Navigating Crisis: Insights into the Depletion and Recovery of Central Java’s Freshwater Eel (Anguilla spp.) Stocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. CMSY Analysis
- (i)
- Initial r–k range determination
- (ii)
- Estimating the initial ranges of relative biomass
- (iii)
- Estimating likely reference points using feasible r–k pairs
2.3. Stock Status and Recovery Scenarios
3. Results
3.1. CMSY Analysis
3.2. Stock Status and Recovery Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jellyman, D.J. An enigma: How can freshwater eels (Anguilla spp.) be such a successful genus yet be universally threatened? Rev. Fish Biol. Fish. 2021, 32, 701–718. [Google Scholar] [CrossRef]
- Gollock, M.; Shiraishi, H.; Carrizo, S.; Crook, V.; Levy, E. Status of Non-CITES Listed Anguillid Eels; ZSL: London, UK, 2018; p. 176. [Google Scholar]
- Arai, T. Do we protect freshwater eels or do we drive them to extinction? SpringerPlus 2014, 3, 534. [Google Scholar] [CrossRef] [PubMed]
- Arai, T. Sustainable Management of Tropical Anguillid Eels in Southeast Asia. In Natural Resources Conservation and Advances for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–480. ISBN 978-0-12-822976-7. [Google Scholar]
- FAO. FAO Fishery and Aquaculture Statistics—Yearbook 2020; FAO: Rome, Italy, 2023; ISBN 978-92-5-138107-6. [Google Scholar]
- SEAFDEC. Enhancing Sustainable Utilization and Management Scheme of Tropical Anguillid Eel Resources in Southeast Asia; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2019; p. 139. [Google Scholar]
- Muthmainnah, D.; Honda, S.; Suryati, N.K.; Prisantoso, B.I. Fish for the People; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2016; pp. 19–25. [Google Scholar]
- Suryati, N.K.; Pamungkas, Y.P.; Muthmainnah, D. Fish for the People; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2019; pp. 19–24. [Google Scholar]
- Muthmainnah, D.; Suryati, N.K.; Pamungkas, Y.P.; Mulyani, Y.S. Fish for the People; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2020; pp. 37–42. [Google Scholar]
- Honda, S.; Muthmainnah, D.; Suryati, N.K.; Oktaviani, D.; Siriraksophon, S.; Amornpiyakrit, T.; Prisantoso, B.I. Current Status and Problems of the Catch Statistics on Anguillid Eel Fishery in Indonesia. Mar. Res. Indones. 2016, 41, 1–14. [Google Scholar] [CrossRef]
- DKP. Jawa Tengah Statistik Perikanan Tangkap Jawa Tengah; Dinas Perikanan dan Kelautan: Semarang, Indonesia, 2020. [Google Scholar]
- Dinas Perikanan. Cilacap Statistik Perikanan Tangkap Perairan Laut Dan Umum Darat; Dinas Perikanan: Kabupaten Cilacap, Indonesia, 2022. [Google Scholar]
- Boesono, H.; Fitri, A.D.P.; Kurohman, F.; Jayanto, B.B. Modifikasi Bubu Paralon untuk Penangkapan Ikan Sidat (Anguila bicolor) di Perairan Segara Anakan, Kabupaten Cilacap; UNDIP Press: Semarang, Indonesia, 2020; ISBN 978-979-097-695-5. [Google Scholar]
- Hakim, A.A.; Kamal, M.M.; A Butet, N.; Affandi, R. Species composition of Freshwater Eels (Anguilla spp.) in Eight Rivers Flowing to Palabuhanratu Bay, Sukabumi, Indonesia. J. Ilmu dan Teknol. Kelaut. Trop. 2016, 7, 573–586. [Google Scholar] [CrossRef][Green Version]
- Kuroki, M.; Miller, M.J.; Tsukamoto, K. Diversity of early life-history traits in freshwater eels and the evolution of their oceanic migrations. Can. J. Zool. 2014, 92, 749–770. [Google Scholar] [CrossRef]
- Hewavitharane, C.A.; Pickering, T.D.; Rico, C.; Mochioka, N. Early life history of tropical freshwater eels (Anguilla spp.) recruiting to Viti Levu, Fiji Islands, in the western South Pacific. Mar. Freshw. Res. 2020, 71, 452–460. [Google Scholar] [CrossRef]
- Harrison, A.J.; Walker, A.M.; Pinder, A.C.; Briand, C.; Aprahamian, M.W. A review of glass eel migratory behaviour, sampling techniques and abundance estimates in estuaries: Implications for assessing recruitment, local production and exploitation. Rev. Fish Biol. Fish. 2014, 24, 967–983. [Google Scholar] [CrossRef]
- Rahmadya, A.; Ridwansyah, I.; Daruati, D.; Rahmat, A.; Triyanto; Dewi, A.P.; Wildan, D.M. A Systematic Literature Review of the Methodology Preference for Analyzing Eel (Anguilla spp.) Habitat. Chiang Mai Univ. J. Nat. Sci. 2022, 21, e2022023. [Google Scholar] [CrossRef]
- Ye, Y.; Cochrane, K.; Bianchi, G.; Willmann, R.; Majkowski, J.; Tandstad, M.; Carocci, F. Rebuilding global fisheries: The World Summit Goal, costs and benefits. Fish Fish. 2012, 14, 174–185. [Google Scholar] [CrossRef]
- Bell, J.D.; Watson, R.A.; Ye, Y. Global fishing capacity and fishing effort from 1950 to 2012. Fish Fish. 2017, 18, 489–505. [Google Scholar] [CrossRef]
- Righton, D.; Piper, A.; Aarestrup, K.; Amilhat, E.; Belpaire, C.; Casselman, J.; Castonguay, M.; Díaz, E.; Dörner, H.; Faliex, E.; et al. Important questions to progress science and sustainable management of anguillid eels. Fish Fish. 2021, 22, 762–788. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Wiedenmann, J.; Deroba, J.J. The refined ORCS approach: A catch-based method for estimating stock status and catch limits for data-poor fish stocks. Fish. Res. 2017, 193, 60–70. [Google Scholar] [CrossRef]
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2016, 18, 506–526. [Google Scholar] [CrossRef]
- Punt, A.E.; Smith, D.C.; Smith, A.D.M. Among-stock comparisons for improving stock assessments of data-poor stocks: The “Robin Hood” approach. ICES J. Mar. Sci. 2011, 68, 972–981. [Google Scholar] [CrossRef]
- Rossenberg, A.A.; Fogarty, M.J.; Cooper, A.B.; Dickey-Collas, M.; Fulton, E.A.; Hyde, K.J.W.; Ye, Y. Developing New Approaches to Global Stock Status Assessment and Fishery Production Potential of the Seas; FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2014; ISBN 978-92-5-107992-8. [Google Scholar]
- Thorson, J.T.; Minto, C.; Minte-Vera, C.V.; Kleisner, K.M.; Longo, C. A new role for effort dynamics in the theory of harvested populations and data-poor stock assessment. Can. J. Fish. Aquat. Sci. 2013, 70, 1829–1844. [Google Scholar] [CrossRef]
- Zhou, S.; Punt, A.E.; Smith, A.D.M.; Ye, Y.; Haddon, M.; Dichmont, C.M.; Smith, D.C. An optimized catch-only assessment method for data poor fisheries. ICES J. Mar. Sci. 2017, 75, 964–976. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Ono, K.; Prince, J.D.; Walters, C.J. A simple length-structured model based on life history ratios and incorporating size-dependent selectivity: Application to spawning potential ratios for data-poor stocks. Can. J. Fish. Aquat. Sci. 2016, 73, 1787–1799. [Google Scholar] [CrossRef]
- Rudd, M.B.; Thorson, J.T. Accounting for variable recruitment and fishing mortality in length-based stock assessments for data-limited fisheries. Can. J. Fish. Aquat. Sci. 2018, 75, 1019–1035. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Palomares, M.L.D.; Dureuil, M.; Pauly, D. Estimating stock status from relative abundance and resilience. ICES J. Mar. Sci. 2019, 77, 527–538. [Google Scholar] [CrossRef]
- Affandi, R. Pengembangan Sumberdaya Ikan Sidat (Anguilla spp.) Di Indonesia. In Teknologi Pengembangan Perikanan dan Kelautan untuk Memperkuat Ketahanan Pangan serta Memacu Perekonomian Nasional Secara Berkelanjutan; IPB Press: Bogor, Indonesia, 2016; pp. 153–198. ISBN 978-979-493-918-5. [Google Scholar]
- Indrawati, A.; Anggoro, S.; Suradi, W.S. Pemetaan Potensi Ikan Sidat (Anguilla bicolor bicolor) Pada Perairan Sungai Di Kabupaten Purworejo. In Proceedings of the Hasil-Hasil Penelitian Perikanan dan Kelautan, June 2016; pp. 669–679. Available online: http://eprints.undip.ac.id/51329/1/89._(F)_Ayuningtyas_Indrawati_-_Pemetaan_Potensi_Ikan_Sidat_(Anguilla_bicolor_bicolor)_pada.pdf (accessed on 13 September 2022).
- Taufiq-Spj, N.; Santos, G.W.; Suryono, S.; Ambariyanto, A. New Perspective of Java Eel Conservation: Case Study in Cilacap Riverine and Coastal Area, Central Java, Indonesia. Egypt. J. Aquat. Biol. Fish. 2023, 27, 671–685. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Anderson, S.C.; Gutierrez, N.L.; Kleisner, K.M.; Longo, C.; Minto, C.; Osio, G.C.; Walsh, J.C. Blood from a stone: Performance of catch-only methods in estimating stock biomass status. Fish. Res. 2019, 223, 105452. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Clavelle, T.; Strauss, C.K.; Hilborn, R.; Melnychuk, M.; Branch, T.A.; Gaines, S.D.; Szuwalski, C.S.; Cabral, R.; et al. Global fishery prospects under contrasting management regimes. Proc. Natl. Acad. Sci. USA 2016, 113, 5125–5129. [Google Scholar] [CrossRef]
- Demirel, N.; Zengin, M.; Ulman, A. First Large-Scale Eastern Mediterranean and Black Sea Stock Assessment Reveals a Dramatic Decline. Front. Mar. Sci. 2020, 7, 103. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and rebuilding of European fisheries. Mar. Policy 2018, 93, 159–170. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, H.; Wang, L.; Lin, L.; He, G.; Fu, P.; Wang, C.; Zhang, Y.; Kang, B. Fishery Status and Rebuilding of Major Economic Fishes in the Largest Freshwater Lake in China Based on Limited Data. Fishes 2022, 7, 47. [Google Scholar] [CrossRef]
- Musinguzi, L.; Bassa, S.; Natugonza, V.; Van Steenberge, M.; Okello, W.; Snoeks, J.; Froese, R. Assessment of exploited fish species in the Lake Edward System, East Africa. J. Appl. Ichthyol. 2021, 37, 216–226. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Liu, Y.; Zhai, L.; Ye, S. Fishery Dynamics, Status, and Rebuilding Based on Catch-Only Data in Coastal Waters of China. Front. Mar. Sci. 2022, 8, 757503. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Xu, Y.; Sun, M.; Chen, Z.; Yuan, M. Application of a catch-based method for stock assessment of three important fisheries in the East China Sea. Acta Oceanol. Sin. 2018, 37, 102–109. [Google Scholar] [CrossRef]
- Alam, M.S.; Liu, Q.; Nabi, R.-U.; Al-Mamun, A. Fish Stock Assessment for Data-Poor Fisheries, with a Case Study of Tropical Hilsa Shad (Tenualosa ilisha) in the Water of Bangladesh. Sustainability 2021, 13, 3604. [Google Scholar] [CrossRef]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Gancitano, V.; Vitale, S.; Fiorentino, F. How Fishery Collapses: The Case of Lepidopus caudatus (Pisces: Trichiuridae) in the Strait of Sicily (Central Mediterranean). Front. Mar. Sci. 2021, 7, 584601. [Google Scholar] [CrossRef]
- Ferragut-Perello, F.; Ramírez-Amaro, S.; Tsikliras, A.C.; Petit-Marty, N.; Dimarchopoulou, D.; Massutí, E.; Serrat, A.; Ordines, F. Exploitation and Conservation Status of the Thornback Ray (Raja clavata) in the Balearic Islands (Western Mediterranean). Fishes 2023, 8, 117. [Google Scholar] [CrossRef]
- Sultana, R.; Liu, Q.; Al-Mamun, A.; Barman, P.P.; Shamsuzzaman, M.; Barua, S. Estimating Stock Status and Biological Reference Points of the Sardine Fishery Using the Surplus Production Model from the Bay of Bengal, Bangladesh. J. Mar. Sci. Eng. 2023, 11, 944. [Google Scholar] [CrossRef]
- Winker, H.; Carvalho, F.; Sharma, R.; Parker, D.; Kerwath, S. Initial Results for North and South Atlantic Shortfin Mako (Isurus oxyrinchus) Stock Assessments Using the Bayesian Surplus Production Model Jabba and The Catch-Resilience Method CMSY. Collect. Vol. Sci. Pap. 2017, 74, 1836–1866. [Google Scholar]
- Liang, C.; Xian, W.; Pauly, D. Assessments of 15 Exploited Fish Stocks in Chinese, South Korean and Japanese Waters Using the CMSY and BSM Methods. Front. Mar. Sci. 2020, 7, 623. [Google Scholar] [CrossRef]
- Zhai, L.; Liang, C.; Pauly, D. Assessments of 16 Exploited Fish Stocks in Chinese Waters Using the CMSY and BSM Methods. Front. Mar. Sci. 2020, 7, 483993. [Google Scholar] [CrossRef]
- Schaefer, M. Some aspects of the dynamics of populations important to the management of the commercial marine fisheries. Bull. Math. Biol. 1991, 53, 253–279. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Department of the Environment, Fisheries and Marine Service: Ottawa, ON, Canada, 1975; ISBN 978-0-662-01440-9. [Google Scholar]
- Muthmainnah, D.; Suryati, N.K.; Fahmi, Z.; Ruchimat, T.; Ame, E.C.; Soe, T.M.; Thruong, G.P. Fish for the People; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2022; pp. 30–34. [Google Scholar]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment; Springer: Boston, MA, USA, 1992; ISBN 978-1-4020-1845-9. [Google Scholar]
- A Hakim, A.; Kamal, M.M.; A Butet, N.; Affandi, R. Determination of fisheries refugia area for freshwater eels (Anguilla spp.) in Palabuhanratu bay, West Java, Indonesia. IOP Conf. Series Earth Environ. Sci. 2020, 404, 012014. [Google Scholar] [CrossRef]
- Wijermans, N.; Schlüter, M. Agent-Based Case Studies for Understanding of Social-Ecological Systems: Cooperation on Irrigation in Bali. In Proceedings of the 9th Conference of the European Social Simulation Association, Warsaw, Poland, 16–20 September 2013; pp. 295–305. [Google Scholar]
- Williams, B.C.; Criddle, K.R.; Kruse, G.H. An agent-based model to optimize transboundary management for the walleye pollock (Gadus chalcogrammus) fishery in the Gulf of Alaska. Nat. Resour. Model. 2021, 34, e12305. [Google Scholar] [CrossRef]
- Duer-Balkind, M. Resilience, Social-Ecological Rules, and Environmental Variability in a Two-Species Artisanal Fishery. Ecol. Soc. 2013, 18, 50. [Google Scholar] [CrossRef]
- Pouso, S.; Borja, Á.; Martín, J.; Uyarra, M.C. The capacity of estuary restoration to enhance ecosystem services: System dynamics modelling to simulate recreational fishing benefits. Estuarine Coast. Shelf Sci. 2018, 217, 226–236. [Google Scholar] [CrossRef]
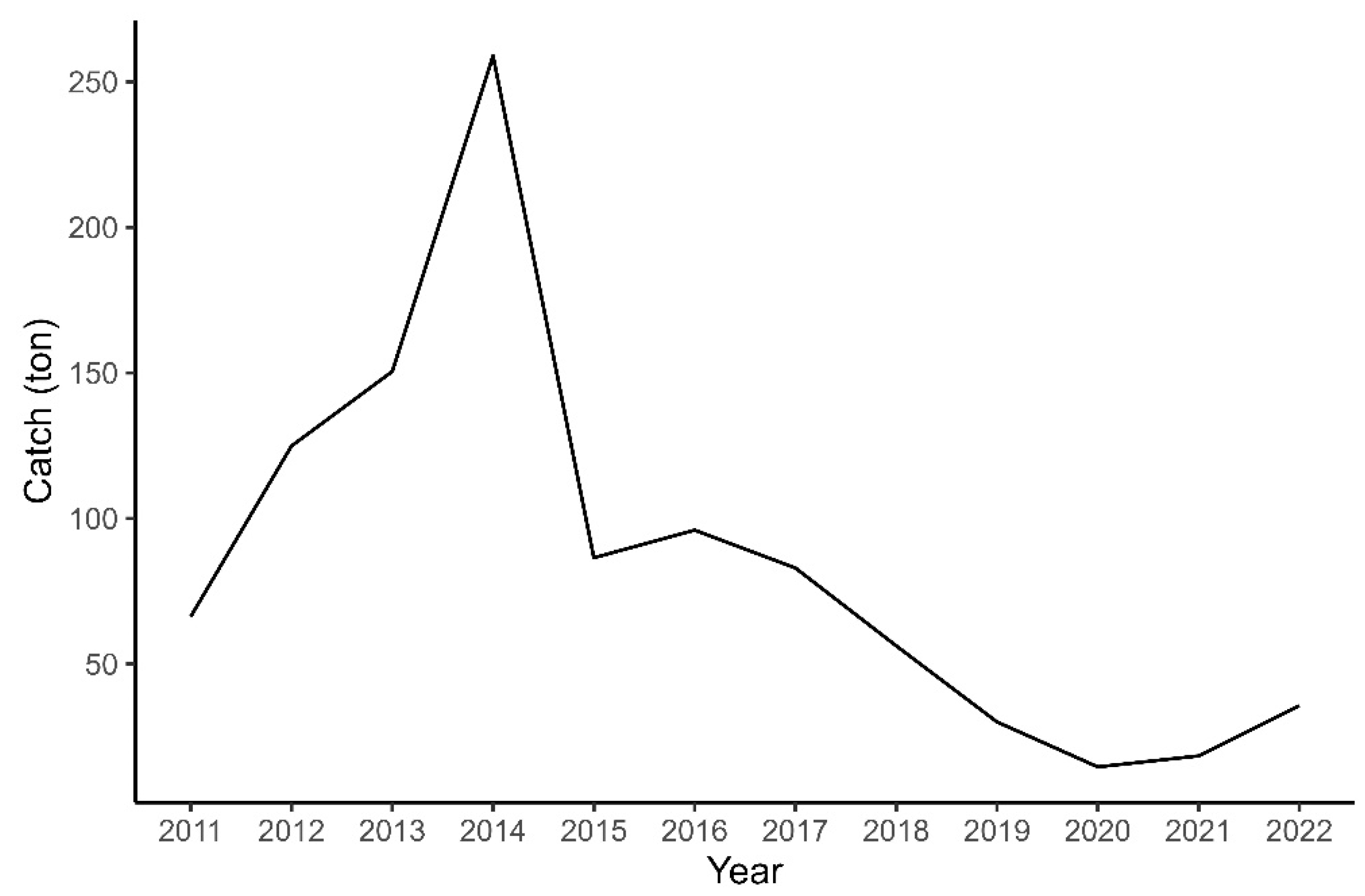
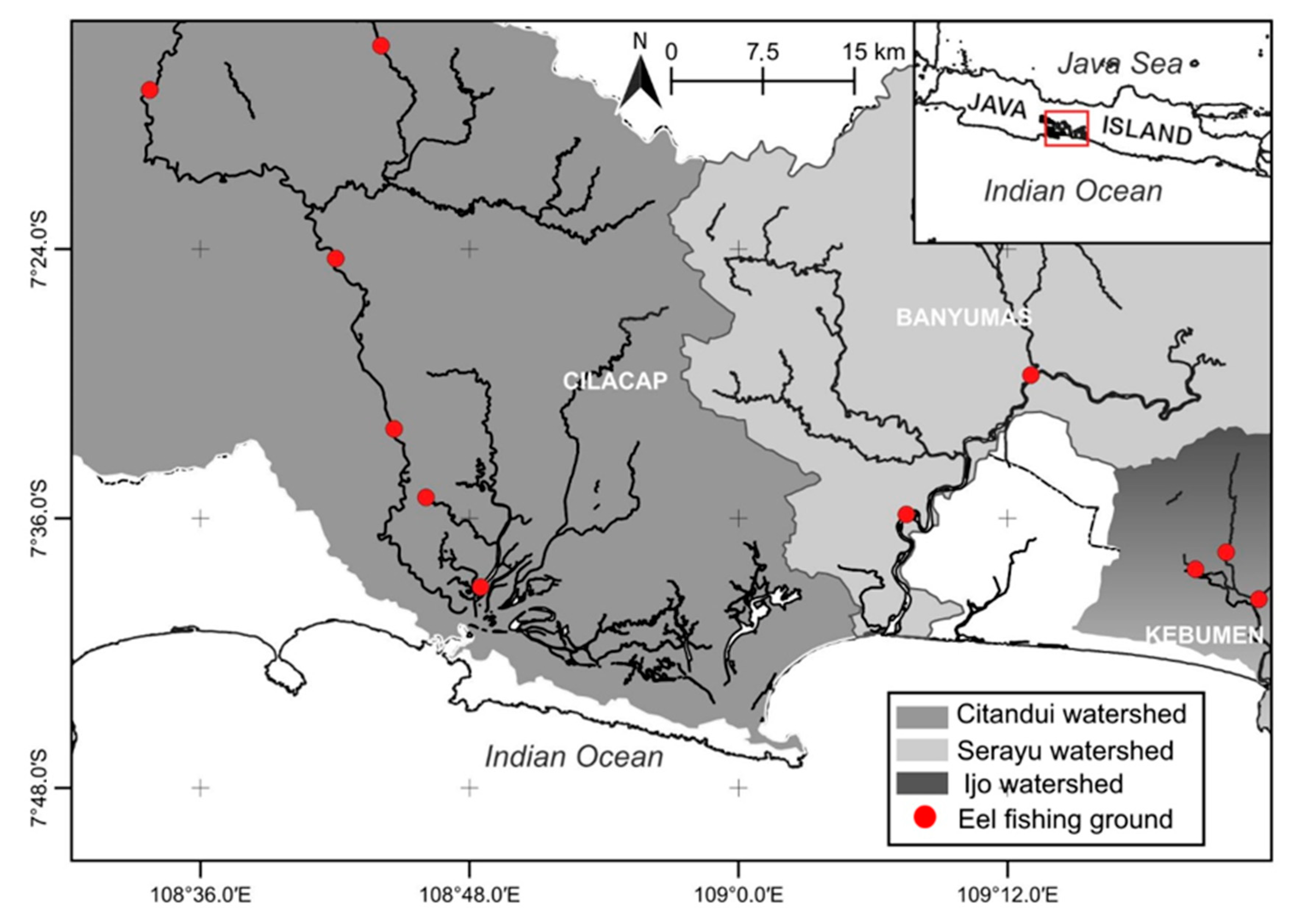
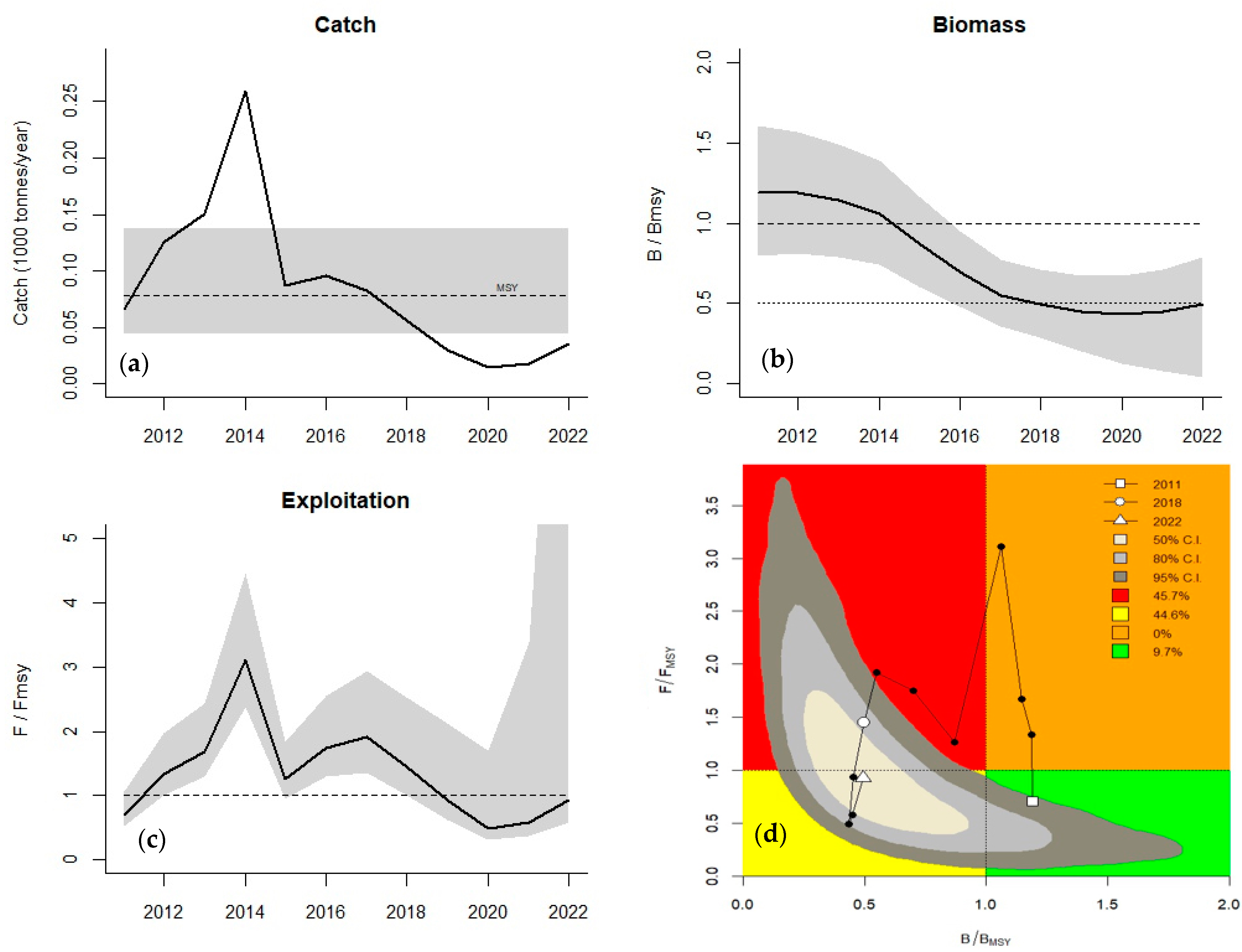

| Resilience | Initial r Range |
|---|---|
| High | 0.6–1.5 |
| Medium | 0.2–0.8 |
| Low | 0.05–0.5 |
| Very low | 0.015–0.1 |
| Strongly Overfished | Overfished | Optimum Fished | Lightly Fished |
|---|---|---|---|
| 0.01–0.2 | 0.01–0.4 | 0.2–0.6 | 0.4–0.8 |
| B/BMSY | F/FMSY | Stock Status |
|---|---|---|
| ≥1 | <1 | Healthy stock |
| 0.5–1.0 | <1 | Recovering stock |
| <0.5 | <1 | Stock beyond safe biological limits |
| 0.5–1.0 | >1 | Overfished stock |
| 0.2–0.5 | >1 | Stock beyond safe biological limits |
| <0.2 | >1 | Severely depleted stock |
| Parameters | Value | 95% CI |
|---|---|---|
| r (1/year) | 0.282 | 0.163–0.487 |
| k (103 MT) | 1.112 | 0.483–2.554 |
| MSY (103 MT) | 0.078 | 0.045–0.137 |
| FMSY (1/year) | 0.141 | 0.082–0.244 |
| BMSY (103 MT) | 0.556 | 0.242–1.277 |
| Parameters | Value | 95% CI |
|---|---|---|
| Biomass in the last year | 0.275 | 0.021–0.438 |
| B/BMSY in the last year | 0.5 | 0.038–0.787 |
| Fishing mortality in the last year | 0.13 | 0.082–1.683 |
| Exploitation F/FMSY | 0.93 | 0.584–12.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugroho, S.; Uehara, T. Navigating Crisis: Insights into the Depletion and Recovery of Central Java’s Freshwater Eel (Anguilla spp.) Stocks. Sustainability 2024, 16, 1578. https://doi.org/10.3390/su16041578
Nugroho S, Uehara T. Navigating Crisis: Insights into the Depletion and Recovery of Central Java’s Freshwater Eel (Anguilla spp.) Stocks. Sustainability. 2024; 16(4):1578. https://doi.org/10.3390/su16041578
Chicago/Turabian StyleNugroho, Supradianto, and Takuro Uehara. 2024. "Navigating Crisis: Insights into the Depletion and Recovery of Central Java’s Freshwater Eel (Anguilla spp.) Stocks" Sustainability 16, no. 4: 1578. https://doi.org/10.3390/su16041578
APA StyleNugroho, S., & Uehara, T. (2024). Navigating Crisis: Insights into the Depletion and Recovery of Central Java’s Freshwater Eel (Anguilla spp.) Stocks. Sustainability, 16(4), 1578. https://doi.org/10.3390/su16041578







