Abstract
Artificial light at night (ALAN) is a major form of anthropogenic pollution, disrupting nocturnal wildlife behaviour and ecosystem function. Large construction sites are typically located at the intersection of urban and natural areas, introducing intense lighting into previously dark natural habitats. This study examines the responses of bats to intense nighttime lighting at a major construction site associated with a linear transport infrastructure (LTI) project. We used passive acoustic monitoring to record bat activity and species richness at the construction site and in adjacent urban and natural areas with different lighting levels. Generalist species, such as Pipistrellus kuhlii and Nyctalus leisleri, were attracted to illuminated areas, likely due to increased prey availability. Conversely, sensitive species, such as those from the Myotis and Plecotus genera, along with Barbastella barbastellus, avoided illuminated areas, particularly the construction site. Species richness was significantly lower at the construction site compared to other environments, reflecting ALAN’s barrier effect on movements and habitat accessibility for more sensitive species. The findings highlight the ecological impacts of introducing ALAN to previously unlit natural environments, underscoring the urgent need for implementing ALAN mitigation strategies in urban planning and construction practices to protect biodiversity in urbanising areas.
1. Introduction
Artificial light at night (ALAN) represents one of the most significant environmental alterations of the last century. It is a ubiquitous phenomenon associated with human activities ranging from urban street lighting to industrial illumination. The global extent of ALAN is such that only the most remote regions, such as Siberia, vast deserts, and the Amazon rainforest remain relatively unaffected [1]. The continuous increase in population, urbanisation, and economic development has led to an amplification in the density and reach of ALAN, affecting ecosystems worldwide [1,2]. Studies have demonstrated that sky background brightness is growing at an annual rate of approximately 6%, with some regions experiencing growth rates as high as 20% [3].
The ecological impacts of ALAN are broad and complex. It disrupts the natural light cycles, crucial for the regulation of many biological processes in both flora and fauna [4]. For instance, ALAN’s impact on plant–pollinator interactions can trigger ripple effects throughout entire ecosystems [5]. For nocturnal animals, especially, ALAN can have detrimental effects on behaviour, influencing foraging, reproduction, and communication [6]. Among these, bats are particularly vulnerable [7]. ALAN can act as a barrier to movements, reducing available environments and forcing bats to use alternative, less optimal travel routes. This shift can lead to increased energy costs and predation risks [5]. Different bat species show varying responses to ALAN. Narrow-space-foraging species consistently avoid ALAN when foraging, whereas open and edge-space-foraging species may exploit insects attracted to artificial lights [8]. However, this shift in foraging behaviour is not without risks. The aggregation of insects around artificial lights can lead to increased prey availability for certain bat species, potentially impacting local food webs [2]. Furthermore, ALAN may disrupt the evasive manoeuvres of moths in response to bat echolocation, making them more susceptible to predation [9]. Such disturbances carry implications for ecosystems at large, potentially altering predator–prey dynamics on a global scale [10].
Despite growing research on the impact of ALAN, substantial gaps still exist in comprehending its multifaceted consequences. One key area needing more study is the way bat species change their habitat selection in response to brightly lit structures. While numerous studies have demonstrated the general impact of ALAN on bats [8] and other taxa [11], the specific habitat selection bats make in the presence of such structures remains unclear. This is particularly relevant for large industrial sites, which are often situated at the juncture of urban and natural areas. These sites introduce intense artificial light into previously unlit natural habitats, potentially upsetting their ecological balance [12]. Understanding bat responses to these lit environments is crucial for reducing ecological disturbances caused by industrial expansion. Accordingly, urban planning should incorporate specific mitigation strategies at these sites, thereby promoting sustainable development in our increasingly urbanised world [13].
This study investigates the changes in bat activity and species richness in environments with high levels of ALAN, focusing on a major, brightly lit construction site for the Turin–Lyon high-speed railway tunnel connecting Italy to France. Conducted eight years after the site’s establishment, our research aims to investigate how bats have responded to the introduction of intense artificial lighting in an environment that was previously naturally dark. Species richness and activity at the construction site are compared to those in adjacent urban and natural areas. We hypothesised that the introduction of high levels of ALAN at the construction site significantly influenced bat activity and community composition in the area. Specifically, we predicted a high level of activity of more generalist species, and low activity levels of more sensitive species. We also predicted that species richness would be lower at the construction site compared to adjacent urban and natural areas due to the disruptive effects of intense artificial lighting.
2. Materials and Methods
2.1. Study Area
The study area was in the Upper Susa Valley (northwestern Italy), crossed by the river Dora Riparia, within a mountain environment. This region is characterised by natural and semi-natural forests [14], predominantly consisting of abandoned traditional chestnut orchards (Castanea sativa) complemented by a widespread presence of oak (Quercus petraea, Q. pubescens) and cherry trees (Prunus avium). Small patches of vineyards, orchards, and grasslands border these forests. The only inhabited areas in the vicinity are small villages with populations ranging from 500 to 900 residents [15].
Established in 2011, the Turin–Lyon high-speed railway construction site is in a forest environment near the village of Chiomonte. It is the largest on the Italian side of the project, spanning 12 ha across the municipalities of Chiomonte and Giaglione. Originally designed for the excavation of an exploratory tunnel, its role evolved due to project modifications, and it now functions as the primary access for the main tunnel excavation from the Italian end [16]. Since 2012, this area has been of designated national strategic importance and undergoes constant police surveillance. The site’s robust nighttime lighting ensures the safety of workers and facilitates regular police patrols. However, by the time of the study in 2019, many of the site’s operations were suspended, resulting in minimal acoustic and human disturbance, particularly during nighttime hours. This unique setting offers a valuable context to study bat responses to high illumination levels after the establishment of the construction site.
2.2. Bat Monitoring
The applied experimental approach compared species richness and activity in the brightly lit construction site with other three areas exhibiting decreasing lighting levels. In each of these four areas, we established five monitoring sites. The four areas were as follows:
- Brightly lit area within the construction site: This area features strong and continuous artificial lighting every night. Three monitoring sites were inside the construction site, while two were outside the construction site but within the outermost fence of the area. The maximum light intensity was 146.58 ± 75.23 lux.
- High-lit urban area: This included four monitoring sites in the centre of the village of Chiomonte and one in the hamlet of Ramats, where artificial lighting levels are relatively high. The maximum light intensity was 66.94 ± 48.95 lux.
- Low-lit urban area: Four monitoring sites were established in the suburbs of Chiomonte and one in the hamlet of Ramats, where artificial lighting is reduced. The maximum light intensity was 20.26 ± 13.31 lux.
- Dark areas: These sites were in unlit natural areas near the construction site, where artificial lighting is nearly absent. Specifically, two sites were in forest environments, two in ecotonal environments, and one in an open environment. The maximum light intensity was 0.48 ± 0.86 lux.
The maximum light intensity was measured once at each site between 00:00 and 03:00 using an IM-720 Light Meter (RS Pro, London, UK).
Bats were recorded using full-spectrum automatic bat detectors (Song Meter SM4BAT FS, Wildlife Acoustics, Maynard, USA) equipped with omnidirectional ultrasonic microphones (SMM-U2, Wildlife Acoustics, Maynard, USA). Bat detectors were set to operate from sunset to sunrise, with a minimum trigger frequency of 8 kHz and maximum recording length of 8 s. During the active bat season from May to September 2019, one night of recording was conducted at each monitoring site approximately every 20 days, resulting in a total of 5–6 nights of recording for each site.
2.3. Acoustic Analysis
Acoustic analysis of bat calls aimed at species identification was conducted through a two-step process. First, we used the automatic classifier Tadarida [17] to remove noise files and categorise bat recordings by potential species. The software employs an extensive reference database of call characteristics from bat species throughout Europe and attributes a species identification to each sequence, along with a confidence index ranging from 0 to 1.
Subsequently, we performed manual verification using BatSound (Pettersson Elektronik AB, Uppsala, Sweden, 2016) following the methodologies described in [18,19,20]. For Pipistrellus pipistrellus, Pipistrellus kuhlii, and Nyctalus leisleri, which have extensive reference collections available, we did not manually verify (and therefore considered valid) recordings with a probability of correct identification greater than 0.9. All remaining recordings were manually verified. Genera Myotis and Plecotus, within which species identification can be challenging, were treated at the genus level. Within the Myotis genus, differentiation was solely made between large Myotis bats (M. myotis/M. blythii) and small Myotis bats (all other Myotis species) [21].
2.4. Statistical Analysis
For each monitoring night, an occurrence dataset was defined by noting the presence or absence of each species. An activity dataset for each species was then derived by calculating the number of bat passes per hour of monitoring (bat passes/hour). Here, a bat pass was defined as any recording containing one or more echolocation calls of the considered species [22]. Additionally, a species richness dataset was derived from the occurrence dataset, providing information on the minimum number of species identified on each monitoring night. Species were categorised into two groups based on their frequency of detection during monitoring hours: common species (detected in at least 30% of the monitoring hours) and rare species (detected in less than 30% of the monitoring hours).
We initially compared the average activity of each species across the four areas with varying illumination levels using the Kruskal–Wallis test, appropriate for the non-normal distribution of our data. For species showing significant differences in the Kruskal–Wallis test, we conducted further analysis through pairwise comparisons between the construction site and each of the other areas using post hoc Wilcoxon tests, with adjustments for multiple comparisons via the Bonferroni method. We then included the Wilcoxon test results in graphs summarising activity data for each species in each area. The same procedure was applied to investigate bat species richness across the areas.
Building on these initial findings, we further explored bat occurrence, activity, and species richness in relation to illumination levels and various environmental variables critical for bat ecology and relevant to our study. Specifically, the following covariates were considered:
- Maximum light intensity;
- Elevation, measured in the field via GPS device (GPSMAP 66s, Garmin, Shaffhouse, Switzerland);
- Linear distance from the construction site;
- Linear distance from the nearest watercourse, using the regional vector dataset from Arpa Piemonte based on the European Water Framework Directive (WFD) 2000/60/EC (https://www.geoportale.piemonte.it/geonetwork/srv/api/records/arlpa_to:01.01.04-D_2011-06-14-16:09; accessed on 10 December 2023);
- Total built-up surface area within a 500 m radius, from the 2015 Imperviousness Density dataset by the European Copernicus Land Monitoring Service (CLMS; https://land.copernicus.eu/en/dataset-catalog; accessed on 10 December 2023);
- Total forest area within 500 m, from the 2015 Tree Cover Density dataset by CLMS;
- Total area occupied by meadows and pastures within 500 m, from the 2015 Grassland dataset by CLMS;
- Overall length of ecotonal strips within 500 m, based on the third-level classification of the 2010 Land Cover Piemonte vector dataset (www.regione.piemonte.it/sit; accessed on 10 December 2023).
Covariates were calculated in ArcGIS version 10.3 [23] and R version 4.3.2 [24], using rgdal and rgeos packages [25,26]. Potential intercorrelation between covariates was assessed using the Pearson correlation coefficient (correlation coefficient ≥ 0.7), retaining those believed to best describe habitat composition and structure while minimising redundancy. The final covariates used in the models were: maximum light intensity, elevation, linear distance from the construction site, total built-up area, total forest area, and total area of meadows and pastures. In addition to these, two temporal factors were introduced: the Julian day and hours after sunset (the latter depending on the species considered, see later). Considering that bat detectors recorded the ambient temperature every minute, the average temperature per hour or per night was also incorporated as a covariate in the analysis.
Data were analysed using generalized linear mixed models (GLMMs), incorporating the identity of the sampling site as a random factor to consider the potential non-independence of the observations within each site. Based on the sufficiency of the data available for analysis, four common and four rare species (or species groups) were selected. Among common species, we chose Hypsugo savii, N. leisleri, P. pipistrellus, and the P. kuhlii/P. nathusii species pair (including all records attributed to P. kuhlii, those attributed to P. nathusii, and those where there was uncertainty between the two species). Among rare species, we selected Barbastella barbastellus, the small Myotis bats, Plecotus genus, and Tadarida teniotis.
For common species, each hour of monitoring was regarded as an observation, and activity (expressed in bat passes/hour) was used as the dependent variable in the models. Consequently, the number of hours after sunset was included as one of the explanatory variables. We also explored the inclusion of a quadratic term for this variable to account for the typical variation in bat activity throughout the night, which often shows a peak whose timing and duration vary among species. These species were analysed using negative binomial GLMMs to better handle overdispersion, a common issue which the Poisson or quasi-Poisson distributions may not adequately address. Since rare species were characterised by a few passes per night, their presence or absence over each monitoring night was considered as the dependent variable instead of the activity, and binomial GLMMs were used. In addition to these models, a negative binomial GLMM was used to analyse species richness, with the number of bat species per night as the dependent variable.
A series of models for each species were produced, encompassing all possible combinations of explanatory variables. The analysis began with simpler models that considered each variable independently, gradually building up to more complex models that included multiple variables. Model selection was based on the Akaike information criterion (AIC). Specifically, the model with the lowest AIC value was selected. When several models had similar AIC values (ΔAIC ≤ 2), the selection process favoured models with fewer variables to prevent overfitting. Secondarily, the explained variance and overdispersion were considered. The same procedure was applied for the analysis of species richness. Statistical analysis was conducted using lme4 and MuMIn packages in R [27,28].
3. Results
3.1. Comparative Analysis across Areas at Different Lighting Levels
During 113 monitoring nights, a total of 144,490 bat recordings were collected. P. kuhlii was the most common species (66,241 recordings, 45.8% of the total), with the highest activity among all species in three of the four monitored areas (Figure 1). Its highest activity was recorded within the construction site (108.81 ± 19.06 bat passes/hour), which was nearly eightfold higher than in dark areas (13.80 ± 6.66 bat passes/hour). Similarly, N. leisleri (n = 22,311, 15.4%) showed a significant selection for the construction site. Its activity there (50.63 ± 9.84 bat passes/hour) was between 2 and almost 20 times greater than in less illuminated areas. H. savii (n = 10,959, 7.6%) was also more active in illuminated areas, but predominantly selected urban environments. In stark contrast, P. pipistrellus, the second most active species in the study area (n = 26,489, 18.3%), showed a clear selection for dark and poorly lit areas, where the average activity was three times higher than in intensely illuminated areas. Another group of species—including B. barbastellus, Eptesicus serotinus, large and small Myotis bats, Pipistrellus pygmaeus, Plecotus species, Rhinolophus ferrumequinum, and T. teniotis—were notably rare within the study area (Figure 2). Most of them were never recorded in the construction site and were either rare or not detected as well in high-lit urban areas. Instead, their activity was concentrated in dark areas or low-lit urban areas. Activity of small Myotis bats (n = 1.004) and Plecotus species (n = 182) was significantly lower in the construction site compared to all the other areas, while B. barbastellus (n = 262) was never recorded in the construction site. Large Myotis bats and R. ferrumequinum were recorded exclusively in dark areas.
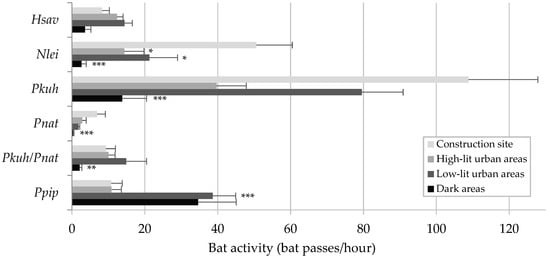
Figure 1.
Average activity (+SD) of common bat species or species groups (i.e., detected in at least 30% of the monitoring hours) across areas with increasing lighting levels, Upper Susa Valley, Italy. For species showing significant activity differences across different areas, significance levels from the pairwise post hoc Wilcoxon tests are indicated to the right of the error bars. Pairwise comparisons were made between the construction site and each of the other areas. Significance codes are as follows: * for 0.01 < p < 0.05; ** for 0.001 < p < 0.01; *** for p < 0.001. Species codes are as follows: Hsav = H. savii; Nlei = N. leisleri; Pkuh = P. kuhlii; Pnat = P. nathusii; Pkuh/Pnat = P. kuhlii/P. nathusii (only records with uncertainty between species); Ppip = P. pipistrellus.
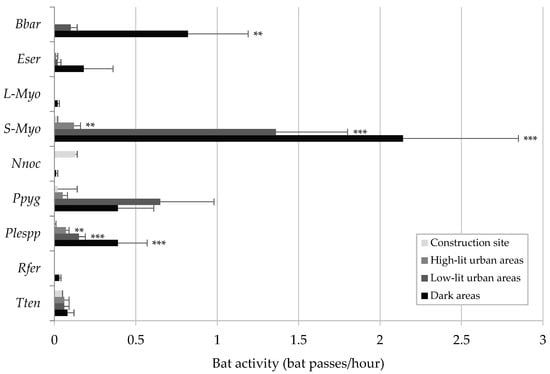
Figure 2.
Average activity (+SD) of rare bat species or species groups (i.e., detected in less than 30% of the monitoring hours) across areas with increasing lighting levels, Upper Susa Valley, Italy. For species showing significant activity differences across different areas, significance levels from the pairwise post hoc Wilcoxon tests are indicated to the right of the error bars. Pairwise comparisons were made between the construction site and each of the other areas. Significance codes are as follows: ** for 0.001 < p < 0.01; *** for p < 0.001. Species codes are as follows: Bbar = B. barbastellus; Eser = E. serotinus; L-Myo = large Myotis bats (M. myotis/M. blythii); S-Myo = small Myotis bats (all other Myotis species); Nnoc = N. noctula; Ppyg = P. pygmaeus; Plespp = Plecotus spp.; Rfer = R. ferrumequinum; Tten = T. teniotis.
Overall, species richness was comparable between dark and low-lit urban areas, with both environments recording an average species richness of 7.4 ± 1.4 (mean ± SD) and 7.4 ± 1.6, respectively, throughout the monitoring period. High-lit urban areas exhibited slightly lower average species richness (6.4 ± 1.2), while the lowest values were found in the construction site area (5.3 ± 1.4). Notably, species richness at the construction site was significantly lower compared to the other three areas with lower levels of illumination (Figure 3).
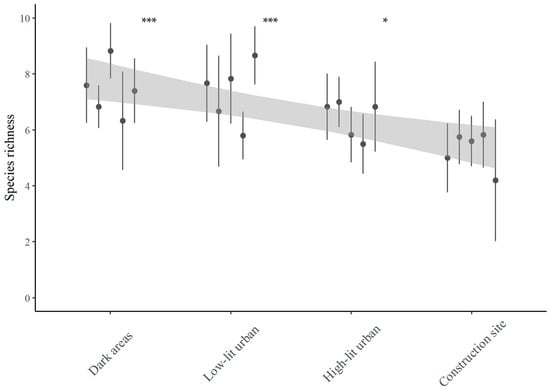
Figure 3.
Average bat species richness across areas with increasing lighting levels, Upper Susa Valley, Italy. Levels of ALAN increase moving from dark areas towards the Turin–Lyon high-speed railway construction site. Each point denotes a monitoring site, and the average species richness (±SD) was computed from all monitoring nights at each site. The significance levels indicated above the first three site groups denote significant differences in species richness in these areas compared to the construction site, as determined by a pairwise post hoc Wilcoxon test. The grey shaded area represents the 95% confidence interval of the linear trend, estimated using a linear regression model. Significance codes are as follows: * for 0.01 < p < 0.05; *** for p < 0.001.
3.2. Statistical Models
Model results for common species (H. savii, N. leisleri, P. kuhlii/P. nathusii, and P. pipistrellus), rare species (B. barbastellus, small Myotis bats, Plecotus species, and T. teniotis) and species richness are summarised in Table 1. Models selected for common species consisted of 5-8 environmental explanatory variables. The distance from the construction site and the time after sunset were always included in the models, with the latter always significant. Three of the four taxa showed a significant positive correlation between their activity and the maximum light intensity. Additionally, both H. savii and the P. kuhlii/P. nathusii species pair showed a significant increase in their activity as the distance from the construction site increased. However, correlations between species activity and variables related to built-up, forest, and open areas did not reach significance.

Table 1.
Model results for the activity of the eight selected species of bats, categorised based on their frequency of detection during monitoring hours. Species detected in at least 30% of monitoring hours were classified as common, while those detected in less than 30% were classified as rare. For common species, negative binomial GLMMs were used, with bat activity (expressed in bat passes/hour) as the dependent variable. For rare species, binomial GLMMs were used, with bat occurrence (presence or absence per night) as the dependent variable. Additionally, model results for species richness are included, employing a negative binomial GLMM with the total number of bat species recorded each night as the dependent variable. In all models, sampling site identity was incorporated as a random factor. If an independent variable was not included in the model, the corresponding cell is left blank. Significant positive correlations are identified by a “+” sign, negative correlations by a “−” sign, and non-significant correlations by “NS”. For quadratic relationships, the shape of the prediction curve is provided. Significance codes are as follows: * for 0.01 < p < 0.05; ** for 0.001 < p < 0.01; *** for p < 0.001. For quadratic relationships, the code refers to the quadratic term. The last two columns report the percentage of variance explained by the model: the first value (R2m, marginal R2) represents the variance explained by the fixed effects, the second value (R2c, conditional R2) represents the variance explained by the entire model, including both fixed and random effects. Species codes are as follows: Hsav = H. savii; Nlei = N. leisleri; Pkuh and Pnat = P. kuhlii and P. nathusii (all records, including those with uncertainty between species); Ppip = P. pipistrellus; Bbar = B. barbastellus; S-Myo = small Myotis bats (all Myotis species except M. myotis/M. blythii); Plespp = Plecotus spp.; Tten = T. teniotis; SR = species richness.
Models selected for rare species consisted of 1–6 environmental variables. The only variable always included in the models was the Julian day, which showed a positive correlation with the presence of three of the four species considered. B. barbastellus and small Myotis bats showed a significant negative correlation between their presence and the maximum light intensity. For Plecotus species, significant correlations were found for all six variables, showing strong positive correlations with built-up, forest, and open areas. The model selected for T. teniotis only included the Julian day and explained the lowest variance (15%) compared to the other seven taxa (47–73% of the variance explained). The model selected for species richness only included the maximum light intensity and the Julian day among environmental explanatory variables, showing a significant negative and a significant positive correlation, respectively.
4. Discussion
To the best of our knowledge, this is the first study assessing how nighttime lighting from a new LTI construction site affected bat activity. Several years after its construction, important differences were found in the bat community composition and activity near the construction site compared to the surrounding high- and low-lit urban areas, and even more compared to dark natural areas.
The construction site area was largely selected by P. kuhlii and N. leisleri. These species are known to be attracted to light sources in urban environments and along illuminated roads, benefitting from insect congregations around the lights [29,30,31]. The high activity levels recorded at the construction site suggest these species likely use the site’s lighting installations as foraging grounds [32,33]. The construction site’s lighting system has therefore altered their habitat selection, with potential dramatic consequences on nocturnal insect populations [34]. P. kuhlii showed a secondary selection for urban areas. This is probably the most urban-linked bat species in Europe, extensively using urban areas for both roosting and foraging [35,36]. In contrast, N. leisleri is a typical forest species, selecting tree cavities for roosting and mainly hunting near the tree canopy or along ponds or streams, even if open areas and streetlights can be used [37]. Alongside these two, other species (P. nathusii, P. pipistrellus, H. savii) also used the construction site for foraging, though to a lesser extent. In accordance with this, a recent study showed an increase in the activity of P. nathusii and H. savii with increasing light radiance only when the proportion of forests became high [38]. In the study area, the construction site is surrounded by forest on three sides.
The statistical model results for P. kuhlii/P. nathusii and H. savii confirm the positive correlation between their activity and light intensity, yet also show an increase in their activity with distance from the construction site. This apparent contradiction can likely be explained by their large use of urban areas [35,39], which are situated away from the construction site. N. leisleri did not follow this trend, in line with its selection for forest habitats [37], thus suggesting that its habitat selection might be more profoundly altered by ALAN at construction sites.
P. pipistrellus was the second most active species in the study, yet it showed a clear selection for dark and poorly lit areas over more illuminated ones. Although frequently defined as a generalist [40], this species is known to be more linked to wooded habitats and water bodies [41,42]. In the study area, dark areas were primarily natural spaces dominated by forest, whereas the poorly lit areas were located along the urban periphery, also in proximity to forest. This likely accounts for the high activity levels of P. pipistrellus in these settings, highlighting the species’ affinity for more natural, less disturbed environments. Insect activity in natural environments may also influence the activity patterns of this species [43].
ALAN has detrimental effects on many insects, particularly moths, which use artificial light instead of the moon for navigation and die from exhaustion flying around lamps or colliding with their incandescent surface [44]. Furthermore, many insects get dazzled and freeze when they approach a light source, settling on the ground or nearby vegetation, becoming easy prey [45]. These phenomena, coupled with the so-called “vacuum cleaner effect”, where light-sensitive species are attracted from a distance to lamps, remove many insects from the ecosystem [46]. Thus, bat predation around light sources only exacerbates the negative effects of light pollution on nocturnal entomofauna. For example, ALAN alters the evasive behaviour of tympanate moths, which would normally detect echolocation signals emitted by bats and avoid predation [9]. This allows bats to easily capture these moths, leading to harmful repercussions on their populations.
While ALAN has led to significant shifts in bat activity patterns, favouring species like P. kuhlii and N. leisleri, it has also contributed to habitat loss and fragmentation for more sensitive species. Myotis and Plecotus species, as well as B. barbastellus, P. pygmaeus and R. ferrumequinum, tended to avoid brightly lit areas, and many taxa were never recorded at the construction site. Activity patterns of small Myotis bats (i.e., all Myotis species except M. myotis/M. blythii) and B. barbastellus are underscored by the model analyses showing a significant negative correlation with the maximum light intensity. Moreover, for these species, model selection favoured those incorporating light intensity as the sole environmental descriptor. This indicates that light intensity alone significantly influences bat activity, a conclusion further supported by the high values of explained variance [47]. Consequently, ALAN not only exacerbates the negative effects caused by the direct loss of natural habitat from the construction site’s establishment but also emerges as the primary factor driving changes in the activity patterns of these species within the study area.
The area surrounding the construction site and the village of Chiomonte features a relatively uniform habitat, predominantly composed of deciduous forests interspersed with small clearings. The absence of trees within both the construction site and the village may restrict the occurrence of forest specialist species [18]. However, small Myotis bats and Plecotus species, along with occasional visits by B. barbastellus, used the village area to some extent. In contrast, the construction site, with its high levels of illumination, remained very difficult to exploit. Activity of more sensitive species in the village area still differed from that in dark natural areas, proving that even minor variation in lighting levels can result in bat activity changes [48,49].
Analysis of species richness confirmed ALAN’s detrimental effects on bat diversity. While dark and low-lit urban areas maintained comparable species richness, indicating some degree of resilience, the construction site’s intense lighting significantly reduced species diversity. This pattern is particularly concerning for rare species, which avoided the construction site, contributing to the observed decrease in species richness in highly illuminated areas. The significant negative correlation between species richness and the maximum light intensity highlights a barrier effect of ALAN, hindering access to suitable habitats for species that rely on dark, forested environments adjacent to the study area [18].
Rare species considered in this study face an unfavourable conservation status [50]. All of them are, to some extent, connected to forest environments [18], and the considerable extent of forest around the study area has likely played a crucial role in sustaining their populations after the site construction [38,51]. However, for many species, high levels of ALAN generated by the construction site have resulted in a barrier effect that can alter bat movements and potentially reduce landscape connectivity, threatening slow fliers and forest-dependent species, such as small Myotis bats [52].
Effective mitigation strategies are essential to minimise the ecological impacts of ALAN in highly illuminated construction sites, particularly for sensitive wildlife like bats. One key approach is the implementation of adaptive lighting systems that reduce intensity or switch off lights during periods of low human activity, thus diminishing light pollution without compromising safety [53]. When lighting systems are primarily installed for surveillance and security purposes, the use of thermal infrared cameras offers an innovative, bat-friendly solution [54]. Where some degree of illumination is necessary, using downward-directed, shielded lighting can significantly reduce light spill into surrounding habitats, concentrating illumination where it’s needed while protecting dark areas [53]. Employing lights with emission spectra to which bats are less sensitive, such as red lights, as well as using low light intensity lamps, can further diminish disturbances [55,56]. Additionally, creating buffer zones around construction sites where lighting is either greatly reduced or entirely absent provides safe corridors and foraging areas for bats and other nocturnal fauna [55]. These measures, combined with community engagement and awareness programs about the importance of dark skies, can form a comprehensive approach to mitigate the adverse effects of ALAN [57], ensuring both operational efficiency and ecological stewardship at construction sites. In the context of impact assessments for the implementation of highly illuminated construction sites, it is crucial to include bat acoustic monitoring protocols. These protocols should be based on the BACI (Before, After, Control, Impact) approach, which allows for the comparison of bat presence and activity before and after the site construction while also considering trends in control areas.
In this study, variations in bat activity and species richness in relation to ALAN at a large construction site were explored. Eight years after the site construction, significant changes in habitat use among both common and rare species were found. Hypsugo, Nyctalus, and Pipistrellus species are likely drawn to the construction site for foraging, exploiting the abundance of insects attracted by its strong lighting system. For some of these species, selection of this environment was stronger than for any other environment in the study. An exception among common species was P. pipistrellus, which selected dark and low-lit areas. Rare bat species from the genera Barbastella, Myotis, and Plecotus avoid the construction site area, which poses a significant barrier effect impacting their movements and available habitats. This trend is further evidenced by the decrease in species richness moving from dark and poorly lit areas towards highly lit areas, culminating in the minimum species richness observed at the construction site. The results of the study underscore the significant ecological consequences of introducing ALAN to previously unlit natural environments, stressing the importance of implementing effective mitigation strategies to minimise these impacts. These may include the implementation of adaptive lighting systems, correct light positioning and shielding, and the preservation of dark corridors. Our study highlights the crucial role of bat acoustic monitoring in impact assessments for construction sites, offering valuable information for the conservation and management of bat populations amid rising challenges from light pollution.
Author Contributions
Conceptualization, S.B. and F.G.; methodology, F.G.; software, F.G.; validation, F.G., S.B. and A.R.; formal analysis, F.G.; investigation, F.G. and C.F.; resources, S.B.; data curation, F.G. and C.F.; writing—original draft preparation, S.B. and F.G.; writing—review and editing, C.F., S.B. and A.R.; visualization, F.G.; supervision, S.B. and A.R.; project administration, S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TELT sas., Agreement Prot. 138, 29/01/2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Falchi, F.; Cinzano, P.; Duriscoe, D.M.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Bruce-White, C.; Shardlow, M. A Review of the Impact of Artificial Light on Invertebrates; Buglife-the Invertebrate Conservation Trust: Buglife, UK, 2011. [Google Scholar]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Shivanna, K.R. Impact of light pollution on nocturnal pollinators and their pollination services. Proc. Natl. Acad. Sci. India–Phys. Sci. 2022, 88, 626–633. [Google Scholar] [CrossRef]
- Russart, K.L.; Nelson, R.J. Artificial light at night alters behavior in laboratory and wild animals. J. Exp. Zoöl. Part A Ecol. Integr. Physiol. 2018, 329, 401–408. [Google Scholar] [CrossRef]
- Stone, E.L.; Harris, S.; Jones, G. Impacts of artificial lighting on bats: A review of challenges and solutions. Mamm. Biol. 2015, 80, 213–219. [Google Scholar] [CrossRef]
- Voigt, C.C.; Dekker, J.; Fritze, M.; Gazaryan, S.; Hölker, F.; Jones, G.; Lewanzik, D.; Limpens, H.J.G.A.; Mathews, F.; Rydell, J.; et al. The Impact of Light Pollution on Bats Varies According to Foraging Guild and Habitat Context. BioScience 2021, 71, 1103–1109. [Google Scholar] [CrossRef]
- Wakefield, A.; Stone, E.L.; Jones, G.; Harris, S. Light-emitting diode street lights reduce last-ditch evasive manoeuvres by moths to bat echolocation calls. R. Soc. Open Sci. 2015, 2, 150291. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, C.; Boyles, J.G.; Minnaar, I.A.; Sole, C.L.; McKechnie, A.E. Stacking the odds: Light pollution may shift the balance in an ancient predator–prey arms race. J. Appl. Ecol. 2014, 52, 522–531. [Google Scholar] [CrossRef]
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D.G. Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems. Front. Neurosci. 2020, 14, 602796. [Google Scholar] [CrossRef] [PubMed]
- Briolat, E.S.; Gaston, K.J.; Bennie, J.; Rosenfeld, E.J.; Troscianko, J. Artificial nighttime lighting impacts visual ecology links between flowers, pollinators and predators. Nat. Commun. 2021, 12, 4163. [Google Scholar] [CrossRef] [PubMed]
- Barré, K.; Kerbiriou, C.; Ing, R.-K.; Bas, Y.; Azam, C.; Le Viol, I.; Spoelstra, K. Bats seek refuge in cluttered environment when exposed to white and red lights at night. Mov. Ecol. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Camerano, P.; Giannetti, F.; Terzuolo, P.G.; Guiot, E. La Carta Forestale Del Piemonte—Aggiornamento 2016. IPLA S.p.A.; Regione Piemonte 2017. Available online: https://www.geoportale.piemonte.it/geonetwork/srv/ita/catalog.search#/metadata/r_piemon:812c28a8-763b-4c74-81a3-c5fe1ed99c68 (accessed on 10 December 2023).
- Istat. Il Censimento Permanente della Popolazione in Piemonte—Anno 2020. 2022. Available online: https://www.istat.it/it/archivio/268878 (accessed on 10 December 2023).
- CIPE. Delibera 21 Marzo 2018. Nuova Linea Ferroviaria Torino-Lione—Sezione Internazionale—Parte Comune Italo-Francese. Sezione Transfrontaliera. Parte in Territorio Italiano. Approvazione Progetto di Variante in Ottemperanza alla Prescrizione 235 della Delibera CIPE 19 del 2015. (CUP: C11J05000030001). (Delibera n. 30/2018). G.U.R.I. 2018, Serie Generale n. 185, 34–36. Available online: www.gazzettaufficiale.it/eli/id/2018/08/10/18A05313/sg (accessed on 10 December 2023).
- Bas, Y.; Bas, D.; Julien, J.-F. Tadarida: A Toolbox for Animal Detection on Acoustic Recordings. J. Open Res. Softw. 2017, 5, 6. [Google Scholar] [CrossRef]
- Barataud, M. Acoustic Ecology of European Bats. In Species Identification, Study of Their Habitats and Foraging Behaviour, 2nd ed.; (Inventaires & biodiversité series); Biotope éditions: Méze, France; Muséum National d’Histoire Naturelle: Paris, France, 2020. [Google Scholar]
- Russ, J. Bat Calls of Britain and Europe: A Guide to Species Identification; Pelagic Publishing: Exeter, UK, 2021. [Google Scholar]
- Middleton, N.; Froud, A.; French, K. Social Calls of the Bats of Britain and Ireland, 2nd ed.; Pelagic Publishing: London, UK, 2022. [Google Scholar]
- Toffoli, R.; Rughetti, M. Bat activity in rice paddies: Organic and conventional farms compared to unmanaged habitat. Agric. Ecosyst. Environ. 2017, 249, 123–129. [Google Scholar] [CrossRef]
- Kerbiriou, C.; Bas, Y.; Le Viol, I.; Lorrilliere, R.; Mougnot, J.; Julien, J.F. Potential of bat pass duration measures for studies of bat activity. Bioacoustics 2018, 28, 177–192. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 December 2023).
- Bivand, R.; Keitt, T.; Rowlingson, B. rgdal: Bindings for the ’Geospatial’ Data Abstraction Library, R Package Version 1.4-8. 2019. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 10 December 2023).
- Bivand, R.; Rundel, C. RGEOS: Interface to Geometry Engine—Open Source (‘GEOS’); R Package Version 0.5-2. 2019. Available online: https://CRAN.R-project.org/package=rgeos (accessed on 10 December 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference; R Package Version 1.47.5. 2023. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 December 2023).
- Mathews, F.; Roche, N.; Aughney, T.; Jones, N.; Day, J.; Baker, J.A.; Langton, S. Barriers and benefits: Implications of artificial night-lighting for the distribution of common bats in Britain and Ireland. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140124. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Ancillotto, L.; Cistrone, L.; Nastasi, C.; Bosso, L.; Smeraldo, S.; Cordero, V.S.; Russo, D. Artificial illumination influences niche segregation in bats. Environ. Pollut. 2021, 284, 117187. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L.; Cistrone, L.; Libralato, N.; Domer, A.; Cohen, S.; Korine, C. Effects of artificial illumination on drinking bats: A field test in forest and desert habitats. Anim. Conserv. 2018, 22, 124–133. [Google Scholar] [CrossRef]
- Slough, B.G.; Reid, D.G.; Schultz, D.S.; Leung, M.C. Little brown bat activity patterns and conservation implications in agricultural landscapes in boreal Yukon, Canada. Ecosphere 2023, 14, e4446. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Ciechanowski, M.; Jaros, R.; Kalcounis-Rüppell, M.; Kmiecik, A.; Kmiecik, P.; Węgiel, J. The foraging activity of bats in managed pine forests of different ages. Eur. J. For. Res. 2019, 138, 383–396. [Google Scholar] [CrossRef]
- Desouhant, E.; Gomes, E.; Mondy, N.; Amat, I. Mechanistic, ecological, and evolutionary consequences of artificial light at night for insects: Review and prospective. Èntomol. Exp. Appl. 2019, 167, 37–58. [Google Scholar] [CrossRef]
- Ancillotto, L.; Tomassini, A.; Russo, D. The fancy city life: Kuhl’s pipistrelle, Pipistrellus kuhlii, benefits from urbanisation. Wildl. Res. 2015, 42, 598. [Google Scholar] [CrossRef]
- Polak, T.; Korine, C.; Yair, S.; Holderied, M.W. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zoöl. 2011, 285, 21–27. [Google Scholar] [CrossRef]
- Boston, E.S.M.; Dechmann, D.K.N.; Ruczyński, I. Leisler’s Noctule Nyctalus leisleri (Kuhl, 1817). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2023; Volume Chiroptera, pp. 423–437. [Google Scholar]
- Barré, K.; Vernet, A.; Azam, C.; Le Viol, I.; Dumont, A.; Deana, T.; Vincent, S.; Challéat, S.; Kerbiriou, C. Landscape composition drives the impacts of artificial light at night on insectivorous bats. Environ. Pollut. 2022, 292, 118394. [Google Scholar] [CrossRef] [PubMed]
- Ancillotto, L.; Budinski, I.; Nardone, V.; Di Salvo, I.; Della Corte, M.; Bosso, L.; Conti, P.; Russo, D. What is driving range expansion in a common bat? Hints from thermoregulation and habitat selection. Behav. Process. 2018, 157, 540–546. [Google Scholar] [CrossRef]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet different: Distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mammal Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Todd, V.L.G.; Williamson, L.D. Habitat usage of Daubenton’s bat (Myotis daubentonii), common pipistrelle (Pipistrellus pipistrellus), and soprano pipistrelle (Pipistrellus pygmaeus) in a North Wales upland river catchment. Ecol. Evol. 2019, 9, 4853–4863. [Google Scholar] [CrossRef] [PubMed]
- Mimet, A.; Kerbiriou, C.; Simon, L.; Julien, J.-F.; Raymond, R. Contribution of private gardens to habitat availability, connectivity and conservation of the common pipistrelle in Paris. Landsc. Urban Plan. 2020, 193, 103671. [Google Scholar] [CrossRef]
- Mendes, E.S.; Fonseca, C.; Marques, S.F.; Maia, D.M.S.; Pereira, M.J.R. Bat richness and activity in heterogeneous landscapes: Guild-specific and scale-dependent? Landsc. Ecol. 2016, 32, 295–311. [Google Scholar] [CrossRef]
- Degen, T.; Mitesser, O.; Perkin, E.K.; Weiß, N.; Oehlert, M.; Mattig, E.; Hölker, F. Street lighting: Sex-independent impacts on moth movement. J. Anim. Ecol. 2016, 85, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Eisenbeis, G. Artificial night lighting and insects: Attraction of insects to streetlamps in a rural setting in Germany. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 281–304. [Google Scholar]
- Eisenbeis, G.; Hänel, A. Light pollution and the impact of artificial night lighting on insects. In Ecology of Cities and Towns; McDonnell, M.J., Hahs, A.H., Breuste, J.H., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 243–263. [Google Scholar]
- Kleinbaum, D.G.; Kupper, L.L.; Nizam, A.; Rosenberg, E.S. Applied Regression Analysis and Other Multivariable Methods; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Azam, C.; Le Viol, I.; Bas, Y.; Zissis, G.; Vernet, A.; Julien, J.-F.; Kerbiriou, C. Evidence for distance and illuminance thresholds in the effects of artificial lighting on bat activity. Landsc. Urban Plan. 2018, 175, 123–135. [Google Scholar] [CrossRef]
- Voigt, C.C.; Azam, C.; Dekker, J.; Ferguson, J.; Fritze, M.; Gazaryan, S.; Hölker, F.; Jones, G.; Leader, N.; Lewanzik, D.; et al. EUROBATS Publication Series No. 8. Guidelines for Consideration of Bats in Lighting Projects; UNEP/EUROBATS Secretariat: Bonn, Germany, 2018. [Google Scholar]
- Barova, S.; Streit, A. Action Plan for the Conservation of All Bat Species in the European Union 2019–2024; European Commission, UNEP Eurobats: Bruxelles, Belgium, 2018. [Google Scholar]
- Straka, T.M.; Wolf, M.; Gras, P.; Buchholz, S.; Voigt, C.C. Tree Cover Mediates the Effect of Artificial Light on Urban Bats. Front. Ecol. Evol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Laforge, A.; Pauwels, J.; Faure, B.; Bas, Y.; Kerbiriou, C.; Fonderflick, J.; Besnard, A. Reducing light pollution improves connectivity for bats in urban landscapes. Landsc. Ecol. 2019, 34, 793–809. [Google Scholar] [CrossRef]
- Cole, M.; DeJohn, M.; McClure, L.; Rogers, S.M. Led Lighting. Minimizing Ecological Impact without Compromising Human Safety. In Proceedings of the 2021 IEEE IAS Petroleum and Chemical Industry Technical Conference (PCIC), San Antonio, TX, USA, 13–16 September 2021. [Google Scholar]
- Andraši, P.; Radišić, T.; Muštra, M.; Ivošević, J. Night-time Detection of UAVs using Thermal Infrared Camera. Transp. Res. Procedia 2017, 28, 183–190. [Google Scholar] [CrossRef]
- Zeale, M.R.K.; Stone, E.L.; Zeale, E.; Browne, W.J.; Harris, S.; Jones, G. Experimentally manipulating light spectra reveals the importance of dark corridors for commuting bats. Glob. Chang. Biol. 2018, 24, 5909–5918. [Google Scholar] [CrossRef]
- Kerbiriou, C.; Barré, K.; Mariton, L.; Pauwels, J.; Zissis, G.; Robert, A.; Le Viol, I. Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity 2020, 12, 165. [Google Scholar] [CrossRef]
- Bjelajac, D.; Đerčan, B.; Kovačić, S. Dark skies and dark screens as a precondition for astronomy tourism and general well-being. Inf. Technol. Tour. 2020, 23, 19–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


