Effects of the Replacement of Chemical Fertilizers with Organic Fertilizers in Different Proportions on Microbial Biomass and Enzyme Activities of Soil Aggregates in Gravel-Mulched Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Experimental Design
- No nitrogen (N) fertilizer, control (CK, N 0 kg∙hm−2);
- Only chemical N fertilizer (CF);
- Organic fertilizer replacing 25% of chemical N fertilizer (OF-25%);
- Organic fertilizer replacing 50% of chemical N fertilizer (OF-50%);
- Organic fertilizer replacing 75% of chemical N fertilizer (OF-75%);
- Organic fertilizer replacing 100% of chemical N fertilizer (OF-100%).
2.3. Sample Collection
2.4. Classification of Soil Aggregates
2.5. Determination of Microbial Biomass
2.6. Measurement of Enzyme Activities
2.7. Statistical Analysis
3. Results
3.1. Effects of Different Fertilizer Treatments on the Distribution of Soil Aggregates
3.2. Effects of Different Fertilizer Treatments on Microbial Biomass Carbon and Nitrogen in Soil Aggregates
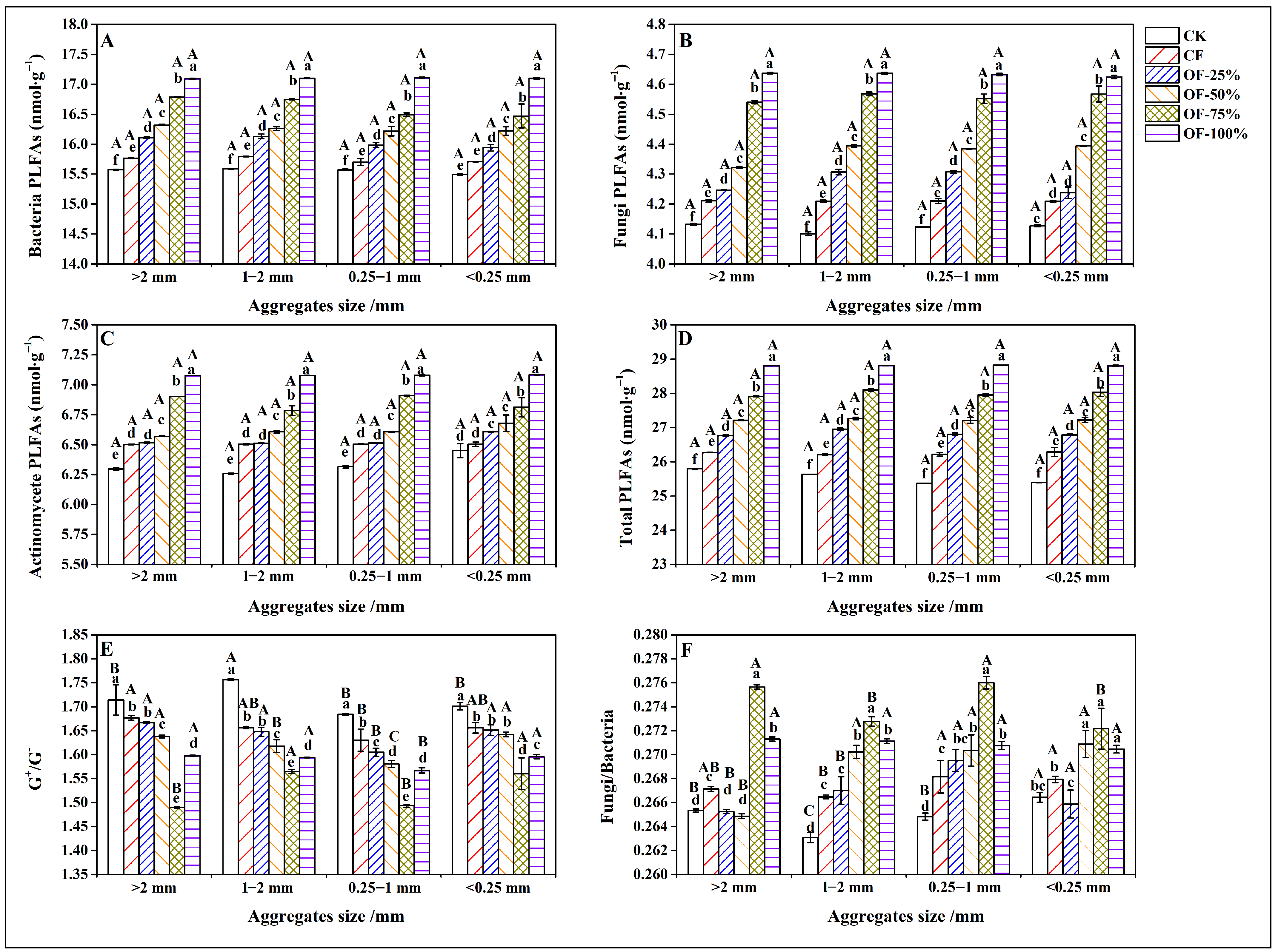
3.3. Effects of Different Fertilization Treatments on Microbial Biomass in Soil Aggregates
3.4. Effects of Different Fertilization Treatments on Microbial Enzyme Activities of Soil Aggregates
3.5. Correlation Analysis
4. Discussion
4.1. Effects of Different Fertilization Treatments on the Distribution of Soil Aggregates of Different Size Fractions
4.2. Effects of Different Fertilization Treatments on Microbial Biomass of Soil Aggregates
4.3. Effects of Different Fertilization Treatments on Enzyme Activities of Soil Aggregates
4.4. Correlation between Soil Aggregate Particle Size and Microbial Biomass and Enzyme Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247, 24–37. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.; Zhu, A.; Lin, X. No-tillage with half-amount residue retention enhances microbial functional diversity, enzyme activity and glomalin-related soil protein content within soil aggregates. Soil Use Manag. 2017, 33, 153–162. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Pokharel, A.K.; Jannoura, R.; Heitkamp, F.; Kleikamp, B.; Wachendorf, C.; Dyckmans, J.; Ludwig, B.; Joergensen, R.G. Development of aggregates after application of maize residues in the presence of mycorrhizal and non-mycorrhizal pea plants. Geoderma 2013, 202, 38–44. [Google Scholar] [CrossRef]
- Jing, L. Organic Carbon and Microbial Characteristics of Soil Aggregates in Cropland Ecosystems with High Carbon and Nitrogen Input. Ph.D. Thesis, China Agricultural University, Beijing, China, 2018. [Google Scholar]
- Nie, M.; Pendall, E.; Bell, C.; Gasch, C.K.; Raut, S.; Tamang, S.; Wallenstein, M.D. Positive climate feedbacks of soil microbial communities in a semi-arid grassland. Ecol. Lett. 2013, 16, 234–241. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Li, Y.; Liu, S.; Chu, G.; Xu, Z.; Liu, J. Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest. Biol. Fertil. Soils 2016, 52, 353–365. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Huang, Z.; Jian, Z.; Ni, Y.; Li, H. Distribution of enzymatic activities within soil aggregates in two types of Pinus massoniana mixed plantations in the Three Gorges Reservoir area. Acta Ecol. Sin. 2020, 40, 6179–6188. [Google Scholar]
- Ma, Z.; Du, S.; Xue, L. Influences of sand-mulching years on soil temperature, water content, and growth and water use efficiency of watermelon. J. Desert Res. 2013, 33, 1433–1439. [Google Scholar]
- Du, S.; Ma, Z.; Xue, L. Effect of manure combined with chemical fertilizers on fruit yield, fruit quality and water and nitrogen use efficiency in watermelon grown in gravel-mulched field. J. Fruit Sci. 2020, 37, 380–389. [Google Scholar]
- Yang, C.; Sainju, U.M.; Li, C.; Fu, X.; Zhao, F.; Wang, J. Long-Term Chemical and Organic Fertilization Differently Affect Soil Aggregates and Associated Carbon and Nitrogen in the Loess Plateau of China. Agronomy 2023, 13, 1466. [Google Scholar] [CrossRef]
- Yu, H.; Ding, W.; Luo, J.; Geng, R.; Cai, Z. Long-term application of organic manure and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil Tillage Res. 2012, 124, 170–177. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; Yu, H.; He, X. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: Result of 20 years compost and inorganic fertilizers repeated application experiment. Biol. Fertil. Soils 2015, 51, 137–150. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Li, F.; Gao, S.; Zhang, J. Effect of compost and inorganic fertilizer on organic carbon and activities of carbon cycle enzymes in aggregates of an intensively cultivated Vertisol. PLoS ONE 2020, 15, e0229644. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Van Agtmaal, M.; Straathof, A.; Termorshuizen, A.; Teurlincx, S.; Hundscheid, M.; Ruyters, S.; Busschaert, P.; Lievens, B.; de Boer, W. Exploring the reservoir of potential fungal plant pathogens in agricultural soil. Appl. Soil Ecol. 2017, 121, 152–160. [Google Scholar] [CrossRef]
- Babin, D.; Deubel, A.; Jacquiod, S.; Sørensen, S.J.; Geistlinger, J.; Grosch, R.; Smalla, K. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol. Biochem. 2019, 129, 17–28. [Google Scholar] [CrossRef]
- Kong, T.; Liu, B.; Henderson, M.; Zhou, W.; Su, Y.; Wang, S.; Wang, L.; Wang, G. Effects of Shelterbelt Transformation on Soil Aggregates Characterization and Erodibility in China Black Soil Farmland. Agriculture 2022, 12, 1917. [Google Scholar] [CrossRef]
- Anis, N.; Arya, A.K. Seasonal variations in microbial diversity in tropical dry deciduous forest of Central Uttar Pradesh. Earth Sci. Ind. 2021, 14, 16–27. [Google Scholar]
- Kumari, P.; Kumar, S.; Singh, R.; Kumar, R.; Shambhavi, S. Effect of different soil conservation practices on soil microbial biomass carbon, nitrogen and phosphorus under rice based cropping system in Bihar. J. Pharmacogn. Phytochem. 2021, 10, 400–403. [Google Scholar]
- Li, C.; Cano, A.; Acosta-Martinez, V.; Veum, K.S.; Moore-Kucera, J. A comparison between fatty acid methyl ester profiling methods (PLFA and EL-FAME) as soil health indicators. Soil Sci. Soc. Am. J. 2020, 84, 1153–1169. [Google Scholar] [CrossRef]
- Francisco, R.; Stone, D.; Creamer, R.E.; Sousa, J.P.; Morais, P.V. European scale analysis of phospholipid fatty acid composition of soils to establish operating ranges. Appl. Soil Ecol. 2016, 97, 49–60. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Xiao, X.; Tang, H. Alterations in soil microbial phospholipid fatty acid profile with soil depth following cropland conversion in karst region, southwest China. Environ. Sci. Pollut. Res. 2022, 30, 1502–1519. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; McMahon, S.K.; Schimel, J.P. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob. Chang. Biol. 2009, 15, 1631–1639. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Z.; Li, T.; Liu, M. Distribution characteristics of soil aggregates and its organic carbon in different tea plantation age. Acta Ecol. Sin. 2014, 34, 6326–6336. [Google Scholar]
- Du, S.; Ma, Z.; Liang, X. Distribution characteristics of soil aggregates and their associated organic carbon in gravel-mulched land with different cultivation years. Chin. J. Appl. Ecol. 2017, 28, 1619–1625. [Google Scholar]
- Kim, H.-Y. Analysis of variance (ANOVA) comparing means of more than two groups. Restor. Dent. Endod. 2014, 39, 74–77. [Google Scholar] [CrossRef]
- Sedgwick, P. Pearson’s correlation coefficient. BMJ 2012, 345, e4483. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Parwada, C.; Van Tol, J. Effects of litter quality on macroaggregates reformation and soil stability in different soil horizons. Environ. Dev. Sustain. 2019, 21, 1321–1339. [Google Scholar] [CrossRef]
- Tisdall, J. Formation of soil aggregates and accumulation of soil organic matter. In Structure and Organic Matter Storage in Agricultural Soils; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–96. [Google Scholar]
- Cui, H.; Zhu, H.; Shutes, B.; Rousseau, A.N.; Feng, W.-D.; Hou, S.-N.; Ou, Y.; Yan, B.-X. Soil aggregate-driven changes in nutrient redistribution and microbial communities after 10-year organic fertilization. J. Environ. Manag. 2023, 348, 119306. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Xiong, H.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, H.; Jiang, T. Influence of long-term fertilization on soil aggregates stability and organic carbon occurrence characteristics in karst yellow soil of Southwest China. Front. Plant Sci. 2023, 14, 1126150. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; An, F.; Su, Y.; Liu, T.; Yang, R.; Du, Z.; Chen, S. Effect of long-term fertilization on aggregate size distribution and nutrient accumulation in aeolian sandy soil. Plants 2022, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Su, Y.; Li, J.; An, F.; Liu, T. Effect of attapulgite application on aggregate formation and carbon and nitrogen content in sandy Soil. Sustainability 2023, 15, 12511. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, Q. Distribution and stability of water-stable aggregates as affected by long-term cattle manure application to saline-sodic soil in the black soil region of northeastern China. Int. J. Agric. Biol. Eng. 2022, 15, 139–145. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhang, B.; Wang, L.; Wang, J.; He, H.; Zhang, X. Long-term manure amendments reduced soil aggregate stability via redistribution of the glomalin-related soil protein in macroaggregates. Sci. Rep. 2015, 5, 14687. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhang, Y.; Wang, K.; Hao, X.; Chen, W.; Huang, Q. Complexity of bacterial and fungal network increases with soil aggregate size in an agricultural Inceptisol. Appl. Soil Ecol. 2020, 154, 103640. [Google Scholar] [CrossRef]
- Li, C.; Ma, S.; Shao, Y.; Ma, S.; Zhang, L. Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat. J. Integr. Agric. 2018, 17, 210–219. [Google Scholar] [CrossRef]
- Du, S.; Ma, Z.; Chen, J.; Xue, L.; Tang, C.; Shareef, T.M.; Siddique, K.H. Effects of organic fertilizer proportion on the distribution of soil aggregates and their associated organic carbon in a field mulched with gravel. Sci. Rep. 2022, 12, 11513. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Salestani, K.; Bahram, M.; Ghanbari Moheb Seraj, R.; Gohar, D.; Tohidfar, M.; Eremeev, V.; Talgre, L.; Khaleghdoust, B.; Mirmajlessi, S.M.; Luik, A.; et al. Cropping systems with higher organic carbon promote soil microbial diversity. Agric. Ecosyst. Environ. 2021, 319, 107521. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, B.; Wang, Y.; Wei, J.; Gao, M.; Zhang, S.; Yang, X. PLFA fingerprint characteristics of an anthropogenic loess soil under long-term different fertilizations. Sci. Agric. Sin. 2017, 50, 94–103. [Google Scholar]
- Hammesfahr, U.; Heuer, H.; Manzke, B.; Smalla, K.; Thiele-Bruhn, S. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biol. Biochem. 2008, 40, 1583–1591. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Gautam, R.K.; Ghosh, A.; Chikara, J.; Jha, B. Effect of nitrogen management on soil microbial community and enzymatic activities in Jatropha curcas L. plantation. CLEAN-Soil Air Water 2015, 43, 1058–1065. [Google Scholar] [CrossRef]
- Wei, W.; Xu, Y.; Zhu, L.; GHan, X. Effects of long-term fertilization on soil microbial community in black soil farmland. Acta Pedol. Sin. 2013, 50, 372–380. [Google Scholar]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Liu, W.; Yang, Q.; Xu, W.; Tang, S. Effects of manure substituting chemical nitrogen fertilizer on rubber seedling growth and soil environment. Chin. J. Appl. Ecol. 2022, 33, 431–438. [Google Scholar]
- Zhang, W. Effects of different proportions of fertilizer and organic fertilizer on soil carbon composition and microbial carbon metabolism. Jiangsu Agric. Sci. 2022, 50, 188–195. [Google Scholar]
- Sinsabaugh, R.L.; Moorhead, D.L.; Xu, X.; Litvak, M.E. Plant, microbial and ecosystem carbon use efficiencies interact to stabilize microbial growth as a fraction of gross primary production. New Phytol. 2017, 214, 1518–1526. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, W.; Di, C.; Su, W.; Liu, X.; Kang, W.; Liu, J.; Zhao, J.; Jing, Y. Effects of organic manure and straw returning on nutrient and microbial quantity of saline soil in river-loop irrigation area. Soil Fertil. Sci. Chin. 2023, 2, 43–45. [Google Scholar]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Chang. 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil aggregate microbial communities: Towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Q.; Sheng, H.; He, T.; Xiong, Z. Effects of biochar on soil aggregate stability and microbial community in paddy field. Acta Pedol. Sin. 2021, 58, 1564–1573. [Google Scholar]
- Liu, Y.; Wang, P.; Wang, J. Formation and stability mechanism of soil aggregates: Progress and Prospect. Acta Pedol. Sin. 2023, 60, 627–643. [Google Scholar]
- Yang, H.; Xiao, Y.; Li, M.; Xu, H.; Shi, X.; Guo, X. Coupling relationship between soil aggregate stability and microbial extracellular enzyme activities in typical urban forests during the dry season. Ecol. Environ. Sci. 2021, 30, 1976–1989. [Google Scholar]


| Microbial Group | Phospholipid Fatty Acid Signatures | Reference |
|---|---|---|
| Bacteria | 14:0, i15:0, a15:0, 15:0, 16:0, i16:0, i17:0, 16:1ω7c, cy17:0, 17:0, 18:0, cy19:0, 20:0 | [25] |
| G+ bacteria | i15:0, a15:0, i16:0, i17:0 | [26] |
| G− bacteria | 16:1ω7c, cy17:0, cy19:0 | [25] |
| Fungi | 18:1ω9c, 18:1ω9t, 18:2ω6 | [27] |
| Actinomycete | 10Me16:0, 10Me18:0 | [25] |
| Soil Enzyme | Substrate | Concentration of Substrate/ (μmol∙L−1) | Measure Wavelength/nm |
|---|---|---|---|
| β-glucosidase (BG) | 4-MUB-β-D-glucoside | 200 | 365, 450 |
| β-Xylosidase (β-XYS) | 4-MUB-β-D-xylosidc | ||
| N-acetyl-β-D- glucosidase (NAG) | 4-MUB-N-acetyl-β-D-glucosaminide | ||
| Leucine aminopeptidase (LAP) | L-Leucine-7-amido-4-methylcoumarin | ||
| Catalase (CAT) | L-3,4-dihydroxyphenylalanine | 25,000 | 465 |
| Polyphenoloxidase (PPO) |
| Treatments | >2 mm | 1–2 mm | 0.25–1 mm | <0.25 mm | MWD (mm) |
|---|---|---|---|---|---|
| CK | 28.62 ± 0.76 dB | 11.01 ± 0.73 cD | 24.43 ± 1.71 aC | 35.95 ± 1.47 aA | 0.78 ± 0.02 d |
| CF | 29.71 ± 0.92 cdB | 11.41 ± 1.33 bcD | 23.57 ± 0.94 abC | 35.30 ± 1.22 aA | 0.79 ± 0.03 d |
| OF-25% | 31.48 ± 1.29 cdA | 12.21 ± 0.80 bcC | 22.99 ± 1.28 abB | 33.33 ± 1.21 aA | 0.81 ± 0.02 cd |
| OF-50% | 34.41 ± 2.96 bcA | 13.66 ± 1.29 abcD | 22.05 ± 1.88 abC | 29.87 ± 1.74 bB | 0.87 ± 0.04 bc |
| OF-75% | 37.20 ± 3.54 abA | 14.10 ± 1.01 abD | 21.47 ± 1.72 abC | 27.24 ± 2.72 bB | 0.90 ± 0.03 ab |
| OF-100% | 40.63 ± 1.95 aA | 14.96 ± 1.33 aC | 21.08 ± 1.10 bB | 23.34 ± 0.39 cB | 0.96 ± 0.03 a |
| Microbial Biomass—C | Microbial Biomass—N | Bacteria | Fungi | Actinomycete | Total PLFA | G+/G− | Fungi/Bacteria | |
|---|---|---|---|---|---|---|---|---|
| MWD | 0.892 * | 0.888 * | 0.986 ** | 0.977 ** | 0.968 ** | 0.972 ** | −0.789 | 0.827 * |
| Catalase (CAT) | Polyphenoloxidase (PPO) | Leucineaminopeptidase (LAP) | N-Acetyl-β-D-Glucosidase (NAG) | β-Glucosidase (BG) | β-Xylosidase (β-XYS) | |
|---|---|---|---|---|---|---|
| MWD | −0.843 * | 0.985 ** | 0.994 ** | 0.967 ** | 0.995 ** | 0.500 |
| Microbial Biomass—C | Microbial Biomass—N | Bacteria | Fungi | Actinomycete | Total PLFA | G+/G− | Fungi/Bacteria | |
|---|---|---|---|---|---|---|---|---|
| Catalase (CAT) | 0.173 | 0.184 | −0.229 | −0.335 | −0.354 | −0.289 | 0.442 * | −0.414 * |
| Polyphenoloxidase (PPO) | 0.512 * | 0.492 * | 0.668 ** | 0.651 ** | 0.609 ** | 0.663 ** | −0.338 | 0.386 |
| Leucineaminopeptidase (LAP) | 0.113 | 0.142 | 0.544 ** | 0.602 ** | 0.620 ** | 0.600 ** | −0.397 | 0.413 * |
| N-acetyl-β-D- glucosidase (NAG) | 0.133 | 0.149 | 0.595 ** | 0.643 ** | 0.669 ** | 0.634 ** | −0.502 * | 0.481 * |
| β-glucosidase (BG) | 0.142 | 0.156 | 0.565 ** | 0.642 ** | 0.629 ** | 0.625 ** | −0.492 * | 0.482 * |
| β-Xylosidase (β-XYS) | 0.143 | 0.123 | 0.326 | 0.370 | 0.402 | 0.420 * | −0.471 * | 0.385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Du, S.; Ma, Z.; Xue, L.; Chen, J.; Hai, L. Effects of the Replacement of Chemical Fertilizers with Organic Fertilizers in Different Proportions on Microbial Biomass and Enzyme Activities of Soil Aggregates in Gravel-Mulched Field. Sustainability 2024, 16, 2483. https://doi.org/10.3390/su16062483
Tang C, Du S, Ma Z, Xue L, Chen J, Hai L. Effects of the Replacement of Chemical Fertilizers with Organic Fertilizers in Different Proportions on Microbial Biomass and Enzyme Activities of Soil Aggregates in Gravel-Mulched Field. Sustainability. 2024; 16(6):2483. https://doi.org/10.3390/su16062483
Chicago/Turabian StyleTang, Chaonan, Shaoping Du, Zhongming Ma, Liang Xue, Juan Chen, and Long Hai. 2024. "Effects of the Replacement of Chemical Fertilizers with Organic Fertilizers in Different Proportions on Microbial Biomass and Enzyme Activities of Soil Aggregates in Gravel-Mulched Field" Sustainability 16, no. 6: 2483. https://doi.org/10.3390/su16062483
APA StyleTang, C., Du, S., Ma, Z., Xue, L., Chen, J., & Hai, L. (2024). Effects of the Replacement of Chemical Fertilizers with Organic Fertilizers in Different Proportions on Microbial Biomass and Enzyme Activities of Soil Aggregates in Gravel-Mulched Field. Sustainability, 16(6), 2483. https://doi.org/10.3390/su16062483





