Evaluation of the Residency of Black Rockfish (Sebastes schlegelii) in Artificial Reef Areas Based on Stable Carbon Isotopes
Abstract
1. Introduction
2. Materials and Methods
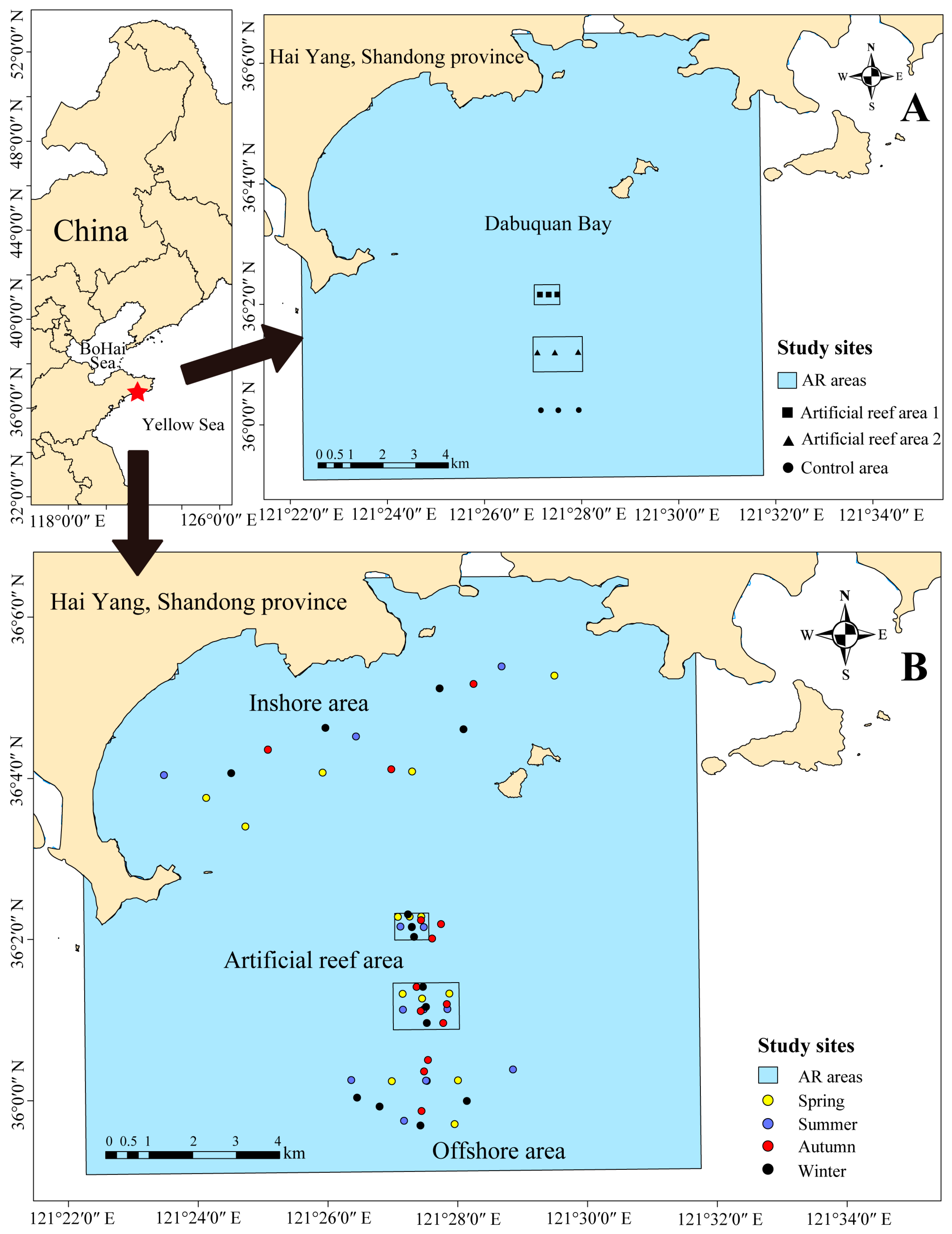
2.1. Study Area
2.2. Sampling Methods
2.3. Construction of the Isoscape
2.4. Stable Isotope Analysis
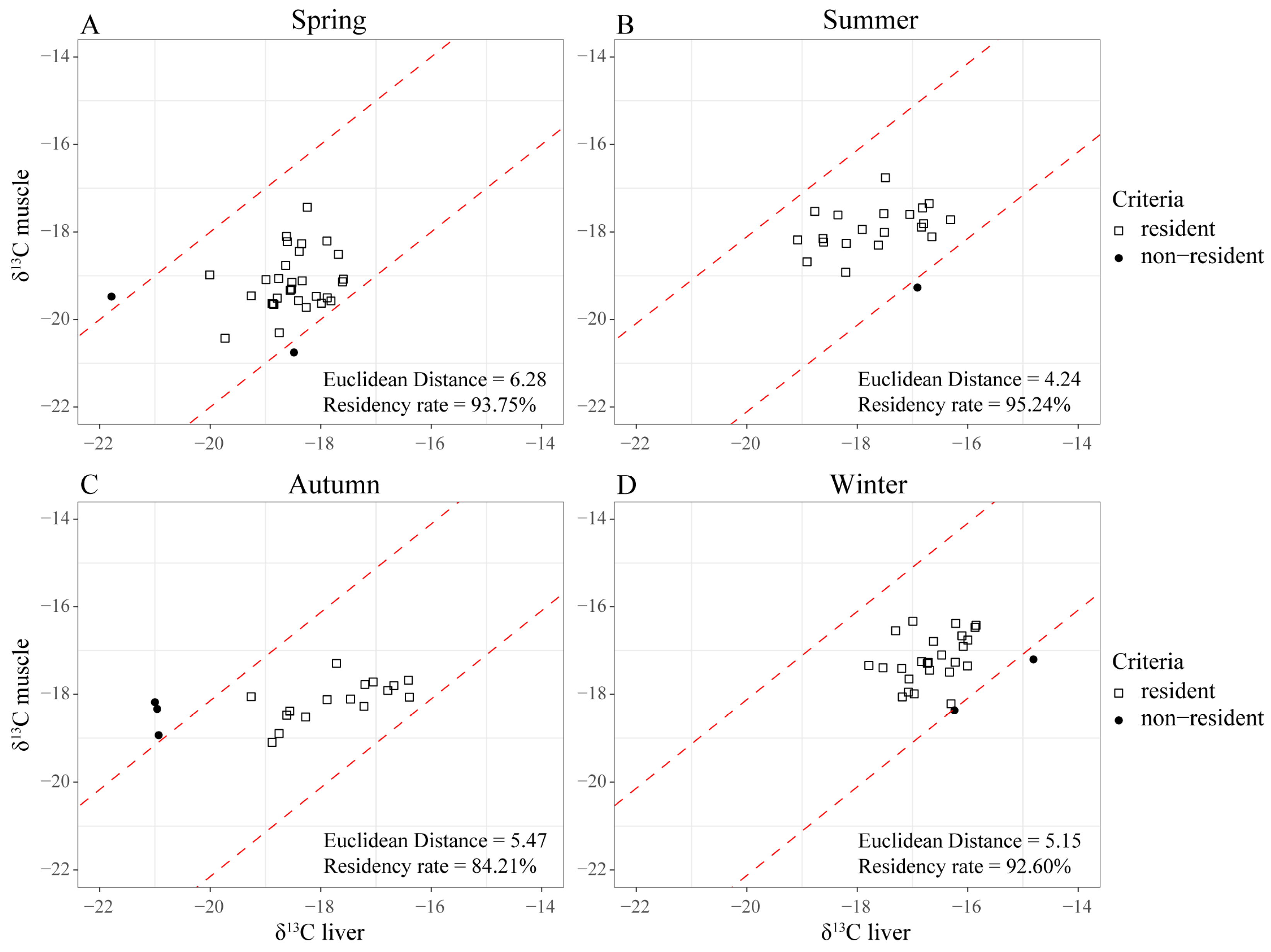
2.5. Determination of Resident Fish
2.6. Sources of Black Rockfish
3. Results
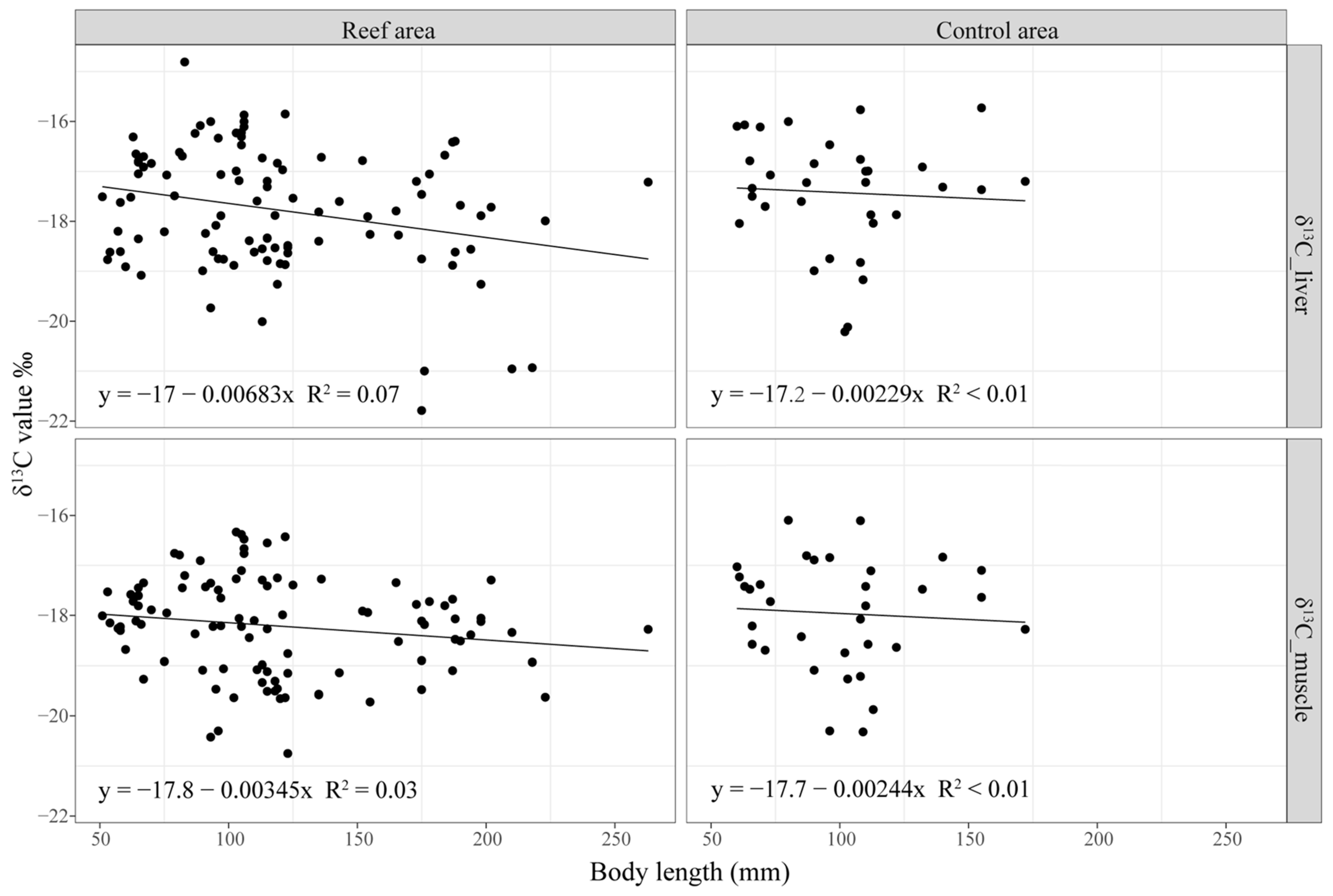
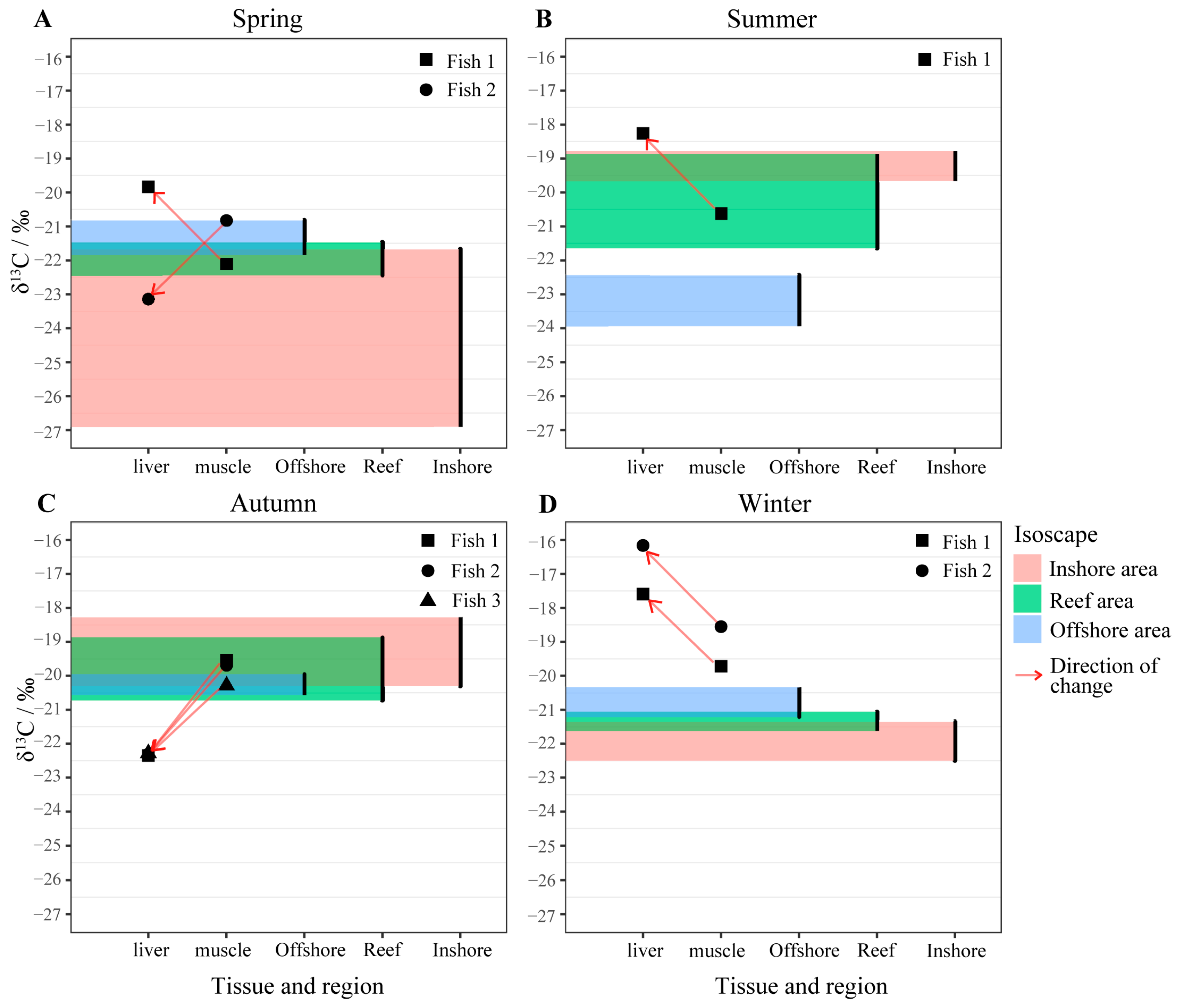
3.1. Residency of Black Rockfish at the Reef Area
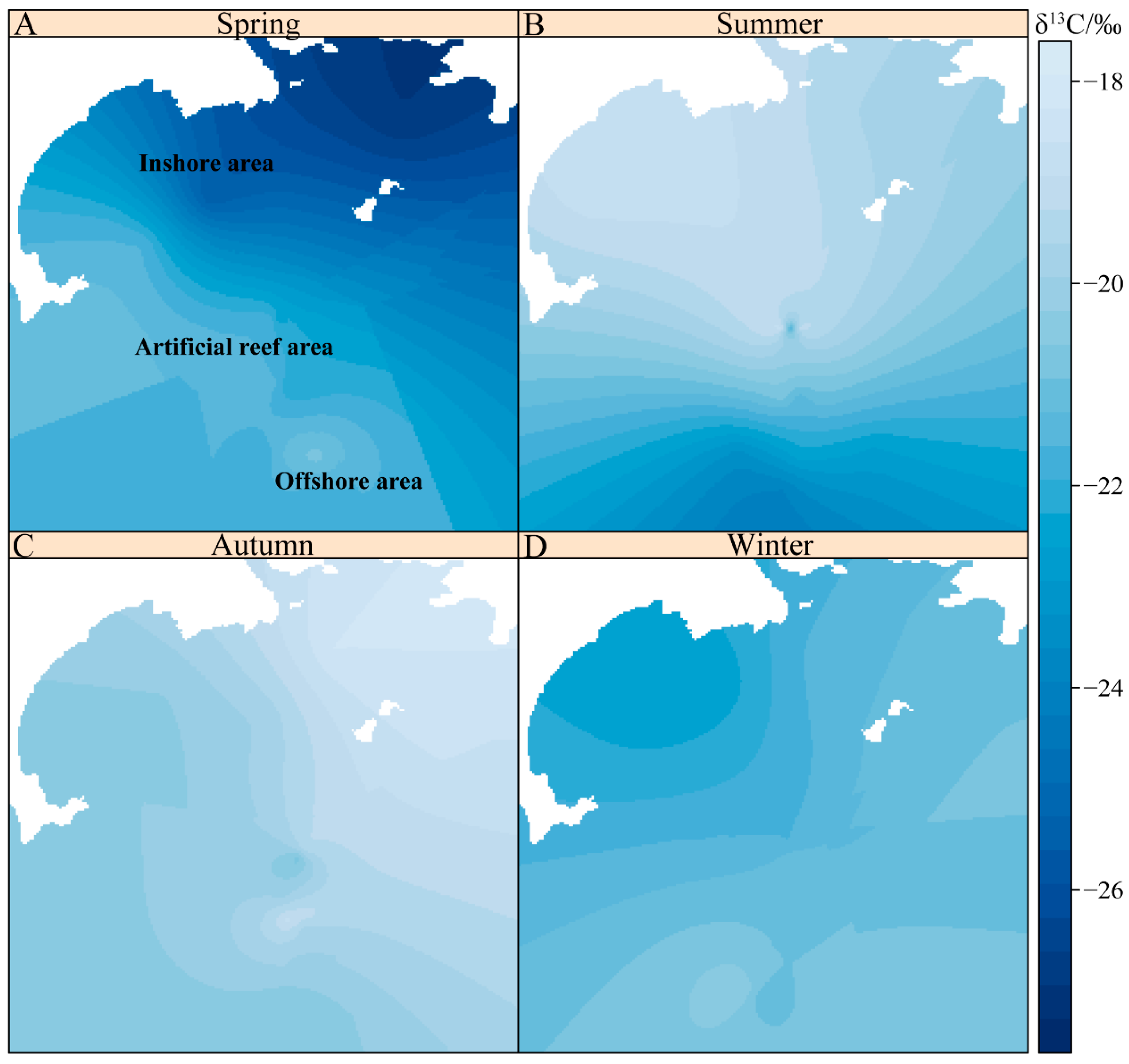
3.2. Spatial Distribution of δ13C Values of Zooplankton
3.3. Traceability of the Non-Resident Black Rockfish
4. Discussion
4.1. Ecological Functions of Reef Area for Black Rockfish
4.2. Potential Sources for Non-Resident Individuals
4.3. Uncertainty
4.4. Implications for Stock Enhancement Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ramm, L.A.W.; Florisson, J.H.; Watts, S.L.; Becker, A.; Tweedley, J.R. Artificial reefs in the Anthropocene: A review of geographical and historical trends in their design, purpose, and monitoring. Bull. Mar. Sci. 2021, 97, 699–728. [Google Scholar] [CrossRef]
- Sean, P.P.; Jonathan, H.G.; Charles, H.P.; William, J.L. Estimating enhancement of fish production by offshore artificial reefs: Uncertainty exhibited by divergent scenarios. Mar. Ecol. Prog. Ser. 2003, 264, 265–277. [Google Scholar]
- Roa-Ureta, R.H.; Santos, M.N.; Leitão, F. Modelling long-term fisheries data to resolve the attraction versus production dilemma of artificial reefs. Ecol. Model. 2019, 407, 108727. [Google Scholar] [CrossRef]
- Bohnsack, J.A.; Sutherland, D.L. Artificial Reef Research: A Review with Recommendations for Future Priorities. Bull. Mar. Sci. 1985, 37, 11–39. [Google Scholar]
- Lindberg, W.J. Can science resolve the attraction-production issue? Fisheries 1997, 22, 10–13. [Google Scholar]
- Smith, J.A.; Lowry, M.B.; Suthers, I.M. Fish attraction to artificial reefs not always harmful: A simulation study. Ecol. Evol. 2015, 5, 4590–4602. [Google Scholar] [CrossRef]
- Bacheler, N.M.; Shertzer, K.W. Catchability of reef fish species in traps is strongly affected by water temperature and substrate. Mar. Ecol. Prog. Ser. 2020, 642, 179–190. [Google Scholar] [CrossRef]
- Cresson, P.; Ruitton, S.; Ourgaud, M.; Harmelin-Vivien, M. Contrasting perception of fish trophic level from stomach content and stable isotope analyses: A Mediterranean artificial reef experience. J. Exp. Mar. Biol. Ecol. 2014, 452, 54–62. [Google Scholar] [CrossRef]
- Davenport, T.M.; Hughes, A.R.; Zu Ermgassen, P.S.E.; Grabowski, J.H. Recruitment enhancement varies by taxonomic group and oyster reef habitat characteristics. Ecol. Appl. 2021, 31, e02340. [Google Scholar] [CrossRef]
- Morais, R.A.; Bellwood, D.R. Principles for estimating fish productivity on coral reefs. Coral Reefs 2020, 39, 1221–1231. [Google Scholar] [CrossRef]
- Smith, J.A.; Lowry, M.B.; Champion, C.; Suthers, I.M. A designed artificial reef is among the most productive marine fish habitats: New metrics to address ‘production versus attraction’. Mar. Biol. 2016, 163, 188. [Google Scholar] [CrossRef]
- Becker, A.; Smith, J.A.; Taylor, M.D.; McLeod, J.; Lowry, M.B. Distribution of pelagic and epi-benthic fish around a multi-module artificial reef-field: Close module spacing supports a connected assemblage. Fish. Res. 2019, 209, 75–85. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, Q.; Alos, J.; Liu, H.; Xu, Q.Z.; Yang, H.S. Short-Term Fidelity, Habitat Use and Vertical Movement Behavior of the Black Rockfish Sebastes schlegelii as Determined by Acoustic Telemetry. PLoS ONE 2015, 10, e0134381. [Google Scholar] [CrossRef]
- Miller, M.W. Using ecological processes to advance artificial reef goals. ICES J. Mar. Sci. 2002, 59, S27–S31. [Google Scholar] [CrossRef]
- Claisse, J.T.; Pondella, D.J.; Love, M.; Zahn, L.A.; Williams, C.M.; Williams, J.P.; Bull, A.S. Oil platforms off California are among the most productive marine fish habitats globally. Proc. Natl. Acad. Sci. USA 2014, 111, 15462–15467. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Ovando, D.; Yang, T.; Dai, F.; Jin, X. Predicting current and future global distribution of black rockfish (Sebastes schlegelii) under changing climate. Ecol. Indic. 2021, 128, 107799. [Google Scholar] [CrossRef]
- Yu, H.; Yang, W.; Liu, C.; Tang, Y.; Song, X.; Fang, G. Relationships Between Community Structure and Environmental Factors in Xixiakou Artificial Reef Area. J. Ocean Univ. China 2020, 19, 883–894. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, H.; Zhang, Q.; Zhang, H.; Zhao, J. Trophic interactions of reef-associated predatory fishes (Hexagrammos otakii and Sebastes schlegelii) in natural and artificial reefs along the coast of North Yellow Sea, China. Sci. Total Environ. 2021, 791, 148250. [Google Scholar] [CrossRef]
- Cybulski, J.D.; Skinner, C.; Wan, Z.; Wong, C.K.M.; Toonen, R.J.; Gaither, M.R.; Soong, K.; Wyatt, A.S.J.; Baker, D.M. Improving stable isotope assessments of inter- and intra-species variation in coral reef fish trophic strategies. Ecol. Evol. 2022, 12, e9221. [Google Scholar] [CrossRef]
- Davis, J.P.; Pitt, K.A.; Fry, B.; Connolly, R.M. Stable isotopes as tracers of residency for fish on inshore coral reefs. Estuar. Coast. Shelf Sci. 2015, 167, 368–376. [Google Scholar] [CrossRef]
- Gelpi, C.G., Jr.; Fry, B.; Condrey, R.E.; Fleeger, J.W.; Dubois, S.F. Using δ13C and δ15N to determine the migratory history of offshore Louisiana blue crab spawning stocks. Mar. Ecol. Prog. Ser. 2013, 494, 205–218. [Google Scholar] [CrossRef]
- Fry, B.; Baltz, D.M.; Benfield, M.C.; Fleeger, J.W.; Gace, A.; Haas, H.L.; Quiñones-Rivera, Z.J. Stable isotope indicators of movement and residency for brown shrimp (Farfantepenaeus aztecus) in coastal Louisiana marshscapes. Estuaries 2003, 26, 82–97. [Google Scholar] [CrossRef]
- Carleton, S.A.; Del Rio, C.M. Growth and catabolism in isotopic incorporation: A new formulation and experimental data. Funct. Ecol. 2010, 24, 805–812. [Google Scholar] [CrossRef]
- Haas, H.L.; Freeman, C.J.; Logan, J.M.; Deegan, L.; Gaines, E.F. Examining mummichog growth and movement: Are some individuals making intra-season migrations to optimize growth? J. Exp. Mar. Biol. Ecol. 2009, 369, 8–16. [Google Scholar] [CrossRef]
- Suresh, B.; Manjappa, S.; Puttaiah, E.T. The contents of zooplankton of the Tungabhadra river, near Harihar, Karnataka and the saprobiological analysis of Water Quality. J. Ecol. Nat. Environ. 2009, 1, 196–200. [Google Scholar]
- Hobson, K.A.; Barnett-Johnson, R.; Cerling, T. Using Isoscapes to Track Animal Migration. In Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping; West, J.B., Bowen, G.J., Dawson, T.E., Tu, K.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 273–298. [Google Scholar]
- Zhao, W. Study on Feeding Ecology and Settlement Rule of Sebastes schlegelii in Artificial Reef Area. Master’s Thesis, Ocean University of China, Qingdao, China, May 2023. [Google Scholar]
- Pinnegar, J.K.; Polunin, N.V.C. Differential fractionation of δ13C and δ15N among fish tissues: Implications for the study of trophic interactions. Funct. Ecol. 1999, 13, 225–231. [Google Scholar] [CrossRef]
- Davis, J.P.; Pitt, K.A.; Fry, B.; Olds, A.D.; Connolly, R.M. Seascape-scale trophic links for fish on inshore coral reefs. Coral Reefs 2014, 33, 897–907. [Google Scholar] [CrossRef]
- Fry, B.; Davis, J. Rescaling stable isotope data for standardized evaluations of food webs and species niches. Mar. Ecol. Prog. Ser. 2015, 528, 7–17. [Google Scholar] [CrossRef][Green Version]
- Andolina, C.; Franzoi, P.; Cavraro, F.; Jackson, A.L.; Mazzola, A.; Vizzini, S. Trophic adaptability shapes isotopic niche of the resident fish Aphanius fasciatus across lagoon habitats. Estuar. Coast. Shelf Sci. 2022, 264, 107685. [Google Scholar] [CrossRef]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Liu, C.; Zhang, W. Improving the accordion-shaped trap selectivity for black rockfish by mounting escape vents: A case study from the small-scale fishery in Shandong, China. Fish. Res. 2019, 219, 105317. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Gong, P.; Guan, C. Effects of the artificial reef and flow field environment on the habitat selection behavior of Sebastes schlegelii juveniles. Appl. Anim. Behav. Sci. 2021, 245, 105492. [Google Scholar] [CrossRef]
- Becker, A.; Lowry, M.B.; Fielder, D.S.; Taylor, M.D. Dispersal of yellowtail kingfish (Seriola lalandi) from a coastal embayment following a recreational fisheries enhancement stocking program: Attempts to integrate aquaculture and habitat-based initiatives. Bull. Mar. Sci. 2021, 97, 615–630. [Google Scholar] [CrossRef]
- Yu, H.; Fang, G.; Rose, K.A.; Lin, J.; Feng, J.; Wang, H.; Cao, Q.; Tang, Y.; Zhang, T. Effects of habitat usage on hypoxia avoidance behavior and exposure in reef-dependent marine coastal species. Front. Mar. Sci. 2023, 10, 1109523. [Google Scholar] [CrossRef]
- Cresson, P.; Le Direach, L.; Rouanet, E.; Goberville, E.; Astruch, P.; Ourgaud, M.; Harmelin-Vivien, M. Functional traits unravel temporal changes in fish biomass production on artificial reefs. Mar. Environ. Res. 2019, 145, 137–146. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, X.; Bi, F.; Zhao, W.; Zhao, L.; Song, H.; Yang, M.; Hu, Z.; Zhou, C.; Shi, P.; et al. Investigating food web structure and system function of an artificial reef ecosystem based on carbon and nitrogen stable isotope analysis: Implications for reef management. Front. Mar. Sci. 2023, 10, 1192173. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bergman, J.N.; Twardek, W.M.; Piczak, M.L.; Casselberry, G.A.; Lutek, K.; Dahlmo, L.S.; Birnie-Gauvin, K.; Griffin, L.P.; Brownscombe, J.W.; et al. The movement ecology of fishes. J. Fish Biol. 2022, 101, 756–779. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Nie, Z.; Wang, L.; Wang, J.; Zhang, J.; Wang, Y.; Guo, Z.; Jiang, Z.; Liang, Z. Evaluation of artificial reef habitats as reconstruction or enhancement tools of benthic fish communities in northern Yellow Sea. Mar. Pollut. Bull. 2022, 182, 113968. [Google Scholar] [CrossRef]
- Fischer, J.L.; Roseman, E.F.; Mayer, C.; Wills, T. If you build it and they come, will they stay? Maturation of constructed fish spawning reefs in the St. Clair-Detroit River System. Ecol. Eng. 2020, 150, 105837. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Griffin, L.P.; Morley, D.; Acosta, A.; Boucek, R.; Adams, A.J.; Danylchuk, A.J.; Cooke, S.J. Spatial-temporal patterns of Permit (Trachinotus falcatus) habitat residency in the Florida Keys, USA. Environ. Biol. Fishes 2023, 106, 419–431. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Li, W.; Zhang, Q.; Liu, B.; Li, M.; Liu, Y.; Tian, T.; Yan, H. Genetic Stock Identification and Adaptability of Hatchery-Reared Black Rockfish, Sebastes schlegelii, Released into the North Yellow Sea waters. Front. Mar. Sci. 2022, 9, 800607. [Google Scholar] [CrossRef]
- Guo, H.; Roques, J.A.C.; Li, M.; Zhang, X. Effects of different feeding regimes on juvenile black rockfish (Sebastes schlegilii) survival, growth, digestive enzyme activity, body composition and feeding costs. Aquac. Res. 2020, 51, 4103–4112. [Google Scholar] [CrossRef]
- Jin, C.; Yan, K.; Wang, M.; Song, W.; Kong, X.; Zhang, Z. Identification, Characterization and Functional Analysis of Fibroblast Growth Factors in Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2023, 24, 3626. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Z.-L.; Liu, Z.-L.; Quan, W.-M.; Zhao, Q.; Zhang, Y.-L.; Liu, H.; Yang, L.-L. Coastal aquaculture farms for the sea cucumber Apostichopus japonicus provide spawning and first-year nursery grounds for wild black rockfish, Sebastes schlegelii: A case study from the Luanhe River estuary, Bohai bay, the Bohai Sea, China. Front. Mar. Sci. 2022, 9, 911399. [Google Scholar] [CrossRef]
- Cusa, M.; St John Glew, K.; Trueman, C.; Mariani, S.; Buckley, L.; Neat, F.; Longo, C. A future for seafood point-of-origin testing using DNA and stable isotope signatures. Rev. Fish Biol. Fish. 2022, 32, 597–621. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Nunn, A.D.; Adams, C.E.; Amundsen, P.-A. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 2019, 94, 539–554. [Google Scholar] [CrossRef]
- Connolly, R.M. Differences in trophodynamics of commercially important fish between artificial waterways and natural coastal wetlands. Estuar. Coast. Shelf Sci. 2003, 58, 929–936. [Google Scholar] [CrossRef]
- MacKenzie, K.M.; Palmer, M.R.; Moore, A.; Ibbotson, A.T.; Beaumont, W.R.C.; Poulter, D.J.S.; Trueman, C.N. Locations of marine animals revealed by carbon isotopes. Sci. Rep. 2011, 1, 21. [Google Scholar] [CrossRef]
- Szpak, P.; Orchard, T.J.; Salomon, A.K.; Gröcke, D.R. Regional ecological variability and impact of the maritime fur trade on nearshore ecosystems in southern Haida Gwaii (British Columbia, Canada): Evidence from stable isotope analysis of rockfish (Sebastes spp.) bone collagen. Archaeol. Anthropol. Sci. 2013, 5, 159–182. [Google Scholar] [CrossRef]
- Olive, P.J.W.; Pinnegar, J.K.; Polunin, N.V.C.; Richards, G.; Welch, R. Isotope trophic-step fractionation: A dynamic equilibrium model. J. Anim. Ecol. 2003, 72, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Sacramento, P.A.; Manetta, G.I.; Benedito, E. Diet-tissue discrimination factors (Δ13C and Δ15N) and turnover rate in somatic tissues of a neotropical detritivorous fish on C3 and C4 diets. J. Fish Biol. 2016, 89, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Canseco, J.A.; Niklitschek, E.J.; Harrod, C. Variability in δ13C and δ15N trophic discrimination factors for teleost fishes: A meta-analysis of temperature and dietary effects. Rev. Fish Biol. Fish. 2022, 32, 313–329. [Google Scholar] [CrossRef]
- Nakagawa, M.; Okouchi, H.; Adachi, J.; Hattori, K.; Yamashita, Y. Effectiveness of stock enhancement of hatchery-released black rockfish Sebastes schlegeli in Yamada Bay—Evaluation by a Fish Market survey. Aquaculture 2007, 263, 295–302. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Wang, Y.; Liu, M.; Song, A.; Liu, H.; You, F. Genetic Assessment of a Black Rockfish, Sebastes schlegelii, Stock Enhancement Program in Lidao Bay, China Based on Mitochondrial and Nuclear DNA Analysis. Front. Mar. Sci. 2020, 7, 94. [Google Scholar] [CrossRef]
- Lee, J.-W.; Min, B.H.; Lee, B.; Kim, K.; Yoon, M. Effects of Stocking Density on Stress, Hematological Responses, and Growth of Black Rockfish Sebastes schlegelii. J. Aquat. Anim. Health 2022, 34, 82–91. [Google Scholar] [CrossRef]
- Olson, A.M.; Trebilco, R.; Salomon, A.K. Expanded consumer niche widths may signal an early response to spatial protection. PLoS ONE 2019, 14, e0223748. [Google Scholar] [CrossRef]
- Bastardie, F.; Angelini, S.; Bolognini, L.; Fuga, F.; Manfredi, C.; Martinelli, M.; Nielsen, J.R.; Santojanni, A.; Scarcella, G.; Grati, F. Spatial planning for fisheries in the Northern Adriatic: Working toward viable and sustainable fishing. Ecosphere 2017, 8, e01696. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Lin, C.; Liu, H.; Chen, M.; Zhang, Y. Structural and Functional Improvements of Coastal Ecosystem Based on Artificial Oyster Reef Construction in the Bohai Sea, China. Front. Mar. Sci. 2022, 9, 829557. [Google Scholar] [CrossRef]
- Gonzalez, E.B.; Umino, T.; Nagasawa, K. Stock enhancement programme for black sea bream, Acanthopagrus schlegelii (Bleeker), in Hiroshima Bay, Japan: A review. Aquac. Res. 2008, 39, 1307–1315. [Google Scholar] [CrossRef]
| Season | Reef Areas | Control Area | ||||
|---|---|---|---|---|---|---|
| δ13Cliver‰ | δ13Cmuscle‰ | Amount | δ13Cliver‰ | δ13Cmuscle‰ | Amount | |
| Spring | −18.60 ± 0.80 C | −19.20 ± 0.70 C | 32 | −19.16 ± 0.77 C | −19.54 ± 0.62 C | 7 |
| Summer | −17.66 ± 0.86 B, a | −17.97 ± 0.56 B, a | 21 | −19.40 ± 0.98 C, b | −19.03 ± 0.49 BC, b | 5 |
| Autumn | −18.21 ± 1.49 BC | −18.19 ± 0.46 B | 19 | −18.02 ± 0.50 B | −18.56 ± 0.59 B | 4 |
| Winter | −16.56 ± 0.63 A | −17.24 ± 0.57 A | 27 | −17.00 ± 0.33 A | −17.50 ± 0.57 A | 9 |
| Season | No. | Body Length (mm) | adj. δ13Cliver‰ | adj. δ13Cmuscle‰ |
|---|---|---|---|---|
| Spring | Fish 1 | 123 | −19.84 | −22.1 |
| Fish 2 | 175 | −23.14 | −20.83 | |
| Summer | Fish 1 | 67 | −18.26 | −20.62 |
| Autumn | Fish 1 | 176 | −22.35 | −19.53 |
| Fish 2 | 210 | −22.31 | −19.69 | |
| Fish 3 | 218 | −22.28 | −20.28 | |
| Winter | Fish 1 | 87 | −17.59 | −19.72 |
| Fish 2 | 83 | −16.16 | −18.55 |
| Season | Areas | ||
|---|---|---|---|
| Inshore Area | Reef Areas | Offshore Area | |
| Spring | −24.23 ± 2.30 B | −22.05 ± 0.37 C | −21.36 ± 0.52 AB |
| Summer | −19.14 ± 0.46 A,a | −20.47 ± 1.18 AB,a | −23.11 ± 0.70 B,b |
| Autumn | −19.47 ± 1.07 AB | −19.66 ± 0.62 AB | −20.17 ± 0.34 A |
| Winter | −21.95 ± 0.61 AB,b | −21.23 ± 0.23 BC,b | −20.77 ± 0.37 AB,a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Feng, J.; Zhao, W.; Zhang, T.; Wang, H.; Ji, Y.; Tang, Y.; Sun, L. Evaluation of the Residency of Black Rockfish (Sebastes schlegelii) in Artificial Reef Areas Based on Stable Carbon Isotopes. Sustainability 2024, 16, 2115. https://doi.org/10.3390/su16052115
Yu H, Feng J, Zhao W, Zhang T, Wang H, Ji Y, Tang Y, Sun L. Evaluation of the Residency of Black Rockfish (Sebastes schlegelii) in Artificial Reef Areas Based on Stable Carbon Isotopes. Sustainability. 2024; 16(5):2115. https://doi.org/10.3390/su16052115
Chicago/Turabian StyleYu, Haolin, Jie Feng, Wei Zhao, Tao Zhang, Haiyan Wang, Yunlong Ji, Yanli Tang, and Liyuan Sun. 2024. "Evaluation of the Residency of Black Rockfish (Sebastes schlegelii) in Artificial Reef Areas Based on Stable Carbon Isotopes" Sustainability 16, no. 5: 2115. https://doi.org/10.3390/su16052115
APA StyleYu, H., Feng, J., Zhao, W., Zhang, T., Wang, H., Ji, Y., Tang, Y., & Sun, L. (2024). Evaluation of the Residency of Black Rockfish (Sebastes schlegelii) in Artificial Reef Areas Based on Stable Carbon Isotopes. Sustainability, 16(5), 2115. https://doi.org/10.3390/su16052115







