Abstract
In many EU countries, a large proportion of domestic effluents is treated in small, decentralized wastewater treatment plants (WWTPs) which often lack appropriate treatment technologies. The low efficiency of these systems and their incorrect maintenance often contribute to environmental deterioration, with a large quantity of inadequately treated sewage dispersed in the soil or discharged into superficial watercourses. In the Abruzzo region (Central Italy), almost all rivers and streams receive wastewater from Imhoff tanks with only primary treatments. The ecological impacts of these effluents have rarely been investigated. This paper aimed to evaluate the response of freshwater invertebrates to Imhoff effluents in receiving watercourses with different ecological status. Our results showed that wastewater from Imhoff plants negatively affected the structure and composition of freshwater communities, with a reduction in the richness and abundance of more sensitive taxa and an increase in the most tolerant ones. These negative effects were more accentuated during low flow periods and in streams with moderate ecological status. To improve the ecological status of rivers and streams and to limit the diffusion of pathogens and micropollutants in freshwater, a more efficient (secondary treatments, possibly with nature-based solutions) and sustainable (water reuse or sewage sludge recycling) approach to wastewater treatment in decentralized WWTPs is urgently needed.
1. Introduction
Discharges from wastewater treatment plants (WWTPs) are recognized as one of the main stressors which directly or indirectly contribute to the deterioration of the ecological status of European streams and rivers [1,2,3]. Furthermore, effluents from WWTPs represent the primary source of micropollutants (pharmaceuticals, personal care products, antibiotic resistance genes, pathogens and microplastics) often found in receiving waterbodies [4,5,6,7,8]. However, most of this evidence comes from large centralized WWTPs, while very little is known about the impacts of small decentralized WWTPs with a capacity below 2000 population equivalent (PE), which often lack appropriate treatment technologies. In many EU countries, a large proportion of domestic effluents is treated in these small decentralized systems [9,10]. Their low efficiency and their incorrect maintenance often contribute to environmental deterioration, with a large quantity of inadequately treated sewage dispersed in the soil or discharged in superficial watercourses [11,12,13]. In Italy, more than 10% of the population is living in small agglomerations served by decentralized WWTPs [14]. In the Abruzzo region, the most common decentralized treatment system is represented by Imhoff tanks with only a primary treatment and with effluents that are discharged directly into superficial waterbodies. In this case, sewage undergoes a simple mechanical/physical treatment including the sedimentation of sludges rich in organic substances and a subsequent partial anaerobic digestion, with a reduction of only 30% in the original organic load (expressed as BOD5 equivalents). The low depurative capacity of these systems, the scarce maintenance (lack of periodic inspection and sludge removal) and the absence of secondary treatments may lead to the discharge of a lot of inadequately treated wastewaters (rich in organic substances, nutrients and micropollutants) into the superficial hydrographic network, with possible negative repercussions on the ecological integrity of freshwater ecosystems [12,13]. In fact, the pressures–impacts analysis conducted during the first revision of the River Basin Management Plan of the Abruzzo region [15] demonstrated that Imhoff plants (number and density) can significantly contribute to the failure to achieve a “good” ecological status of most waterbodies. Therefore, the regional authority envisaged the decommissioning of the Imhoff plants in the regional territory. It should be noted that in the Abruzzo region, about 1500 Imhoff plants, with a total capacity of 385,886 PE, were operating before the adoption of the ongoing decommission plan [16]. Considering a volume of about 200 L per person per day, we have more than 77,000 m3 of almost untreated wastewaters discharged every day into superficial watercourses. The ecological impacts of these discharges on receiving waterbodies have rarely been investigated and most of the previous research has concentrated on large plants with a design capacity > 3000 PE (see Hamdhani et al. for a review [17]). Similar studies on smaller decentralized WWTPs (from <50 to 500 PE) are limited [18] or mainly focus on water quality elements rather than biological ones [12,13,19]. The main objective of this study is, therefore, to evaluate the effects of wastewaters from small Imhoff plants (<250 PE, with only a primary treatment) on the structure and composition of freshwater communities. Our second objective is to assist water managers in setting priorities for the regional decommission plan and to suggest some alternatives for a more sustainable approach to sewage treatment in decentralized systems. Considering the low depurative efficiency of these systems and their scarce management, we hypothesized that the richness and abundance of more sensitive taxa would be negatively affected by Imhoff effluents, with changes in community structure and composition downstream of sewage discharges. We also assumed that the extent of the impacts would be directly related to the self-depurative capacity and to the flow regime of the recipient waterbody.
2. Methods
2.1. Study Area
This study was conducted in two river basins of the Abruzzo region (Central Italy): the Tordino River and the Vibrata River. Four small Imhoff thanks (<250 PE capacity) whose effluents are discharged into superficial watercourses with different ecological status were selected for the study (Table 1).

Table 1.
Main characteristics of the four Imhoff plants investigated.
The Padula plant discharges into the Tordino River (waterbody Tordino 2), a mid-sized second-order stream flowing at high altitude in a low-anthropized and semi-pristine landscape. About 10 km downstream, the Tordino River (waterbody Tordino 3) receives the effluents of the Imhoff Travazzano. In this reach, the ecological status of the river is moderate, although it flows in a low-urbanized territory at mid-altitude, with most of its course inside a Natura 2000 protected site. The Fiumicello stream is an unpolluted first-order stream not included in the regional monitoring network (watershed < 10 km2); it receives the effluents of the Imhoff Faieto and flows for about 6 km before discharging into Tordino 2. The Imhoff plant Casette discharges into the Vibrata River (waterbody Vibrata 2), a heavily polluted lowland stream with poor ecological status. Waterbodies were identified and classified according to the River Basin Management Plan (RBMP) of the Abruzzo region [20].
2.2. Sampling
Sampling was carried out between December 2022 and September 2023. Quantitative benthic macroinvertebrate samples were collected with a Surber net (area 0.06 m2; mesh size 200 µm). Three Surber samples were taken about 10 m upstream and 50 m downstream of the discharge point of Imhoff tanks in the receiving watercourses and in two different seasons: winter and summer, with a high and low-flow regime, respectively. A total of 48 biological samples (3 Surber × 2 sampling points × 4 Imhoff × 2 seasons) were collected. On each sampling occasion, some physicochemical (temperature, dissolved oxygen, pH and conductivity) and hydrologic (current velocity, depth, stream section and flow discharge) parameters were also recorded by using a multiparameter probe (Hach, HQ 4300, Hach Lange srl, Milano, Italy) and a digital flowmeter (FP 101, Global Water, Gold River, CA, USA).
2.3. Data Analysis
We compared the structure and composition of macroinvertebrate assemblages upstream and downstream of Imhoff plant discharge. Some community metrics, such as total taxa richness and density, EPT richness and density, and oligochaetes and chironomids density, were chosen as dependent variables.
Multiple two-way ANOVAs were applied to detect differences in macroinvertebrate community metrics upstream and downstream of the discharge point of each Imhoff plant and in two different flow regimes (summer low-flow and winter high-flow). Differences in community composition were assessed by comparing the Bray–Curtis distance on log-transformed abundance of taxa cumulatively collected at each sampling point. Distances were ordered and graphically represented by applying the non-Metric Multidimensional Scaling (nMDS) method. Abundances were always expressed as densities (ind. m−2). Statistical analyses were performed using Addinsoft™ XLSTAT v 1.09 and PRIMER v 6.1.16.
3. Results
3.1. Physicochemical and Environmental Parameters
No marked differences were found for temperature, pH, conductivity and dissolved oxygen upstream and downstream of the discharge of wastewater. The main differences were observed on the Vibrata River, with a reduction in dissolved oxygen and an increase in conductivity values below the Imhoff plant Casette (Table 2). Water temperature was very low in winter samples, mainly along the course of the Tordino River. Seasonal differences in stream flow values were not accentuated for the main course of the Tordino River (−30% in summer months) but were more substantial for the Fiumicello stream and the Vibrata River (Table 3). The reduction in stream flow in summer was accompanied by a substantial increase in the volume of Imhoff effluents. The presence of odor nuisance around the discharge point was detected in all of the Imhoff plants investigated. Moreover, in summer, an abundant growth of filamentous green algae was observed below the point of Imhoff plants discharge.

Table 2.
Values of some physicochemical parameters recorded upstream and downstream of Imhoff plants’ discharge in winter and summer months.

Table 3.
Seasonal variation in volume of Imhoff effluents and flow discharge of receiving waterbodies.
3.2. Freshwater Invertebrates
A total of 73 taxa and 18,829 individuals were sampled and identified during the two seasons of investigation in the four watercourses (Table S1). Almost the same number of individuals were sampled upstream and downstream of Imhoff discharges and a total of 68 and 61 taxa were identified upstream and downstream, respectively. The autochthonous crayfish Austropotamobius pallipes and some trichopteran families (Leptoceridae, Phylopotamiidae and Psychomiidae) were sampled only upstream. Plecoptera, Ephemeroptera and Oligochaeta were proportionally more abundant in upstream samples, while the relative abundance of Chironomidae and Simuliidae was higher downstream (Figure 1). It should be noted that almost all Simuliidae were sampled in the Vibrata River (Imhoff Casette), where Plecoptera were totally absent.
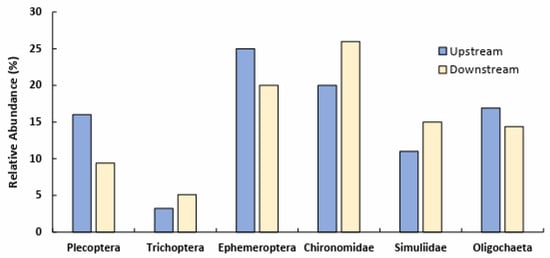
Figure 1.
Percentage of the most abundant freshwater macroinvertebrates cumulatively collected upstream and downstream of Imhoff plants’ discharge.
Taxa richness tended to be higher in summer (except for in the Vibrata River at Casette) and lower below the confluence of Imhoff discharges, except for in the Tordino River (Imhoff Padula) and the Vibrata River in winter (Figure 2). However, the ANOVA results indicated that the reduction in the number of taxa was significant only downstream of the Imhoff plant Faieto (Table S2). Significant seasonal differences were detected for Faieto (higher richness in summer) and Casette (higher richness in winter).
Densities generally increased downstream, except for in the Fiumicello stream (Imhoff Faieto). However, in all watercourses investigated, upstream/downstream differences in the total abundance of macroinvertebrate communities were not significant (p > 0.05). Significant seasonal differences were detected only for Padula (higher density in summer) and Travazzano (higher density in winter) (Table S2 and Figure 3). In general, the number of EPT taxa was lower below Imhoff plant discharges (Figure 4); winter samples of the Tordino River at Padula were an exception. Effluents from Faieto and Travazzano led to a significant reduction (p < 0.05) in EPT taxa in the receiving watercourses (Table S2). On the other hand, the degraded freshwater community of the Vibrata River, composed of few and less sensitive EPT taxa (Baetis sp., Caenis sp., Cleon dipterum and Hydropsyche sp.), seemed to not be particularly affected by sewage from the Imhoff plant Casette.
The density of more tolerant taxa (oligochaetes and chironomids) generally increased below Imhoff plant discharges, except for below Faieto (Figure 5). Upstream/downstream differences were significant (p < 0.05) for the Tordino River at Padula and Travazzano (Table S2), but not for the Vibrata River at Casette where Chironimidae, Oligochaeta and Simuliidae dominated the assemblages also upstream of Imhoff discharges.
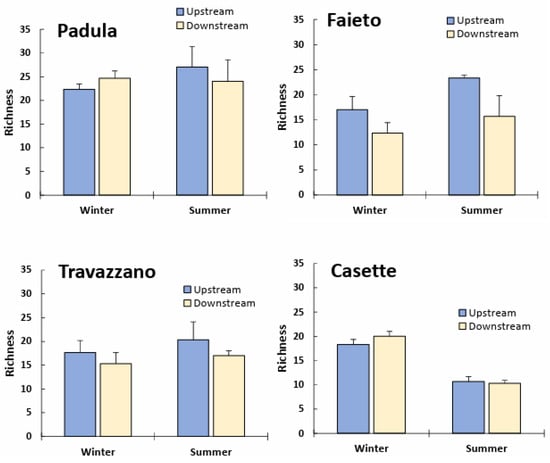
Figure 2.
Taxa richness (mean + SD) of freshwater communities upstream and downstream of Imhoff plants’ discharge in winter and summer months.
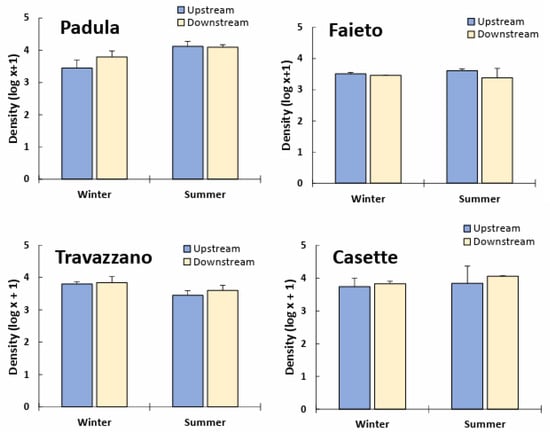
Figure 3.
Log-transformed density (mean + SD) of freshwater communities upstream and downstream of Imhoff plants’ discharge in winter and summer months.
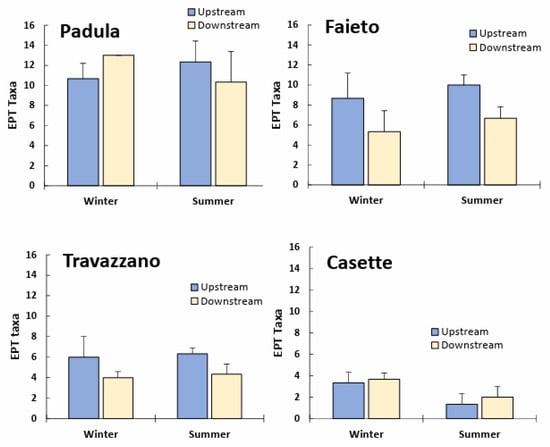
Figure 4.
Ephemeroptera, Plecoptera and Trichoptera (EPT) richness (mean + SD) of freshwater communities upstream and downstream of Imhoff plants’ discharge in winter and summer months.
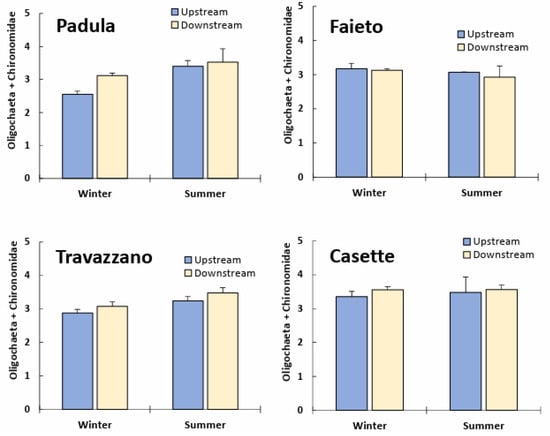
Figure 5.
Log-transformed density (mean + SD) of Oligochaeta and Chironomidae in winter and summer samples of freshwater communities upstream and downstream of Imhoff plants’ discharge.
Other than community metrics, Imhoff effluents also affected the composition of assemblages in the receiving streams. The general ordination pattern of Bray–Curtis distances (Figure 6) reflects the compositional shift of communities from semi-pristine (the Tordino River at Padula and the Fiumicello stream at Faieto) and moderately impacted conditions (the Tordino River at Travazzano) to degraded conditions (the Vibrata River at Casette). However, for all Imhoff plants investigated, communities downstream of wastewater discharges were rather different when compared with their upstream compositional asset, notwithstanding that sampling points were located at short distance from each other. These differences were particularly relevant for the Tordino River below the Imhoff Travazzano plant in both seasons and for the Fiumicello stream (Imhoff Faieto) in winter.
Seasonal upstream/downstream differences in the total richness and abundance of the most sensitive taxa seem more relevant by examining the composition of the whole community at each sampling point (cumulated samples). In winter (high flow), a net reduction in taxa richness downstream of sewage discharges was noted only for the Fiumicello stream, below the Imhoff plant Faieto (Figure 7). In contrast, in summer, richness declined downstream in all of the watercourses investigated. A slightly worse condition in summer was also detected for the difference in the number of EPT taxa upstream and downstream of Imhoff discharges, except for Faieto. In addition, seasonal differences were detected for the relative abundance of two of the most sensitive freshwater taxa (plecopterans and heptageniid ephemeropterans). This was particularly evident in the Tordino River at Padula, where their proportion in summer decreased from about 50% upstream to 17% downstream (Figure 8), while in winter, Plecoptera and Heptageniidae were slightly more abundant downstream of the Imhoff discharge.
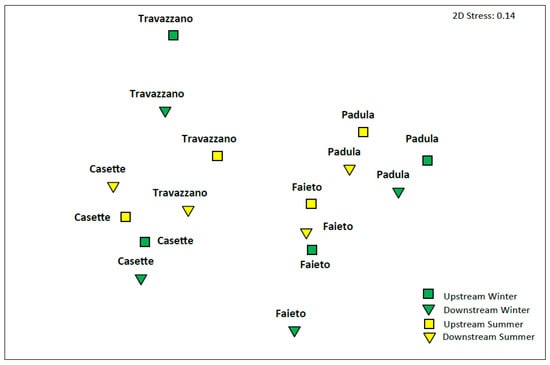
Figure 6.
NMDs’ ordination pattern of community composition (Bray–Curtis distance on log-transformed density) upstream and downstream of Imhoff plants’ discharge in winter and summer months.
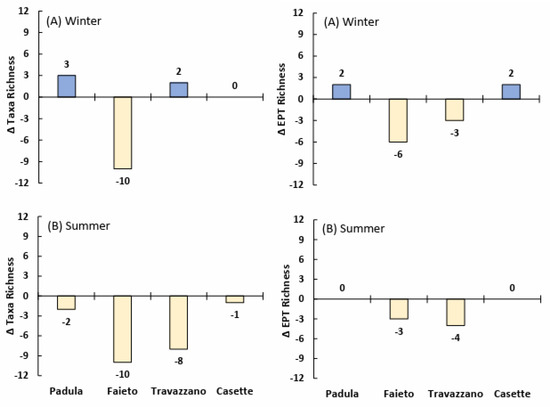
Figure 7.
Difference in taxa richness and EPT richness of freshwater communities (cumulated samples) upstream and downstream of Imhoff plants’ discharge in winter and summer months. Negative values indicate lower richness downstream.
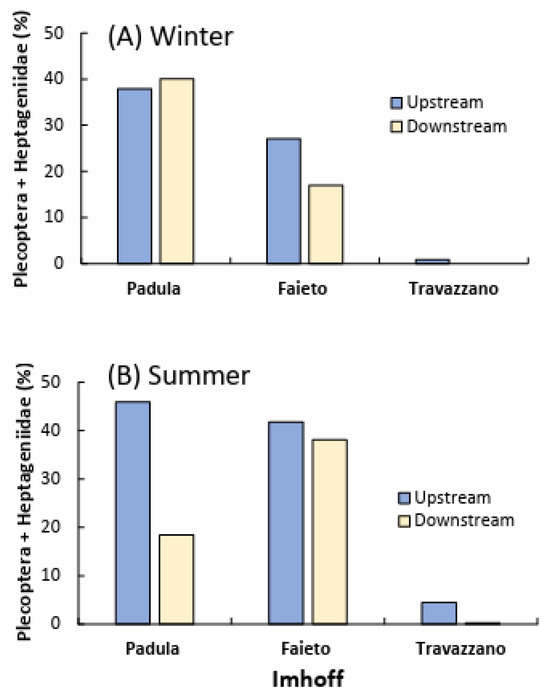
Figure 8.
Relative abundance of Plecoptera and Ephemeroptera Heptageniidae cumulatively sampled upstream and downstream of Imhoff plants’ discharge in (A) winter and (B) summer months. The Imhoff plant Casette was excluded because of the complete absence of both taxa.
4. Discussion
Although with some exceptions, our findings show that effluents from small Imhoff tanks have negative impacts on the richness and abundance of more sensitive taxa, while they have a positive effect on the more tolerant ones. In all of the situations examined, Imhoff effluents determined a shift in community composition both in the winter and summer samples. These effects were more pronounced in receiving watercourses that already showed some signs of ecological alteration and in streams with a better ecological status mainly during low-flow periods.
As hypothesized, the extent of the impacts is directly related to the self-depurative capacity of the receiving watercourses and to their ecological status. For example, in the upper reach of the Tordino River, the semi-pristine conditions of the watercourse and the almost total absence of anthropogenic pressures allowed for the presence of a highly diversified and ecologically relevant freshwater community (near-reference condition). Here, the effects of sewage from the Imhoff plant Padula in the winter months are negligible, and the apport of organic matter and nutrients contribute to favoring the presence of more taxa and more abundant populations below the sewage discharge point (bottom-up effect). This situation is completely reversed in the summer months when the number and the relative abundance of more sensitive taxa significantly decreased downstream. The concomitant effects of the roughly 100-fold increase in the resident population and the lower dilution power of the stream may be responsible for the observed seasonal differences. In contrast, Aristone et al. [21] showed that the impacts on benthic communities of wastewater from large centralized WWTPs were largely similar between winter and summer months, while Richards et al. [19] documented a worse quality of stream water below septic tank effluents during summer low-flow periods. Other than the life-cycle characteristics of the resident benthic fauna, the volume of effluents and flow dilution power, local factors may influence the seasonal response of freshwater invertebrates to Imhoff discharge [18]. In fact, the community of the unpolluted first-order stream Fiumicello was negatively affected by effluents from the Imhoff plant Faieto, with a significant reduction in taxa richness, EPT richness and plecopterans abundance, both in winter and summer months. However, in summer, the differences were less accentuated despite a drastic reduction in stream flow (−80%, Table 2) and a marked increase in the volume of discharged effluents (Table 3). This could be mainly explained by the fact that the short collecting channel (from the Imhoff outflow to the receiving stream) was almost dry during the summer months and part of the effluents were dispersed in the soil before reaching the waterbody.
The main upstream/downstream differences in community structure and composition were recorded in the Tordino River below the Imhoff plant Travazzano. In both seasons, the total richness and number of EPT taxa declined substantially downstream, while chironomids and oligochaetes increased significantly. In this reach, the river flows inside a poorly urbanized and protected area with low anthropogenic pressures. Therefore, we cannot exclude the possibility that the impacts of Imhoff effluents on macroinvertebrates and other biological quality elements may increase the probability of failing to achieve a good ecological status.
In contrast, no significant effects of Imhoff effluents were detected for the Vibrata River with poor ecological status. Here, impacts from urbanization, agriculture and industry are responsible for the already degraded condition of the watercourse. Impoverished freshwater assemblages were completely composed of tolerant taxa also upstream wastewater discharges. Therefore, there were no evident ecological effects of Imhoff effluents, even though some authors demonstrated that wastewater discharges from large WWTPs may significantly impact freshwater communities of streams with poor/bad ecological status [22].
Our findings, though preliminary and limited to a few case studies, confirm and integrate results of similar research conducted on larger WWTPs [18,21,22,23,24,25,26]. We can affirm that discharges from small Imhoff plants with only a primary treatment can considerably affect the structure and composition of freshwater communities, causing a reduction in taxa richness, EPT richness and abundance, and an increase in more tolerant taxa. Imhoff effluents also have negative effects on the most sensitive macroinvertebrates and may negatively affect the ecological status of receiving waterbodies. These effects are more evident when wastewater was discharged into streams with moderate ecological status and with a lower self-depurative capacity. However, since we evaluated the ecological impacts of Imhoff plants just below their discharge point into the receiving watercourses, we cannot exclude the possibility of a recovery of community structure and composition at some distance downstream [27]. We should also consider that our study evaluated the impacts of a single WWTP; the effects on freshwater communities could be more severe and diffuse when a waterbody receives the effluents of several Imhoff plants.
5. Conclusions
Our study demonstrated that wastewater from Imhoff plants can negatively affect freshwater communities, with a reduction in more sensitive taxa and an increase in the most tolerant ones. These effects were more accentuated during low-flow periods and in streams with moderate ecological status. These results will help water managers to set priorities for the Imhoff decommission plan and suggest that they concentrate their efforts on plants discharging into waterbodies with moderate ecological status. Special attention should also be paid to receiving streams with good ecological status since they host a rich and variegated community, mainly composed of rare and sensitive taxa of high ecological and conservation value. Furthermore, the presence of odor nuisance in proximity to Imhoff discharges may limit the fruition of important esthetic, recreative and cultural services in these pristine or semi-pristine freshwater ecosystems [28].
In conclusion, considering the characteristics of the regional territory, the diffuse and sparse pattern of human settlements, especially in rural and montane areas, and the problematics (high costs, high energy consumption and environmental risks) concerning large centralized WWTPs [10,26,29], we suggest an upgrade of Imhoff tanks rather than their complete dismission [30]. More stringent and certified management procedures (periodic inspection and sludge removal), as well as the adjunct of secondary treatments, possibly with nature-based solutions, will surely contribute to improving the ecological status of rivers and streams and will also limit the diffusion of pathogens and micropollutants into freshwaters [31,32,33]. It is also desirable to update and integrate both regional and national legislation to ensure a more efficient and sustainable (water reuse or sewage sludge recycling) approach to wastewater treatment in small decentralized WWTPs [34]. The higher quality of effluents from these upgraded systems may have positive environmental effects and may also contribute to ensuring a minimal superficial flow in receiving small ephemeral streams [17,18,35] or in larger perennial watercourses which are increasingly becoming intermittent [36,37].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16062452/s1, Table S1. List and abundance of taxa sampled upstream (Up) and downstream (Down) of Imhoff plants’ discharge on the receiving watercourses. Numbers refer to three cumulated Surber samples (area sampled = 0.18 m−2). Table S2. Results of multiple ANOVAs testing differences in selected community metrics upstream and downstream (sampling point) of Imhoff discharges and in two different seasons (winter and summer). Significant differences (p < 0.05) are in bold.
Author Contributions
Conceptualization, A.D.S., G.D. and L.R.; methodology, A.D.S.; validation, A.D.S. and L.R.; formal analysis, A.D.S.; investigation, A.D.S., L.R., G.D. and G.E.; data curation, A.D.S., G.E. and F.R.; writing—original draft preparation, A.D.S., G.D. and L.R.; writing—review and editing, A.D.S., G.D., L.R., G.E. and F.R.; visualization, G.E. and F.R.; supervision, A.D.S. and L.R.; funding acquisition, A.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ERSI Abruzzo (contract No 06/DISAB).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are included in the article and Supplementary Materials; further inquiries can be directed to the corresponding author.
Acknowledgments
We are greatly indebted to the personnel of Ruzzo Reti Spa (Teramo) and ERSI Abruzzo (L’Aquila) for their technical and logistic support. We also thank the three anonymous reviewers for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Büttner, O.; Jawitz, J.W.; Birk, S.; Borchardt, D. Why wastewater treatment fails to protect stream ecosystems in Europe. Water Res. 2022, 217, e118382. [Google Scholar] [CrossRef]
- European Environment Agency. European Waters—Assessment of Status and Pressures 2018; EEA Report n° 7; European Environment Agency: Copenhagen, Denmark, 2018; 85p.
- Vigiak, O.; Udias, A.; Pistocchi, A.; Zanni, M.; Aloe, A.; Grizzetti, B. Probability maps of anthropogenic impacts affecting ecological status in European rivers. Ecol. Ind. 2021, 126, 107684. [Google Scholar] [CrossRef]
- Woodward, J.C.; Li, J.; Rothwell, J.J.; Hurley, R. Acute riverine microplastic contamination due to avoidable releases of untreated wastewater. Nat. Sustain. 2021, 4, 793–802. [Google Scholar] [CrossRef]
- Phillips, P.; Schubert, C.; Argue, D.; Fisher, I.; Furlong, E.; Foreman, W.; Gray, J.; Chalmers, A. Concentrations of hormones, pharmaceuticals and other micropollutants in groundwater affected by septic systems in New England and New York. Sci. Total Environ. 2015, 512, 43–54. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, L.; Fatra_Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 147, 345–360. [Google Scholar] [CrossRef]
- Segatore, B.; Piccirilli, A.; Setacci, D.; Cicolani, B.; Di Sabatino, A.; Miccoli, F.P.; Perilli, M.; Amicosante, G. First ldentification of ß-Lactamases in Antibiotic-Resistant Escherichia coli, Citrobacter freundii, and Aeromonas spp. isolated in Stream Macroinvertebrates in a Central Italian Region. Microb. Drug Resist. 2020, 26, 976–981. [Google Scholar] [CrossRef]
- Langergraber, G.; Pressl, A.; Kretschmer, F.; Weissenbacher, N. Small wastewater treatment plants in Austria—Technologies, management and training of operators. Ecol. Eng. 2018, 120, 164–169. [Google Scholar] [CrossRef]
- Libralato, G.; Volpi Ghirardini, A.; Avezzù, F. To centralise or to decentralise: An overview of the most recent trends in wastewater treatment management. J. Environ. Manag. 2012, 94, 61–68. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P.; Stoate, H. Quantifying the impact of septic tank systems on eutrophication risk in rural headwaters. Environ. Int. 2011, 37, 644–653. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jordan, P.; May, L.; Jarvie, H.P.; Deal, N.E. Do septic tank systems pose a hidden threat to water quality? Front. Ecol. Environ. 2014, 12, 123–130. [Google Scholar] [CrossRef]
- Richards, S.; Withers, P.J.A.; Paterson, E.; McRoberts, C.W.; Stutter, M. Temporal variability in domestic point source discharges and their associated impact on receiving waters. Sci. Total Environ. 2016, 571, 1275–1283. [Google Scholar] [CrossRef]
- Pistocchi, A.; Parravicini, V.; Langergraber, G.; Mas, F. How Many Small Agglomerations Do Exist in the European Union, and How Should We Treat Their Wastewater? Water Air Soil Pollut. 2022, 233, 431. [Google Scholar] [CrossRef]
- Regione Abruzzo. DGR 852, 2019. Available online: https://www.regione.abruzzo.it/content/aggiornamento-piano-di-tutela-delle-acque (accessed on 20 February 2024).
- Regione Abruzzo. DGR 111, 2021. Available online: https://www.regione.abruzzo.it/content/aggiornamento-piano-di-tutela-delle-acque (accessed on 20 February 2024).
- Hamdhani, H.; Eppehimer, D.E.; Bogan, M.T. Release of treated effluent into streams: A global review of ecological impacts with a consideration of its potential use for environmental flows. Freshw. Biol. 2020, 65, 1657. [Google Scholar] [CrossRef]
- Mor, J.R.; Doledec, S.; Acuña, V.; Sabater, S.; Muñoz, I. Invertebrate community responses to urban wastewater effluent pollution under different hydro-morphological conditions. Environ. Poll. 2019, 252, 483–492. [Google Scholar] [CrossRef]
- Richards, S.; Withers, P.J.A.; Paterson, E.; McRoberts, C.W.; Stutter, M. Potential tracers for tracking septic tank effluent discharges in watercourses. Environ. Pollut. 2017, 228, 245–255. [Google Scholar] [CrossRef]
- Regione Abruzzo. DGR 905, 2022. Available online: https://www.regione.abruzzo.it/content/aggiornamento-piano-di-tutela-delle-acque (accessed on 20 February 2024).
- Aristone, C.; Mehdi, H.; Hamilton, J.; Bowen, K.L.; Currie, W.J.S.; Kidd, K.A.; Balshine, S. Impacts of wastewater treatment plants on benthic macroinvertebrate communities in summer and winter. Sci. Total Environ. 2022, 820, 153224. [Google Scholar] [CrossRef]
- Jesus, T.; Abreu, I.; Guerreiro, M.J.; Monteiro, A. Study of the effect of two wastewater treatment plants (WWTP’s) discharges on the benthic macroinvertebrate communities’ structure of the River Tinto (Portugal). Limnetica 2020, 39, 353–372. [Google Scholar] [CrossRef]
- Ortiz, J.D.; Puig, A. Point source effects on density, biomass and diversity of benthic macroinvetebrates in a mediterranean stream. River Res. Appl. 2007, 23, 155–170. [Google Scholar] [CrossRef]
- Grantham, T.E.; Cañedo-Argüelles, M.; Perrée, I.; Rieradevall, M.; Prat, N. A mesocosm approach for detecting stream invertebrate community responses to treated wastewater effluent. Environ. Pollut. 2012, 160, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Álvarez-Troncoso, R.; Martínez-Barciela, J.; Polina, A.; Garrido, J. Influence of wastewater discharges on benthic macroinvertebrate communities in a cantabrian-atlantic coastal river. J. Limnol. 2014, 81. [Google Scholar] [CrossRef]
- González, J.M.; de Guzmán, I.; Elosegi, A.; Larrañaga, A. Tertiary wastewater treatment combined with high dilution rates fails to eliminate impacts on receiving stream invertebrate assemblages. Sci. Total Environ. 2023, 859, 160425. [Google Scholar] [CrossRef]
- Ortiz, J.D.; Martí, E.; Puig, A. Recovery of the macroinvertebrate community below a wastewater treatment plant input in a Mediterranean stream. Hydrobiologia 2004, 545, 289–302. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Coscieme, L.; Vignini, P.; Cicolani, B. Scale and ecological dependence of ecosystem services evaluation: Spatial extension and economic value of freshwater ecosystems in Italy. Ecol. Ind. 2014, 32, 259–263. [Google Scholar] [CrossRef]
- Hammond, P.; Suttie, M.; Lewis, V.T.; Smith, A.P.; Singer, A.C. Detection of untreated sewage discharges to watercourses using machine learning. NPJ Clean Water 2021, 4, 18. [Google Scholar] [CrossRef]
- Eggimann, S.; Truffer, B.; Maurer, M. To connect or not to connect? Modelling the optimal degree of centralisation for wastewater infrastructures. Water Res. 2016, 84, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Senante, M.; Gómez, T.; Garrido-Baserba, M.; Caballero, R.; Sala-Garrido, R. Assessing the sustainability of small wastewater treatment systems: A composite indicator approach. Sci. Total Environ. 2014, 497–498, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.; Graham, D.W. A Simple Approach to Predicting the Reliability of Small Wastewater Treatment Plants. Water 2019, 11, 2397. [Google Scholar] [CrossRef]
- Engstler, E.; Kerschbaumer, D.J.; Langergraber, G. Evaluating the Performance of Small Wastewater Treatment Plants. Front. Environ. Sci. 2022, 10, 948366. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Luthy, R.G.; Sedlak, D.L.; Plumlee, M.H.; Austin, D.; Resh, V.H. Wastewater-effluent-dominated streams as ecosystem-management tools in a drier climate. Front. Ecol. Environ. 2015, 13, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Coscieme, L.; Cristiano, G. Effects of antecedent drying events on structure, composition and functional traits of invertebrate assemblages and leaf-litter breakdown in a former perennial river of Central Apennines (Aterno River, Abruzzo, Central Italy). Ecohydrology 2021, 15, e2358. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Coscieme, L.; Cristiano, G. No post-drought recovery of the macroinvertebrate community after five months upon rewetting of an irregularly intermittent Apennine River (Aterno River). Ecohydrol. Hydrobiol. 2023, 23, 141–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).