 0.23 Bb | 0.31 Bb | 0.99 ns | 0.82 Bb | 1 ns |
| E + N | 1 ns | 1 ns | 0.21 Ba | 0.48 Ba | 0.99 ns | 0.84 Bb | 1 ns | |
| A | 1 ns | 1 ns | 0.46 Aa | 0.88 Aa | 0.99 ns | 0.80 Bb | 1 ns | |
| E + A | 1 ns | 1 ns | 0.52 Aa | 0.82 Aa | 0.99 ns | 0.88 Ab | 1 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.P.A.; Cherubin, M.R.; de Araujo, A.S.F.; Santana, M.C.; de Medeiros, E.V.; da Costa, D.P.; de Souza, A.J.; Lima, A.Y.V.; da Silva, D.F.; Estrada, P.A.C.; et al. Soil Quality Evaluation in Mono and Mixed Eucalypt Plantation. Sustainability 2024, 16, 2534. https://doi.org/10.3390/su16062534
Pereira APA, Cherubin MR, de Araujo ASF, Santana MC, de Medeiros EV, da Costa DP, de Souza AJ, Lima AYV, da Silva DF, Estrada PAC, et al. Soil Quality Evaluation in Mono and Mixed Eucalypt Plantation. Sustainability. 2024; 16(6):2534. https://doi.org/10.3390/su16062534
Chicago/Turabian StylePereira, Arthur P. A., Maurício R. Cherubin, Ademir S. F. de Araujo, Maiele C. Santana, Erika V. de Medeiros, Diogo P. da Costa, Adijailton J. de Souza, Antonio Y. V. Lima, Danilo F. da Silva, Paula A. C. Estrada, and et al. 2024. "Soil Quality Evaluation in Mono and Mixed Eucalypt Plantation" Sustainability 16, no. 6: 2534. https://doi.org/10.3390/su16062534
APA StylePereira, A. P. A., Cherubin, M. R., de Araujo, A. S. F., Santana, M. C., de Medeiros, E. V., da Costa, D. P., de Souza, A. J., Lima, A. Y. V., da Silva, D. F., Estrada, P. A. C., Mendes, L. W., Verma, J. P., Koutika, L. S., & Cardoso, E. J. B. N. (2024). Soil Quality Evaluation in Mono and Mixed Eucalypt Plantation. Sustainability, 16(6), 2534. https://doi.org/10.3390/su16062534







