Addressing Hydrogen Sulfide Corrosion in Oil and Gas Industries: A Sustainable Perspective
Abstract
1. Introduction
2. H2S Corrosion in Refinery Operations
2.1. Hydrogen Sulfide (H2S)
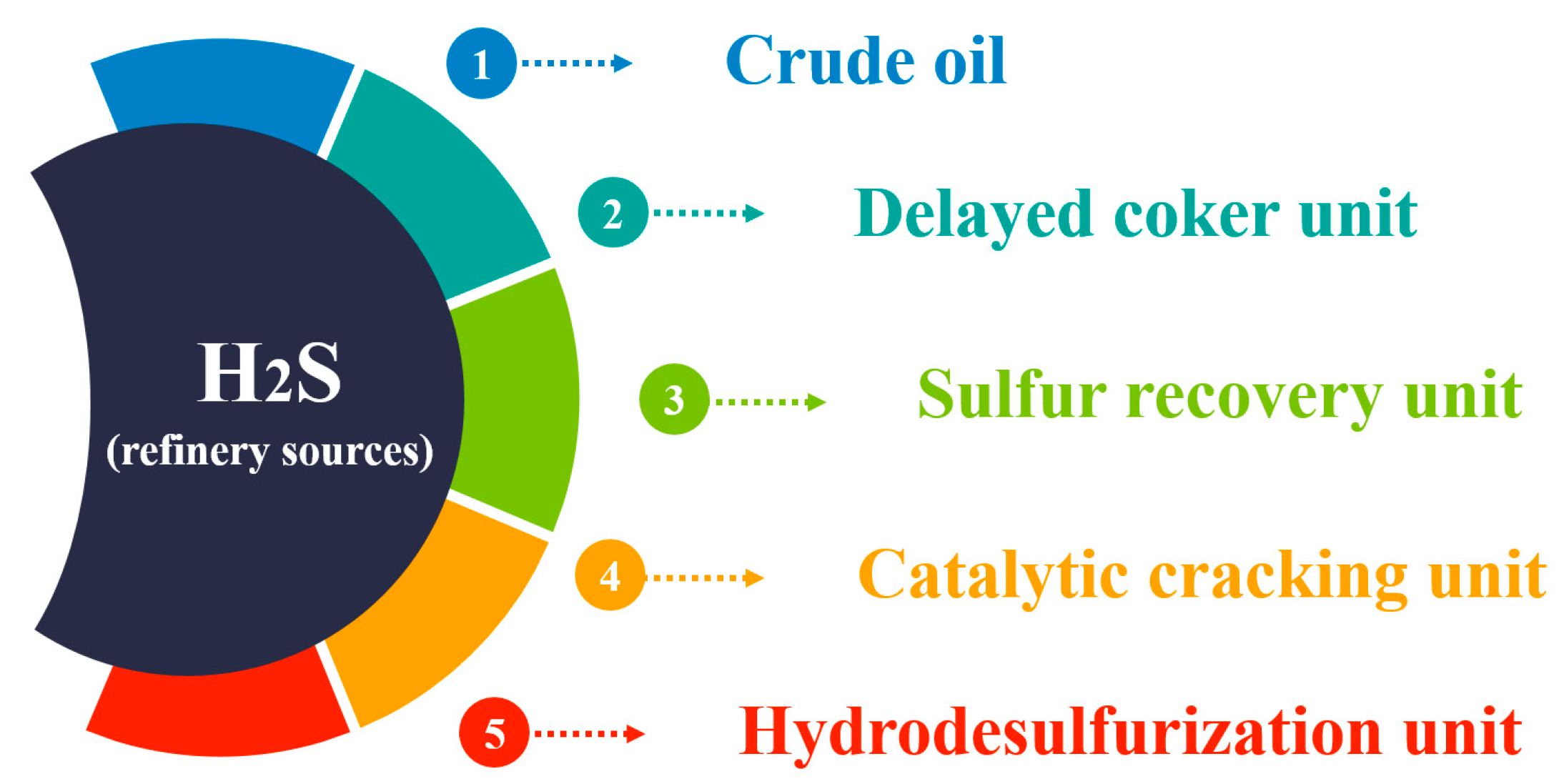
2.2. H2S Sources
- Crude oil serves as the primary source of H2S within refineries. During processing, naturally occurring sulfur compounds in crude oil release H2S gas [14]. Crude oil is typically categorized as “sweet” or “sour” based on its sulfur content, with sour crude oil containing higher levels of sulfur compounds, including H2S.
- Various other sources within refinery facilities can also contribute to H2S production. Refineries utilize hydrodesulfurization units to efficiently remove sulfur from products such as jet fuel, diesel, and gasoline, converting sulfur compounds into H2S [15].
- Catalytic reforming is a crucial process in refineries for converting low-octane hydrocarbons into high-octane gasoline blending components. This process can potentially generate H2S if sulfur-containing compounds are present in the feedstock [16].
- Hydrotreating units utilize hydrogen gas to react with hydrocarbon streams, removing impurities such as sulfur and converting sulfur compounds into H2S [17].
- Delayed coker units facilitate the conversion of heavy residuals into lighter products, including petroleum coke, which can also release H2S [18].
- Sulfur recovery units (SRUs) in refineries extract elemental sulfur from sour gases produced during refining processes. H2S is typically converted into elemental sulfur or sulfuric acid in these units. However, incomplete conversion or operational inefficiencies can result in H2S emissions [19].
- Sulfuric acid alkylation units, during the alkylation process, produce high-octane alkylate using sulfuric acid. While sulfuric acid primarily acts as a catalyst and is not consumed in the reaction, sulfur-containing impurities in the feedstock can lead to the formation of H2S as a byproduct [20].
- Tank vents and storage facilities, especially those containing sulfur-containing products such as sour crude oil or intermediate products from desulfurization processes, may emit H2S when vented, particularly during filling or maintenance activities [21].
- Wastewater treatment processes in refineries generate wastewater containing various contaminants, including sulfur compounds. During wastewater treatment, such as biological or chemical processes, H2S may be produced due to microbial activity or chemical reactions [22].
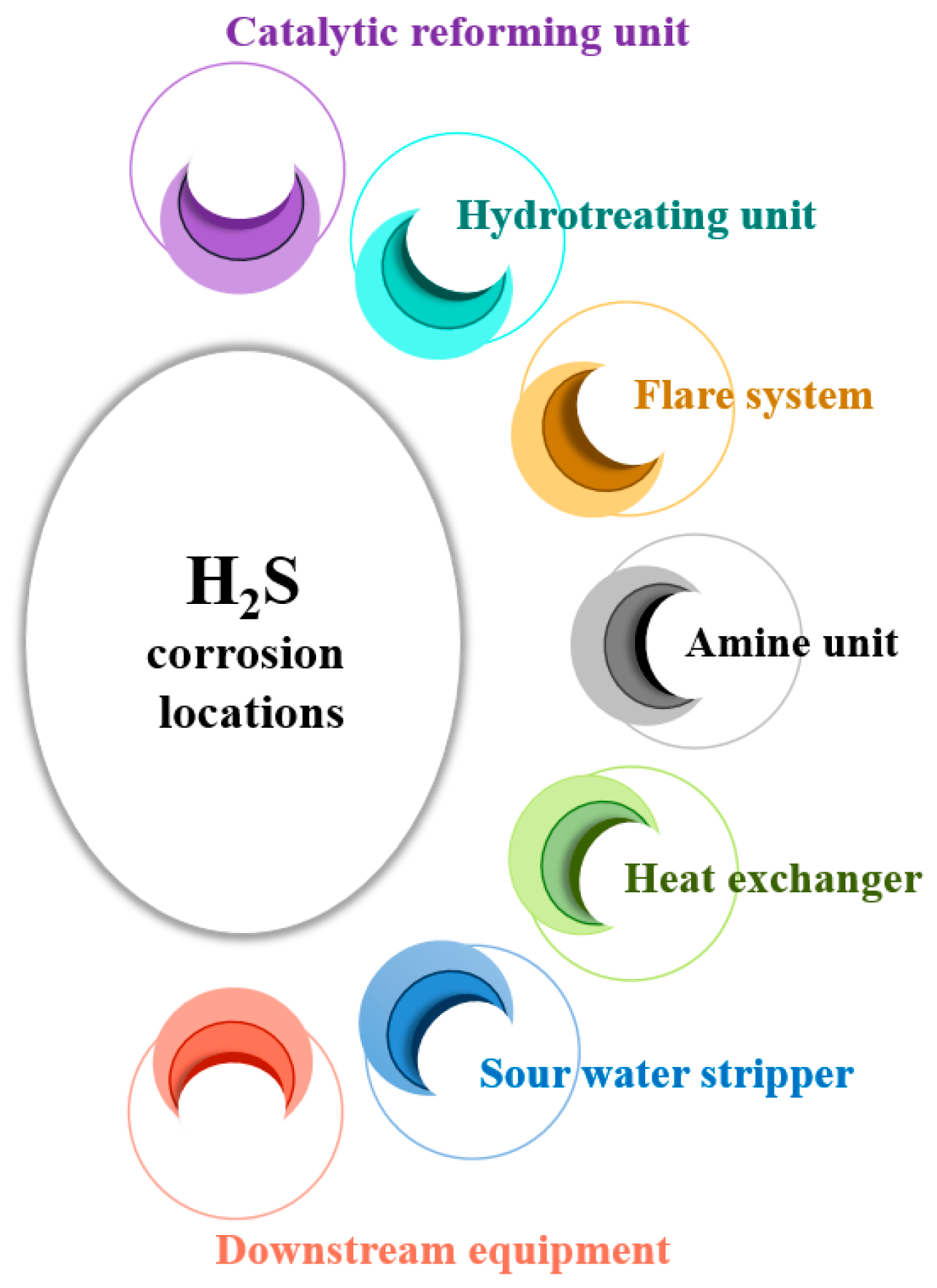
2.3. H2S Corrosion Locations
2.4. H2S Corrosion Mechanism
- (a)
- The formation of a hydrogen atom contributes to hydrogen embrittlement (HE) in steel. When H2S and/or are present, the conversion of hydrogen atoms into molecules is hindered, leading to an accumulation of excess hydrogen atoms and increased pressure.
- (b)
- Elevated partial pressure of H2S leads to decreased pH values in the solution, potentially worsening metal corrosion.
2.5. H2S Corrosion Products
3. H2S Corrosion Type
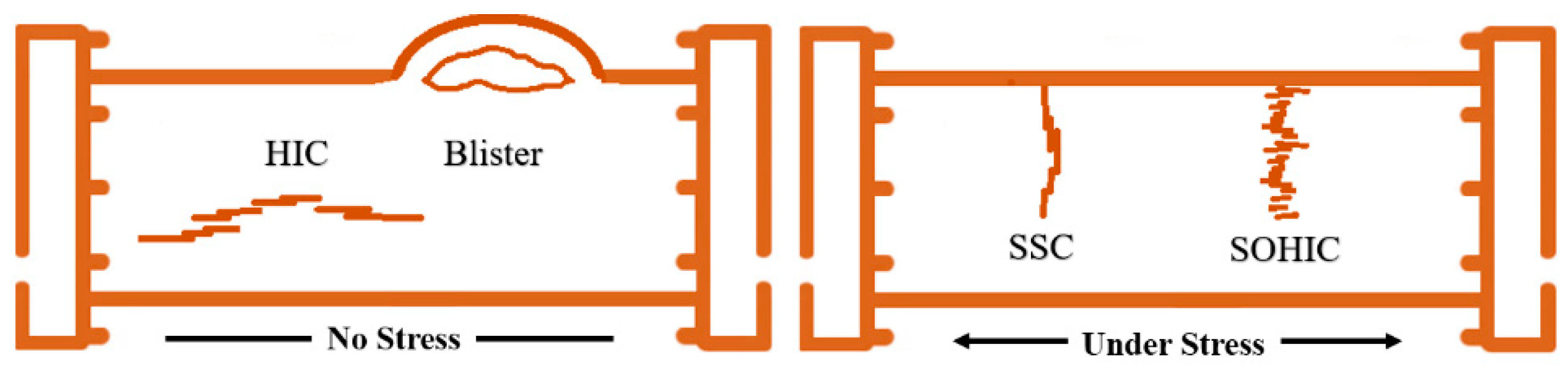
- Hydrogen atoms tend to recombine at voids and interfaces of inclusions within the steel matrix, forming molecular hydrogen that becomes trapped and is unable to desorb. This can lead to an accumulation of hydrogen partial pressure, potentially resulting in blister formation. Blisters are a common failure mode, particularly observed in low-carbon steel containing elongated inclusions near the steel surface.
- When hydrogen becomes confined within parallel lamination planes, it can initiate small cracks associated with HIC. These microcracks may accumulate and align along residual stresses, facilitating crack propagation. This phenomenon is known as stress-oriented hydrogen-induced cracking (SOHIC).
- Even small amounts of hydrogen, typically measured in parts per million (ppm), can cause embrittlement in high-strength steels under external or residual stress, leading to SSC.
3.1. Hydrogen-Induced Cracking (HIC)
3.2. Sulfide Stress Cracking (SSC)
3.3. Stress-Oriented Hydrogen-Induced Cracking (SOHIC)
4. Factors Affecting H2S Corrosion
4.1. Effect of Temperature
4.2. Effect of Flow Rate
4.3. Effect of pH
4.4. Effect of H2S Concentration
5. Monitoring
5.1. Continuous Monitoring
5.2. Corrosion Coatings
5.3. Cathodic Protection
5.4. Corrosion Inhibitor
5.5. Material Selection
5.6. Data Analysis and Reporting
5.7. Training
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muthukumar, N. Petroleum products transporting pipeline corrosion—A review. In The Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–571. [Google Scholar]
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lashari, N.; Lu, H.; Sambo, C. Integrity assessment of corroded oil and gas pipelines using machine learning: A systematic review. Eng. Fail. Anal. 2022, 131, 105810. [Google Scholar] [CrossRef]
- Senouci, A.; Elabbasy, M.; Elwakil, E.; Abdrabou, B.; Zayed, T. A model for predicting failure of oil pipelines. Struct. Infrastruct. Eng. 2014, 10, 375–387. [Google Scholar] [CrossRef]
- Pournara, A.-E. Structural Integrity of Steel Hydrocarbon Pipelines with Local Wall Distortions. Master’s Thesis, University of Thessaly, Volos, Greece, 2015. [Google Scholar]
- Obot, I.B.; Sorour, A.A.; Verma, C.; Al-Khaldi, T.A.; Rushaid, A.S. Key parameters affecting sweet and sour corrosion: Impact on corrosion risk assessment and inhibition. Eng. Fail. Anal. 2023, 145, 107008. [Google Scholar] [CrossRef]
- Song, C.; Li, Y.; Wu, F.; Luo, J.; Li, L.; Li, G. Failure analysis of the crack and leakage of a crude oil pipeline under CO2-steam flooding. Processes 2023, 11, 1567. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Wang, Y.; Qiao, J.; Wang, Z. Corrosion failure mechanism of associated gas transmission pipeline. Materials 2018, 11, 1935. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Wu, L.; Li, X.; Zhang, Z. Corrosion law of metal pipeline in tahe oilfield and application of new materials. Coatings 2021, 11, 1269. [Google Scholar] [CrossRef]
- Videm, K.; Koren, A. Corrosion, passivity, and pitting of carbon steel in aqueous solutions of HCO3−, CO2, and Cl−. Corrosion 1993, 49, 746–754. [Google Scholar] [CrossRef]
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulfide intoxication. In Handbook of Clinical Neurology; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 111–133. [Google Scholar]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on plant functioning. Front. Plant Sci. 2019, 10, 743. [Google Scholar] [CrossRef]
- Ahmad, W.; Sethupathi, S.; Kanadasan, G.; Lau, L.C.; Kanthasamy, R. A review on the removal of hydrogen sulfide from biogas by adsorption using sorbents derived from waste. Rev. Chem. Eng. 2021, 37, 407–431. [Google Scholar] [CrossRef]
- Kraia, T.; Varvoutis, G.; Marnellos, G.E.; Konsolakis, M. Unveiling the role of in situ sulfidation and H2O excess on H2S decomposition to carbon-free H2 over cobalt/ceria catalysts. Catalysts 2023, 13, 504. [Google Scholar] [CrossRef]
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A.P. Hydrogen sulfide adsorption by iron oxides and their polymer composites: A case-study application to biogas purification. Materials 2020, 13, 4725. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Ahmad, I.; Irfan, M.; Mursal, S.N.F.; Legutko, S.; Kruszelnicka, I.; Ginter-Kramarczyk, D. Catalytic hydrogen evolution from H2S cracking over CrxZnS Catalyst in a cylindrical single-layered dielectric barrier discharge plasma reactor. Materials 2022, 15, 7426. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Xu, W.; Li, Y.; Tian, J.; Wu, L. Energy consumption analysis of a diesel hydrotreating unit using an aspen simulation. Processes 2022, 10, 2055. [Google Scholar] [CrossRef]
- Magomedov, R.; Popova, A.; Maryutina, T.; Kadiev, K.M.; Khadzhiev, S. Current status and prospects of demetallization of heavy petroleum feedstock. Pet. Chem. 2015, 55, 423–443. [Google Scholar] [CrossRef]
- Jagannath, A.; Ibrahim, S.; Raj, A. Heat integration in straight through sulfur recovery units to increase net high pressure steam production. Chem. Eng. Technol. 2021, 44, 164–173. [Google Scholar] [CrossRef]
- Salah, H.B.; Nancarrow, P.; Al-Othman, A. Ionic liquid-assisted refinery processes—A review and industrial perspective. Fuel 2021, 302, 121195. [Google Scholar] [CrossRef]
- Yang, R.; Zhirong, W.; Juncheng, J.; Shuoxun, S.; Peipei, S.; Yawei, L. Cause analysis and prevention measures of fire and explosion caused by sulfur corrosion. Eng. Fail. Anal. 2020, 108, 104342. [Google Scholar] [CrossRef]
- Jiad, M.M.; Abbar, A.H. Petroleum refinery wastewater treatment using a novel combined electro-Fenton and photocatalytic process. J. Ind. Eng. Chem. 2024, 129, 634–655. [Google Scholar] [CrossRef]
- Asadian, M.; Sabzi, M.; Anijdan, S.H.M. The effect of temperature, CO2, H2S gases and the resultant iron carbonate and iron sulfide compounds on the sour corrosion behaviour of ASTM A-106 steel for pipeline transportation. Int. J. Press. Vessels Pip. 2019, 171, 184–193. [Google Scholar] [CrossRef]
- Liu, W.; Lyu, Y.; Duan, Z.; Li, W.; Yu, W. Investigation of corrosion sequence in the overhead pipeline of H2S stripper column through CFD models. Eng. Fail. Anal. 2022, 136, 106187. [Google Scholar] [CrossRef]
- Lins, V.F.C.; Guimarães, E.M. Failure of a heat exchanger generated by an excess of SO2 and H2S in the sulfur recovery unit of a petroleum refinery. J. Loss. Prev. Process. Ind. 2007, 20, 91–97. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, C.; Chen, X.; Zhang, L.; Zheng, J.; Zhao, Y. Dependence of the abnormal protective property on the corrosion product film formed on H2S-adjacent API-X52 pipeline steel. Int. J. Hydrogen Energy 2014, 39, 13919–13925. [Google Scholar] [CrossRef]
- Lucio-Garcia, M.A.; Gonzalez-Rodriguez, J.G.; Casales, M.; Martinez, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. Effect of heat treatment on H2S corrosion of a micro-alloyed C–Mn steel. Corros. Sci. 2009, 51, 2380–2386. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, L.; Yang, J.; Lu, M.; Ding, J.; Li, H. Polymorphous FeS corrosion products of pipeline steel under highly sour conditions. Corros. Sci. 2016, 102, 103–113. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Afroukhteh, S. A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J. Nat. Gas Sci. Eng. 2019, 71, 102971. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. High resolution transmission electron microscopic study of synthetic nanocrystalline mackinawite. Earth Planet. Sci. Lett. 2006, 241, 227–233. [Google Scholar] [CrossRef]
- Boursiquot, S.; Mullet, M.; Abdelmoula, M.; Génin, J.M.; Ehrhardt, J.J. The dry oxidation of tetragonal FeS1-xmackinawite. Phys. Chem. Miner. 2001, 28, 600–611. [Google Scholar] [CrossRef]
- Pearce, C.I.; Pattrick, R.A.; Vaughan, D.J. Electrical and magnetic properties of sulfides. Rev. Mineral. Geochem. 2006, 61, 127–180. [Google Scholar] [CrossRef]
- Davison, W. The solubility of iron sulphides in synthetic and natural waters at ambient temperature. Aquat. Sci. 1991, 53, 309–329. [Google Scholar] [CrossRef]
- Kvarekvål, J.; Moloney, J. 6—Sour corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Boston, MA, USA, 2017; pp. 113–147. [Google Scholar]
- Wen, X.; Bai, P.; Luo, B.; Zheng, S.; Chen, C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 2018, 139, 124–140. [Google Scholar] [CrossRef]
- Ren, C.; Liu, D.; Bai, Z.; Li, T. Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. Phys. 2005, 93, 305–309. [Google Scholar] [CrossRef]
- Skála, R.; Císařová, I.; Drábek, M. Inversion twinning in troilite. Am. Mineral. 2006, 91, 917–921. [Google Scholar] [CrossRef]
- Wang, H.; Salveson, I. A review on the mineral chemistry of the non-stoichiometric iron sulphide, Fe1−xS (0 ≤ x ≤ 0.125): Polymorphs, phase relations and transitions, electronic and magnetic structures. Phase Transit. 2005, 78, 547–567. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Menguy, N.; Abreu, F.; Lins, U.; Pósfai, M.; Prozorov, T.; Pignol, D.; Frankel, R.B.; Bazylinski, D.A. A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science 2011, 334, 1720–1723. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Ceder, G. Evaluating structure selection in the hydrothermal growth of FeS2 pyrite and marcasite. Nat. Commun. 2016, 7, 13799. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, J.H.; Hayes, K.F. Characterization of synthetic nanocrystalline mackinawite: Crystal structure, particle size, and specific surface area. Geochim. Cosmochim. Acta. 2008, 72, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cheng, X.; Chen, S.; Li, G.; Chen, X.; Lei, S.; Yang, H. Theoretical interpretation on impedance spectra for anodic iron dissolution in acidic solutions containing hydrogen sulfide. Corrosion 1998, 54, 634–640. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Sun, W.; Nesic, S.; Papavinasam, S. Kinetics of iron sulfide and mixed iron sulfide/carbonate scale precipitation in CO2/H2S corrosion. In Nace Corrosion; NACE: Bethlehem, PA, USA, 2006; p. NACE-06644. [Google Scholar]
- Zheng, Y.; Ning, J.; Brown, B.; Young, D.; Nesic, S. Mechanistic study of the effect of iron sulfide layers on hydrogen sulfide corrosion of carbon steel. In Nace Corrosion; NACE: Bethlehem, PA, USA, 2015; p. NACE-2015-5933. [Google Scholar]
- King, R.A. 11—Sulfide stress cracking. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Boston, MA, USA, 2017; pp. 271–294. [Google Scholar]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Ossai, C.I.; Boswell, B.; Davies, I.J. Pipeline failures in corrosive environments—A conceptual analysis of trends and effects. Eng. Fail. Anal. 2015, 53, 36–58. [Google Scholar] [CrossRef]
- Anijdan, S.M.; Arab, G.; Sabzi, M.; Sadeghi, M.; Eivani, A.R.; Jafarian, H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an API X65 pipeline steel in H2S containing environment: Influence of heat treatment and its subsequent microstructural changes. J. Mater. Res. Technol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Abdelshafeek, K.A.; Abdallah, W.E.; Elsayed, W.M.; Eladawy, H.A.; El-Shamy, A. Vicia faba peel extracts bearing fatty acids moieties as a cost-effective and green corrosion inhibitor for mild steel in marine water: Computational and electrochemical studies. Sci. Rep. 2022, 12, 20611. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, J.C.B.; Simoni, L.; Tagliari, M.R.; Scheid, A.; Paes, M.T.P.; Kwietniewski, C.E.F. Effects of thermal spray aluminium coating on SSC and HIC resistance of high strength steel in a sour environment. Surf. Coat. Technol. 2020, 399, 126156. [Google Scholar] [CrossRef]
- Traidia, A.; Alfano, M.; Lubineau, G.; Duval, S.; Sherik, A. An effective finite element model for the prediction of hydrogen induced cracking in steel pipelines. Int. J. Hydrogen Energy 2012, 37, 16214–16230. [Google Scholar] [CrossRef]
- Martin, M.L.; Sofronis, P. Hydrogen-induced cracking and blistering in steels: A review. J. Nat. Gas Eng. 2022, 101, 104547. [Google Scholar] [CrossRef]
- Campos, E.S.; de Sá, J.D.S.; Campos, T.S.; de Souza, E.A.; Gomes, J.A.D.C.P. Influence of metallurgical factors on the hydrogen induced cracking of carbon steel wires in H2S-containing environments. Eng. Fail. Anal. 2024, 155, 107739. [Google Scholar] [CrossRef]
- Toribio, J.; Lorenzo, M.; Vergara, D.; Aguado, L. Residual stress redistribution induced by fatigue in cold-drawn prestressing steel wires. Constr. Build. Mater. 2016, 114, 317–322. [Google Scholar] [CrossRef]
- De Giorgi, M. Residual stress evolution in cold-rolled steels. Int. J. Fatigue 2011, 33, 507–512. [Google Scholar] [CrossRef]
- Domizzi, G.; Anteri, G.; Ovejero-García, J. Influence of sulphur content and inclusion distribution on the hydrogen induced blister cracking in pressure vessel and pipeline steels. Corros. Sci. 2001, 43, 325–339. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163–175. [Google Scholar] [CrossRef]
- Lee, H.-L.; Lap-Ip Chan, S. Hydrogen embrittlement of AISI 4130 steel with an alternate ferrite/pearlite banded structure. Mater. Sci. Eng. A 1991, 142, 193–201. [Google Scholar] [CrossRef]
- Tau, L.; Chan, S.L.I. Effects of ferrite/pearlite alignment on the hydrogen permeation in a AISI 4130 steel. Mater. Lett. 1996, 29, 143–147. [Google Scholar] [CrossRef]
- Roccisano, A.; Nafisi, S.; Ghomashchi, R. Stress corrosion cracking observed in ex-service gas pipelines: A comprehensive study. Metall. Mater. Trans. A 2020, 51, 167–188. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Shi, G.; Meng, K.; Wang, Q.; Zhang, F. Effect of niobium on sulfide stress cracking behavior of tempered martensitic steel. Corros. Sci. 2020, 165, 108387. [Google Scholar] [CrossRef]
- Zvirko, O.; Gabetta, G.; Tsyrulnyk, O.; Kret, N. Assessment of in-service degradation of gas pipeline steel taking into account susceptibility to stress corrosion cracking. Procedia Struct. Integr. 2019, 16, 121–125. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Abbas, A.T.; Gopi, D.; El-Shamy, A. Corrosion and corrosion inhibition of high strength low alloy steel in 2.0 M sulfuric acid solutions by 3-amino-1,2,3-triazole as a corrosion inhibitor. J. Chem. 2014, 2014, 538794. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Abbas, A.T.; Halfa, H.; El-Shamy, A.M. Corrosion of high strength steel in concentrated sulfuric acid pickling solutions and its inhibition by 3-amino-5-mercapto-1,2,3-triazole. Int. J. Electrochem. Sci. 2015, 10, 1777–1791. [Google Scholar] [CrossRef]
- Chong, T.-V.S.; Kumar, S.B.; Lai, M.O.; Loh, W.L. Effects of wet H2S containing environment on mechanical properties of NACE grade C–Mn steel pipeline girth welds. Eng. Fract. Mech. 2014, 131, 485–503. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; Abdelfattah, I.; Elshafey, O.I.; Shehata, M.F. Potential removal of organic loads from petroleum wastewater and its effect on the corrosion behavior of municipal networks. J. Environ. Manag. 2018, 219, 325–331. [Google Scholar] [CrossRef]
- Suman, S.; Biswas, P. Comparative study on SAW welding induced distortion and residual stresses of CSEF steel considering solid state phase transformation and preheating. J. Manuf. Process. 2020, 51, 19–30. [Google Scholar] [CrossRef]
- Haidemenopoulos, G.N.; Kamoutsi, H.; Polychronopoulou, K.; Papageorgiou, P.; Altanis, I.; Dimitriadis, P.; Stiakakis, M. Investigation of stress-oriented hydrogen-induced cracking (SOHIC) in an amine absorber column of an oil refinery. Metals 2018, 8, 663. [Google Scholar] [CrossRef]
- Al-Anezi, M.A.; Rao, S. Failures by SOHIC in sour hydrocarbon service. J. Fail. Anal. Prev. 2011, 11, 363–371. [Google Scholar] [CrossRef]
- El-Shamy, A.M. Fabrication of commercial nanoporous alumina by low voltage anodizing. Egypt. J. Chem. 2018, 61, 175–185. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Mousavi, H.; Pourazizi, R.; Szpunar, J.A. Finite element modeling of HIC propagation in pipeline steel with regard to experimental observations. Int. J. Hydrogen Energy 2020, 45, 23122–23133. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Zhang, L. Effect of high temperature on the corrosion behavior and passive film composition of 316 L stainless steel in high H2S-containing environments. Corros. Sci. 2020, 174, 108844. [Google Scholar] [CrossRef]
- Medvedeva, M. Specifics of high-temperature corrosion processes during oil recovery. Chem. Pet. Eng. 2000, 36, 749–754. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, X.; Wang, Z.; Li, T.; Zhang, Z.; Lu, M. The corrosion behavior of 316L stainless steel in H2S environment at high temperatures. Int. J. Electrochem. Sci. 2017, 12, 8806–8819. [Google Scholar] [CrossRef]
- Silva, C.C.; Farias, J.P.; de Sant’Ana, H.B. Evaluation of AISI 316L stainless steel welded plates in heavy petroleum environment. Mater. Des. 2009, 30, 1581–1587. [Google Scholar] [CrossRef]
- Gao, S.; Jin, P.; Brown, B.; Young, D.; Nešić, S.; Singer, M. Effect of high temperature on the aqueous H2S corrosion of mild steel. Corrosion 2017, 73, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Nešic, S.; Papavinasam, S. Kinetics of corrosion layer formation. Part 2—Iron sulfide and mixed iron sulfide/carbonate layers in carbon dioxide/hydrogen sulfide corrosion. Corrosion 2008, 64, 586–599. [Google Scholar] [CrossRef]
- Asmara, Y.P. The roles of H2S gas in behavior of carbon steel corrosion in oil and gas environment: A review. J. Tek. Mesin 2018, 7, 37–43. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, D.; Zhang, Z.; Zhao, W.; Yao, G. Effect of flow velocity on pipeline steel corrosion behaviour in H2S/CO2 environment with sulphur deposition. Corros. Eng. Sci. Technol. 2018, 53, 370–377. [Google Scholar] [CrossRef]
- Martelo, D.; Sampath, D.; Monici, A.; Morana, R.; Akid, R. Correlative analysis of digital imaging, acoustic emission, and fracture surface topography on hydrogen assisted cracking in Ni-alloy 625+. Eng. Fract. Mech. 2019, 221, 106678. [Google Scholar] [CrossRef]
- Farhadian, A.; Go, W.; Yun, S.; Rahimi, A.; Reza Nabid, M.; Iravani, D.; Seo, Y. Efficient dual-function inhibitors for prevention of gas hydrate formation and CO2/H2S corrosion inside oil and gas pipelines. J. Chem. Eng. 2022, 431, 134098. [Google Scholar] [CrossRef]
- Kermani, M.; Morshed, A. Carbon dioxide corrosion in oil and gas productiona compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Nešic, S.; Li, H.; Huang, J.; Sormaz, D. An open source mechanistic model for CO2/H2S corrosion of carbon steel. In Nace Corrosion; NACE: Bethlehem, PA, USA, 2009; p. NACE-09572. [Google Scholar]
- Brown, B.; Nešic, S. Aspects of localized corrosion in an H2S/CO2 environment. In Nace Corrosion; NACE: Bethlehem, PA, USA, 2012; p. NACE-2012-1559. [Google Scholar]
- Liu, R.; Li, J.; Liu, Z.; Du, C.; Dong, C.; Li, X. Effect of pH and H2S concentration on sulfide stress corrosion cracking (SSCC) of API 2205 duplex stainless steel. Int. J. Mater. Res. 2015, 106, 608–613. [Google Scholar] [CrossRef]
- Tang, J.; Shao, Y.; Guo, J.; Zhang, T.; Meng, G.; Wang, F. The effect of H2S concentration on the corrosion behavior of carbon steel at 90 °C. Corros. Sci. 2010, 52, 2050–2058. [Google Scholar] [CrossRef]
- Taheri, H.; Kakooei, S.; Ismail, M.C.; Dolati, A. The effect of H2S concentration and temperature on corrosion behavior of pipeline steel A516-Gr70. Casp. J. Appl. Sci. 2012, 1, 41–47. [Google Scholar]
- Qi, Y.; Luo, H.; Zheng, S.; Chen, C.; Lv, Z.; Xiong, M. Effect of H2S partial pressure on the tensile properties of A350LF2 steel in the absence and presence of pre-immersion. Mater. Sci. Eng. A 2014, 609, 161–167. [Google Scholar] [CrossRef]
- Musayeva, N.; Khalilova, H.; Izzatov, B.; Trevisi, G.; Ahmadova, S.; Alizada, M. Highly selective detection of hydrogen sulfide by simple Cu-CNTs nanocomposites. C 2023, 9, 25. [Google Scholar] [CrossRef]
- Khalid, H.U.; Ismail, M.C.; Nosbi, N. Permeation damage of polymer liner in oil and gas pipelines: A review. Polymers 2020, 12, 2307. [Google Scholar] [CrossRef]
- Ali, M.A.H.; Baggash, M.; Rustamov, J.; Abdulghafor, R.; Abdo, N.A.-D.N.; Abdo, M.H.G.; Mohammed, T.S.; Hasan, A.A.; Abdo, A.N.; Turaev, S.; et al. An automatic visual inspection of oil tanks exterior surface using unmanned aerial vehicle with image processing and cascading fuzzy logic algorithms. Drones 2023, 7, 133. [Google Scholar] [CrossRef]
- Cheng, L.; Lou, F.; Guo, W. Corrosion protection of the potassium silicate conversion coating. Vacuum 2020, 176, 109325. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Groysman, A. Corrosion problems and solutions in oil, gas, refining and petrochemical industry. KOM Corros. Mater. Prot. J. 2017, 61, 100–117. [Google Scholar] [CrossRef]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 35. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. Corrosion inhibitors for sour oilfield environment (H2S corrosion). In Corrosion Inhibitors in the Oil and Gas Industry; Wiley: Hoboken, NJ, USA, 2020; pp. 229–254. [Google Scholar]
- Zhao, X.; Huang, W.; Li, G.; Feng, Y.; Zhang, J. Effect of CO2/H2S and applied stress on corrosion behavior of 15Cr tubing in oil field environment. Metals 2020, 10, 409. [Google Scholar] [CrossRef]
- Dong, C.; Luo, H.; Xiao, K.; Sun, T.; Liu, Q.; Li, X. Effect of temperature and Cl− concentration on pitting of 2205 duplex stainless steel. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 641–647. [Google Scholar] [CrossRef]
- Liu, H.; Hua, Y.; Shi, S.; Lin, X.; Neville, A.; Wang, Y.; Sun, J. Stability of passive film and pitting susceptibility of 2205 duplex stainless steel in CO2/H2S-containing geothermal environment. Corros. Sci. 2023, 210, 110832. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Chen, C.F.; Chen, L.Q. Corrosion characteristics of 2205 duplex stainless steel in high temperature and high pressure environment containing H2S/CO2. Appl. Mech. Mater. 2012, 236, 95–98. [Google Scholar] [CrossRef]
- Thorhallsson, A.I.; Karlsdottir, S.N. Corrosion Behaviour of Titanium Alloy and Carbon Steel in a High-Temperature, Single and Mixed-Phase, Simulated Geothermal Environment Containing H2S, CO2 and HCl. Corros. Mater. Degrad. 2021, 2, 190–209. [Google Scholar] [CrossRef]
- Karlsdottir, S.N.; Ragnarsdottir, K.R.; Thorbjornsson, I.O.; Einarsson, A. Corrosion testing in superheated geothermal steam in Iceland. Geothermics 2015, 53, 281–290. [Google Scholar] [CrossRef]
- Kang, Y.; Leng, X.; Zhao, L.; Bai, B.; Wang, X.; Chen, H. Review on the corrosion behaviour of nickel-based alloys in supercritical carbon dioxide under high temperature and pressure. Crystals 2023, 13, 725. [Google Scholar] [CrossRef]
- Silva, C.C.; de Miranda, H.C.; Motta, M.F.; Farias, J.P.; Afonso, C.R.M.; Ramirez, A.J. New insight on the solidification path of an alloy 625 weld overlay. J. Mater. Res. Technol. 2013, 2, 228–237. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Qian, J.; Yu, H.; Chen, C. Study of the pitting corrosion at welding joints of Inconel 625 alloy under high temperature and high H2S/CO2 partial pressure. Int. J. Electrochem. Sci. 2017, 12, 8929–8943. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.m.p.; Ferreira, M.G.S.; Belo, M.D.C. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 1999, 41, 17–34. [Google Scholar] [CrossRef]
- Tomio, A.; Sagara, M.; Doi, T.; Amaya, H.; Otsuka, N.; Kudo, T. Role of alloyed molybdenum on corrosion resistance of austenitic Ni–Cr–Mo–Fe alloys in H2S–Cl− environments. Corros. Sci. 2015, 98, 391–398. [Google Scholar] [CrossRef]
- Gao, X.; Liu, M. Corrosion behavior of high-strength C71500 copper-nickel alloy in simulated seawater with high concentration of sulfide. Materials 2022, 15, 8513. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Zhang, L.; Hao, Y.; Li, C.; Hu, S. Intergranular-stress corrosion cracking mechanism of brass in H2S environment: A DFT study. Comput. Mater. Sci. 2019, 170, 109193. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chu, H.; Qi, G.; Ding, H.; Gao, X.; Meng, J. Molecular simulation on permeation behavior of CH4/CO2/H2S mixture gas in PVDF at service conditions. Polymers 2022, 14, 545. [Google Scholar] [CrossRef]
- Maraveas, C. Durability issues and corrosion of structural materials and systems in farm environment. Appl. Sci. 2020, 10, 990. [Google Scholar] [CrossRef]
- Hussain, M.; Zhang, T.; Chaudhry, M.; Jamil, I.; Kausar, S.; Hussain, I. Review of prediction of stress corrosion cracking in gas pipelines using machine learning. Machines 2024, 12, 42. [Google Scholar] [CrossRef]
- Xie, M.; Tian, Z. A review on pipeline integrity management utilizing in-line inspection data. Eng. Fail. Anal. 2018, 92, 222–239. [Google Scholar] [CrossRef]
- Vakili, M.; Koutník, P.; Kohout, J. Corrosion by Polythionic Acid in the Oil and Gas Sector: A Brief Overview. Materials 2023, 16, 7043. [Google Scholar] [CrossRef]
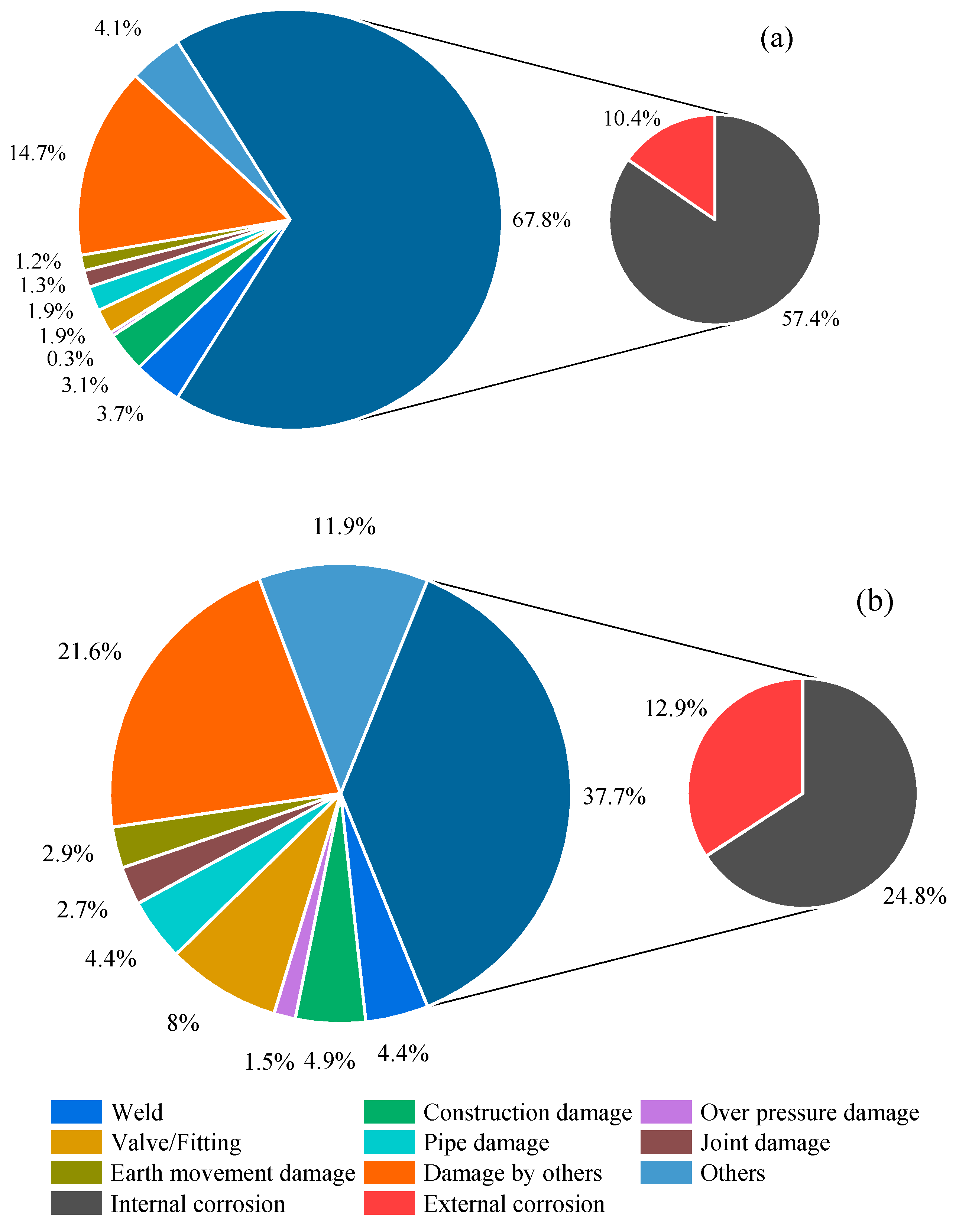
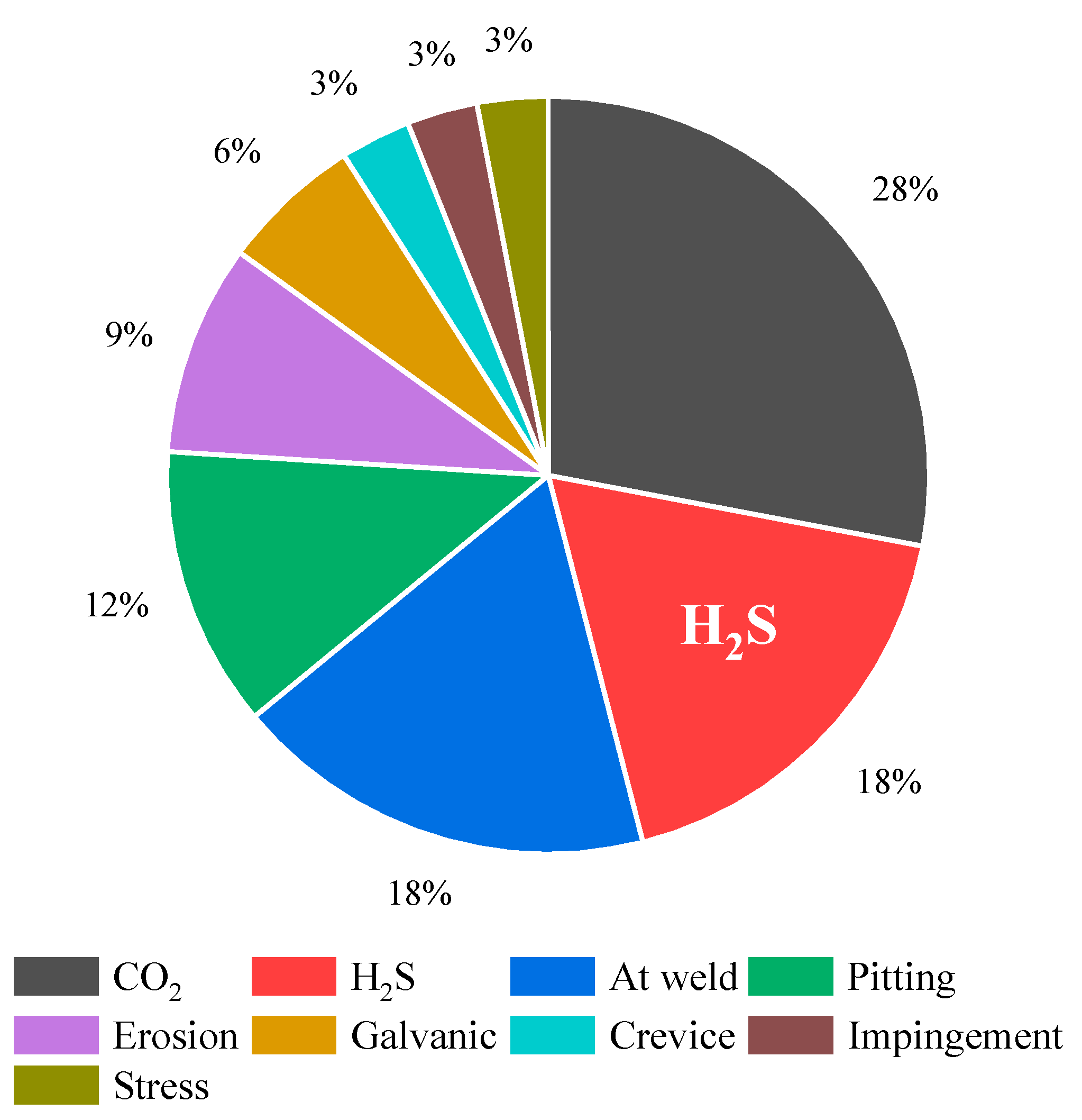


| Characteristic | Detail |
|---|---|
| Chemical structure | 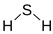 |
| Molar weight | 34.08 g mol−1 |
| Odor | Offensive and strong odor of rotten eggs |
| Color | Colorless |
| Taste | Sweetish taste |
| Density | 1.5392 g/L |
| Specific gravity | 1.189 |
| Boiling point | −60.25 °C |
| Melting point | −82 °C |
| Physical state | Gas |
| Upper explosive limit (UEL) | 44% |
| Lower explosive limit (LEL) | 4% |
| Auto-ignition temperature | 500 °F (260 °C) |
| Henry’s law constant at 25 °C | 0.0098 atm-m3/mol |
| Vapor pressure at 25 °C | 13,600 mmHg |
| Solubility in water (H2O) | 4 g dm−3 (at 20 °C) |
| Name | Formula | Lattice Structure |
|---|---|---|
| Amorphous FeS | Fe(HS)2, FeSx | Nan-crystalline |
| Mackinawite | Fe1+xS, x = 0.005–0.025 | Tetragonal |
| Pyrite | FeS2 | Cubic |
| Greigite | Fe3S4 | Cubic |
| Cubic FeS | FeS | Cubic |
| Marcasite | FeS2 | Orthorhombic |
| Pyrrhotite | Fe1−xS Fe7S8 | Hexagonal Monoclinic |
| Smythite | Fe9S11, Fe7S8 | Hexagonal |
| Troilite | FeS | Hexagonal |
| SSC | HIC | |
|---|---|---|
| Material strength | Mainly in high-strength steel | Mainly in low-strength steel |
| Applied stress | Affects severely | No effect |
| Crack direction | Perpendicular to stress | Dependent on microstructure |
| Location | Anywhere | Ingot core |
| Environment | Can occur even in mildly corrosive media | Highly corrosive conditions, appreciable hydrogen uptake |
| Microstructure | Critical effect, Q and T treatment enhances SSC resistance | Cleanliness and nonmetallic inclusions are critical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakili, M.; Koutník, P.; Kohout, J. Addressing Hydrogen Sulfide Corrosion in Oil and Gas Industries: A Sustainable Perspective. Sustainability 2024, 16, 1661. https://doi.org/10.3390/su16041661
Vakili M, Koutník P, Kohout J. Addressing Hydrogen Sulfide Corrosion in Oil and Gas Industries: A Sustainable Perspective. Sustainability. 2024; 16(4):1661. https://doi.org/10.3390/su16041661
Chicago/Turabian StyleVakili, Mohammadtaghi, Petr Koutník, and Jan Kohout. 2024. "Addressing Hydrogen Sulfide Corrosion in Oil and Gas Industries: A Sustainable Perspective" Sustainability 16, no. 4: 1661. https://doi.org/10.3390/su16041661
APA StyleVakili, M., Koutník, P., & Kohout, J. (2024). Addressing Hydrogen Sulfide Corrosion in Oil and Gas Industries: A Sustainable Perspective. Sustainability, 16(4), 1661. https://doi.org/10.3390/su16041661







